Introduction
Statins, the 3-hydroxy-3-methylglutaryl co-enzyme A
reductase inhibitors, are widely used clinically. They exert
numerous beneficial effects, particularly in the prevention of
cardiovascular diseases. It was previously reported that they can
improve endothelial function, alleviate inflammatory reactions,
repress ventricular hypertrophy, inhibit the proliferation of
fibroblasts and reduce the deposition of interstitial tissue
collagen (1–7).
Transforming growth factor (TGF)-β1 is now
considered to be the quintessential multifunctional cytokine. A
considerable number of previous studies showed that TGF-β1
intracellular signaling mediated the development of cardiomyocyte
hypertrophy and interstitial fibrosis (8,9).
TGF-activated kinase (TAK)1 and drosophila mothers against
decapentaplegic (Smad) proteins, including Smad1–8, which have been
shown to regulate cardiac hypertrophy and remolding, are crucial
mediators of the cytostatic effect of TGF-β1 (8–11).
Ventricular remodeling in post-myocardial infarction
(MI) is a common pathophysiological alteration, as well as an
important factor responsible for chronic heart failure (CHF), and
CHF is the leading major cause of mortality in all developed
countries. Due to the extraordinary clinical importance of
cardiovascular disease and the role of the TGF-β1 signaling pathway
in cardiac hypertrophy and remodeling, the present study
hypothesized that statins mediate their effects via the TGF-β1
signaling pathway, however, direct evidence in support of this
hypothesis is insufficient. Therefore, the present study aimed to
determine whether statins ameliorate ventricular remodeling in
post-MI rats via the TGF-β1 signaling pathway. For this purpose,
simvastatin was widely used in clinical patients used in the
present study.
Materials and methods
Experimental animals and drugs
Male Sprague-Dawley rats (age, 6–8 weeks; weight,
160–240 g) were provided by the Animal Experimental Center of
Chongqing Medical University (Sichuan, China) were housed in groups
of five on a 12 h light/dark cycle, with ad libitum access
to standard rat chow and water. The rats were subjected to
sham-surgery or left coronary artery ligation. The experiments were
performed following the approval of the Animal Ethical Committee of
the Chongqing Medical University for the use of experiment animals
and conform to the Guide for Care and Use of Laboratory Animals.
Simvastatin was purchased from Merck Sharp and Dohme (Rahway, NJ,
USA).
Establishment of the MI model
All rats were anesthetized using 3.5% of 10 ml/kg
chloral hydrate (Qingdao Yulong Algae Co., Ltd., Shandong, China).
Tracheotomy was performed for ventilation by a respirator (ALC-V8B;
Shanghai Alcott Biotech Co., Ltd., Shanghai, China) with a stroke
volume of 28 ml/kg, air pressure of 10 mmHg, respiration rate of
1:1 and at a rate of 86 strokes/min. The electrocardiogram of lead
II was monitored. Thoracotomy was performed and the left anterior
descending coronary artery was ligated using 6-0 silk (Shanghai
Pudong Jinhuan Medical Products Co., Ltd., Shanghai, China). Fifty
rats are randomly divided into five groups (n=10 in each group),
the MI group, the Sim1 group, the Sim2 group,the Sim4 group, and
the sham-surgery group. All rats underwent MI by left anterior
descending coronary artery ligation except for the sham rats, which
underwent identical surgery, with the exception of the coronary
artery ligation. At 24 h after MI, one rat had died in the MI
group, two rats had died in the Sim1 group and one rat had died in
the Sim4 group. At 24 h after MI the groups were treated as
follows: MI (n=9), 10 mg/kg/d simvastatin (Sim1; n=8), 20 mg/kg/d
simvastatin (Sim2; n=10); 40 mg/kg/d simvastatin (Sim4; n=9) and
sham-surgery animals (n=10). At 48 h after the operation, all the
Sim-treated groups were administered intragastrically with 4%
simvastatin for 4 consecutive weeks, while the MI and Sham groups
were administered intragastrically with the identical quantity of
normal saline at 17:30–18:00 on a daily basis.
Hemodynamics measurements
All rats had a recorded body mass (BM; g). The right
common carotid artery and left femoral artery were isolated, and a
polystyrene PE-20 catheter was inserted into the left ventricle
(LV) via the right common carotid artery, with one end connected to
the MPA-2000 multichannel physiologic recorder via energy converter
to measure heart rate (HR), left ventricular systolic pressure
(LVSP), left ventricular end-diastolic pressure (LVEDP) and the
rates of maximum positive and negative left ventricular pressure
development (±LVdp/dtmax). Additionally, femoral arterial
cannulation was performed for simultaneous recording of systolic
(SBP) and diastolic arterial pressure (DBP).
Serum lipid analysis
Upon completion, thoracotomy was performed for
sampling blood (2 ml) from the left ventricle. This was obtained by
centrifugation to determine serum lipids, including total
cholesterol (TC), triglyceride (TG), low-density lipoprotein
(LDL-C) and high-density lipoprotein (HDL-C). An AU640 automatic
biochemistry analyzer and serum-lipid kit (Wako Pure Chemical.
Industries Ltd., Osaka, Japan) were used, according to the
manufacturer's protocol, for the detection of serum levels of TC,
TG, LDL-C and HDL-C.
Histological analysis
The LV mass (LVM; mg), and the ratio of left
ventricular mass/body mass (LVMI; mg/g) were measured.
Subsequently, the LV was sectioned at a thickness of 3 mm for
paraffin sectioning and the other tissue sections were stored at
−80°C for further examinations. The paraffin sections were
subjected to hematoxylin and eosin (HE; Beyotime Institute of
Biotechnology, Haimen, China) staining. A total of 50
nucleus-centered cardiomyocytes were selected from each specimen
and these cardiomyocytes underwent measurement of their
cross-sectional area (CSA) using Computer Pathological Image
Analytical system (Beijing University of Aeronautics and
Astronautics, Beijing, China), and the mean value for each specimen
was obtained.
Determination of the collagen volume
fraction (CVF)
Paraffin sections of rat LV were measured for
collagen deposition by Picric-Sirius Red Polarimetry. The slides
were placed in 1% picric-sirius red solution (Sigma-Aldrich, St.
Louis, MO, USA) for 45 min. Under polarization microscopy, red or
yellow staining is representative of the type I collagen, which is
arranged tightly and with strong double refraction, where as green
indicated type III collagen, which is porous and thin, with weak
double refraction. A total of four visual fields in non-infarction
zone (NIZ) were randomly selected on each slide under polarization
microscopy (X51-P, Olympus, Tokyo, Japan). The averages of the type
I CVF and type III CVF were determined using the Image Pro Plus 6.0
image analysis system (Media Cybernetics, Inc., Rockville, MD, USA)
and the collagen I/III ratio was calculated.
Western blot analysis
Protein samples were isolated from the left
ventricular myocardium of rats. Left ventricular myocardium lysates
were prepared by homogenization in cell lysis buffer (Beyotime
Institute of Biotechnology). The protein concentration was
determined using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology). The protein samples (40/20 µg) were mixed
with 2X sodium dodecyl sulfate sample loading buffer (Beyotime
Institute of Biotechnology) and were subsequently separated on a
12% polyacrylamide gel and blotted on a nitrocellulose membrane
(Beyotime Institute of Biotechnology, Inc). The membranes were
blocked with 5% non-fat milk, followed by incubation (at 4°C for 24
h) with antibodies specific for TGF-β1 (1:100, sc-146), TAK1
(1:100, sc-7162), Smad3 (1:100, sc-169248), Smad7 (1:100,
sc-100140) or β-actin (1:100; sc-47778) from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The membranes were
subsequently incubated at 37°C for 30 min with horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. ZDR-5306), rabbit
anti-goat (cat. no. ZDR-5308) and goat anti-mouse (cat. no.
ZDR-5307) immunoglobulin G (1:1,000; all from Zhongshan
Goldenbridge Biotechnology Corporation, Beijing, China) and
enhanced chemiluminescence detection system (Bio-Rad Laboratories,
Hercules, CA, USA) was used for visualization. The grey value was
measured using Quantity One software.
Immunohistochemistry
Immunohistochemistry was performed, according to the
Streptavidin-Biotin Complex methods (12). Briefly, paraffin sections of rat
myocardial tissue were dehydrated and dewaxing, then permeabilized
with methanol and heated with a microwave to 100°C for 5 min.
Following heating, the tissue sections were blocked with goat serum
(Beyotime Institute of Biotechnology, Inc.) for 30 min at room
temperature. The samples were then incubated with rabbit anti-Smad7
(1:200; Santa Cruz Biotechnology Inc.,) antibody overnight at 4°C.
The sections were subsequently incubated with horseradish
peroxidase conjugated-goat anti-rabbit immunoglobulin G (1:150;
Zhongshan Goldenbridge Biotechnology Corporation) at room
temperature for 30 min. The samples were then counterstained with
HE and washed with phosphate-buffered saline.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was extracted using the Biozol kit,
according to the manufacturer's protocol (Beijing Jiamei Niunuo
Biotechnology Co., Ltd., Beijing, China). A total of 1 µl
RNA was subjected to RT using an RT-PCR kit (Beijing Jiamei Niunuo
Biotechnology Co., Ltd.). The cDNA was PCR-amplified using
appropriate primers. Primer sequences used as follows: TGF-β1,
forward: GACTACGCCAAAGAAGTCAC and reverse: AAGCCACTCAGGCGTATCAG;
TAK1, forward: ACA AGT CCC TGC CAC AAA C and reverse:
GATGGATCTACGCCTTGGTT; Smad3, forward: ATCTACTGCCG-CTTGTGG and
reverse: CTGTGAAGCGTGGAATGT; Smad7, forward: GGCATTCCTCGGAAGTCA and
reverse: AGAAGTTGGGAATCTGAAAGC (Beijing Parkson Gene Technology
Co., Ltd., Beijing, China). β-actin was used as an internal control
in RT-qPCR. The PCR products were separated on agarose gels and
stained with ethidium bromide. The bands were quantified using
Quantity One software 4.62 (Bio-Rad Laboratories) and normalized
against β-actin.
Statistical analysis
SPSS 17.0 software was used for statistical
analysis. The data are presented as the mean ± standard deviation.
Differences between the values were determined using Student's
t-test. Grouped data were analyzed using a one-way analysis of
variance, followed by the Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
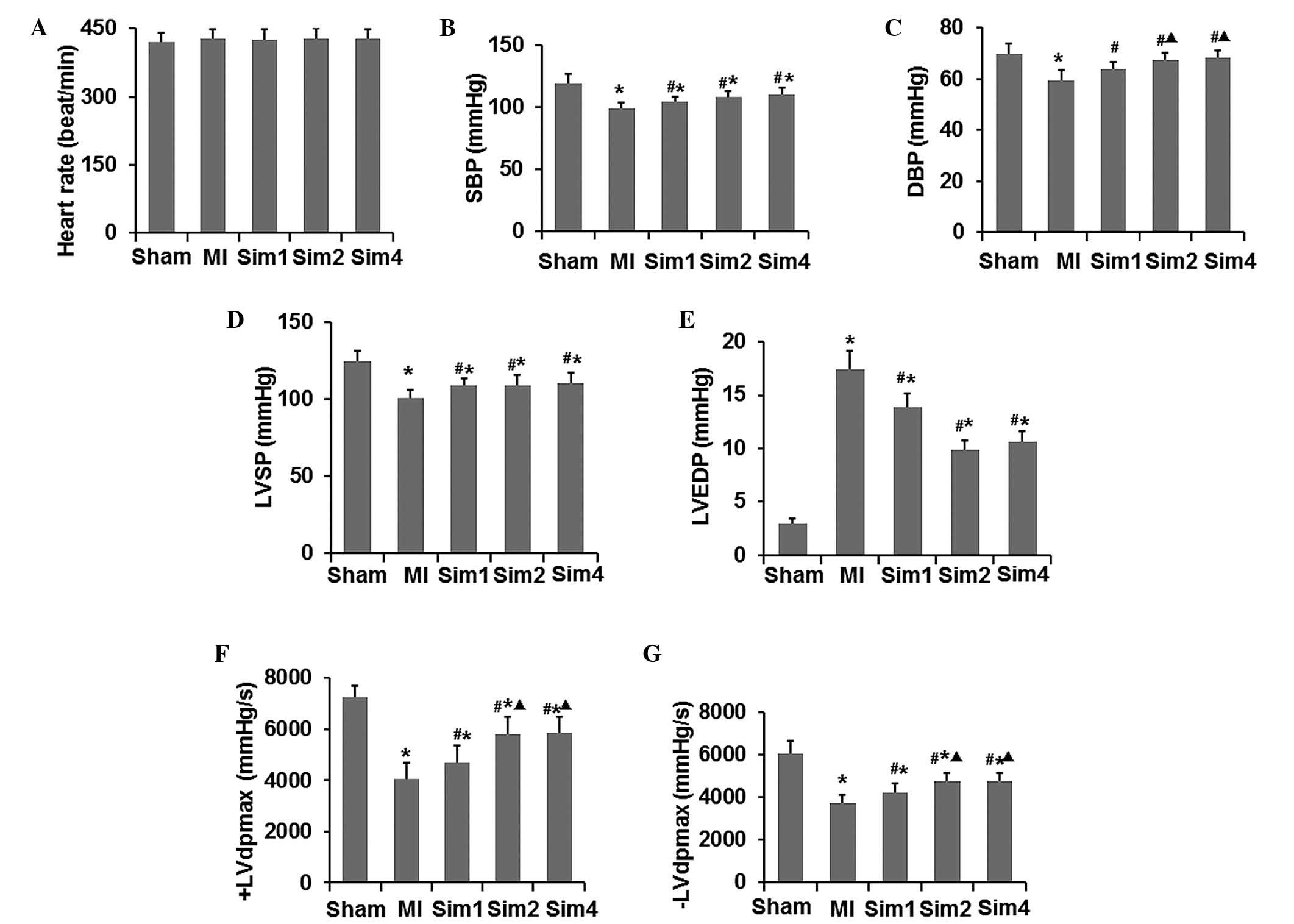
Simvastatin enhances left ventricular
function following myocardial injury in MI rats
Firstly, the effects of simvastatin on the
hemodynamics were assessed using an MPA-2000 multichannel
physiologic recorder, analyzing the HR, LVSP, LVEDP, SBP, DBP and
±LVdp/dtmax. As shown in Fig. 1,
treatment of the rats with simvastatin increased the LVSP, SBP,
DBP, ±LVdp/dtmax and decreased LVEDP; however, these remained lower
compared with the rats in the sham group. Additionally, the Sim2
and Sim4 groups revealed an improved LVSP, SBP, DBP ±LVdp/dtmax and
LVEDP compared with the Sim1 gorup. No differences between the Sim2
and Sim4 group were observed in any hemodynamic parameters. Taken
together, these data indicated that left ventricular function was
enhanced by simvastatin in MI rats with myocardial injury.
Simvastatin causes no affect on the
levels of serum lipids
In order to role out the possible lipid-regulating
effects of simvastatin, the basic clinical features of rats were
assessed following simvastatin treatment. As shown in Fig. 2, the Sim groups exhibited lower
levels of serum lipids compared with the rats in the Sham and MI
groups. However, no significant difference was observed in the
levels of serum lipid among all groups. These results suggested
that the effect of simvastatin in improving post-MI remodeling in
rats was independent of its lipid-regulating effect.
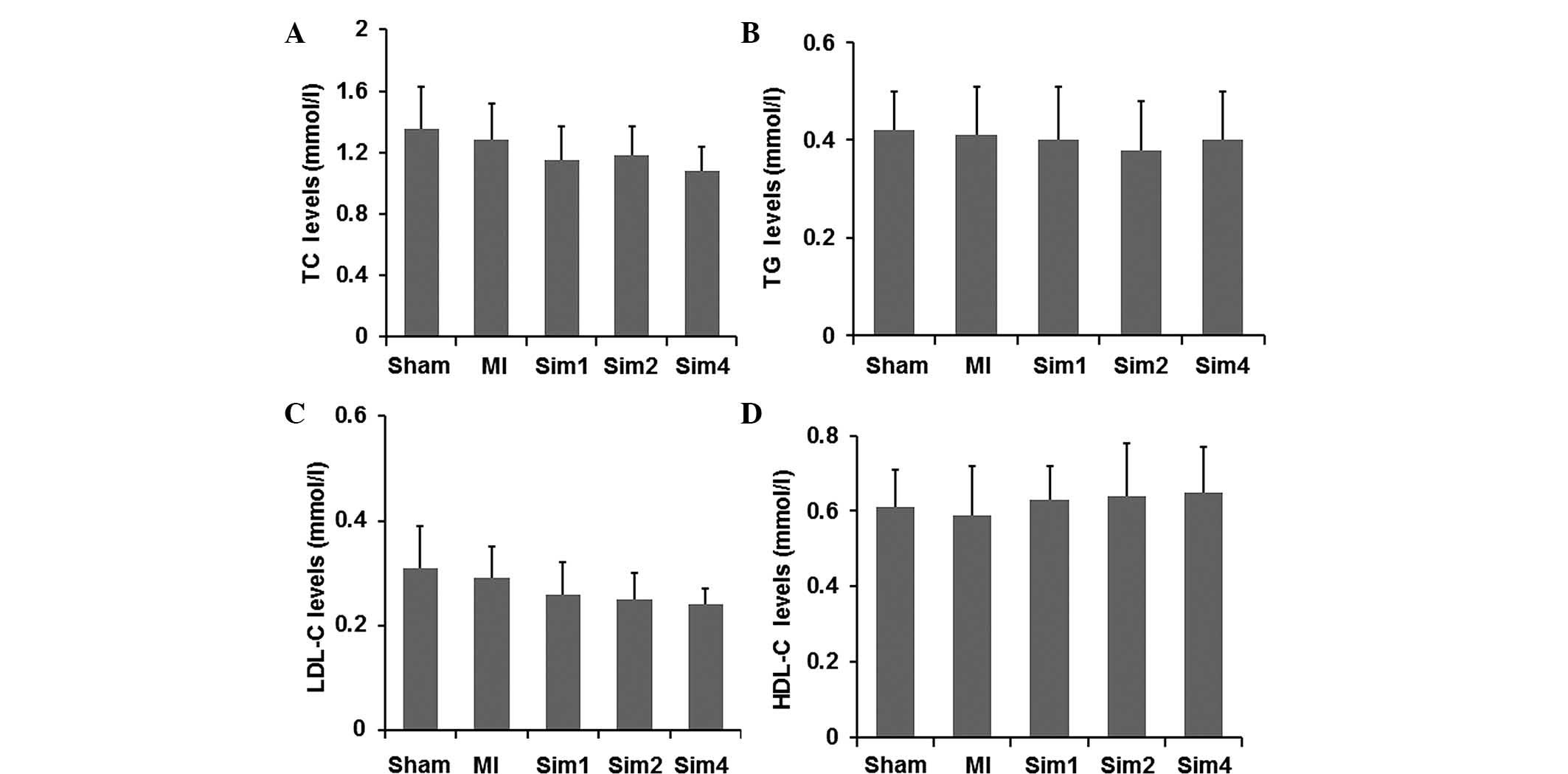 | Figure 2Effects of simvastatin on the serum
levels of (A) TC, (B) TG, (C) LDL-C and (D) HDL-C. The data are
expressed as the mean ± standard deviation. MI, myocardial
infarction; Sim1, group treated with simvastatin (10 mg/kg/day);
Sim2, group treated with simvastatin (20 mg/kg/day); Sim4, group
treated with simvastatin (40 mg/kg/day); TC, total cholesterol; TG,
triglyceride; LDL-C, low-density lipoprotein; HDL-C, high-density
lipoprotein. |
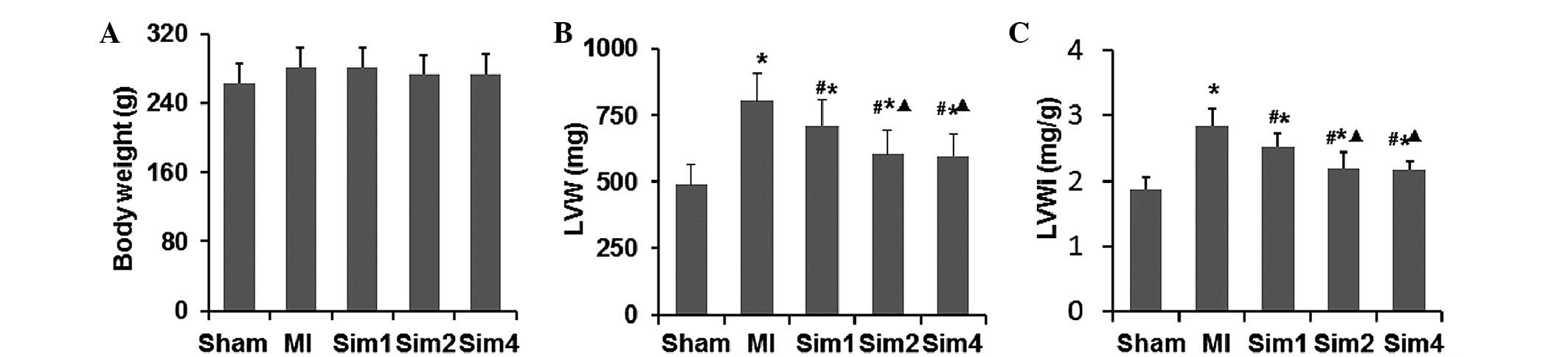
Simvastatin ameliorates ventricular
remodeling following myocardial injury in MI rats
LVMI is a marker of left ventricular hypertrophy.
The LV was sieved and the LVM and LVMI were recorded (Fig. 3). No difference was observed
between any of the groups in BM. The LVM and LVMI in the MI and Sim
groups were significantly higher compared with the Sham-operated
group, while the values were lower in the Sim groups compared with
the MI group. The LVM and LVMI in the Sim2 and Sim4 groups were
decreased more markedly compared with in the Sim1 group; however,
no difference between the Sim2 and Sim4 was detected.
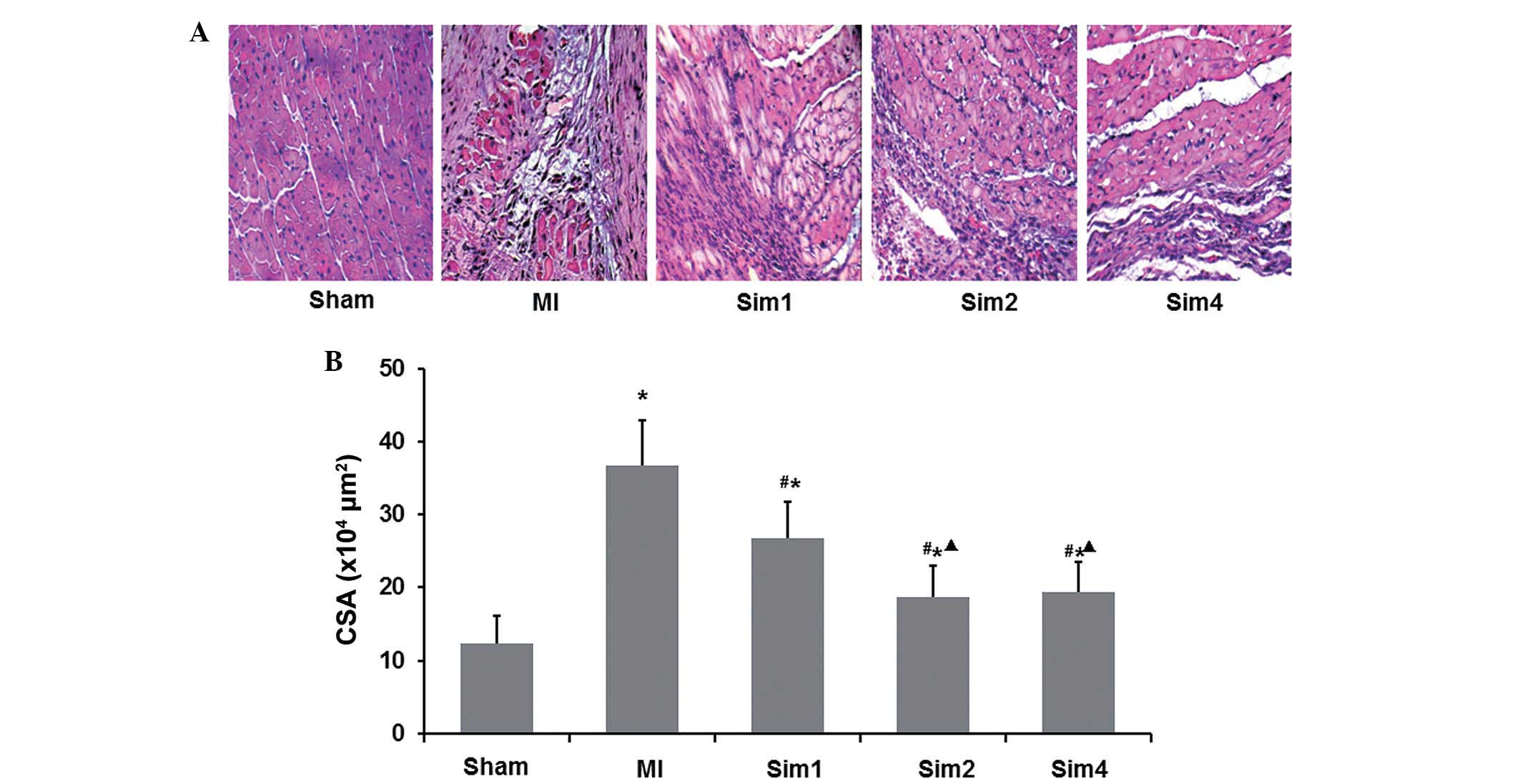
The histopathology and cardiomyocyte CSA
(×104 µm2) of the myocardium tissues
were also assessed (Fig. 4A). HE
staining revealed that the cardiomyocytes in the Sham group were
regularly in good condition. The MI group exhibited large
infarction size, thin ventricle wall on the infracted region and
significantly reduced cardiomyocytes, which were substituted by
numerous strip-like fasciculation collagen fibers and were
structurally disordered. In the non-infarcted region of MI group,
infiltration of considerable inflammatory granulocytes and
monocytes, proliferation of protofibrocytes, and compensatory
hypertrophy of the survival cardiomyocytes were noted. Compared
with the MI group, the Sim groups exhibited significant attenuation
of cell degeneration and necrosis along with infiltration of
inflammatory cells and less collagen fiber in the infracted region,
while in the non-infarcted region, infiltration of numerous
inflammatory cells and smaller cardiomyocytes were noted.
Functional assays, shown in Fig. 4B, demonstrated that compared with
the MI group, simvastatin reduced the cardiomyocyte CSA
(×104 µm2). It was also observed that
the CSA was lower in the Sim2 and Sim4 groups compared with in Sim1
group, while no difference was observed between the Sim2 and Sim4
groups.
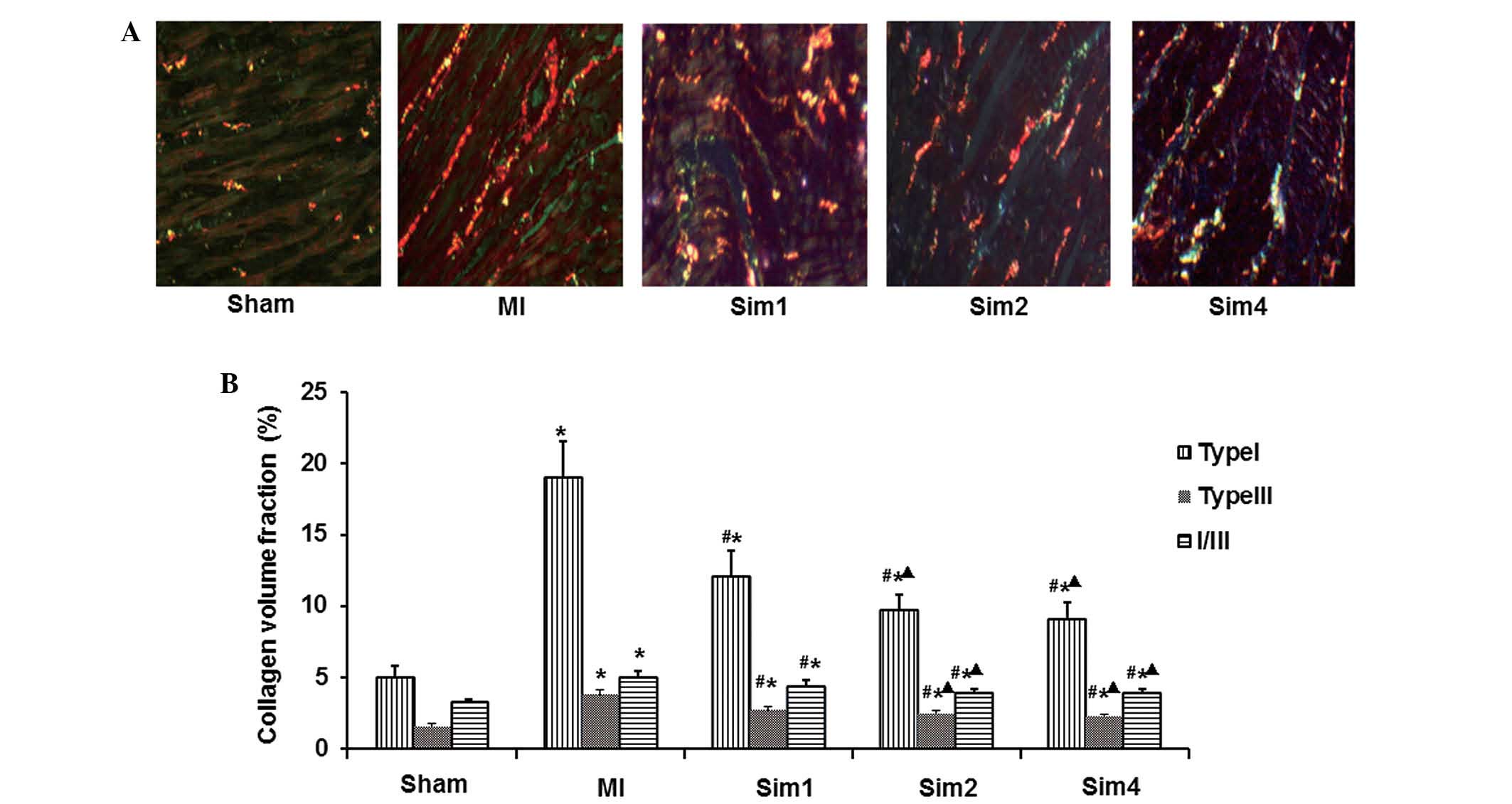
The collagen volume fraction (CVF) was altered in
the non-infarcted zone of the LV of all groups. Fig. 5A shows the results of Picric-Sirius
Red Polarimetry for type I and type III collagen fiber in the
non-infarcted zone of the LV. Quantification of the data (Fig. 5B) revealed that the levels of the
type I, type III collagen fiber and I/III were increased
significantly in the MI rats compared with those from Sham- and
Sim-treatment rats. The data in the Sim2 and Sim4 groups were also
significantly reduced compared with those in the Sim1 group;
however, no differences were observed between the Sim2 and Sim4
groups.
Taken together, these data indicated that
simvastatin can ameliorate ventricular remodeling following
myocardial injury in MI rats.
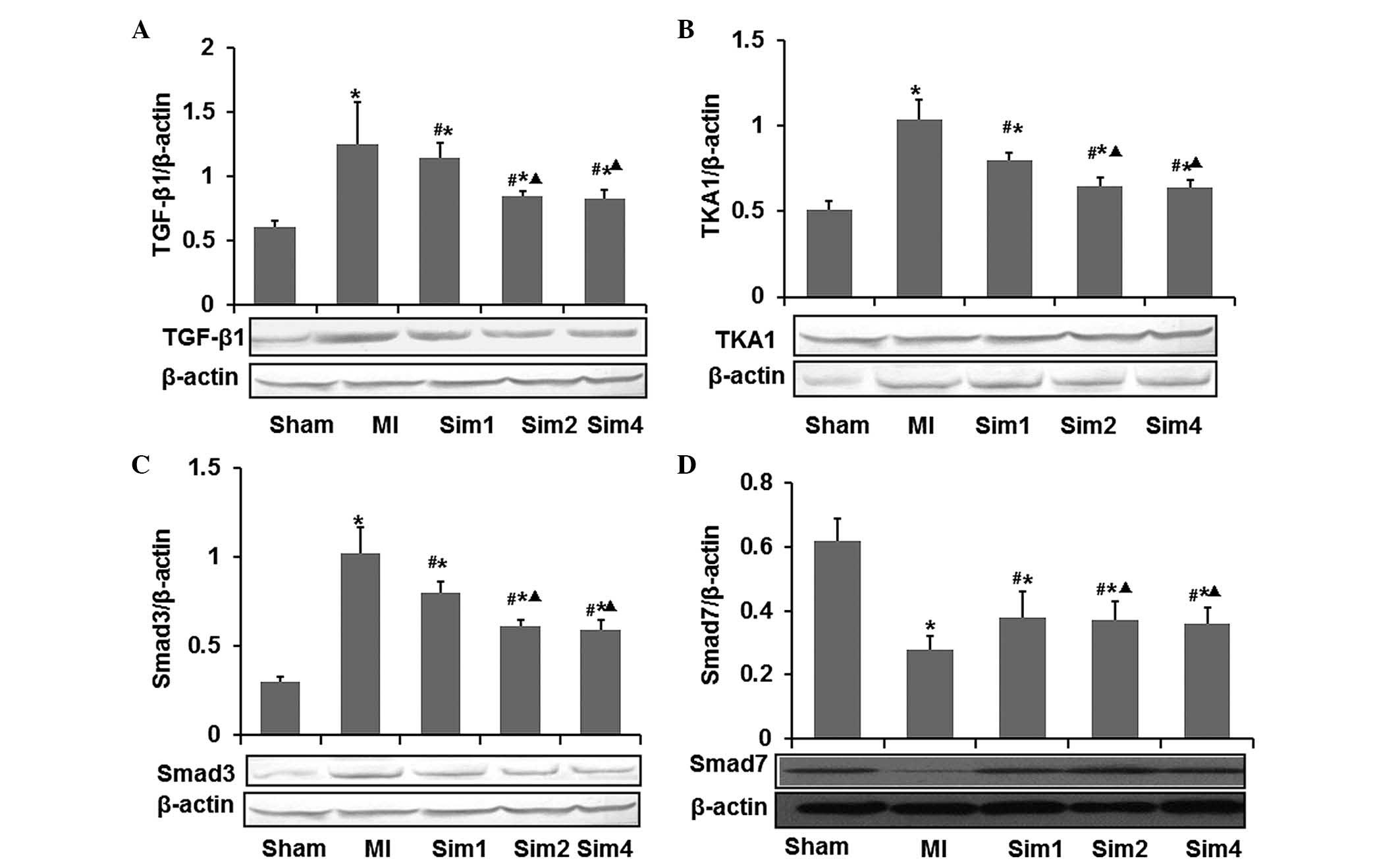
Simvastatin inhibits the expression
levels of TGF-β1, TAK1 and Smad3, and increases the expression of
Smad7 following myocardial injury in MI rats
The effects of simvastatin on the mRNA and protein
expression levels of TGF-β1, TAK1, Smad3 and Smad7 were examined by
RT-qPCR and western blotting (Figs.
6 and 7). As shown in Figs. 6 and 7, the mRNA and protein expression levels
of TGF-β1, TAK1 and Smad3 were higher in the non-infarcted zone of
the LV in the MI group compared with those from the Sham and Sim
groups. While the mRNA and protein expression levels of Smad7 were
lower in the MI group compared with those in the Sham and Sim
groups. Following treatment with simvastatin, the mRNA and protein
expression levels of TGF-β1, TAK1 and Smad3 were elevated compared
with those in the MI group, while the mRNA and protein expression
levels of Smad7 in the Sim groups were significantly decreased
compared with those in the MI group. Notably, both the mRNA and
protein expression levels revealed no difference between the Sim2
and Sim4 groups.
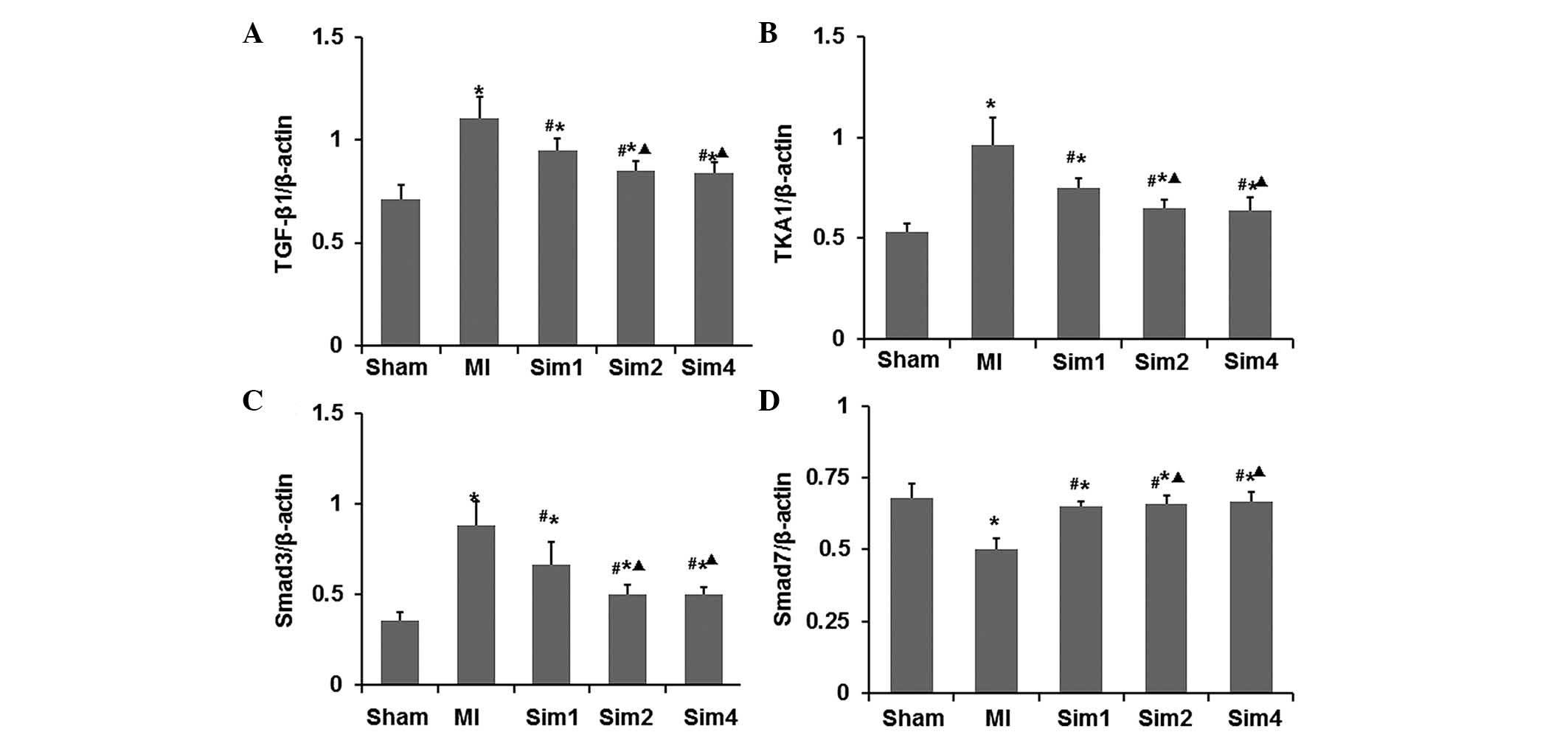 | Figure 6Inhibitory effects of simvastatin on
the mRNA expression levels of TGF-β1, TAK1 and Smad3 in the left
ventricles. The mRNA expression levels of (A) TGF-β1, (B) TAK1, (C)
Smad3 and (D) Smad7 were determined by RT-qPCR and the data are
expressed as the ratio against β-actin. The data are expressed as
the mean ± standard deviation (*P<0.05 vs.
Sham-operated group; #P<0.05 vs. MI group;
∆P<0.05 vs. Sim1 group). MI, myocardial infarction;
Sim1: simvastatin (10 mg kg−1·d−1) treatment
group; Sim2, simvastatin (20 mg·kg−1·d−1)
treatment group; Sim4, simvastatin (40
mg·kg−1·d−1) treatment group; TGF,
transforming growth factor; TAK, TGF-activated kinase; Smad,
drosophila mothers against decapentaplegic protein; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
Therefore, the present study speculated that
simvastatin ameliorated ventricular remodeling via the TGF-β1
signaling pathway in post-MI rats.
Discussion
The present study investigated the protective
effects of simvastatin treatment on ventricular remodeling in the
MI rat model. Following pre-treatment with simvastatin in MI rats,
it was revealed that the left ventricular function was enhanced.
Additionally, the ventricular remodeling markers, including LVMI,
CSA and CVF were inhibited. These data indicated that simvastatin
meliorated ventricular remodeling in the MI rat model. RT-qPCR and
western blotting also showed that the expression levels of TGF-β1,
TAK1 and Smad3 were reduced, and the expression of Smad7 was
increased. Therefore, it was speculated that simvastatin reduced
ventricular remodeling in MI rats via TGF-β1, TAK1 and Smad3
decrease, and Smad7 increase.
Several lines of evidence have demonstrated that the
TGF-β1 signaling pathway is key role in the pathogenesis of
ventricular remodeling following MI (8–11).
In the present study, simvastatin was used to demonstrate the
positive effects of statins on the ventricular remodeling following
MI and provided a novel role of TGF-β1 signaling in the positive
effect of statins on ventricular remodeling.
In the present study, the effects of simvastatin on
the hemodynamics were assessed and revealed that the LVSP and
±LVdp/dtmax were decreased, while LVEDP increased significantly
following MI. This indicated that both systolic and diastolic
functions in MI rats were seriously damaged. However, these
pathological changes were improved in the simvastatin treated rats.
Structurally, in the MI rats, necrosis and apoptosis of
cardiomyocytes was observed, ventricular remodeling was confirmed
by increased LVMI and interstitial collagen deposition, and it was
directly correlated to functional alteration in respective groups
mentioned above. Furthermore, treatment with simvastatin
ameliorated all these changes.
LVMI, a marker of left ventricular hypertrophy, was
increased in the MI and simvastatin-treated groups compared with
the Sham-operated group. These occurrences signified the presence
of post-MI remodeling, evidenced by increased LVMI, and
interstitial collagen deposition was decreased by the treatment of
simvastatin for 4 weeks compared with the MI group. Guo et
al (13) also demonstrated
decreased LVMI by treatment of simvatatin for 4 weeks in post-MI
rats, which is consistent with the present findings, demonstrating
the effect of simvastatin in improving post-MI ventricular
remodeling.
The present study also demonstrated increased CSA in
the non-infarcted region following MI; however, this was decreased
by simvastatin treatment. This finding is consistent with other
previous studies (4,14), suggesting the effect of simvastatin
in improving post-MI ventricular function, and this effect may be
attributed to its activities in inhibiting ventricular hypertrophy
post-MI and improving the compliance of ventricular wall. The
deposition of myocardial interstitial collagen and the
disproportion of collagen type I/III are partly responsible for
decreased compliance of ventricular wall and impairment of heart
function. The present data suggested that in the post-MI group
compared with the Sham group, the type I and type III CVFs were
significantly elevated, with the collagen type I/III ratio altered
in NIZ. This resulted in increased proliferation, synthesis and
secretion of myocardial fibroblasts, which resulted in the
disproportion of collagen types and remodeling of myocardial
collagen lattice spatial structure. Compared with the MI group,
type I and type III CVFs in the NIZ were significantly decreased,
accompanied by normalization of collagen ratio, after 4 weeks
treatment with simvastatin. This suggested the effect of
simvastatin in decreasing collagen content, ameliorating the ratio
of collagen types, improving interstitial remodeling and increasing
compliance of ventricular wall, in accordance to that reported
previously (15–17). Serum levels of TG, TC, HDL-C and
LDL-C in the post-MI rats after 4 weeks remained significantly
unaltered in all simvastatin-treated groups as compared with those
in the MI or Sham-operated groups. This suggested that the
treatment with simvastatin for 4 weeks had little impact on serum
lipids in post-MI rats, and this finding is consistent with those
reported overseas (18,19), confirming that the effect of
simvastatin in improving post-MI remodeling in rats is independent
of its lipid-regulating effect.
TGF-β is a type of multifunctional cytokine,
including three highly homologous TGF-β isoforms (TGF-β1, 2 and 3).
TGF-β1 is the prevalent isoform and is found almost ubiquitously,
whereas the other isoforms are expressed in a more limited spectrum
of cells and tissues (20). TGF-β1
signaling has a wide variety of biological and pathological
actions, and it is one of the most important factors responsible
for cardiac remodeling in cases of myocardial damage or cardiac
overload (5).
TAK1, a member of the mitogen-activated protein
kinase kinase kinase and the downstream substrate of TGF-β1 signal
transduction, has a close correlation with cardiomyocyte
hypertrophy and cardiac failure (6,10).
The present study positively demonstrated a significantly increased
expression of TGF-β1 and TAK1 at the transcriptional and
translational level in the non-infarcted region in post-MI rats as
compared with the Sham-operated rats. However, it was significantly
downregulated by the simvastatin treatment. Previous studies
elsewhere found that the TGF-β1 and TAK1 expression in the
non-infarcted region following MI increased significantly as
compared with the control, and subsequent immunohistochemical
staining revealed that TAK1 was chiefly located in cardiomyocytes.
This implied that the activation of the TGF-β1/TAK1 signaling
pathway in the non-infarcted region accounted for cardiomyocyte
hypertrophy following MI (21).
The present study suggested that pleiotropic effects of simvastatin
may be attributed to its role in downregulating the expression of
TGF-β1/TAK1 and inhibiting intracellular signaling of the
TGF-β1/TAK1 pathway, and by doing so, improved post MI-associated
ventricular remodeling.
TGF-β1/Smads signal transduction is induced and
rapidly activated in cases of cardiac cell damage or infarct
healing, and may be important in modulating inflammatory reaction
and fibrosis (5,11). With regards to the association
between the activation of the TGF-β1/Smad3 signaling pathway
myocardial interstitial remodeling, the present study demonstrated
low level expression of TGF-β1 and Smad3 in normal myocardium and
significantly increased the expression of TGF-β1 and Smad3 in the
non-infarcted region in post-MI rats. This was accompanied with
increased collagen content and collagen type I/III ratio
alteration. It was previously demonstrated that gene knockdown
regulating Smad3 expression prevents interstitial fibrosis in the
non-infarcted myocardium and attenuates further cardiac remodeling
(6). Accordingly, SIS3, a reagent
with selective inhibition of Smad3, can inhibit excessive
extracellular matrix production by inhibiting TGF-β1 signaling
(22). The expression of TGF-β1
and Smad3 in the non-infarcted region was remarkably decreased by
simvastatin for 4 weeks, accompanied by decreased collagen content
and the amelioration of collagen type I/III ratio. This expression
altogether augmented hemodynamic parameters, suggesting that
simvastatin improves post-MI ventricular remodeling via its effects
in downregulating the expression of TGF-β1/Smad3 and inhibiting
intracellular signaling of the TGF-β1/Smad3 pathway.
Smad7 is different from Smad3, in that it is an
inhibitory type subunit in TGF-β1/Smads signal transduction and is
negatively regulated by this pathway (9). The present study demonstrated that
the alteration of Smad7 is conversed with TGF-β1 and Smad3 in all
groups, and increased expression of Smad7 accompanied by the
amelioration of ventricular remodeling, suggesting that simvastatin
improves post-MI ventricular remodeling attributed to its effects
in upregulating the expression of Smad7.
It had been proved medically that early
administration of large doses of statins as an intensive therapy
for patients with acute coronary syndrome can lower the incidence
of coronary artery events (23–27).
In the present study, three groups with different simvastatin
dosages were set up to observe their effects on ventricular
remodeling. The present study demonstrated that Sim2 and Sim4 were
superior compared with Sim1 in efficacy by improving ventricular
remodeling and hemodynamic parameters, with comparable Sim2 and
Sim4 potency, suggesting that for MI model rats, simvastatin
exhibited a dose-dependent manner with 20
mg.kg−1.d−1 as potential therapeutic range,
however, not exceeding this dose.
In conclusion, simvastatin treatment may
significantly improve ventricular remodeling following MI, inhibit
cardiomyocyte hypertrophy and interstitial fibrosis, improve the
compliance of ventricular wall and improve cardiac function. These
outcomes proved to be independent of its lipid-regulating effect,
but achieved through intracellular signal transduction of TGF-β1
inhibition.
Acknowledgments
The present study was supported by the National
Natural Science Fund (no. 81570212), the Natural Science Foundation
Project of CQ CSTC (no. cstc2011jjA10008), the Chongqing Municipal
Health Bureau fund (nos. 2010-1-07, 2012-2-125 and ZY20132124), and
the National key Clinical Specialties Construction Program of China
(no. 2011-170). The authros would like to thank Mr. Jianyong Wu and
Mr. Dezhang Zhao (Institute of Life Sciences, Chongqing Medical
University, Chongqing, China) for their excellent technical support
for the flow cytometry analysis.
References
|
1
|
Wassmann S, Laufs U, Bäumer AT, Müller K,
Ahlbory K, Linz W, Itter G, Rösen R, Böhm M and Nickenig G: HMG-CoA
reductase inhibitors improve endothelial dysfunction in
normocholesterolemic hypertension via reduced production of
reactive oxygen species. Hypertension. 37:1450–1457. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stumpf C, Petzi S, Seybold K, Wasmeier G,
Arnold M, Raaz D, Yilmaz A, Daniel WG and Garlichs CD: Atorvastatin
enhances interleukin-10 levels and improves cardiac function in
rats after acute myocardial infarction. Clin Sci (Lond). 116:45–52.
2009. View Article : Google Scholar
|
|
3
|
Porter KE and Turner NA: Statins and
myocardial remodelling: Cell and molecular pathways. Expert Rev Mol
Med. 13:e222011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correale M, Abruzzese S, Greco CA,
Concilio M, Biase MD and Brunetti ND: Pleiotropic effects of statin
in therapy in heart failure: A review. Curr Vasc Pharmacol.
12:873–884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonifacio A, Mullen PJ, Mityko IS,
Navegantes LC, Bouitbir J and Krähenbühl S: Simvastatin induces
mitochondrial dysfunction and increased atrogin-1 expression in
H9c2 cardiomyocytes and mice in vivo. Arch Toxicol. 2014.Epub ahead
of print. PubMed/NCBI
|
|
6
|
Node K, Fujita M, Kitakaze M, Hori M and
Liao JK: Short-term statin therapy improves cardiac function and
symptoms in patients with idiopathic dilated cardiomyopathy.
Circulation. 108:839–843. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horwich TB, MacLellan WR and Fonarow GC:
Statin therapy is associated with improved survival in ischemic and
non-ischemic heart failure. J Am Coll Cardio. 43:642–648. 2004.
View Article : Google Scholar
|
|
8
|
Li L, Chen Y, Doan J, Murray J, Molkentin
JD and Liu Q: Transforming growth factor β-activated kinase 1
signaling pathway critically regulates myocardial survival and
remodeling. Circulation. 130:2162–2172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang BW, Wu GJ, Cheng WP and Shyu KG:
Mechanical stretch via transforming growth factor-β1 activates
microRNA-208a to regulate hypertrophy in cultured rat cardiac
myocytes. J Formos Med Assoc. 112:635–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding YF, Peng YR, Li J, Shen H, Shen MQ
and Fang TH: Gualou xiebai decoction prevents myocardial fibrosis
by blocking TGF-beta/Smad signalling. J Pharm Pharmacol.
65:1373–1381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang HY, Chu JF, Li P, Li N and Lv ZH:
Expression and diagnosis of transient receptor potential vanilloid1
in urothelium of patients with overactive bladder. J Biol Regul
Homeost Agents. 29:875–879. 2015.
|
|
13
|
Guo Y, Shi DZ, Yin HJ and Chen KJ: Effects
of tribuli saponins on ventricular remodeling after myocardial
infarction in hyper-lipidemic rats. Am J Chin Med. 35:309–316.
2007. View Article : Google Scholar
|
|
14
|
Nishikawa H, Miura S, Zhang B, Shimomura
H, Arai H, Tsuchiya Y, Matsuo K and Saku K: Statins induce the
regression of left ventricular mass in patients with angina. Circ
J. 68:12l–125. 2004. View Article : Google Scholar
|
|
15
|
Li TS, Takahashi M, Suzuki R, Kobayashi T,
Ito H, Mikamo A and Hamano K: Pravastatin improves remodeling and
cardiac function after myocardial infarction by an antiinflammatory
mechanism rather than by the induction of angiogenesis. Ann Thorac
Surg. 81:2217–2225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin J, Denver R, Bailey M and Krum H:
In vitro inhibitory effects of atorvastatin on cardiac fibroblasts:
Implications for ventricular remodeling. Clin Exp Pharmacol
Physiol. 32:697–701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang DY, Qin S, Tang XJ and Yang H:
Effect of simvastatin on left ventricular remodeling and heart
function in rats with myocardial infarction. Chin Pharmacol Bull.
22:814–818. 2006.
|
|
18
|
Bauersachs J, Galuppo P, Fraccarollo D,
Christ M and Ertl G: Improvement of left ventricular remodeling and
function by hydroxymethylglutaryl coenzyme a reductase inhibition
with cerivastatin in rats with heart failure after myocardial
infarction. Circulation. 104:982–985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Cheng X, Liao YH, Lu B, Yang Y,
Li B, Ge H, Wang M, Liu Y, Guo Z and Zhang L: Simvastatin regulates
myocardial cytokine expression and improves ventricular remodeling
in rats after acute myocardial infarction. Cardiovasc Drugs Ther.
19:13–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flanders KC: Smad3 as a mediator of the
fibrotic response. Int J Exp Pathol. 85:47–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumoto-Ida M, Takimoto Y, Aoyama T,
Akao M, Takeda T and Kita T: Activation of TGF-beta1-TAK1-p38 MAPK
pathway in spared cardiomyocytes is involved in left ventricular
remodeling after myocardial infarction in rats. Am J Physiol Heart
Circ Physiol. 290:H709–H715. 2006. View Article : Google Scholar
|
|
22
|
Jinnin M, Ihn H and Tamaki K:
Characterization of SIS3, a novel specific inhibitor of smad3 and
its effect on transforming growth factor-beta1-induced
extracellular matrix expression. Mol Pharmacol. 69:597–607. 2006.
View Article : Google Scholar
|
|
23
|
Johnson C, Waters DD, DeMicco DA, Breazna
A, Bittner V, Greten H, Grundy SM and LaRosa JC: Comparison of
effectiveness of atorvastatin 10 mg versus 80 mg in reducing major
cardiovascular events and repeat revascularization in patients with
previous percutaneous coronary intervention (post hoc analysis of
the Treating to New Targets [TNT] Study). Am J Cardiol.
102:1312–1317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reddy G and Bittner V: LDL lowering after
acute coronary syndrome: Is lower better? Curr Treat Options
Cardiovasc Med. 15:33–40. 2013. View Article : Google Scholar
|
|
25
|
Colivicchi F, Tubaro M and Santini M:
Clinical implications of switching from intensive to moderate
statin therapy after acute coronary syndromes. Int J Cardiol.
152:56–60. 2011. View Article : Google Scholar
|
|
26
|
Shepherd J, Kastelein JJ, Bittner V,
Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A and Wenger
NK: TNT (Treating to New Targets) Investigators. Intensive lipid
lowering with atorvastatin in patients with coronary heart disease
and chronic kidney disease: The TNT (Treating to New Targets)
study. J Am Coll Cardiol. 51:1448–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Apiyasawat S, Sritara P, Ngarmukos T,
Sriratanasathavorn C and Kasemsuwan P: Association of statin
therapy with ventricular arrhythmias among patients with acute
coronary syndrome. Heart Asia. 5:39–41. 2013. View Article : Google Scholar : PubMed/NCBI
|