Introduction
Diabetic nephropathy (DN) is a major complication of
diabetes and a frequent cause of end-stage renal disease (1). The predominant pathological
alterations associated with DN include mesangial expansion,
podocyte loss, increased thickness of the basement membrane, and
glomerular and tubular cell injury, resulting in glomerulosclerosis
and interstitial fibrosis (2,3).
Hyperglycemia is key in renal cell injury and extracellular matrix
overproduction in DN (4,5).
Podocytes are terminally differentiated cells
present on the outer surface of the glomerular basement membrane,
which maintain the structure and function of the glomerular
filtration barrier. Previous studies have demonstrated that
podocyte injury is involved in the generation and progression of DN
(6–8). Furthermore, a decrease in the number
of podocytes in the glomeruli is the strongest predictor of DN
progression (7,8). Previous studies have indicated that
apoptosis contributes to a reduction in podocytes, and high glucose
(HG) induces podocyte apoptosis (9,10).
Transient receptor potential channel 6 (TRPC6) is a
member of the large TRP superfamily of nonselective cation
channels. This superfamily comprises a group of six trans-membrane
domain-containing ion channels (11–13).
It has been demonstrated that mutations in the TRPC6 channel result
in familial focal segmental glomerulosclerosis (14). In addition, TRPC6 is involved in
the physiological and pathophysiological roles of podocytes
(15,16), including HG-induced podocyte
apoptosis (17–19).
The downstream signaling of TRPC6 includes the
activation of two calcium-dependent transcription factors, the
nuclear factor of activated T cells (NFAT) and cAMP response
element binding protein (20–23).
NFAT is the substrate for calcineurin (CaN) and belongs to the
family of Ca2+-dependent transcription factors (24). In inactivated cells, NFAT
transcription factors are highly phosphorylated and are located in
the cytoplasm. NFAT is translocated to the nucleus upon
dephosphorylation where it stimulates gene transcription. Previous
studies have demonstrated that NFAT activation is associated with
podocyte injury and glomerulosclerosis (25,26).
Excessive activation of the CaN/NFAT signaling pathway has been
identified as a potential mechanism underlying TRPC6-induced renal
disease, including podocyte injury (27,28).
Astragalus membranaceus is a widely used
traditional Chinese medicine, particularly in renal diseases. Its
extracts are typically classified as saponins, polysaccharides or
flavonoids (29). Astragaloside IV
(AS-IV) is an active ingredient of Astragalus, which exerts
numerous effects, including antihypertensive, positive inotropic,
anti-inflammatory and immunoregulatory activities (30,31).
Previous studies have demonstrated that AS-IV prevents podocyte
apoptosis and ameliorates renal injury in DN (32–34).
However, few studies have determined an association between AS-IV
and ion channels. The present study aimed to investigate whether
AS-IV prevents HG-induced podocyte apoptosis via TRPC6, thus
providing a possible novel therapeutic strategy for the clinical
treatment of DN.
Materials and methods
Reagents
Polyclonal rabbit anti-TRPC6 antibody (cat. no.
PAB13220) was purchased from Abnova (Taiwan) Corporation (Taipei,
Taiwan), the polyclonal rabbit anti-B-cell lymphoma 2-associated X
protein (Bax; cat. no. 2772) and monoclonal rabbit glyceraldehyde
3-phosphate dehydrogenase (GAPDH; cat. no. 2118) antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA),
the polyclonal rabbit anti-NFAT2 antibody (cat. no. ab25916) and
polyclonal rabbit anti-histone H3 (cat. no. ab18521) were obtained
from Abcam (Cambridge, MA, USA), and the horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody
(cat. no. 7074) was purchased from Cell Signaling Technology, Inc.
A nuclear and cytoplasmic extraction kit was purchased from Cayman
Chemical Company (Ann Arbor, MI, USA), and a bicinchoninic acid
(BCA) protein assay kit was from Pierce Biotechnology, Inc.
(Rockford, IL, USA). Primers were synthesized by Sangon Biotech
Co., Ltd. (Shanghai, China). RPMI-1640 medium and fetal bovine
serum (FBS) were obtained from Hyclone (GE Healthcare Life
Sciences, Logan, UT, USA), and interferon-γ was obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and treatment
Conditionally immortalized mouse podocytes were
verified by Professor Mundel (Division of Nephrology, Massachusetts
General Hospital, Harvard Medical School) and donated by Professor
Hao Chuanming (Huashan Hospital, Fudan University, Shanghai,
China). The cells were inoculated into flasks coated with collagen
type I (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), and were cultured at 33°C in RPMI-1640 medium supplemented
with 10 U/ml recombinant mouse interferon-γ and 10% FBS. Once the
cells reached 85% confluence they were digested and passaged using
0.05% trypsin-EDTA. To induce differentiation, the podocytes were
cultured at 37°C in the same medium deprived of interferon-γ for 14
days. Differentiated podocytes were cultured for 24 h in RPMI-1640
medium containing 1% FBS prior to exposure to the various
experimental conditions. AS-IV (Shanghai Tauto Biotech Co., Ltd.,
Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO) to
produce a stock solution; the final DMSO concentration did not
exceed 0.1% (v/v). The cells were divided into the following
groups: i) Normal glucose (NG) control group, in which the cells
were incubated in RPMI-1640 containing 5 mM glucose; ii) mannitol
(MA) group, in which the cells were incubated in NG medium
supplemented with 25 mM D-mannitol as an osmotic control; iii) HG
group, in which the cells were incubated in RPMI-1640 containing 30
mM glucose; and iv) AS-IV + HG group, in which the cells were
pre-incubated with AS-IV (10, 20 or 40 µM) for 1 h and were
then incubated in HG medium for 24 h. All the glucose used in the
present study was D-glucose and each reaction was repeated in
triplicate.
3-[4,5]-Dimethylthiazol-2,5-diphenyltetrazolium bromide (MTT)
assay
Podocyte viability was assessed by MTT assay. Cells
were plated in a 96-well plate, 24 h after stimulation 20 µl
MTT was added (5 mg/ml; Sigma-Aldrich), the cells were cultured for
4 h and the supernatant was carefully discarded. DMSO (50
µl) was added to dissolve the crystals and the absorbance
was measured at a wavelength of 570 nm using a SpectraMax 190
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). The
cell activity was calculated as follows: Inhibition rate = (1 -
treated group/control group) x 100%.
Apoptosis assay
The suspended cells were collected and centrifuged
for 5 min. The adherent cells were digested with EDTA-free trypsin
and centrifuged at 800 x g for 10 min. The cells were washed with
cold phosphate-buffered saline (PBS), resuspended, and centrifuged
at 800 x g for a further 10 min. Binding buffer (1X; 300 µl)
was added and the cells were resuspended. Subsequently, 5 µl
Annexin V-fluorescein isothiocyanate (FITC; BioVision, Mountain
View, CA, USA) was added to the cells, and the cells were agitated
and incubated at room temperature in the dark for 15 min. Propidium
iodide (PI; 5 µl; Sigma-Aldrich) was added for staining and
200 µl 1X binding buffer was added. The stained cells were
analyzed for apoptosis using fluorescence-activated cell sorting
with a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). Cells positive for Annexin V-FITC and negative for PI
were considered to be apoptotic.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the treated cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Reverse transcription was
performed at 37°C for 15 min, then 85°C for 5 sec using a One Step
PrimeScript® cDNA Synthesis kit (Clontech; Takara
Biotechnology Co., Ltd., Dalian, China) with 1 µl RNA per
reaction, and with a total reaction volume of 20 µl. To
determine the quantity of mRNA, the cDNA was amplified by qPCR with
SYBR® Premix Ex Taq™ II (Takara Biotechnology Co., Ltd.)
using an iCycler® thermal cycler (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The qPCR reaction consisted of 12.5
µl SYBR® Premix Ex Taq™ II, 1 µl each of
forward and reverse primers, 2 µl cDNA, and made up to 25
µl with distilled water. The PCR primer sequences were as
follows: TRPC6, sense 5′-TGT ACG GAT TGT GGA GGCT-3′, antisense
5′-GAT TGG GGT CAC ATC GTG-3′; Bax, sense 5′-GCA AAC TGG TGC TCA
AGGC-3′, antisense 5′-GGT CCC GAA GTA GGA AAGG-3′; NFAT2, sense
5′-GCC CAG CGA TGA GTA TGAA-3′, antisense 5′-ATG CAC CAG CAC AGA
ACG-3′; and GAPDH, sense 5′-ACC ACA GTC CAT GCC ATCAC-3′ and
antisense 5′-TCC ACC ACC CTG TTG CTGTA-3′. The housekeeping gene
GAPDH served as the internal control for mRNA expression levels.
PCR cycling conditions consisted of an initial denaturation (95°C,
30 sec), followed by 40 cycles of incubation at 95°C for 5 sec,
then at 60°C for 30 sec. The relative expression levels of each
group were measured using the 2−ΔΔCq method (35).
Protein extraction and western blot
analysis
Podocytes subjected to the different experimental
conditions were lysed with radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China). The
samples were centrifuged, and the supernatants were collected as
the total cell extracts. Nuclear and cytoplasmic proteins from the
cells were extracted using the nuclear and cytoplasmic protein
extraction kit, according to the manufacturer's protocol. Protein
concentration of the samples were then determined by BCA assay, and
50 µg total protein was used for gel electrophoresis.
Proteins were separated by 10% sodium dodecyl
sulfate-poly-acrylamide gel electrophoresis. Proteins were
transferred to a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA), blocked in 5% bovine serum albumin at 37°C for
2 h and were subjected to immunoblot analysis with antibodies
against TRPC6, NFAT2, Bax, histone H3 and GAPDH overnight at 4°C.
All antibodies were used at a working concentration of 1 mg/ml
(1:1,000) in PBS containing 5% nonfat milk powder. The blots were
then incubated with HRP-conjugated goat anti-rabbit IgG (dilution,
1:5,000) for 1 h at room temperature and visualized using the
Enhanced Chemiluminescence kit (EMD Millipore). Optical density of
the bands was measured using a Bio-Rad Molecular Imager VersaDoc MP
gel imaging system with Bio-Rad Quantity One software and corrected
by reference to the optical density of GAPDH bands.
Immunofluorescence staining and
fluorescence microscopy
To determine NFAT2 localization, podocytes were
placed on cover slips in a six-well plate. Following exposure to
the various treatments, the differentiated podocytes were fixed
with 4% paraformaldehyde at room temperature for 15 min. The cells
were blocked with 5% bovine serum albumin (Abcam) for 30 min at
room temperature and were then incubated with rabbit anti-NFAT2
overnight at 4°C. Following three washes with PBS, the cells were
incubated with the secondary antibody in the dark for 1 h at room
temperature, washed three times with PBS, rinsed once with
distilled water, and were then incubated with Hoechst 33342
(Anaspec, Fremont, CA, USA). The cells were inspected under an
Olympus IX51 fluorescence microscope (Olympus Corporation, Tokyo,
Japan).
Intracellular Ca2+ level
assay
The podocytes were cultured in a 96-well
fluorescence plate under routine conditions. After 24 h, the cells
were rinsed with Hank's balanced salt solution (HBSS; GE Healthcare
Life Sciences) and were then incubated with Fluo-3/AM (final
concentration, 5 µM; Invitrogen; Thermo Fisher Scientific,
Inc.) in the dark for 45 min at 37°C. The cells were centrifuged at
800 x g for 5 min at 37°C, the medium was aspirated, and the cells
were washed twice with HBSS and incubated for 15 min at 37°C.
Ca2+ labeling with Fluo-3/AM was detected at an
excitation wavelength of 488 nm and an emission wavelength of 526
nm with an LSM 880 confocal microscope (Zeiss AG, Oberkochen,
Germany).
Statistical analysis
Statistical analyses were conducted using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). All data are presented as
the mean ± standard deviation. The statistical significance was
evaluated using one-way analysis of variance and Newman-Keuls
multiple comparisons post hoc analysis, and P<0.05 was
considered to indicate a statistically significant difference.
Results
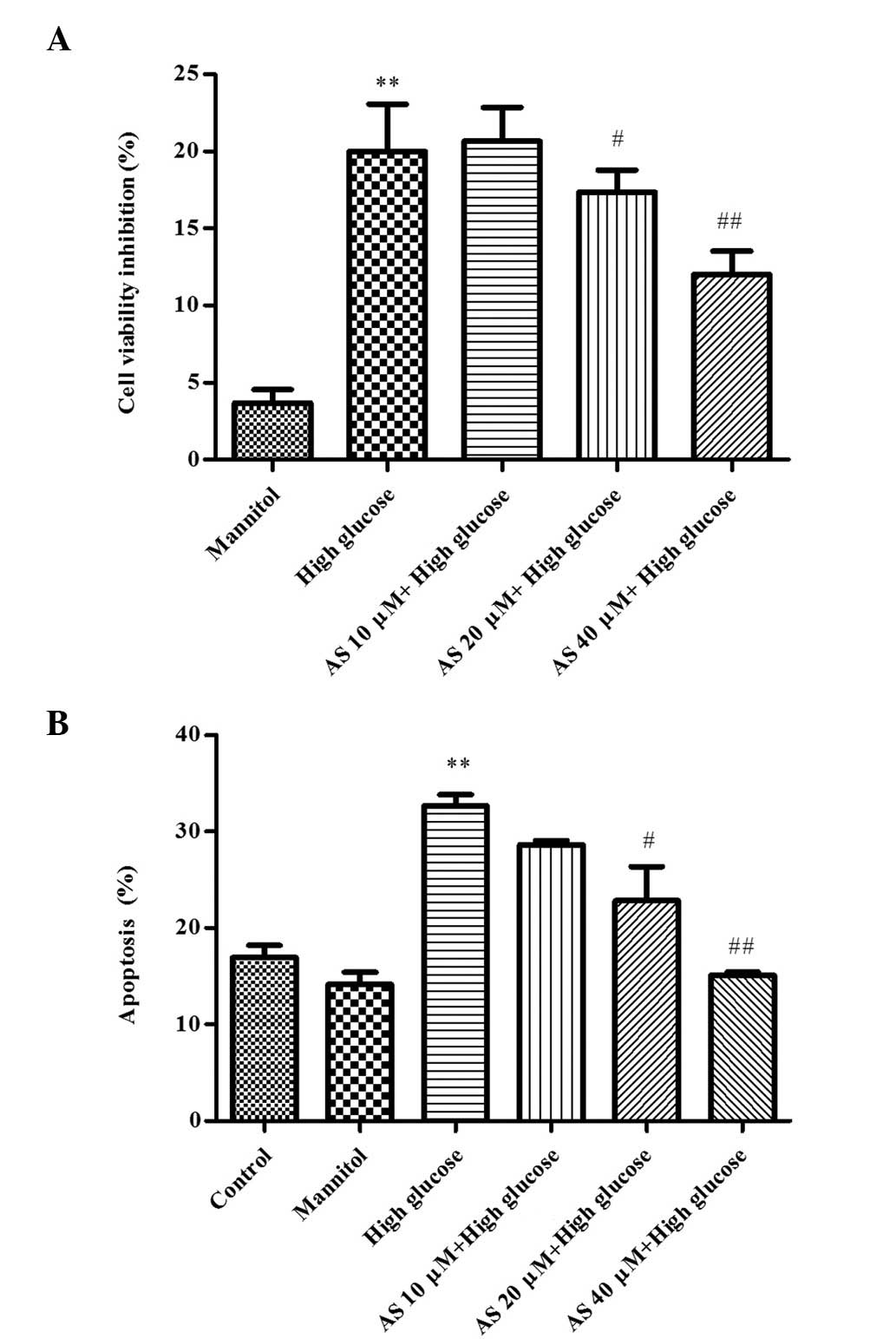
HG decreases podocyte viability, which is
attenuated by AS-IV
To investigate the effects of AS-IV on the viability
of HG-stimulated podocytes, an MTT assay was performed. As
presented in Fig. 1A, exposure to
HG for 24 h significantly inhibited the viability of podocytes by
20.19±4.26% (P=0.0006; n=3). As expected, MA had no significant
effect on podocyte viability, suggesting that the decreased cell
viability in the HG group was not due to high osmolarity.
Conversely, 20 or 40 µM AS-IV significantly enhanced
podocyte viability, and cell viability inhibition was reduced to
18.04±2.01% (P=0.03; n=3) and 13.21±1.78% (P=0.0008; n=3),
respectively, as compared with the HG group.
HG induces podocyte apoptosis, which is
inhibited by AS-IV
The effects of AS-IV on apoptosis were determined by
flow cytometry. As presented in Fig.
1B, HG significantly increased the rate of podocyte apoptosis
to 32.66±1.99% (P=0.0004; n=3) and MA resulted in no significant
change. AS-IV (20 or 40 µM) reduced the rate of podocyte
apoptosis to 22.15±6.15% (P=0.03; n=3) and 15.09±0.57% (P=0.0007;
n=3), respectively, compared with the HG group.
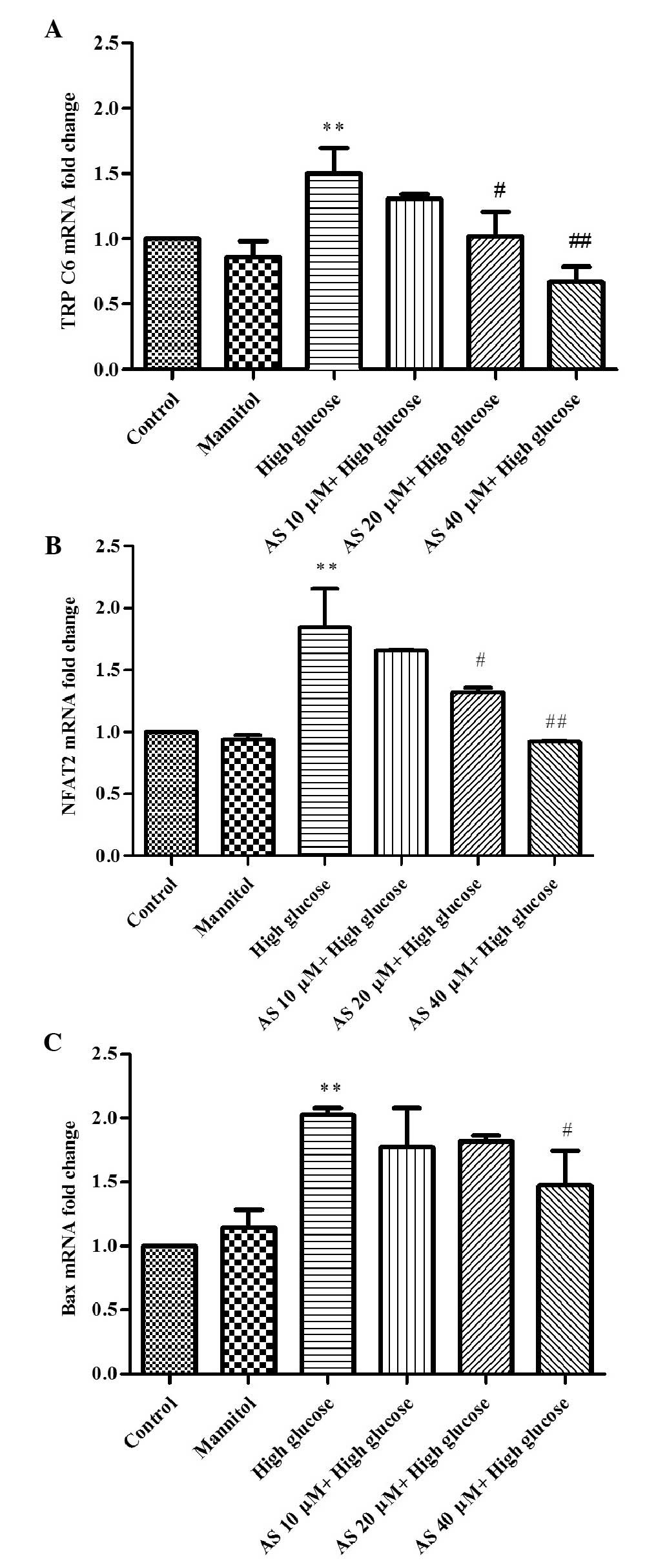
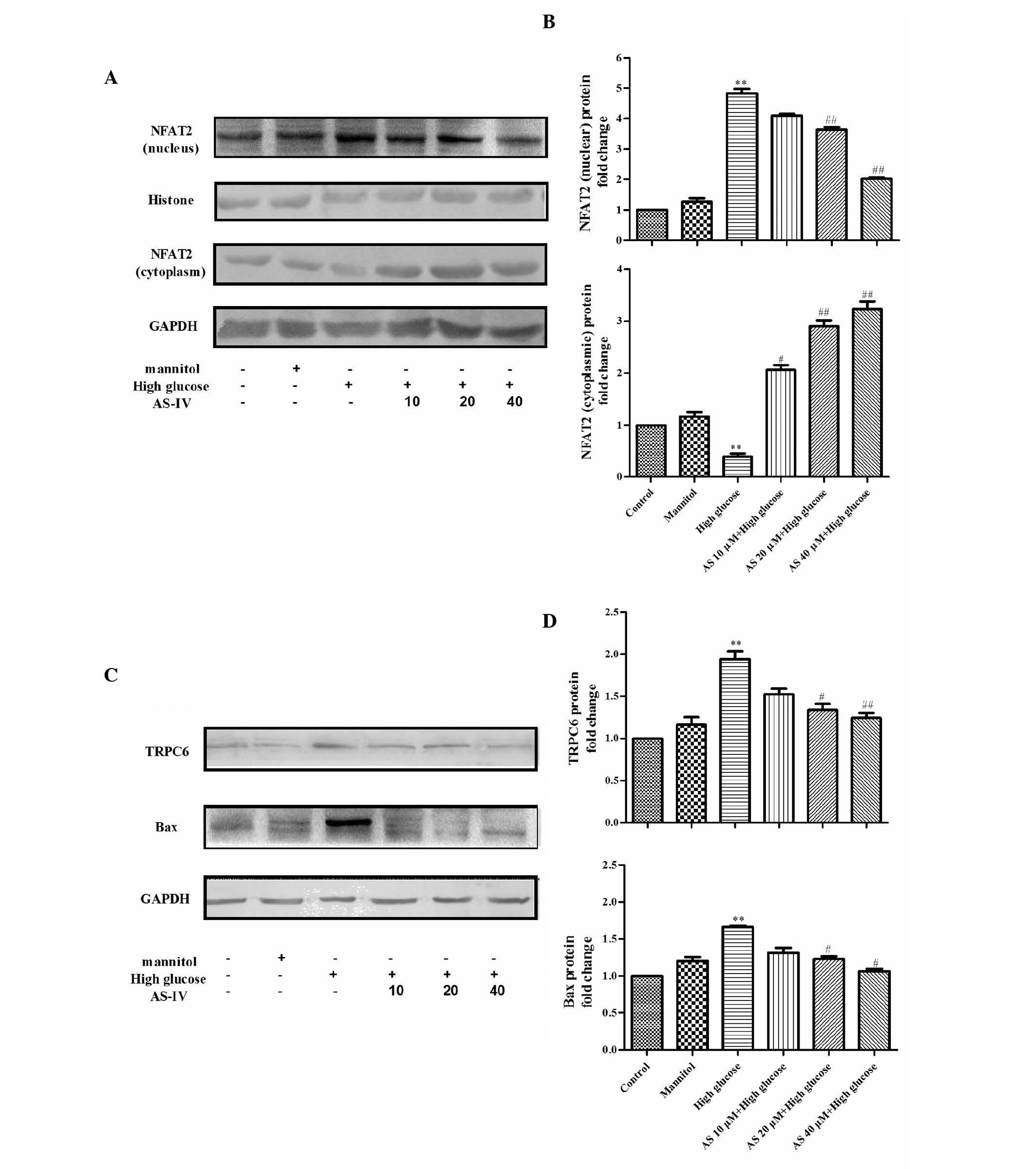
AS-IV inhibits the upregulation of TRPC6
in HG-stimulated podocytes
To determine whether AS-IV reduces TRPC6 expression
in HG-stimulated podocytes, the effects of different treatments
were assessed by RT-qPCR and western blotting (Figs. 2 and 3). HG increased TRPC6 mRNA and protein
expression levels by 151±0.37% and 89.2±13.4%, respectively
(P=0.0006 and P=0.0007, respectively; n=3). Conversely, treatment
with 20 or 40 µM AS-IV reduced the mRNA (P=0.02, n=3;
P=0.0005, n=3, respectively) and protein expression levels of TRPC6
(P=0.01, n=3; P=0.0004, n=3, respectively; Figs. 2A, 3C
and D).
HG increases NFAT expression, which is
suppressed by AS-IV
Using RT-qPCR and western blot analysis (Figs. 2 and 3), the mRNA and protein expression levels
of NFAT2, a downstream protein in the TRPC6 signaling pathway, were
determined. As presented in Figs.
2B, 3A and B, HG significantly
elevated NFAT2 mRNA expression levels by 182±0.33% (P=0.0005; n=3)
and nuclear protein expression levels by 389.2±19.8% (P=0.0004;
n=3), in addition to decreasing NFAT2 expression in the cytoplasm
by 313.6±8.7% (P=0.0005; n=3); however, MA did not result in
significant changes. Furthermore, the effects were suppressed by
AS-IV, AS-IV (20 and 40 µM) decreased NFAT2 mRNA expression
levels (P=0.04, n=3; P=0.0003, n=3, respectively), upregulated
NFAT2 cytoplasmic protein expression levels (P=0.0004, n=3; and
P=0.0002, n=3, respectively), and decreased NFAT2 nuclear protein
expression levels (P=0.0002, n=3; and P=0.0001, n=3, respectively)
in a dose-dependent manner.
AS-IV inhibits Bax expression in
HG-stimulated podocytes
To further investigate the prevention of podocyte
apoptosis by AS-IV, alterations in Bax expression levels were
observed (Figs. 2 and 3). HG upregulated Bax mRNA and protein
expression levels by 203±0.01% and by 73.2±6.8% (P=0.0005 and
P=0.0001, respectively; n=3), respectively. Conversely, 40
µM AS-IV reduced Bax gene and protein expression levels
(P=0.03 and P=0.01, respectively; n=3), as compared with the HG
group (Fig. 2C, 3C and D).
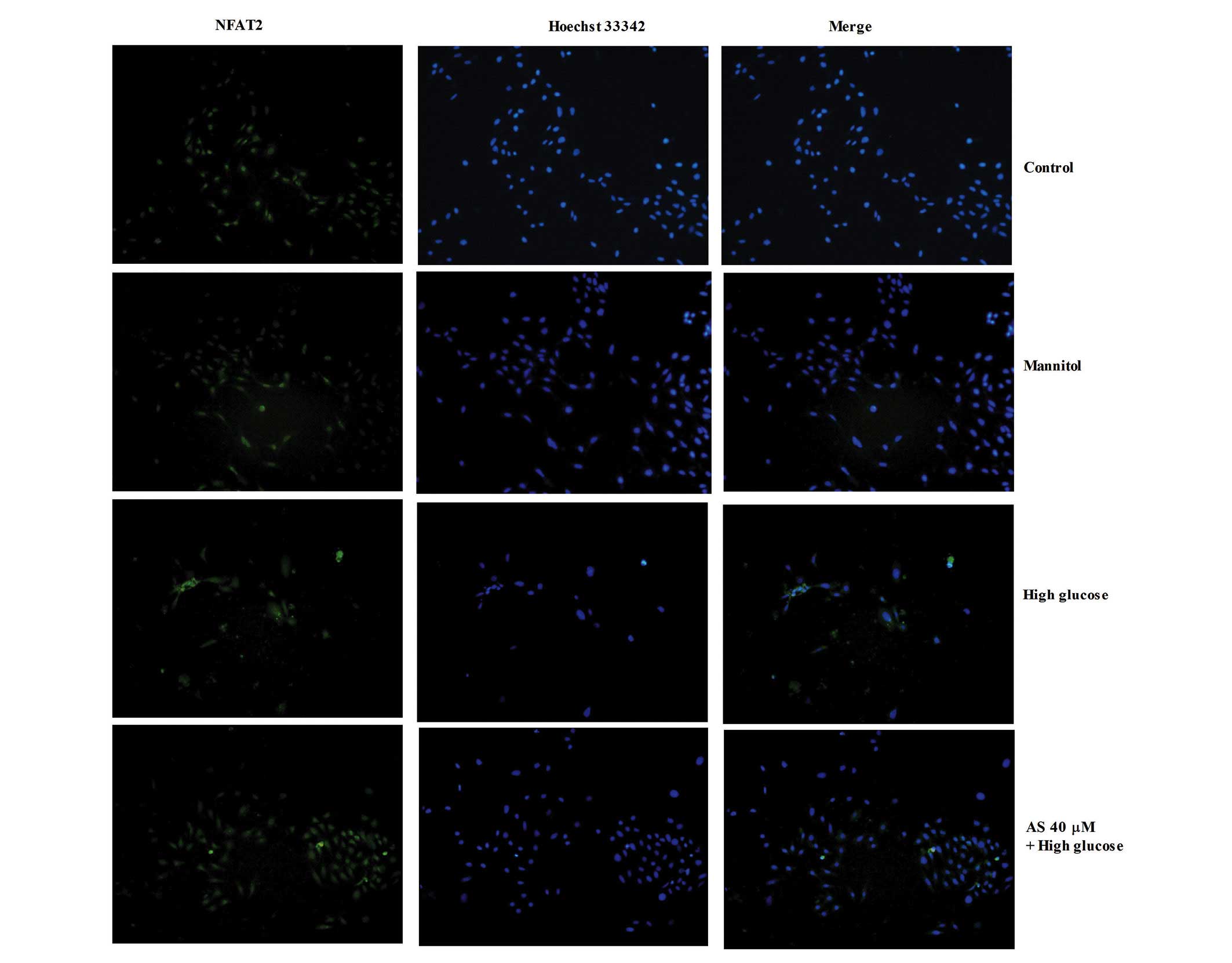
HG induces the nuclear translocation of
NFAT2 in podocytes, which is suppressed by AS-IV
Immunoblotting experiments were performed to assess
the nuclear translocation of NFAT2 in podocytes. As presented in
Fig. 4, NFAT2 was predominantly
distributed in the cytoplasm, and HG markedly increased NFAT2
nuclear accumulation in podocytes. However, 40 µM AS-IV
inhibited nuclear NFAT2 accumulation in the HG-stimulated
podocytes.
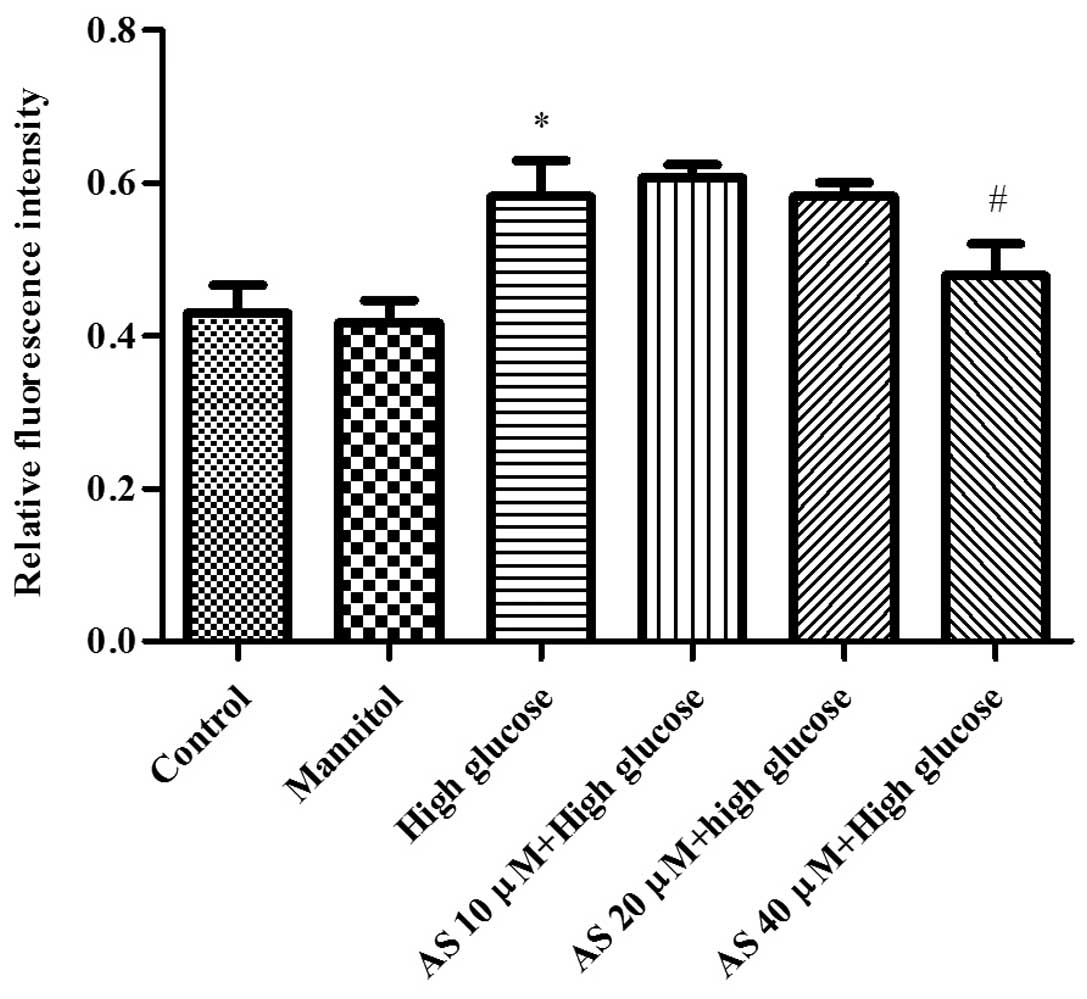
HG increases the intracellular
Ca2+ concentration in cultured podocytes, which is
attenuated by AS-IV
To measure the changes in intracellular
Ca2+ concentration in podocytes, the levels of
Ca2+ labeled with Fluo-3/AM were determined by confocal
laser scanning microscopy. The results demonstrated that HG
treatment, but not MA treatment, significantly increased the
Ca2+ level to 0.58±0.08% (P=0.01; n=3) in cultured
podocytes. Treatment with 40 µM AS-IV reduced the
intracellular Ca2+ influx level to 0.48±0.07% in the
HG-stimulated podocytes (P=0.02; n=3; Fig. 5).
Discussion
The present study is, to the best of our knowledge,
the first to demonstrate that AS-IV prevents glucose-induced
apoptosis of podocytes via ion channels. Upregulation of TRPC6
expression and increased intracellular Ca2+ levels in
HG-stimulated podocytes were observed. In addition, it was
demonstrated that TRPC6 was involved in the HG-induced apoptosis of
podocytes. AS-IV significantly attenuated the HG-induced apoptosis
of podocytes and induced a significant decrease in TRPC6 expression
and intracellular Ca2+ levels. Furthermore, AS-IV
inhibited the activation of NFAT2, a downstream protein of TRPC6.
AS-IV prevented the HG-induced apoptosis of podocytes via the
downregulation of TRPC6, which was possibly mediated via the
CaN/NFAT signaling pathway.
DN is an important and common complication of type 1
and type 2 diabetes, which results in end-stage renal disease
(1). Podocyte apoptosis is key in
the generation and progression of DN (6–8).
Therefore, the prevention or inhibition of podocyte apoptosis may
be a promising therapeutic strategy for the treatment of DN.
Podocytes express a family of nonselective cation
channels termed TRPC that may contribute to calcium influx
(15). TRPC6, one of the important
Ca2+-permeable ion channels in podocytes, is closely
associated with hereditary and acquired kidney diseases (36,37).
Numerous studies have demonstrated that TRPC6 is involved in
cytoskeletal rearrangement, NFAT-dependent transcription, and
apoptosis in podocytes (16,27,37–39).
Recent investigations have demonstrated that HG increases TRPC6
expression in podocytes and that TRPC6 is involved in HG-induced
podocyte apoptosis (17–19). In the present study, HG was
observed to induce podocyte apoptosis and significantly increase
TRPC6 mRNA and protein expression, and intracellular
Ca2+ in podocytes. Exposure to MA had no significant
effects, suggesting that the changes as a result of HG did not
result from high osmolarity. These results were also consistent
with those of previous studies (17–19).
NFAT2, which is a downstream protein of TRPC6, was
also investigated. The NFAT transcription factors are
well-researched CaN substrates and the major regulators of
transcription in response to Ca2+/CaN signaling
(40,41). It has been demonstrated that the
TRPC6/CaN/NFAT signaling pathway is involved in renal diseases,
such as in podocyte injury (27,28),
and that HG-induced podocyte apoptosis is mediated via the
CaN/NFAT2/Bax signaling pathway (42). Results from the present study
indicated that HG induced NFAT2 nuclear translocation in podocytes.
Furthermore, in the present study, Bax, an important indicator of
apoptosis (43,44), was observed to be activated in
response to HG stimulation in podocytes.
AS-IV is considered a characteristic active saponin
compound that mediates numerous pharmacological properties of
Astragalus (29).
Increasing evidence suggests that AS-IV has renal protective roles
in DN, including attenuating podocyte injury and ameliorating
podocyte apoptosis via anti-inflammatory signaling pathways, or
inhibiting oxidative stress (32–34).
Although TRPC6 participates in HG-induced podocyte apoptosis, the
protective role of AS-IV in ion channels remains to be elucidated.
In the present study, AS-IV was demonstrated to inhibit podocyte
apoptosis induced by HG, downregulate TRPC6, NFAT2 and Bax
expression levels, and suppress intracellular Ca2+
levels in HG-stimulated podocytes.
In conclusion, these results demonstrated that AS-IV
prevented HG-induced podocyte apoptosis via the down-regulation of
TRPC6, which was possibly mediated by the CaN/NFAT signaling
pathway. These findings provide novel insights into the treatment
of DN with AS-IV. There were, however, certain limitations to the
present study. Although there are a number of subtypes of TRPC,
TRPC6 was focused on in the current study. Changes in TRPC6
expression do not guarantee the involvement of this signaling
pathway during cell injury and protection. Therefore, in a future
investigation, the use of a more specific inhibitor or a genetic
method to knockdown TRPC6 is required.
Acknowledgments
The present study was supported by the General
Medicine of Key Discipline Construction Project, State
Administration of Traditional Chinese Medicine of the People's
Republic of China (grant no. 2013PT210), and the Budget Project
(grant no. 2013JW59) and the Putuo Hospital Project, Shanghai
University of Traditional Chinese Medicine (grant no.
2013PT028).
References
|
1
|
Collins AJ, Foley RN, Chavers B,
Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St
Peter W, et al: United States Renal Data System 2011 Annual Data
Report: Atlas of chronic kidney disease & end-stage renal
disease in the United States. Am J Kidney Dis. 59(Suppl 1):
e1–e420. 2012.
|
|
2
|
Hovind P, Rossing P, Tarnow L, Smidt UM
and Parving HH: Progression of diabetic nephropathy. Kidney Int.
59:702–709. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parving HH: Diabetic nephropathy:
Prevention and treatment. Kidney Int. 60:2041–2055. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanwar YS, Wada J, Sun L, Xie P, Wallner
EI, Chen S, Chugh S and Danesh FR: Diabetic nephropathy: Mechanisms
of renal disease progression. Exp Biol Med (Maywood). 233:4–11.
2008. View Article : Google Scholar
|
|
5
|
Ban CR and Twigg SM: Fibrosis in diabetes
complications: Pathogenic mechanisms and circulating and urinary
markers. Vasc Health Risk Manag. 4:575–596. 2008.PubMed/NCBI
|
|
6
|
Wiggins RC: The spectrum of
podocytopathies: A unifying view of glomerular diseases. Kidney
Int. 71:1205–1214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drummond K and Mauer M; International
Diabetic Nephropathy Study Group: The early natural history of
nephropathy in type 1 diabetes: II. Early renal structural changes
in type 1 diabetes. Diabetes. 51:1580–1587. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pagtalunan ME, Miller PL, Jumping-Eagle S,
Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L and Meyer TW:
Podocyte loss and progressive glomerular injury in type II
diabetes. J Clin Invest. 99:342–348. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schiffer M, Bitzer M, Roberts IS, Kopp JB,
ten Dijke P, Mundel P and Böttinger EP: Apoptosis in podocytes
induced by TGF-beta and Smad7. J Clin Invest. 108:807–816. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Susztak K, Raff AC, Schiffer M and
Böttinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar
|
|
11
|
Venkatachalam K and Montell C: TRP
channels. Annu Rev Biochem. 76:387–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montell C: The TRP superfamily of cation
channels. Sci STKE. 2005:re32005.PubMed/NCBI
|
|
13
|
Ramsey IS, Delling M and Clapham DE: An
introduction to TRP channels. Annu Rev Physiol. 68:619–647. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Winn MP, Conlon PJ, Lynn KL, Farrington
MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S,
Burchette JL, et al: A mutation in the TRPC6 cation channel causes
familial focal segmental glomerulosclerosis. Science.
308:1801–1804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dryer SE and Reiser J: TRPC6 channels and
their binding partners in podocytes: Role in glomerular filtration
and pathophysiology. Am J Physiol Renal Physiol. 299:F689–F701.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Möller CC, Wei C, Altintas MM, Li J, Greka
A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, et al:
Induction of TRPC6 channel in acquired forms of proteinuric kidney
disease. J Am Soc Nephrol. 18:29–36. 2007. View Article : Google Scholar
|
|
17
|
Yang H, Zhao B, Liao C, Zhang R, Meng K,
Xu J and Jiao J: High glucose-induced apoptosis in cultured
podocytes involves TRPC6-dependent calcium entry via the RhoA/ROCK
pathway. Biochem Biophys Res Commun. 434:394–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Xu J, Xu P, Liu S and Yang Z:
Wnt/β-catenin signalling pathway mediates high glucose induced cell
injury through activation of TRPC6 in podocytes. Cell Prolif.
46:76–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu BC, Song X, Lu XY, Li DT, Eaton DC,
Shen BZ, Li XQ and Ma P: High glucose induces podocyte apoptosis by
stimulating TRPC6 via elevation of reactive oxygen species. Biochim
Biophys Acta. 1833:1434–1442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuwahara K, Wang Y, McAnally J, Richardson
JA, Bassel-Duby R, Hill JA and Olson EN: TRPC6 fulfills a
calcineurin signaling circuit during pathologic cardiac remodeling.
J Clin Invest. 116:3114–3126. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishida M, Onohara N, Sato Y, Suda R,
Ogushi M, Tanabe S, Inoue R, Mori Y and Kurose H:
Galpha12/13-mediated up-regulation of TRPC6 negatively regulates
endothelin-1-induced cardiac myofibroblast formation and collagen
synthesis through nuclear factor of activated T cells activation. J
Biol Chem. 282:23117–23128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onohara N, Nishida M, Inoue R, Kobayashi
H, Sumimoto H, Sato Y, Mori Y, Nagao T and Kurose H: TRPC3 and
TRPC6 are essential for angiotensin II-induced cardiac hypertrophy.
EMBO J. 25:5305–5316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia Y, Zhou J, Tai Y and Wang Y: TRPC
channels promote cerebellar granule neuron survival. Nat Neurosci.
10:559–567. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao A, Luo C and Hogan PG: Transcription
factors of the NFAT family: Regulation and function. Annu Rev
Immunol. 15:707–747. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Jarad G, Tripathi P, Pan M,
Cunningham J, Martin DR, Liapis H, Miner JH and Chen F: Activation
of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc
Nephrol. 21:1657–1666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Chang JH, Paik SY, Tang Y, Eisner
W and Spurney RF: Calcineurin (CN) activation promotes apoptosis of
glomerular podocytes both in vitro and in vivo. Mol Endocrinol.
25:1376–1386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schlöndorff J, Del Camino D, Carrasquillo
R, Lacey V and Pollak MR: TRPC6 mutations associated with focal
segmental glomerulosclerosis cause constitutive activation of
NFAT-dependent transcription. Am J Physiol Cell Physiol.
296:C558–C569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nijenhuis T, Sloan AJ, Hoenderop JG,
Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer
RA, Möller CC, et al: Angiotensin II contributes to podocyte injury
by increasing TRPC6 expression via an NFAT-mediated positive
feedback signaling pathway. Am J Pathol. 179:1719–1732. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu QT, Qi LW, Li P, Yi L, Zhao J and Bi Z:
Determination of seventeen main flavonoids and saponins in the
medicinal plant Huang-qi (Radix astragali) by HPLC-DAD-ELSD. J Sep
Sci. 30:1292–1299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu LH, Xie XJ and Zhang BQ: Astragaloside
IV improves homocysteine-induced acute phase endothelial
dysfunction via antioxidation. Biol Pharm Bull. 33:641–646. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao J, Yang P, Li F, Tao L, Ding H, Rui
Y, Cao Z and Zhang W: Therapeutic effects of astragaloside IV on
myocardial injuries: Multi-target identification and network
analysis. PLoS One. 7:e449382012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gui D, Huang J, Guo Y, Chen J, Chen Y,
Xiao W, Liu X and Wang N: Astragaloside IV ameliorates renal injury
in streptozotocin-induced diabetic rats through inhibiting
NF-κB-mediated inflammatory genes expression. Cytokine. 61:970–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Gui D, Chen Y, Mou L, Liu Y and
Huang J: Astragaloside IV improves high glucose-induced podocyte
adhesion dysfunction via alpha3beta1 integrin upregulation and
integrin-linked kinase inhibition. Biochem Pharmacol. 76:796–804.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gui D, Guo Y, Wang F, Liu W, Chen J, Chen
Y, Huang J and Wang N: Astragaloside IV, a novel antioxidant,
prevents glucose-induced podocyte apoptosis in vitro and in vivo.
PLoS One. 7:e398242012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Reiser J, Polu KR, Möller CC, Kenlan P,
Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C,
et al: TRPC6 is a glomerular slit diaphragm-associated channel
required for normal renal function. Nat Genet. 37:739–744. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mundel P, Reiser J, Borja AZM, Pavenstädt
H, Davidson GR, Kriz W and Zeller R: Rearrangements of the
cytoskeleton and cell contacts induce process formation during
differentiation of conditionally immortalized mouse podocyte cell
lines. Exp Cell Res. 236:248–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Z, Wei X, Zhang Y, Ma X, Li B, Zhang
S, Du P, Zhang X and Yi F: NADPH oxidase-derived ROS contributes to
upregulation of TRPC6 expression in puromycin
aminonucleoside-induced podocyte injury. Cell Physiol Biochem.
24:619–626. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang H, Ding J, Fan Q and Liu S: TRPC6
upregulation in Ang II-induced podocyte apoptosis might result from
ERK activation and NF-κB translocation. Exp Biol Med (Maywood).
234:1029–1036. 2009. View Article : Google Scholar
|
|
40
|
Wu H, Peisley A, Graef IA and Crabtree GR:
NFAT signaling and the invention of vertebrates. Trends Cell Biol.
17:251–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Crabtree GR: Calcium, calcineurin, and the
control of transcription. J Biol Chem. 276:2313–2316. 2001.
View Article : Google Scholar
|
|
42
|
Li R, Zhang L, Shi W, Zhang B, Liang X,
Liu S and Wang W: NFAT2 mediates high glucose-induced glomerular
podocyte apoptosis through increased Bax expression. Exp Cell Res.
319:992–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: Mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kriz W, Gretz N and Lemley KV: Progression
of glomerular diseases: Is the podocyte the culprit? Kidney Int.
54:687–697. 1998. View Article : Google Scholar : PubMed/NCBI
|