Introduction
Aquaporins are integral membrane channel proteins
that facilitate transcellular water movements (1). To date, 13 AQPs (AQP0-AQP12) have
been cloned in mammals. AQP3, 7, 9 and 10 constitute the
aquaglyceroporin (AQP) subfamily of the aquaporin family, and are
permeable to both water and glycerol (2). Apart from the transport of small
molecules, the AQPs are involved in a variety of biological
processes, including tissue swelling (3), glucolipid metabolism (4), neural signal transduction (5) and cell migration (6). AQP9 is a unique AQP channel in
hepatocytes, while AQP3 and AQP7 act as glycerol channels in
adipocytes (7). The three AQP
subtypes serve key roles in glucolipid metabolism (8,9).
Accumulating evidence indicates a close association
between the expression of AQPs and carcinogenesis (10). For instance, AQP3 is expressed in
human esophageal and oral squamous cell carcinoma, and contributes
to tumor cell growth (11). Tan
et al (12) reported that
the expression of AQP9 is significantly higher in human astrocytic
tumors compared with that in normal brain tissues, and is
positively correlated with pathological grade. Hepatocellular
carcinoma (HCC) is one of the most common gastrointestinal
malignancies worldwide, with a particularly high incidence in Asian
countries (13). It has been
previously documented that combined overexpression of AQP3 and AQP5
is an independent poor prognostic factor for HCC (14). Decreased expression levels of AQP8
and AQP9 has been revealed to confer apoptosis resistance in a rat
HCC line (15). These previous
studies suggest that AQPs are involved in the development and
progression of HCC. However, the expression and clinical
significance of glycolipid metabolism-associated AQPs (AQP3, 7 and
9) in HCC remains to be fully elucidated.
Therefore, the present study assessed the mRNA and
protein expression levels of AQP3, 7 and 9 in human HCCs and
adjacent non-tumorous liver (NTL) tissues, and explored the
association of AQP proteins with the clinicopathological features
of HCC.
Materials and methods
Tissue specimens
The present study enrolled a total of 68 HCC
patients who underwent hepatectomy at the affiliated hospitals of
Chongqing Medical University (Chongqing, China) between October
2009 and May 2013. All patients were pathologically diagnosed with
HCC. Fresh tumor samples, coupled with adjacent NTL tissues, were
collected from each patient. Some of the excised tissues were
placed immediately in liquid nitrogen and stored at −80°C until
gene expression analysis. The other tissue samples were fixed,
paraffin-embedded and sectioned (4 μm; OML-QPA/QPB; Hubei
OML Medical Science & Technology Co., Ltd., Xiaogan, China) for
immunohistochemistry. Written informed consent was obtained from
each patient and the study protocol was approved by the Ethics
Committee of Chongqing Medical University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the tissue samples
using the RNAiso Plus reagent (Takara Bio, Inc., Tokyo, Japan).
cDNA was synthesized from the total RNA using the PrimeScript RT
reagent Kit (Takara Bio, Inc.). RT-qPCR was performed on a CFX96
Real-Time System PCR detecting system (Bio-Rad Laboratories,
Hercules, CA, USA) using the SYBR Premix Ex Taq II kit (Takara Bio,
Inc.). The primers used are as follows: AQP3, sense: 5′-CCT CTG GAC
ACT TGG ATA TGA T-3′ and antisense: 5′-GGG ACG GGG TTG TTG TAG-3′;
AQP7, sense: 5′-CCG CAT CTT CAC CTT CAT TG-3′ and antisense: 5′-CAC
CCA CCA CCA GTT CTC-3′; AQP9, sense: 5′-ATC CAC CAG AAG TTG TTT-3′
and antisense 5′-AGC AAT GAC AAT AAT CAG GAGGC-3′. For the control,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified in a
parallel reaction, with the following primers: GAPDH, sense: 5′-GGT
GGT CTC CTC TGA CTT CAA CA-3′ and antisense: 5′-GTT GCT GTA GCC AAA
TTC GTT GT-3′. The cycling conditions were as follows: Initial
denaturation at 95°C for 3 min, followed by 40 cycles of
denaturation at 95°C for 10 sec and annealing at 60°C for 30 sec.
The data were analyzed using the 2−ΔΔCq method (16). The relative mRNA levels were
calculated following normalization against GAPDH mRNA levels.
Western blot analysis
Tissue samples were homogenized in
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) containing 1% sodium dodecyl
sulfate (SDS) and 1% phenylmethylsulfonyl fluoride (Sigma-Aldrich,
St. Louis, MO, USA), a potent protease inhibitor. The protein
concentrations were measured using the bicinchoninic acid Protein
Assay kit (Pierce, Rockford, IL, USA). Equal quantities of the
total protein (~100 μg) were separated by 12%
SDS-polyacrylamide gel electrophoresis and were transferred onto a
polyvinylidene fluoride membrane. The membrane was blocked at room
temperature for 1 h with 5% fat-free milk, and incubated overnight
with primary antibodies at 4°C. The following primary antibodies
were used: Mouse monoclonal anti-GAPDH (1:500; cat. no. TA-08;
ZSGB-BIO Co., Ltd., Beijing, China), rabbit polyclonal anti-AQP3
(1:500; cat. no. LS-B8185; LifeSpan Biosciences, Inc., Seattle, WA,
USA), rabbit polyclonal anti-AQP7 (1:300; cat. no. ab85907;Abcam,
Cambridge, MA, USA) and rabbit polyclonal anti-AQP9 (1:500; cat.
no. ab85910; Abcam). The membranes were washed with Tris-buffered
saline and Tween 20 buffer (ZSGB-BIO Co., Ltd.) and incubated for 1
h at room temperature with goat anti-rabbit (cat. no. ZB-2301) or
anti-mouse immunoglobulin G (ZB-2305)(1:3,000; ZSGB-BIO Co., Ltd.).
The proteins were visualized using an enhanced chemiluminescence
detection kit (ECL plus; Beyotime Institute of Biotechnology).
Relative band intensities (AQP/GAPDH protein ratios) were
determined by densitometry using the Quality One software (version
4.62; Bio-Rad Laboratories).
Immunohistochemistry
Tissue specimens were embedded in paraffin and cut
into 4 μm sections. The tissue sections were dewaxed in
xylene, rehydrated, and heated in citrate buffer (ZSGB-BIO Co.,
Ltd.) for 20 min at 100°C to retrieve antigen. Following the
elimination of endogenous peroxidase, the tissue sections were
blocked with 3% hydrogen peroxidase diluted with methyl alcohol for
20 min and washed with phosphate-buffered saline (PBS), and
incubated overnight at 4°C with anti-AQP3 (1:100), anti-AQP7
(1:200) or anti-AQP9 (1:200) primary antibodies. Negative controls
were included by omitting the primary antibody. The membranes were
then washed with PBS for 10 min, and the secondary antibody
reaction was performed using the Polink-2 plus Polymer Horseradish
Peroxidase Detection system (GBI Labs, Mukilteo, WA, USA),
according to the manufacturer's protocol. The tissue sections were
developed with 3,3-diaminobenzidine (ZSGB-BIO Co., Ltd.) and were
counterstained with hematoxylin. The stained sections were
independently assessed by two pathologists in a blinded manner. The
median percentage of immunostained tumor cells (10%) was used as a
cutoff. High expression of AQPs was defined as nuclear staining of
≥10% of the tumor cells and low expression of AQPs was defined as
nuclear staining of <10% of the tumor cells or no nuclear
staining.
Statistical analysis
The data are presented as the mean ± standard
deviation. All statistical calculations were performed using SPSS
version 18.0 (IBM SPSS, Chicago, IL, USA). Continuous data were
compared using the paired Student's t-test. The correlation between
the expression levels of AQP3, 7 and 9 and the association between
AQP expression and the clinicopathological features of HCC were
analyzed using the χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
mRNA expression levels of AQPs in
HCC
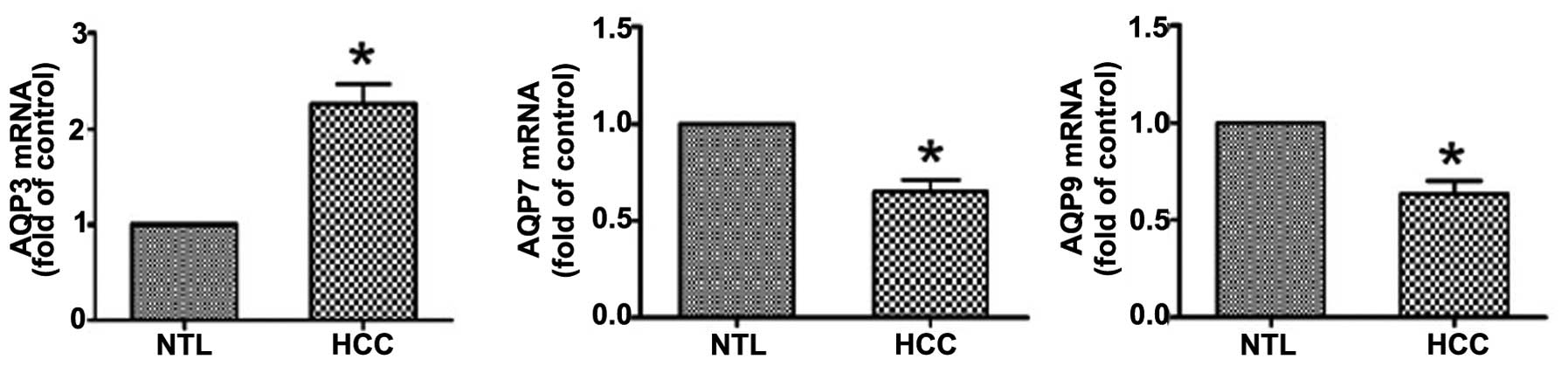
RT-qPCR analysis revealed that compared with NTL
tissue, HCC tissues exhibited a significant (P<0.05) increase in
the AQP3 mRNA level and a concomitant reduction in the mRNA
expression levels of AQP7 and AQP9 (Fig. 1).
Protein expression levels of AQPs in
HCC
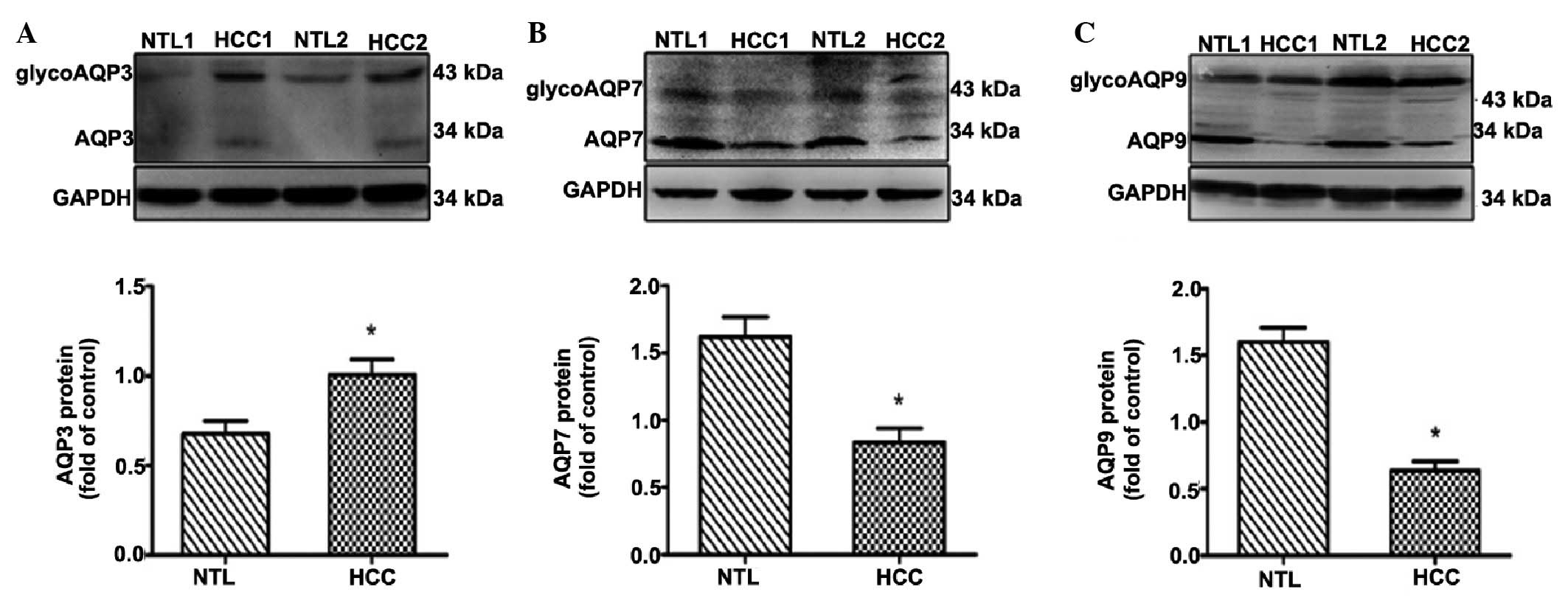
Western blot analysis confirmed an upregulation of
AQP3 and downregulation of AQP7 and AQP9 in HCC compared with in
the NTL tissue (Fig. 2).
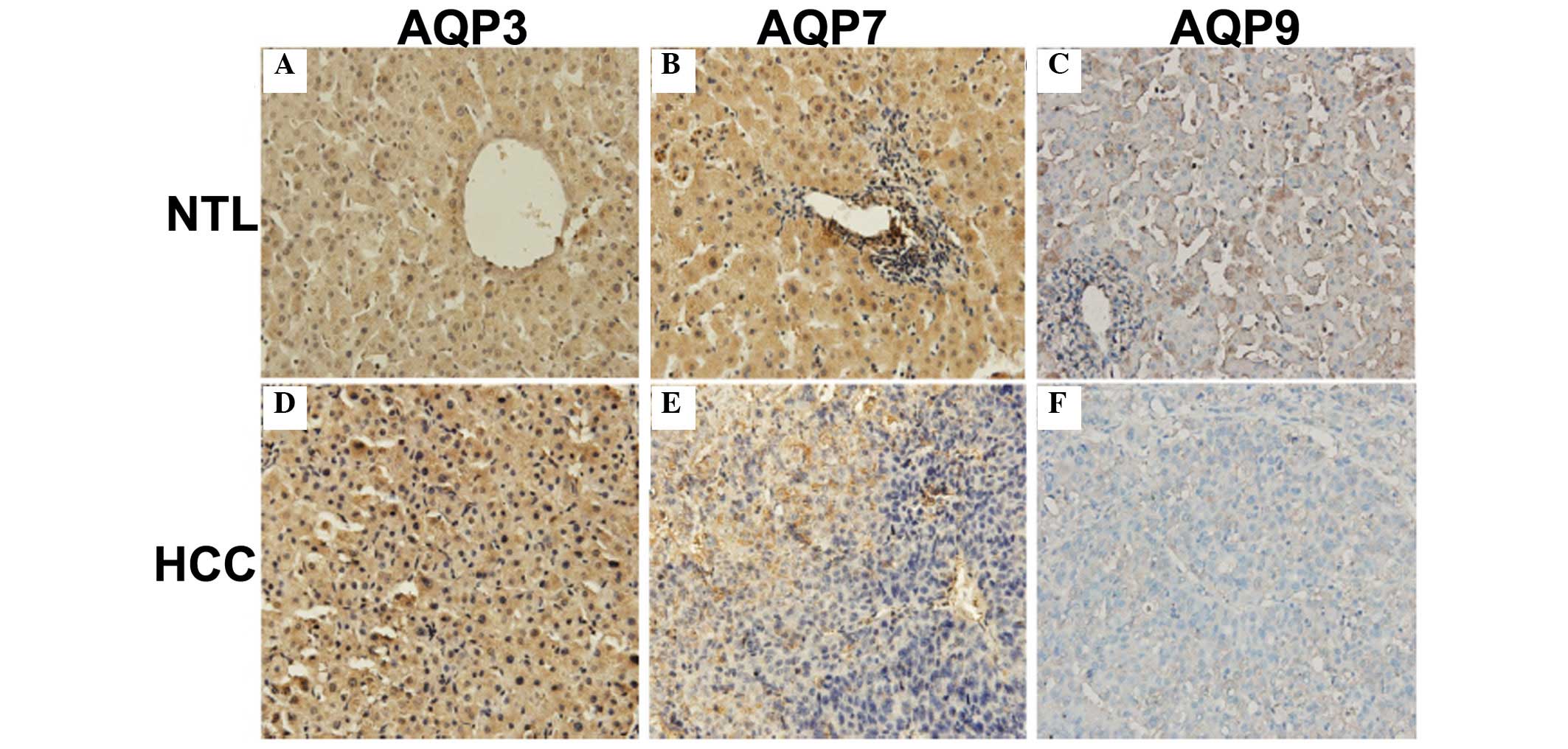
Immunohistochemistry was performed to determine the location and
distribution of AQPs in HCC. AQP9 was localized on the plasma
membrane and in the cytoplasm of hepatocytes, while AQP3 and AQP7
showed predominantly cytoplasmic and nuclear distribution (Fig. 3). The majority of HCC tissues
exhibited a significant decrease in the expression levels of AQP7
and AQP9, compared with adjacent NTL tissues (P<0.0001 for each
comparison; Table I). By contrast,
an increased expression of AQP3 was observed in HCC compared with
the NTL tissues (P=0.0035; Table
I).
 | Table IAltered mRNA expression levels of
AQP3, 7 and 9 in human HCC tissues and pair-matched NTL
tissues. |
Table I
Altered mRNA expression levels of
AQP3, 7 and 9 in human HCC tissues and pair-matched NTL
tissues.
| Group | AQP3 | AQP7 | AQP9 |
|---|
| NTL (n=68) | 1.000±0.000 | 1.000±0.000 | 1.000±0.000 |
| HCC (n=68) | 2.259±0.210 | 0.652±0.487 | 0.636±0.069 |
| t-test | 3.025 | 4.928 | 6.596 |
| P-value | <0.0001 | <0.0001 | <0.0001 |
| R-value | 0.120 | 0.266 | 0.394 |
| 95% CI |
(−0.546)–(−0.112) | 0.467–1.104 | 0.672–1.256 |
Correlation between the expression levels
of AQP3, 7 and 9
To determine whether a correlation exists between
the protein expression levels of AQP3, 7 and 9 in HCC tissues, a
χ2 test was performed. As shown in Table II, high expression of AQP3 was
significantly (P<0.05) associated with low expression of AQP7
(r=0.479; P<0.05) and AQP9 (r=0.448; P<0.05) in HCC tissues.
However, no significant correlation was observed between AQP7 and
AQP9 (data not shown)
 | Table IICorrelation analysis of the protein
expression levels of AQP3, AQP7 and AQP9 in HCC tissues (n=68). |
Table II
Correlation analysis of the protein
expression levels of AQP3, AQP7 and AQP9 in HCC tissues (n=68).
| AQP3
| χ2 | r | P-value |
|---|
| High (n=46) | Low (n=22) |
|---|
| AQP7 | | | | | |
| High (n=12) | 5 | 7 | 4.494 | −0.479 | <0.05 |
| Low (n=56) | 41 | 15 | | | |
| AQP9 | | | | | |
| High (n=10) | 4 | 6 | 4.095 | −0.448 | <0.05 |
| Low (n=58) | 42 | 16 | | | |
Correlation between AQP proteins and the
clinicopathological parameters of HCC
Clinicopathological features and the expression of
AQP proteins in the 68 HCC patients were summarized in Table III. High expression of AQP3
correlated with tumor grade (χ2=5.740; P=0.017), tumor
stage (χ2=6.680; P=0.010) and lymphatic metastasis
(χ2=4.636; P=0.031). Low expression of AQP7 was
correlated with tumor grade (χ2=4.091; P=0.043).
However, the expression of AQP9 protein revealed no association
with any of the clinicopathological factors studied.
 | Table IIICorrelation between AQP proteins and
clinicopathological parameters of HCC (n=68). |
Table III
Correlation between AQP proteins and
clinicopathological parameters of HCC (n=68).
| Variable | AQP3 protein
| AQP7 protein
| AQP9 protein
|
|---|
| Low (n=22) | High (n=46) | χ2 | P-value | Low (n=56) | High (n=12) | χ2 | P-value | Low (n=58) | High (n=10) | χ2 | P-value |
|---|
| Age (year) | | | | | | | | | | | | |
| <50 | 8 (36.4%) | 19 (41.3%) | 0.152 | 0.697 | 21 (37.5%) | 6 (50.0%) | 0.229 | 0.633 | 23 (39.7%) | 4 (40%) | 0.109 | 0.742 |
| ≥50 | 14 (63.6%) | 27 (58.7%) | | | 35 (62.5%) | 6 (50.0%) | | | 35 (60.3%) | 6 (10%) | | |
| Gender | | | | | | | | | | | | |
| Male | 15 (68.2%) | 37 (80.4%) | 0.654 | 0.419 | 41 (73.2%) | 11 (91.7%) | 0.985 | 0.321 | 44 (75.9%) | 8 (80%) | 0.014 | 0.906 |
| Female | 7 (31.8%) | 9 (19.6%) | | | 15 (26.8%) | 1 (8.3%) | | | 14 (24.1%) | 2 (20%) | | |
| Tumor size
(cm) | | | | | | | | | | | | |
| <3 | 6 (27.3%) | 15 (32.6%) | 0.199 | 0.656 | 18 (32.1%) | 3 (25.0%) | 0.020 | 0.887 | 19 (32.8%) | 2 (20%) | 0.190 | 0.663 |
| ≥3 | 16 (72.7%) | 31 (67.4%) | | | 38 (67.9%) | 9 (75.0%) | | | 39 (67.2%) | 8 (80%) | | |
| Tumor number | | | | | | | | | | | | |
| Single | 18 (81.8%) | 39 (84.8%) | 0.002 | 0.967 | 46 (82.1%) | 11 (91.7%) | 0.145 | 0.703 | 47 (81.0%) | 10 (100%) | 1.080 | 0.299 |
| Multiple | 4 (18.2%) | 7 (15.2%) | | | 10 (17.9%) | 1 (8.3%) | | | 11 (19.0%) | 0 (0%) | | |
| Tumor grade | | | | | | | | | | | | |
| Moderate or
well | 20 (90.9%) | 29 (63.0%) | 5.740 | 0.017 | 37 (66.1%) | 12 (100.0%) | 4.091 | 0.043 | 39 (67.2%) | 10 (100%) | 3.065 | 0.080 |
| Poor | 2 (9.1%) | 17 (37.0%) | | | 19 (33.9%) | 0 (0.0%) | | | 19 (32.8%) | 0 (0%) | | |
| Tumor stage | | | | | | | | | | | | |
| I–II | 19 (86.4%) | 25 (54.3%) | 6.680 | 0.010# | 33 (58.9%) | 11 (91.7%) | 3.315 | 0.069 | 37 (63.8%) | 7 (70%) | 0.000 | 0.983 |
| III–IV | 3 (13.6%) | 21 (45.7%) | | | 23 (41.1%) | 1 (8.3%) | | | 21 (36.2%) | 3 (30%) | | |
| PVTT | | | | | | | | | | | | |
| Yes | 2 (9.1%) | 8 (17.4%) | 0.290 | 0.591 | 10 (17.9%) | 0 (0.0%) | 1.290 | 0.256 | 10 (17.2%) | 0 (0%) | 0.881 | 0.348 |
| No | 20 (90.9%) | 38 (82.6%) | | | 46 (82.1%) | 12 (100.0%) | | | 48 (82.8%) | 10 (100%) | | |
| LM | | | | | | | | | | | | |
| Yes | 0 (0.0%) | 11 (23.9%) | 4.636 | 0.031 | 11 (19.6%) | 0 (0.0%) | 1.550 | 0.213 | 10 (17.2%) | 1 (10%) | 0.012 | 0.913 |
| No | 22 (100.0%) | 35 (76.1%) | | | 45 (80.4%) | 12 (100.0%) | | | 48 (82.8%) | 9 (90%) | | |
| TM | | | | | | | | | | | | |
| Yes | 1 (4.5%) | 13 (28.3%) | 3.772 | 0.052 | 13 (23.2%) | 1 (8.3%) | 0.583 | 0.445 | 13 (22.4%) | 1 (10.0%) | 0.224 | 0.636 |
| No | 21 (95.5%) | 33 (71.7%) | | | 43 (76.8%) | 11 (91.7%) | | | 45 (77.6%) | 9 (90.0%) | | |
| HBsAg | | | | | | | | | | | | |
| Positive | 19 (86.4%) | 36 (78.3%) | 0.217 | 0.642 | 45 (80.4%) | 10 (83.3%) | 0.028 | 0.868 | 46 (79.3%) | 9 (90%) | 0.129 | 0.720 |
| Negative | 3 (13.6%) | 10 (21.7%) | | | 11 (19.6%) | 2 (16.7%) | | | 12 (20.7%) | 1 (10%) | | |
| AFP (µg/l) | | | | | | | | | | | | |
| <400 | 19 (86.4%) | 36 (78.3%) | 0.217 | 0.642 | 46 (82.1%) | 9 (75.0%) | 0.028 | 0.868 | 46 (79.3%) | 9 (90%) | 0.129 | 0.720 |
| ≥400 | 3 (13.6%) | 10 (21.7%) | | | 10 (17.9%) | 3 (25.0%) | | | 12 (20.7%) | 1 (10%) | | |
| Liver
cirrhosis | | | | | | | | | | | | |
| Yes | 6 (27.3%) | 18 (39.1%) | 0.471 | 0.493 | 17 (30.4%) | 7 (58.3%) | 2.273 | 0.132 | 20 (34.5%) | 4 (40%) | 0.000 | 0.983 |
| No | 16 (72.7%) | 28 (60.9%) | | | 39 (69.6%) | 5 (41.7%) | | | 38 (65.5%) | 6 (60%) | | |
Discussion
As water transporters, AQPs are expressed in a
variety of tissues and cells (1,17).
Accumulating evidence indicates that AQPs are frequently
dysregulated in cancer and serve critical roles in tumor
development and progression (10).
The expression of AQP3 has been shown to be upregulated in
colorectal carcinoma (18),
gastric cancer (19), cervical
cancer (20) and HCC (14). Guo et al (14) reported that elevated expression of
AQP3 and AQP5, as determined by immunohistochemistry, is
significantly associated with tumor progression and prognosis in
patients with HCC. The present data confirmed the upregulation of
AQP3 in HCC compared with that in adjacent NTL tissue.
Additionally, it was revealed that the mRNA and protein expression
levels of AQP3 were consistently increased in HCC, suggesting that
the upregulation of AQP3 likely occurs at the transcriptional
level. It has been documented that the expression of AQP9 is
significantly decreased in HCC tissues compared with NTL tissues
(21). In agreement with this
previous study, the present results demonstrated that both the mRNA
and protein expression levels of AQP9 were reduced in HCC compared
with the adjacent NTL tissue. The downregulation of AQP9 has been
shown to induce apoptosis resistance in HCC cells (21). Notably, AQP7 has been identified in
human urothelial carcinoma (22).
The present study provided the first evidence, to the best of our
knowledge, that AQP7 was downregulated in HCC compared with
adjacent NTL tissue. This downregulation may be due to
transcriptional inhibition, since the mRNA and protein expression
levels of AQP7 were consistently decreased. Taken together, the
present data demonstrated that HCCs exhibited coordinated
expression of AQPs. However, the exact mechanisms for their
dysregulation require further clarified.
By immunohistochemistry, AQPs exhibited different
localization patterns in HCC. It was found that AQP9 was localized
on the plasma membrane and in the cytoplasm of hepatocytes, while
AQP3 and AQP7 exhibited cytoplasmic and nuclear distribution. Nihei
et al (23) reported that
AQP9 is normally localized on the surface of rat hepatocytes and
Leydig cells. Similarly, Elkjaer et al (24) demonstrated an immunolocalization of
AQP9 on the plasma membrane of liver hepatocytes. The data from
knockout mice support the essential role for AQP9 in glycerol
transport (25). Previous studies,
combined with the present findings, suggested that AQP9 may
facilitate the transport of water, glycerol and other small
molecules in HCC cells. The cytoplasmic and nuclear expression
pattern of AQP3 and AQP7 suggested that the two proteins may be
involved in the regulation of gene expression. Indeed, Xie et
al (26) demonstrated that
AQP3 has a protective activity against ultraviolet A-induced human
skin fibroblast apoptosis via the upregulation of B-cell
lymphoma-2. AQP3 depletion has been shown to induce the expression
of p21 and FAS in cancer cells (27). Forced expression of AQP7 leads to
improved insulin resistance by increasing the phosphorylation of
protein kinase B (28). Taken
together, different expression and localization patterns of AQPs in
HCC indicate their distinct roles in tumor progression.
The present data demonstrated that high expression
of AQP3 correlated with tumor grade, tumor stage and lymphatic
metastasis in HCC, suggesting its favorable role in HCC
progression. The tumor-promoting effects of AQP3 have also been
previously described in several other cancer types. For instance,
Chen et al (29) reported
that AQP3 facilitates the epithelial-mesenchymal transition in
gastric cancer. Li et al (18) revealed that AQP3 overexpression
promotes colorectal carcinoma cell migration and is significantly
associated with tumor metastasis. Different from AQP3, low
expression of AQP7 was found to be significantly correlated with
tumor grade in HCC patients, implying that AQP7 may exert
suppressive effects on HCC. Although the protein expression of AQP9
exhibited no significant association with HCC clinicopathological
features, low expression of AQP9 was significantly associated with
high expression of AQP3 in patients with HCC. It has been
documented that AQP9 and AQP7 are implicated in the uptake of
certain chemotherapeutic agents into cancer cells (30,31).
Decreased expression of AQP9 is associated with increased
resistance to apoptosis in HCC cells (15). Therefore, the dysregulation of
AQP3, 7 and 9 may cooperatively contribute to the pathogenesis of
HCC. However, their biological functions in HCC requires further
elucidation.
In conclusion, the present data demonstrated that
AQP3 is upregulated, and that AQP7 and AQP9 are downregulated in
HCC. It was also revealed that the three investigated AQPs exhibit
different intracellular localizations in HCC hepatocytes. High
expression of AQP3 was significantly associated with tumor grade,
tumor stage and lymphatic metastasis in patients with HCC, while
low expression of AQP7 was significantly correlated with tumor
grade. The present results suggest a complex role for AQPs in the
development and progression of HCC. Additional direct studies are
required to determine the biological functions of AQPs in this
malignancy.
References
|
1
|
Ishibashi K, Hara S and Kondo S: Aquaporin
water channels in mammals. Clin Exp Nephrol. 13:107–117. 2009.
View Article : Google Scholar
|
|
2
|
Mukhopadhyay R, Bhattacharjee H and Rosen
BP: Aquaglyceroporins: Generalized metalloid channels. Biochim
Biophys Acta. 1840:1583–1591. 2014. View Article : Google Scholar :
|
|
3
|
Papadopoulos MC and Verkman AS:
Aquaporin-4 gene disruption in mice reduces brain swelling and
mortality in pneumococcal meningitis. J Biol Chem. 280:13906–13912.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calamita G, Gena P, Ferri D, Rosito A,
Rojek A, Nielsen S, Marinelli RA, Frühbeck G and Svelto M:
Biophysical assessment of aquaporin-9 as principal facilitative
pathway in mouse liver import of glucogenetic glycerol. Biol Cell.
104:342–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amiry-Moghaddam M, Williamson A, Palomba
M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre
P and Ottersen OP: Delayed K+ clearance associated with aquaporin-4
mislocalization: Phenotypic defects in brains of
alpha-syntrophin-null mice. Proc Natl Acad Sci USA.
100:13615–13620. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hara-Chikuma M and Verkman AS: Aquaporin-1
facilitates epithelial cell migration in kidney proximal tubule. J
Am Soc Nephrol. 17:39–45. 2006. View Article : Google Scholar
|
|
7
|
Rodríguez A, Catalán V, Gómez-Ambrosi J,
García-Navarro S, Rotellar F, Valentí V, Silva C, Gil MJ, Salvador
J, Burrell MA, et al: Insulin- and leptin-mediated control of
aquaglyceroporins in human adipocytes and hepatocytes is mediated
via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab.
96:E586–E597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeda N: Implications of aquaglyceroporins
7 and 9 in glycerol metabolism and metabolic syndrome. Mol Aspects
Med. 33:665–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodríguez A, Catalán V, Gómez-Ambrosi J
and Frühbeck G: Aquaglyceroporins serve as metabolic gateways in
adiposity and insulin resistance control. Cell Cycle. 10:1548–1556.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribatti D, Ranieri G, Annese T and Nico B:
Aquaporins in cancer. Biochim Biophys Acta. 1840:1550–1553. 2014.
View Article : Google Scholar
|
|
11
|
Kusayama M, Wada K, Nagata M, Ishimoto S,
Takahashi H, Yoneda M, Nakajima A, Okura M, Kogo M and Kamisaki Y:
Critical role of aquaporin 3 on growth of human esophageal and oral
squamous cell carcinoma. Cancer Sci. 102:1128–1136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan G, Sun SQ and Yuan DL: Expression of
the water channel protein aquaporin-9 in human astrocytic tumours:
Correlation with pathological grade. J Int Med Res. 36:777–782.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15(Suppl
4): S5–S13. 2010. View Article : Google Scholar
|
|
14
|
Guo X, Sun T, Yang M, Li Z, Li Z and Gao
Y: Prognostic value of combined aquaporin 3 and aquaporin 5
overexpression in hepatocellular carcinoma. Biomed Res Int.
2013:2065252013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jablonski EM, Mattocks MA, Sokolov E,
Koniaris LG, Hughes FM Jr, Fausto N, Pierce RH and McKillop IH:
Decreased aquaporin expression leads to increased resistance to
apoptosis in hepatocellular carcinoma. Cancer Lett. 250:36–46.
2007. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Verkman AS, Anderson MO and Papadopoulos
MC: Aquaporins: Important but elusive drug targets. Nat Rev Drug
Discov. 13:259–277. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li A, Lu D, Zhang Y, Li J, Fang Y, Li F
and Sun J: Critical role of aquaporin-3 in epidermal growth
factor-induced migration of colorectal carcinoma cells and its
clinical significance. Oncol Rep. 29:535–540. 2013.
|
|
19
|
Chen J, Wang T, Zhou YC, Gao F, Zhang ZH,
Xu H, Wang SL and Shen LZ: Aquaporin 3 promotes
epithelial-mesenchymal transition in gastric cancer. J Exp Clin
Cancer Res. 33:382014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen R, Shi Y, Amiduo R, Tuokan T and
Suzuk L: Expression and prognostic value of aquaporin 1, 3 in
cervical carcinoma in women of Uygur ethnicity from Xinjiang,
China. PLoS One. 9:e985762014. View Article : Google Scholar
|
|
21
|
Jablonski EM, Mattocks MA, Sokolov E,
Koniaris LG, Hughes FM Jr, Fausto N, Pierce RH and McKillop IH:
Decreased aquaporin expression leads to increased resistance to
apoptosis in hepatocellular carcinoma. Cancer Lett. 250:36–46.
2007. View Article : Google Scholar
|
|
22
|
Rubenwolf PC, Otto W, Denzinger S,
Hofstädter F, Wieland W and Georgopoulos NT: Expression of
aquaporin water channels in human urothelial carcinoma: Correlation
of AQP3 expression with tumour grade and stage. World J Urol.
32:991–997. 2014. View Article : Google Scholar
|
|
23
|
Nihei K, Koyama Y, Tani T, Yaoita E,
Ohshiro K, Adhikary LP, Kurosaki I, Shirai Y, Hatakeyama K and
Yamamoto T: Immunolocalization of aquaporin-9 in rat hepatocytes
and Leydig cells. Arch Histol Cytol. 64:81–88. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elkjaer M, Vajda Z, Nejsum LN, Kwon T,
Jensen UB, Amiry-Moghaddam M, Frøkiaer J and Nielsen S:
Immunolocalization of AQP9 in liver, epididymis, testis, spleen,
and brain. Biochem Biophys Res Commun. 276:1118–1128. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Promeneur D, Rojek A, Kumar N,
Frøkiaer J, Nielsen S, King LS, Agre P and Carbrey JM: Aquaporin 9
is the major pathway for glycerol uptake by mouse erythrocytes,
with implications for malarial virulence. Proc Natl Acad Sci USA.
104:12560–12564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie H, Liu F, Liu L, Dan J, Luo Y, Yi Y,
Chen X and Li J: Protective role of AQP3 in UVA-induced NHSFs
apoptosis via Bcl2 up-regulation. Arch Dermatol Res. 305:397–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trigueros-Motos L, Pérez-Torras S, Casado
FJ, Molina-Arcas M and Pastor-Anglada M: Aquaporin 3 (AQP3)
participates in the cytotoxic response to nucleoside-derived drugs.
BMC Cancer. 12:4342012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen FX, Gu X, Pan W, Li WP, Li W, Ye J,
Yang LJ, Gu XJ and Ni LS: Over-expression of AQP7 contributes to
improve insulin resistance in adipocytes. Exp Cell Res.
318:2377–2384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Wang T, Zhou YC, Gao F, Zhang ZH,
Xu H, Wang SL and Shen LZ: Aquaporin 3 promotes
epithelial-mesenchymal transition in gastric cancer. J Exp Clin
Cancer Res. 33:382014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Shen J, Carbrey JM, Mukhopadhyay R,
Agre P and Rosen BP: Arsenite transport by mammalian
aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA.
99:6053–6058. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhattacharjee H, Carbrey J, Rosen BP and
Mukhopadhyay R: Drug uptake and pharmacological modulation of drug
sensitivity in leukemia by AQP9. Biochem Biophys Res Commun.
322:836–841. 2004. View Article : Google Scholar : PubMed/NCBI
|