Introduction
The prevalence of food allergies have increased in
the last few decades (1), however,
the pathogenesis remains unknown. The 'hygiene hypothesis'
postulated that limited exposure to bacterial and viral pathogens
during early childhood results in an insufficient stimulation of T
helper (Th) 1 cells, which in turn cannot counterbalance the
expansion of Th2 cells, leading to a predisposition towards allergy
(2,3). However, multiple environmental
factors with currently unrecognized interactions contribute to the
atopic status (4). The prevalence
of atopic diseases may be closely associated with increased
environmental pollution and industrialization (5,6). In
addition to classical allergy-triggering factors, toxic
environmental agents are increasingly implicated as causal factors
in allergic diseases (7). The
'hapten-atopy hypothesis' suggested that oral and cutaneous
exposure to environmental chemicals, and in particular to haptens,
may have additionally contribute to the increased prevalence of
atopic disease (8,9).
In contrast with protein antigens, haptens are low
molecular weight (usually <500 Da) chemicals, which are able to
covalently bind to peptides and proteins and thus alter their
immunogenic profiles, with these modifications resulting in atopic
diseases when recognized by the immune system. Allergic reactions
with different Th cell phenotypes may occur when chemical haptens
come into direct contact with the skin, via binding with skin
proteins (10). In addition,
certain drugs, such as haptens, induce adverse reactions including
drug hypersensitivity, with this occurring in approximately 7% of
the population when drugs are absorbed and combined with
self-protein (11,12). Trinitrobenzene sulfonic acid
(TNBS), as a chemical hapten, and is frequently used in the
induction of atopic disease in the laboratory (13), and has been demonstrated to result
in a T cell-mediated immune response in the colonic mucosa in
susceptible mice when administered rectally (14,15).
With the increase of atopic diseases, dietary hapten exposure has
additionally increased via different means, including processed
food, oral antibiotics, formula milk and drug use (8,16).
If haptens do bind to dermal proteins, then it may possible that
these haptens may additionally bind to food antigens (8).
Cluster of differentiation (CD)4+ T cells have a
pivotal role in the initiation of the allergic response by
activating B cells to produce antigen specific IgE. Th2 cells and
associated cytokines, including interleukin (IL)-4, IL-5 and IL-13,
are considered as key factors in the production of IgE antibodies,
which can be crossregulated by Th1 cytokines such as interferon
(IFN)-γ and IL-12 (17). T
regulatory cells (Treg) serve an important role in maintaining oral
immune tolerance and suppressing allergic sensitization to food
allergen (18). As the body's
largest immunologic organ, the gastrointestinal tract receives
multiple daily exposure to a variety of proteins, chemicals and
microorganisms (19). It remains
unknown whether haptens are associated with the pathogenesis of
food allergy, and further studies are required to elucidate the
CD4+ T cell response and the cytokines in the intestine associated
with the hypersensitivity response that is induced by haptens and
food antigens. We have previously established a mouse model of food
allergy induced by TNBS in the presence of a food antigen,
ovalbumin (OVA). The aim of the present study was to further
elucidate the CD4+ T cell response and their cytokine profile in
the intestine. The present study indicated that skewed Th2
polarization and higher IL-4 expression in the intestinal mucosa
were involved in the hapten induced food allergy.
Materials and methods
Reagents
RNeasy Mini kit was obtained from Qiagen, Inc.
(Valencia, CA, USA). IScript™ cDNA Synthesis kit and SYBR Green
Supermix were purchased from Bio-Rad Laboratories, Inc. (Hercules,
CA, USA). X-ray films were obtained from Kodak (Rochester, NY,
USA). Antibodies against IL-4, IFN-γ, forkhead box protein P3
(Foxp3) were from Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
while rat phycoerythrin (PE)-Cy7-conjugated-anti-mouse IL-4, rat
PE-conjugated-anti-mouse IFN-γ, rat allophycocyanin
(APC)-anti-mouse CD4, rat APC-anti-mouse CD11c (cat. no. 17-0114;
used at 1:100 dilution), rat fluorescein isothiocyanate-conjugated
anti-mouse CD86 (cat. no. 11-0862; used at 1:200 dilution), rat
PET-conjugated anti-mouse T-cell immunoglobulin and mucin
domain-containing molecule (TIM4; cat. no. 12-5866; used at 1:100
dilution as well as rat APC anti-mouse tumor necrosis factor ligand
superfamily member 4 (OX40L; cat. no. 17-5905; used at 1:200
dilution) were obtained from eBioscience, Inc. (San Diego, CA,
USA). The remaining reagents in the current study were purchased
from Sigma-Aldrich (St. Louis, MO, USA).
Bone marrow derived dendritic cells
(BmDCs) generation
Two male BALB/c mice (20–24 g), 6–8 weeks old were
provided by Guangdong Medical Laboratory Animal Center (Guangzhou,
China) and housed in animal cages at 25±1°C with 65±5% humidity for
at least one week prior to experiments. The mice were anesthetized
with isoflurane (2%; Sigma-Aldrich) prior to sacrification by
cervical dislocation. Following sterilization with 75% alcohol, the
hind legs were opened and the femurs were separated with tweezers
and scissors in a biosafety cabinet. Bone marrow cells were
obtained by flushing the femurs of BALB/c mice with
phosphate-buffered saline (PBS). The bone marrow cells were
resuspended in lysis buffer for 2–4 min to lyse red blood cells.
The remaining bone marrow cells were washed twice in Roswell Park
Memorial Institute (RPMI) 1640 medium. Bone marrow cells were then
cultured at 1×106cells/well in RPMI 1640 medium with 10
ng/ml of recombinant murine granulocyte-macrophage
colony-stimulating factor (GM-CSF) and IL-4. The culture medium was
refreshed with fresh medium supplemented with GM-CSF and IL-4 on
days 3 and 5. The cells were harvested on day 7. The purity of
CD11c+ DCs was greater than 85%.
Effect of TNBS on the properties of
DCs
BmDCs were cultured in the presence of TNBS (40
ng/ml), OVA (10 ng/ml) or lipo-polysaccharide (LPS, 10 ng/ml) for 3
days. The cells were collected and analyzed by flow cytometry (BD
FACSCanto II; BD Biosciences) for TIM4, CD86 and OX40L
expression.
Mice and sensitization
A total of 42 male BALB/c mice (weight, 20–24 g;
age, 6–8 weeks) were provided by the Guangdong Medical Laboratory
Animal Center (Guangzhou, China) and were randomly divided into
eight groups (16 mice for the establishment of the mouse model as
shown in the schematic in Fig 2;
24 mice in another separate experiment, used for the analysis of
CD4+T-cell responses and the two mice remaining for
isolation of bone marrow-derived dendritic cells as described
above. Animals were fed on an OVA-free diet and kept at 25±1°C with
65±5% humidity under a 12-h light/dark cycle (lights on between
07:30 and 19:30) for at least one week prior to experiments. All
experimental procedures used in the present study were approved by
the Ethics Committee for Animal Experimentation at Shenzhen
Institute of Ear, Nose and Throat (Shenzhen, China). According to a
previous study (20), the
procedures to establish a food allergy model induced by TNBS and
OVA are presented in Fig. 2a. The
mixture of TNBS and OVA was prepared as follows: 1,000 μg
TNBS and 1,000 μg OVA were dissolved in 10 ml sterile saline
at 4°C overnight using a magnetic stirrer. Following
centrifugation, the supernatants were dialyzed to remove the extra
TNBS. BALB/c mice (six mice/group) fed on an OVA-free diet were
randomly divided into four groups: Control group; TNBS+OVAgroup;
OVA group and TNBS group. Mice were sensitized by intraperitoneal
injection (ip) with TNBS (1 mg/mouse), OVA (100 μg/mouse),
or both TNBS and OVA on days 0, 1, 2, 3 and 4. Subsequently, mice
were boosted with OVA (100 μg/mouse) in 0.1 ml of saline
intragastrically on days 9, 11 and 13. Control groups were treated
with normal saline (NS) by ip and then gavage. At 24 h following
the last gavage, mice were sacrificed by cervical dislocation.
Parameters of the intestinal hypersensitivity status were examined
following previously described established protocols (21) which included: Levels of intestinal
OVA-specific IgE antibody, intestinal histamine, numbers of mast
cells, eosinophils and mononuclear cells in the lamina propria.
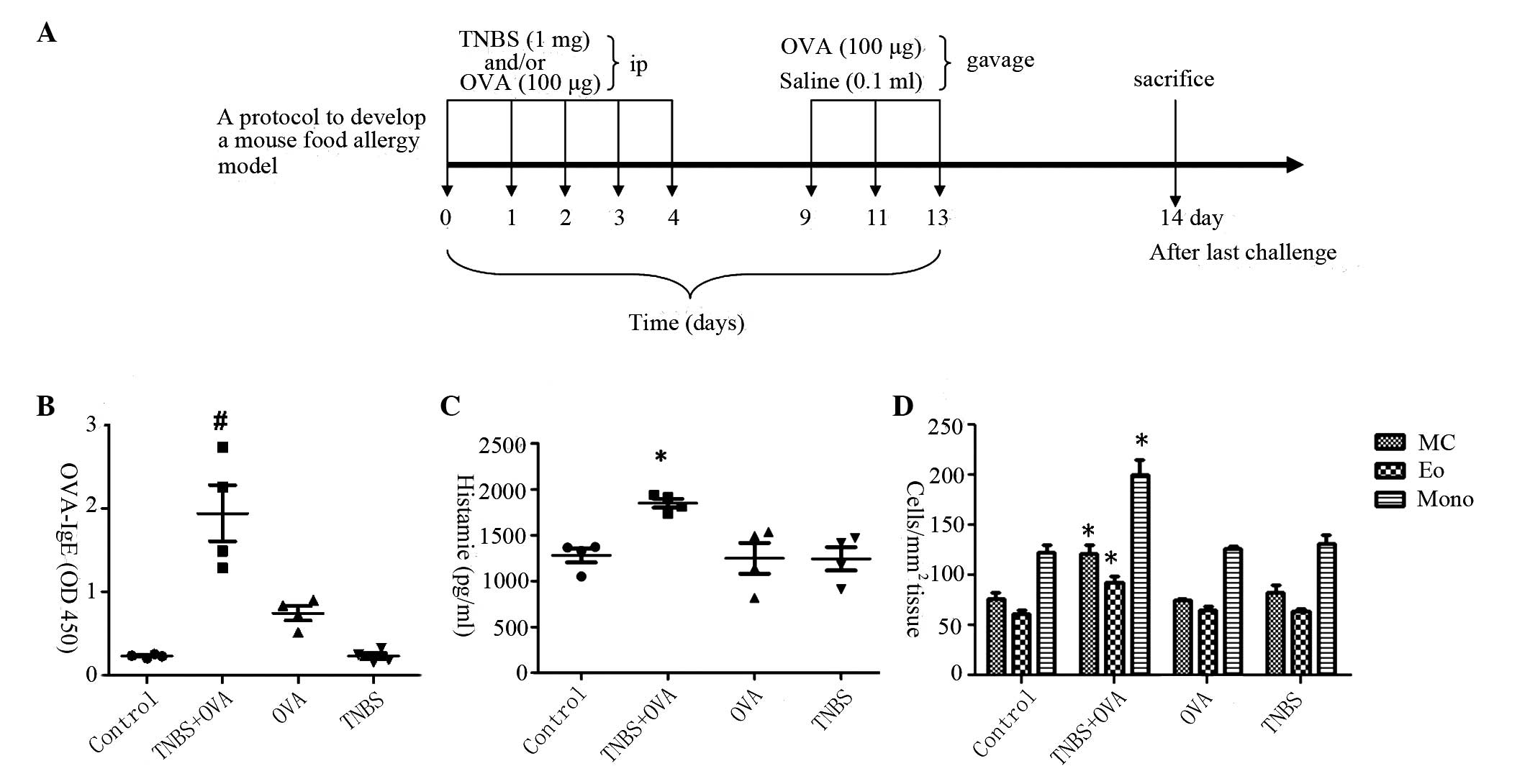 | Figure 2Hypersensitivity response induced in
the small intestine. (A) Protocol to develop food allergy
inflammation in the intestine. BALB/c mice (4 per group) fed on an
OVA-free diet were randomly divided into four groups: Control;
TNBS+OVA; OVA; and TNBS. Mice were sensitized by ip with TNBS (1
mg/mouse), OVA (100 μg/mouse), or both TNBS and OVA on days
0, 1, 2, 3 and 4. Mice were then boosted with OVA (100
μg/mouse) in 0.1 ml of saline intragastrically on days 9, 11
and 13. Control groups were treated with normal saline by ip and
then gavage. At 24 h following the last gavage, mice were
sacrificed by cervical dislocation. (B) Graphs presenting the
levels of OVA-specific IgE in the intestinal tissue as measured by
ELISA. (C) Graphs presenting the intestinal levels of histamine as
determined by ELISA. (D) Numbers of MC, Eo and Mono cells in the
intestinal mucosa. *P<0.05 vs. the control group,
#P<0.01 vs. the OVA group. OVA, ovalbumin; TNBS,
trinitrobenzene sulfonic acid; ip, intraperitoneal injection; IgE,
immunoglobulin E; MC, mast cells; Eo, eosinophils; Mono,
mononuclear cells. |
Assessment of intestinal CD4+ T cell
phenotype and proliferation
Lamina propria mononuclear cells (LPMCs) were
prepared following previously described procedures (10) with minor modifications. Briefly,
jejunal segments were opened and washed with PBS, mucus was removed
by the incubation with predigestion buffer for 20 min under
rotation (40 × g) at 37°C, following which the tissue was cut into
small pieces and incubated with digestion buffer for 20 min under
rotation (40 × g) at 37°C. Following grinding into single cell
suspensions with two sterile glass slides, cells were passed
through a cell-strainer (100 μm). Resulting cells were
centrifuged over a Percoll gradient to enrich for mononuclear
cells. Cells (1×106/well) were cultured in the presence
of OVA, and IL-4 and IFN-γ secreting cells were differentiated by
flow cytometry. A proportion of cells (1×106/well) were
also labeled with carboxyfluoresceinsuccinimidyl ester (CFSE) (10
mmol/l) at 37°C for 10 min. Labeling was stopped with 1 ml of
autologous plasma and excess dye was washed away. CFSE-labeled
cells (1×106/ml) were cultured with OVA (5 μg/ml)
in RPMI 1640 media for 4 days. Following culture, cells were
labeled with rat APC-conjugated anti-mouse CD4 (cat. no. 17-0041,
1:200 dilution; eBioscience, Inc.), rat PE-cy7-conjugated
anti-mouse IL-4 (cat. no. 25-7042-82, 1:200 dilution; eBioscience,
Inc.), rat PE-conjugated anti-mouse IFN-γ (cat. no. 12-7311; 1:200
dilution; eBioscience Inc.) and Rat Alexa Fluor® 647
anti-mouse Foxp3 (cat. no. 560401; 1:200 dilution; BD Biosciences,
Franklin Lakes, NJ, USA), and Th1, Th2 and Treg cellular
proliferation was assessed by flow cytometry (BD FACSCanto II; BD
Biosciences).
Flow cytometry
BmDCs and LPMCs were collected from the culture and
fixed with 1% formaldehyde and 0.1% Triton X-100) and
permeabilization buffer if necessary for 30 min at 4°C, washed with
1% bovine serum albumin (BSA)/PBS 3 times, and blocked for 30 min
at 4°C with 1% BSA. Cells were incubated with the indicated
fluorescein-conjugated antibodies (10 μg/ml; rat
PE-Cy7-conjugated-anti-mouse IL-4, rat PE-conjugated-anti-mouse
IFN-γ, rat APC-anti-mouse CD4, rat APC-anti-mouse CD11c, rat
fluorescein isothiocyanate-conjugated anti-mouse CD86, rat
PET-conjugated anti-mouse TIM4 or rat APC anti-mouse OX40L) for 1 h
at 4°C. Following three washes, cells were resuspended in 400
μl PBS. The mean intensity of fluorescence was determined
for 10,000 cells using a FACScan flow cytometer (BD Biosciences).
An isotype IgG was used as a negative control. All experiments were
performed a minimum of 3 times.
Western blot analysis
The total proteins were extracted from jejunal
segments in protein extraction buffer, which consisted of 20 mmol/l
Tris-Cl buffer (pH 7.5), containing 1 mmol/l
ethylenediaminetetraacetic acid (EDTA), a protease inhibitor
cocktail (complete, Mini, EDTA-free, 1 tablet in 10-ml buffer), 1%
sodium dodecyl sulfate (SDS), 10% Triton X-100 and 2 mol/l
dithiothreitol. Following 30 min on ice, the samples were
centrifuged (17,600 × g, 10 min, 4°C), and protein concentration of
the resulting supernatant was measured using the Bradford method
with BSA as a standard. Sample proteins were denatured in a 250
mmol/l Tris-Cl loading buffer (pH 6.8), containing 100 mmol/l EDTA,
2% SDS, 10% glycerol, 1% β-mercaptoethanol and bromphenol blue,
heated at 100°C for 10 min. Each aliquot was loaded in duplicate
onto a 10% SDS-polyacrylamide gel and proteins were separated by
electrophoresis, prior to transfer to nitrocellulose membranes. The
membranes were blocked with 5% skim milk in Tris-buffered saline
(pH 8) for 1 h at room temperature and then incubated overnight at
4°C with the following primary antibodies: Goat polyclonal
anti-mouse IL-4 (1:200 dilution; cat. no. sc-1260), rat monoclonal
anti-mouse IFN-γ (1:300 dilution; cat. no. sc-69910), or rat
polyclonal anti-mouse Foxp3 (1:200 dilution; cat. no. sc-28705)
(all from Santa Cruz Biotechnology, Inc.). Subsequently, membranes
were incubated with horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin (Ig)G (cat. no. sc-2006) or donkey
anti-goat IgG (cat. no. sc-2020) (both at 1:5,000 dilution and from
Santa Cruz Biotechnology, Inc.) secondary antibodies for 1 h at
room temperature. Following washing, the hybridized bands were
detected using enhanced chemiluminescence detection kits and
Hyperfilm ECL reagents (cat. nos. GERPN2134 and GE28-9068-35,
respectively; Sigma-Aldrich).
Reverse-transcription quantitative
polymerase chain reaction(RT-qPCR)
Jejunal mucosa was removed from intestinal tissue
and total RNA was extracted using an RNeasy mini kit (Qiagen,
Inc.). A total of 1 μg RNA was reverse transcribed into cDNA
using the IScript™ cDNA Synthesis kit (cat no. 170-8891, Bio-Rad
Laboratories, Inc.) according to the manufacturer's recommended
protocol. The resulting complementary DNA was then subjected to
RT-qPCR using the iQ™ SYBR® Green Supermix (cat. no.
1708880; Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. The primers used for the RT-qPCR
amplification of IL-4, IFN-γ are presented in Table I, β-actin was used as an internal
control. RT-qPCR reactions were conducted using a MiniOpticon
thermal cycler (Bio-Rad Laboratories, Inc.) in triplicate. The
amplification protocol was a follows: 1 cycle at 98°C for 1 min
followed by 40 cycles at 98°C for 10 sec, 55°C for 20 sec, 72°C for
30 sec. A standard curve was generated for the determination of the
linear range and amplification efficiency. The relative cytokine
gene expression compared to a house keeping gene was analyzed by
using the comparative quantification cycle method (22) according to the standard curve.
 | Table IOligonucleotide sequences (5′ to 3′)
of the forward and reverse primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Oligonucleotide sequences (5′ to 3′)
of the forward and reverse primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward | Reverse |
|---|
| Interleukin-4 |
CCTCACAGCAACGAGAACA |
ATCGAAAAGCCCGAAAGAGT |
| Interferon-γ |
GGCCATCAGCAACAACATAA |
TGAGCTCATTGAATGCTTGG |
| β-actin |
CTGTCCCTGTATGCCTCTG |
TGATGTCACGCACGATTT |
Statistical analysis
All values were presented as the mean ± standard
deviation of a minimum of three independent experiments. The values
were analyzed by one-way analysis of variance, followed by Tukey's
test for multiple comparisons. SPSS 18.0 (International Business
Machines, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
OX40L and TIM4 expression on DCs was
regulated by TNBS
DCs serve a key role in the initiation and induction
of T cell proliferation and differentiation. Following generation
and cultured with LPS, OVA or TNBS, the CD11c+ BmDCs were gated by
flow cytometry for further analysis. The expression of OX40L and
TIM4 in the BmDCs increased following the addition of TNBS to the
culture compared with the control. However, the CD86 expression was
only slightly affected by the stimulation with TNBS (Fig. 1).
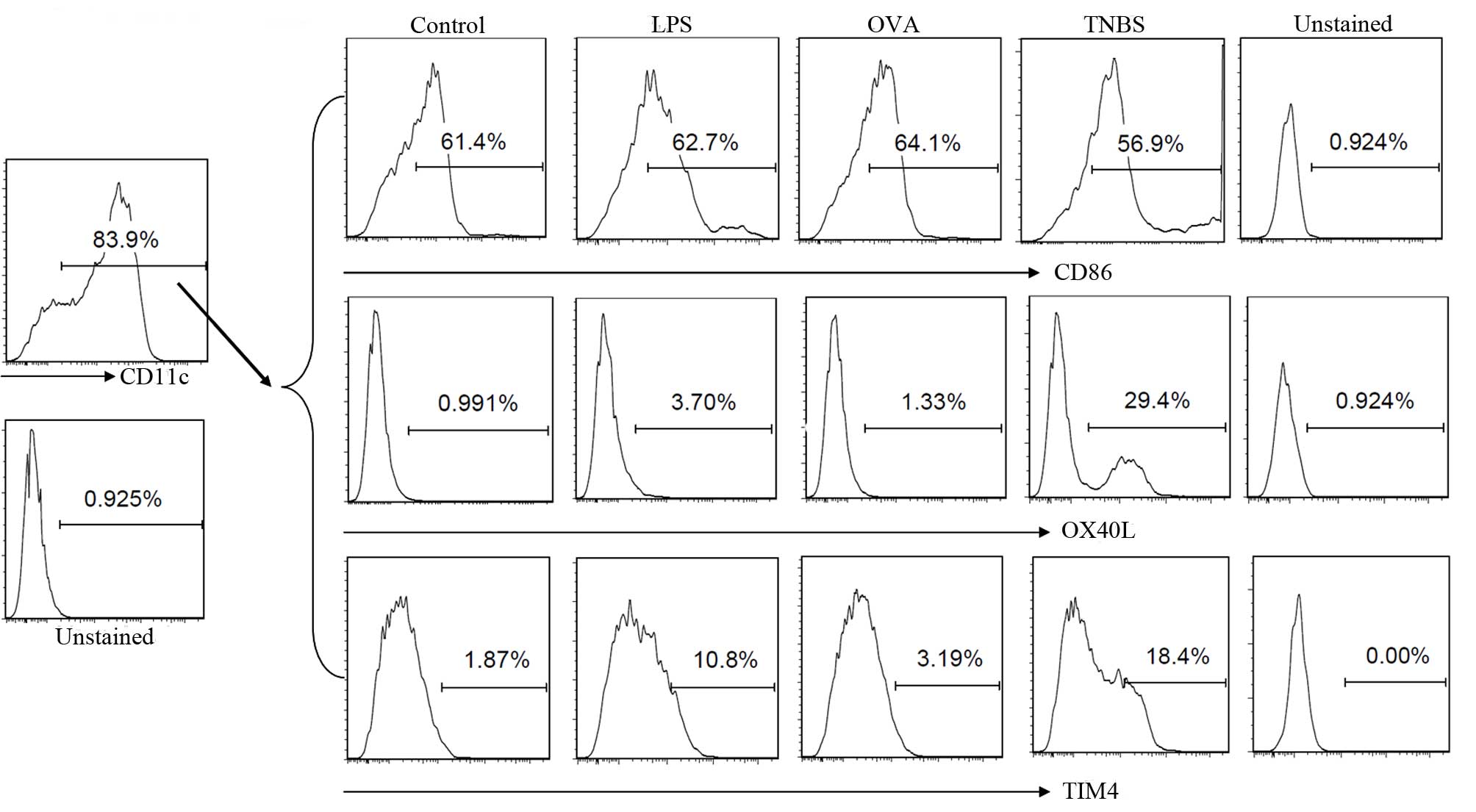 | Figure 1TNBS increased the expression levels
of TIM4 and OX40L in BmDCs. BmDCs were prepared and exposed to
saline (control), LPS, OVA or TNBS in culture for 72 h. Cells were
collected and analyzed by flow cytometry. CD11c+ BmDCs were gated
for further analysis. The histograms indicate the levels of CD86,
OX40L and TIM4 expression in each group. Data are from 3 three
independent experiments. TNBS, trinitrobenzene sulfonic acid; TIM4,
T-cell immunoglobulin and mucin domain-4; OX40L, tumor necrosis
factor ligand superfamily member 4 ; BmDCs, bone marrow derived
dendritic cells; LPS, lipopolysaccharide; OVA, ovalbumin; CD,
cluster of differentiation. |
Skewed Th2 polarization was detected in
the intestinal mucosa sensitized by TNBS and OVA
The skewed Th2 polarization in the intestine is
considered as an important factor in the pathogenesis of food
allergy, however, the CD4+ T cell response in hapten induced food
allergy remains unclear. To elucidate the Th1/Th2 phenotypic
response and the cytokine profile in the intestine following
challenge with TNBS and OVA, a model of food allergy was generated
(Fig. 2A). As presented in
Fig. 2B–D, hypersensitivity status
was induced in the intestinal mucosa, resulting in significant
higher IgE (Fig. 1B), histamine
expression levels (Fig. 2C) and
increased infiltration of mast cells, eosinophils and mononuclear
cells (Fig. 2D) following exposure
to TNBS and OVA simultaneously, while there was no significant
difference compared with control group when challenged with TNBS or
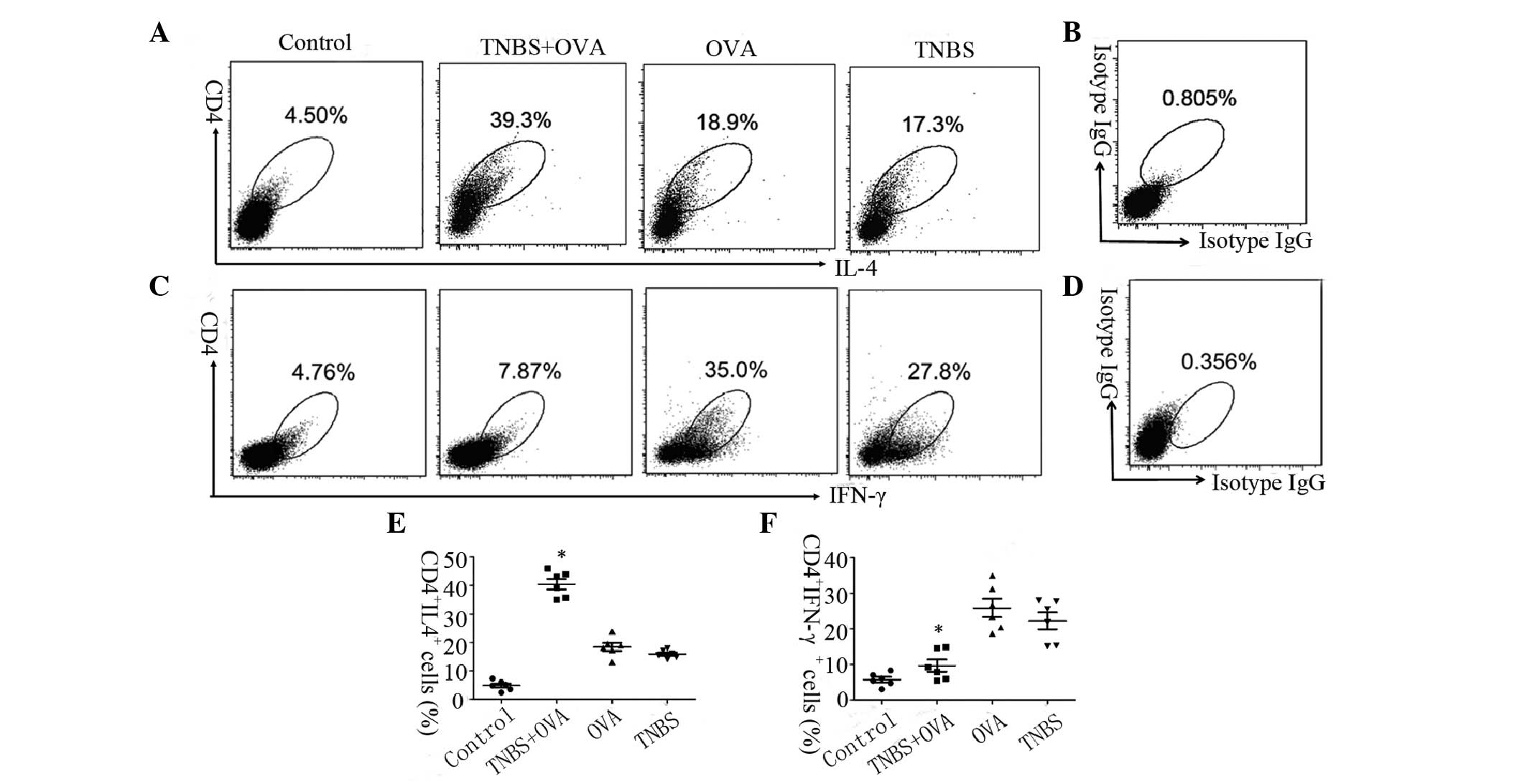
OVA alone. The CD4+ IL-4+ T cells and CD4+ IFN-γ+ T cells in the
LPMCs were detected by flow cytometry (Fig. 3). The frequency of the CD4+ IL-4+ T
cells in mouse LPMCs from the TNBS+OVA group was increased 2-fold
compared with the OVA and TNBS alone groups (Fig. 3A and C). The frequency of the CD4+
IFN-γ+ T cell phenotype in the mouse LPMCs from the TNBS+OVA group
was greater than 3-fold lower compared with the OVA and TNBS alone
groups (Fig. 3B and D). These
results may indicate that there is a Th2 polarization in the
intestine mucosa induced by TNBS combined with OVA.
TNBS combined with OVA promotes OVA
specific Th2 proliferation in the intestine
To further identify the OVA specific Th2
proliferation in the intestinal mucosa, a population of the
isolated LPMCs from each group was stained with CFSE and cultured
for 4 days in the presence of the specific antigen (OVA, 5
μg/ml). Cells were collected following culture and analyzed
by flow cytometry. The histograms show the results of the CFSE
dilution, the gated portion is the proliferated cells (Fig. 4A). CD4+ IL-4+ or CD4+ IFN-γ+ T
cells were gated for further proliferation analysis. The frequency
of OVA specific CD4+ IL-4+ T cell proliferation was significantly
increased in cells from the TNBS+OVA group (66.3%) compared with
the OVA group (17.5%) or the TNBS group (16.8%; Fig. 4B and C), while the frequency of
CD4+ IFN-γ+ T cell proliferation in the TNBS+OVA group showed a
significant reduction (greater than 3-fold) in the LPMCs compared
with the other two groups (Fig. 4B and
D). Together, these results suggest that TNBS combined with OVA
as a specific antigen may facilitate the differentiation of the Th2
phenotype in the intestine, while differentiation of Th1 cells can
be elicited by TNBS or OVA alone.
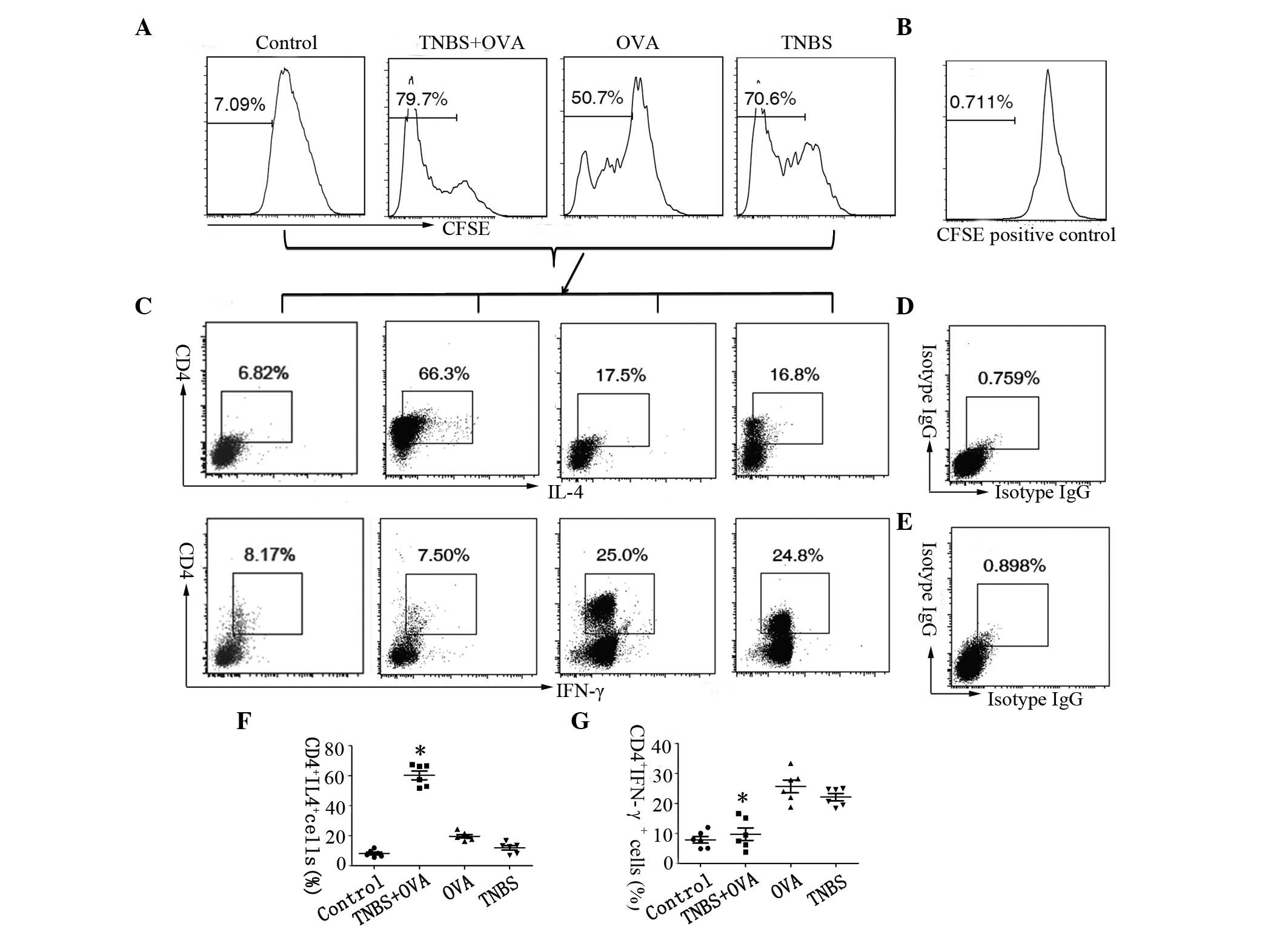 | Figure 4OVA specific Th2 and Th1 proliferation
in the intestine. LPMCs were isolated from the small intestine of
the mice and analysed by flow cytometry. Cells were stained with
CFSE and cultured in the presence of OVA for 4 days. (A) Graphs
showing the proliferation rate of LPMCs, (B) shows the CFSE
positive control. (C) Plots showing the frequency of CD4+ and IL-4+
cells and CD4+ and IFN-γ+ cells in the LPMCs, (D and E) show the
isotype controls. (F) Graph showing CD4+ IL-4+ cells. (G) Graph
showing CD4+ IFN-γ+ cells. The data represent six separate
experiments. *P<0.05 vs. the OVA or TNBS alone group.
OVA, ovalbumin; Th, T helper; LMPCs, lamina propria mononuclear
cells; CFSE, carboxyfluoresceinsuccinimidyl ester; CD, cluster of
differentiation; IL, interleukin; IFN, interferon; TNBS,
trinitrobenzene sulfonic acid; IgG, immunoglobulin G. |
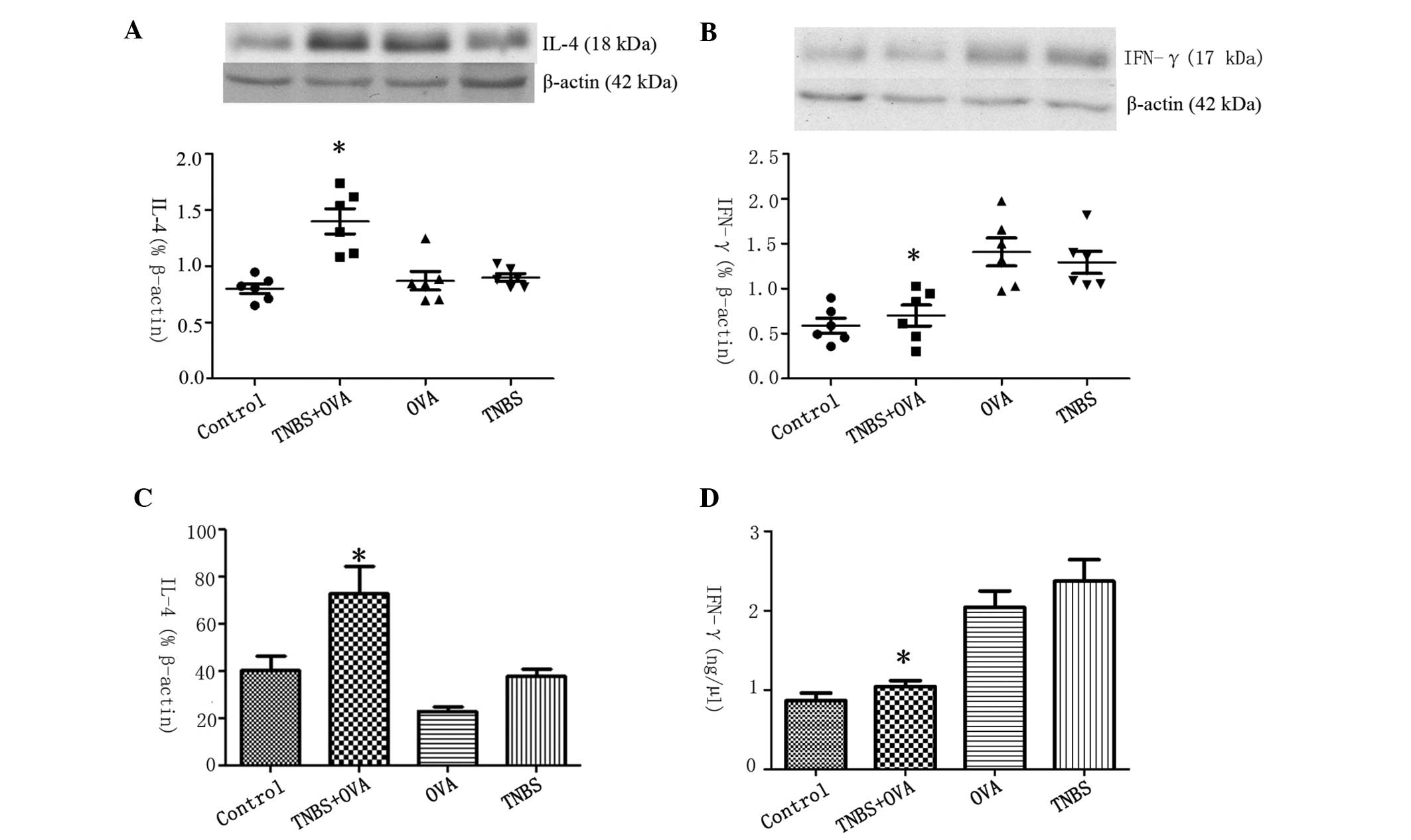
Cytokine profiles were altered in the
intestinal mucosa when challenged with both TNBS and OVA
IL-4 was selected to represent Th2-type cytokines
and IFN-γ to represent Th1 cytokines. To further investigate the
cytokine profile of CD4+ T cells in the intestine, the expression
levels of IL-4 and IFN-γ in the intestinal extracts were measured
by western blotting and RT-qPCR. The results indicated that the
protein expression levels of IL-4 increased significantly in
TNBS+OVA group compared with the OVA, TNBS and control groups
(Fig. 5A). In comparison, the
levels of IFN-γ were significantly lower in the TNBS+OVA group
compared with the other groups (Fig.
5B). There was increased IL-4 mRNA expression and reduced
expression of IFN-γ mRNA in the intestinal mucosa of the mice in
the TNBS+OVA group, with this difference significant (P<0.05)
when compared with the control group (Fig. 5C and D). This may indicate that
there was a skewed Th2 phenotype cytokine response in the intestine
when challenged with TNBS combined with OVA, while this response
was not elicited when challenged with TNBS or OVA alone.
OVA specific Treg proliferation and Foxp3
expression in the intestine
Tregs are a subpopulation of T cells which maintain
oral-tolerance and downregulate the immune system. The Foxp3 gene
is identified as the master transcriptional factor of Tregs, and
serves an important role in the development and function of
regulatory T cells (23).
Therefore, the present study determined the Treg proliferation in
the intestinal mucosa by FACS and measured Foxp3 expression in the
intestinal extracts by western blotting. The histograms show the
CFSE dilution and the proportion of proliferated cells gated
(Fig. 6A). CD4+ Foxp3+ cells were
gated for further proliferation analysis. The rate of proliferation
of OVA specific Tregs was significantly reduced in LPMCs from the
TNBS+OVA group compared with the control, OVA and TNBS alone groups
(Fig. 6B and C). The expression of
Foxp3 in the mouse intestinal tissues from the TNBS+OVA group was
significantly reduced compared with the other groups (Fig. 6D). This may indicate that there is
a functional deficiency in Tregs in the intestine induced by TNBS
and OVA.
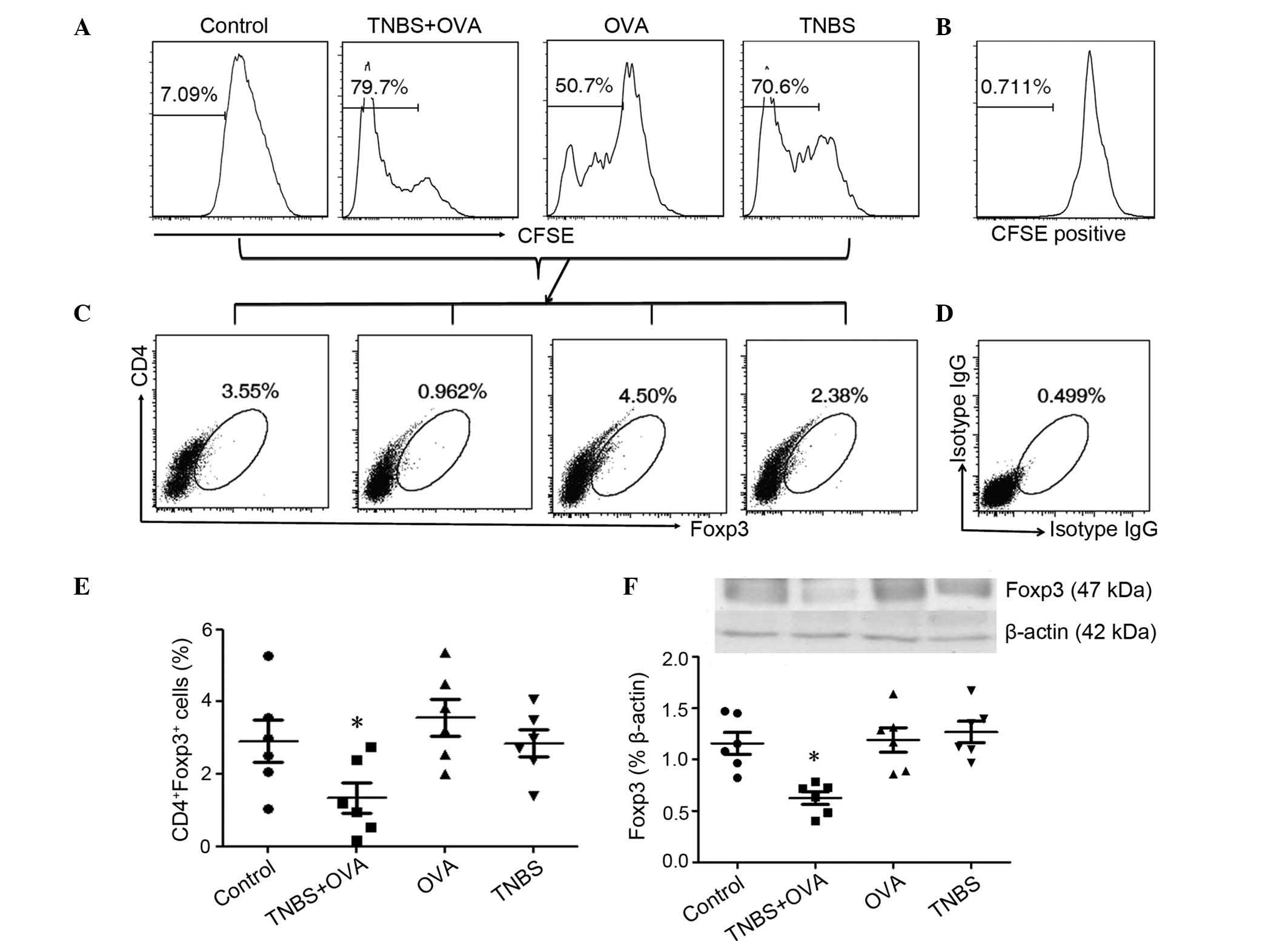 | Figure 6OVA specific Treg proliferation and
Foxp3 expression in the intestine. LPMCs were isolated from the
small intestines of the mice and analysed by flow cytometry. Cells
were stained with CFSE and cultured with OVA for 4 days. (A) The
graphs show the proliferation rate in LPMCs, (B) shows the CFSE
positive control. (C) The plots show the frequency of CD4+ and
Foxp3+ cells in the LPMCs, (D) shows the isotype controls. (E)
Graph showing the CD4+ Foxp3+ cells. (F) Foxp3 expression levels in
the intestinal tissue was measured by western blotting. Values are
presented as the mean ± standard deviation. The data represent six
separate experiments. *P<0.05 vs. the OVA or TNBS
alone group. OVA, ovalbumin; Treg, regulatory T cells; Foxp3,
forkhead box protein P3; LMPCs, lamina propria mononuclear cells;
CFSE, carboxyfluoresceinsuccinimidyl ester; CD, cluster of
differentiation; TNBS, trinitrobenzene sulfonic acid; IgG,
immunoglobulin G. |
Discussion
With the increase in the incidence of atopic
diseases, there has additionally been an increase in dietary hapten
exposure (16). The 'hapten-atopy
hypothesis' suggests that oral and cutaneous exposure to
environmental haptens may contribute to the increase of atopic
disease (24). Further studies are
required to elucidate the role of haptens in the pathogenesis of
food allergy. A previous study indicated that in an established
mouse model of food allergy induced by TNBS, as a typical hapten,
in the presence of a food antigen OVA, there was a hypersensitive
status in the intestine, with higher OVA specific IgE, histamine
expression and increased infiltration of inflammatory cells. In the
present study, the CD4+ T cell response and the cytokine profile in
the intestine were investigated. The results indicated that TNBS is
able to facilitate the expression of TIM4 and OX40L on DCs, with
skewed Th2 polarization and increased IL-4 expression in the
intestinal mucosa observed to be induced by TNBS in the presence of
OVA, with a reduction in Tregs and the expression of Foxp3. This
may indicate that haptens combined with food antigens may
facilitate Th2 phenotypic differentiation in the intestinal mucosa,
which is different from a Th1 response caused by TNBS alone.
Haptens are small molecules that are able to elicit
an immune response only when attached to a large carrier such as a
protein (25,26). Hapten sensitization is common in
2–15% of allergic emergencies that are caused by chemicals
(25). Cutaneous exposure to
haptens may produce immune responses with different Th cell
phenotypes according to different hapten exposure regimen and doses
(27). Allergic contact dermatitis
is generally regarded as a delayed-type hypersensitivity reaction
which is mediated by selective Th1 cells (28). Atopic dermatitis is characterized
by preferential Th2 cell responses with a Th2 cytokine shift
(29). The identity of the T cell
response and cytokine profile following hapten exposure via other
surfaces, such as the intestinal tract or airways, remains unknown.
The results of the present study demonstrate that there was a
skewed Th2 phenotype, with proliferation of OVA-specific Th2 cells
in the intestinal mucosa when concurrently exposed to TNBS and OVA
as a food antigen, while the skewed Th2 response in the intestine
was not elicited following exposure to TNBS or OVA alone. This may
provide evidence for the first time that haptens may also
covalently bind to food antigens and thus alter their immunogenic
profile and serve an important role in initiating a Th2 response in
the intestine. In addition, the mechanism of TNBS-induced Th2
polarization and whether DCs can be activated by TNBS remain
unclear. The present study indicates that TNBS is able to stimulate
DCs to express TIM-4 and OX40L, which are the ligands of TIM1 and
CD134, respectively, on T cells that enable the amplification of
Th2 cell differentiation.
A key feature of food allergy is a Th2-predominant
allergen-specific immune response, with the production of allergen
specific IgE antibodies (30). As
a Th2 cytokine, IL-4 has the effect of regulating B cell growth, T
cell growth and function, and thus is a critical factor for the
development of Th2 type responses (31), while it can be antagonized by the
Th1 type cytokine, IFN-γ. In the present study, the cytokine
expression levels in the intestine were investigated, with the
results indicating increased IL-4 expression and reduced IFN-γ
expression levels following exposure to TNBS and OVA
simultaneously. This may indicate that TNBS can facilitate the
intestinal sensitization to luminal antigens. As with
hapten-induced atopic dermatitis, the results of the current study
show that the exposure of haptens in the presence of food antigens
in the intestinal tract may additionally be characterized as a Th2
response with increased Th2 cytokines. Tregs serve a critical role
in the maintenance of immune homeostasis in the body. Reductions in
Treg numbers and the impairment of Treg function have been noted in
patients with allergic diseases by an unknown mechanism (32). Tregs may prevent immunopathological
reactions and maintain peripheral tolerance to haptens by acting
via a cell-to-cell contact mechanism (33). In the present study, the results
indicated that OVA-specific Treg proliferation was reduced, and, as
a marker of Tregs, Foxp3 expression was reduced in the intestine
following the challenge with TNBS in the presence of OVA compared
with the other groups. This suggests that there is a functional
deficiency in Tregs in the intestinal mucosa when concurrently
exposed to haptens and food antigens.
TNBS-induced colitis is generally regarded as Th1
cell-mediated inflammation (14,15),
while another previous study reported that hapten-induced colitis
may additionally display features of intestinal hypersensitivity
which may be observed during food allergy (34). Consistent with previous studies, a
Th2 cell-mediated hypersensitivity in the intestine was elicited in
the present study following the mice being challenged with TNBS and
OVA via intraperitoneal injection and subsequent treatment with OVA
as a specific food antigen via gavage. These data imply that TNBS
may possess an adjuvant-like effect on the intestinal allergic
reaction to food antigens when treated via intraperitoneal
injection, while it may result in a Th1 response in the intestinal
mucosa by itself. This may be supported by that haptens may act as
immune response-stimulating adjuvants and immune response-steering
adjuvants to alter the type of Th1/Th2 cellular response (35). Further studies are required to
reveal the mechanisms associated with the adjuvant effect.
In summary, the data indicated that a skewed Th2
polarization and higher IL-4 expression with reduced Tregs in the
intestinal mucosa were involved in the TNBS-induced food allergy.
This may provide novel insight into the study of the pathogenesis
of food allergy occurring as a result of environmental haptens.
Acknowledgments
The present study was supported by the Natural
Science Foundation of China (grant nos. 81370494 and 81403160), the
Medical Research Foundation of Guangdong Province (grant no.
A2014515), Shenzhen Health Committee Foundation (grant no.
201401097), the Longgang District Science and Technology Plan
(grant no. YLWS20140609120004346) and the Innovation of Science and
Technology Commission of Shenzhen Municipality (grant nos.
JCYJ20140411150916749 and ZDSYS201506050935272).
References
|
1
|
Lomidze N, Gotua T and Gotua M:
Ige-mediated food allergy-current problems and future perspectives
(review). Georgian Med News. 238:73–78. 2015.
|
|
2
|
Rook GA: Hygiene and other early childhood
influences on the subsequent function of the immune system. Dig
Dis. 29:144–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bloomfield SF, Stanwell-Smith R, Crevel RW
and Pickup J: Too clean, or not too clean: The hygiene hypothesis
and home hygiene. Clin Exp Allergy. 36:402–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiarchiaro J, Schuster RA, Ernecoff NC,
Barnato AE, Arnold RM and White DB: Developing a simulation to
study conflict in intensive care units. Ann Am Thorac Soc.
12:526–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Giampaolo L, Quecchia C, Schiavone C,
et al: Environmental pollution and asthma. Int J Immunopathol
Pharmacol. 24(Suppl 1): S31–S38. 2011.
|
|
6
|
Peden DB and Bush RK: Advances in
environmental and occupational respiratory disease in 2010. J
Allergy Clin Immunol. 127:696–700. 2011. View Article : Google Scholar
|
|
7
|
Ionescu JG: New insights in the
pathogenesis of atopic disease. J Med Life. 2:146–154. 2009.
|
|
8
|
McFadden JP, Dearman RJ, White JM,
Basketter DA and Kimber I: The hapten-atopy hypothesis II: The
'cutaneous hapten paradox'. Clin Exp Allergy. 41:327–337. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McFadden JP, Basketter Da, Dearman RJ,
Puangpet P and Kimber I: The haptenatopy hypothesis III: The
potential role of airborne chemicals. Br J Dermatol. 170:45–51.
2014. View Article : Google Scholar
|
|
10
|
Feng BS, Zheng PY, Chen X, Liao XQ and
Yang PC: Investigation of the role of cholera toxin in assisting
the initiation of the antigen-specific Th2 response. Immunol
Invest. 37:782–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yawalkar N: Drug hypersensitivity. Acta
Clin Belg. 64:529–533. 2009. View Article : Google Scholar
|
|
12
|
Dodiuk-Gad RP, Laws PM and Shear NH:
Epidemiology of severe drug hypersensitivity. Semin Cutan Med Surg.
33:2–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ott H, Baron JM, Heise R, Skazik C and
Merk HF: Tacrolimus modulates dendritic cell activation in the
sensitization phase of allergic contact dermatitis. Skin Pharmacol
Physiol. 23:53–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X and Wang J: Anti-inflammatory
effects of iridoid glycosides fraction of Folium syringae leaves on
TNBS-induced colitis in rats. J Ethnopharmacol. 133:780–787. 2011.
View Article : Google Scholar
|
|
15
|
Monk JM, Turk HF, Fan YY, Callaway E,
Weeks B, Yang P, McMurray DN and Chapkin RS: Antagonizing
arachidonic acid-derived eicosanoids reduces inflammatory Th17 and
Th1 cell-mediated inflammation and colitis severity. Mediators
Inflamm. 2014:9171492014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madsen C: Chemicals in food and allergy:
Fact and fiction. Environ Toxicol Pharmacol. 4:115–120. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McFadden JP, Thyssen JP, Basketter DA,
Puangpet P and Kimber I: T helper cell 2 immune skewing in
pregnancy/early life: Chemical exposure and the development of
atopic disease and allergy. Br J Dermatol. 172:584–591. 2015.
View Article : Google Scholar
|
|
18
|
Berin MC and Sicherer S: Food allergy:
Mechanisms and therapeutics. Curr Opin Immunol. 23:794–800. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chehade M and Mayer L: Oral tolerance and
its relation to food hypersensitivities. J Allergy Clin Immunol.
115:3–12; quiz 13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang PC, Xing Z, Berin CM, Soderholm JD,
Feng BS, Wu L and Yeh C: TIM-4 expressed by mucosal dendritic cells
plays a critical role in food antigen-specific Th2 differentiation
and intestinal allergy. Gastroenterology. 133:1522–1533. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang PC, Jury J, Söderholm JD, Sherman PM,
McKay DM and Perdue MH: Chronic psychological stress in rats
induces intestinal sensitization to luminal antigens. Am J Pathol.
168:104–114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Haque R, Lei F, Xiong X and Song J: The
regulation of FoxP3-expressing regulatory T cells. Endocr Metab
Immune Disord Drug Targets. 11:334–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McFadden JP, White JM, Basketter DA and
Kimber I: Does hapten exposure predispose to atopic disease? The
hapten-atopy hypothesis. Trends Immunol. 30:67–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fodey TL, Greer NM and Crooks SR: Antibody
production: Low dose immunogen vs. low incorporation hapten using
salmeterol as a model. Anal Chim Acta. 637:328–332. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gordon BR: Approaches to testing for food
and chemical sensitivities. Otolaryngol Clin North Am. 36:917–940.
2003. View Article : Google Scholar
|
|
27
|
Man MQ, Hatano Y, Lee SH, Man M, Chang S,
Feingold KR, Leung DY, Holleran W, Uchida Y and Elias PM:
Characterization of a hapten-induced, murine model with multiple
features of atopic dermatitis: Structural, immunologic and
biochemical changes following single versus multiple oxazolone
challenges. J Invest Dermatol. 128:79–86. 2008. View Article : Google Scholar
|
|
28
|
Tončić RJ, Lipozenčić J, Martinac I and
Gregurić S: Immunology of allergic contact dermatitis. Acta
Dermatovenerol Croat. 19:51–68. 2011.
|
|
29
|
Caubet JC and Eigenmann PA: Allergic
triggers in atopic dermatitis. Immunol Allergy Clin North Am.
30:289–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vickery BP, Chin S and Burks AW:
Pathophysiology of food allergy. Pediatr Clin North Am. 58:363–376.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cardoso CR, Provinciatto PR, Godoi DF,
Ferreira BR, Teixeira G, Rossi MA, Cunha FQ and Silva JS: IL-4
regulates susceptibility to intestinal inflammation in murine food
allergy. Am J Physiol Gastrointest Liver Physiol. 296:G593–G600.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nouri-Aria KT and Durham SR: Regulatory T
cells and allergic disease. Inflamm Allergy Drug Targets.
7:237–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zielinski CE, Zuberbier T and Maurer M:
Immunoregulation in cutaneous allergy: Prevention and control. Curr
Opin Allergy Clin Immunol. 12:498–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bailón E, Cueto-Sola M, Utrilla P, Nieto
A, Garrido-Mesa N, Celada A, Zarzuelo A, Xaus J, Gálvez J and
Comalada M: DNFB-DNS hapten-induced colitis in mice should not be
considered a model of inflammatory bowel disease. Inflamm Bowel
Dis. 17:2087–2101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoo Y and Perzanowski MS: Allergic
sensitization and the environment: Latest update. Curr Allergy
Asthma Rep. 14:4652014. View Article : Google Scholar : PubMed/NCBI
|