Introduction
Despite numerous previous advances in the treatment
of cardiovascular disease, sudden cardiac death caused by ischemic
arrhythmias remains a major reason of mortality worldwide (1). Numerous intensive studies have been
conducted on researching and developing drugs to treat arrhythmias
over the course of the last few past decades, and only a few of
them have assisted in enabling patients to live longer whereas, in
effect, the majority of them may have led to mortality due to their
proarrhythmic potentials (2). The
underlying disease causing ischemic arrhythmias is usually
atherosclerotic coronary artery disease, which narrows or occludes
the coronary artery and induces myocardial ischemia and infarction
(3). A previous study has shown
that various mechanisms trigger ischemic arrhythmias during
myocardial ischemia, including reconstruction of the cardiac
sympathetic nerve, alterations of the transmembrane ion current and
intracellular Ca2+ ([Ca2+]i)
dysregulation (4). Myocardial
ischemia affects the electrophysiological properties of the cardiac
cells, inducing the generation and conduction of abnormal impulses,
reducing the fibrillation threshold and initiating ectopic
impulses. These ectopic beats cause abnormal current flow across
the boundary between ischemic and non-ischemic myocardium (5). In addition, Ca2+ overload
during the myocardial ischemia diastolic period causes
afterdepolarizations and aftercontractions, which are the primary
causes of electrical instability (6). Changes in the resting potential and
the inward and outward currents of the action potentials lead to
alterations in conduction, refractoriness and automaticity, all of
which contribute to the occurrence of ischemic arrhythmias.
Traditional Chinese medicines (TCMs) have been
extensively used to treat arrhythmias due to their stable curative
effects and low toxicity. In addition, TCMs mediate their effects
via different pathways and multiple targets simultaneously, due to
their various components. Shensong Yangxin capsules (SSYX) are
clinically used to treat coronary heart disease (7). The bioactive components of SSYX
include sodium danshensu, chlorogenic acid, paeoniflorin, spinosin,
salvianolic acid B, berberine hydrochloride, gensenoside
Rb1 and schisantherin A (8,9).
Several studies demonstrated that SSYX inhibited multiple ion
channels in cardiac cells, including the potassium/sodium
hyperpolarization-activated cyclic nucleotide-gated channel 4
(HCN4) and L-type calcium channel, which may affect the electrical
activity of myocardial cells and contribute to their antiarrhythmic
effects (10–12). The present study aimed to establish
whether SSYX prevents ischemic arrhythmias, and to explore the
underlying mechanisms.
Materials and methods
Establishment of ischemic
arrhythmias
SSYX (cat. no. Z20030058; Yiling Phamaceutical Co.,
Ltd., Shijiazhuang, China) and amiodarone (cat. no.
P20080122150958640; Harvest Pharmaceutical Co., Ltd., Shanghai,
China) were dissolved in distilled water and administered to rats
(13). Amiodarone was used for the
purposes of comparison. All experiments were approved by the ethics
committee of Harbin Medical University, and the health guide for
the use of experimental animals of the National Institutes was
observed. Sprague-Dawley rats (male; 180±20 g; 6–8 weeks) were
purchased from the Animal Center of the Second Affiliated Hospital
of Harbin Medical University (certificate no. SCXK (Ha) 2012–010).
The animals were housed under standard laboratory conditions
(temperature, 20–22°C; humidity, 40–70%; 12 h/12 h day-night cycle)
and divided into four groups at random: Sham (n=8) rats had the
chest opened without ligating the coronary artery; for the control
(n=8) rats, distilled water was administered intragastrically once
a day for 7 days prior to ligation of the coronary artery; for the
SSYX (n=8) rats, a suspension of SSYX (1.8 g/kg) was administered
to rats once a day for 7 days intragastrically prior to ligation of
the coronary artery; and for the amiodarone (n=8) rats, a
suspension of amiodarone (0.12 g/kg) was administered
intragastrically once a day for 7 days prior to ligation of the
coronary artery.
Ischemic arrhythmias induced by acute myocardial
ischemia (AMI) were performed as previously described (14,15).
At 1 h following the final administration of the respective drugs,
rats were anesthetized with 3% sodium pentobarbital (0.12 ml/100
g), and the left anterior descending branch of the coronary artery
was subsequently ligated. Electrocardiograms and the heart rate
were monitored using the BL-420F Data Acquisition and Analysis
system (TME Technology Co., Ltd, Chengdu, China). Evident S-T
elevation, as shown in the electrocardiogram, and a darkened
myocardium under the ligature indicated that the AMI model had been
successfully constructed. The incidence and onset of arrhythmias
were monitored on the electrocardiogram. The arrhythmia score was
calculated to assess the severity of arrhythmias, according to
Lambeth Conventions (16). The
infarct region was measured by Evans Blue (Sigma-Aldrich, St Louis,
USA) staining and 2,3,5-triphenyltetrazolium chloride (Sigma, St
Louis, USA) staining after ligation for 6 h. The area dyed grey was
defined as the infarction area, whereas the red coloration
indicated ischemic tissue, and blue indicated the presence of
normal myocardium (17). The
infarction area of each heart was cut and weighed to calculate the
ratio of the myocardial infarction region to the whole heart.
Whole-cell patch-clamp recording Serum
preparation
Serum containing SSYX was prepared according to a
previously described method (18).
Distilled water and suspensions of SSYX were administered to rats
by gavage (1.8 g/kg) once a day for 7 days. At 2 h following the
final administration, the blood was collected from the abdominal
aorta of rats and maintained for 1 h at 25°C. Subsequently, the
blood was centrifuged at 3,000 × g for 10 min to separate the
serum. The serum was then centrifuged at 3,000 × g for 30 min in a
3 kDa tube (Millipore Corp., Billerica, MA, USA) to remove
proteins. The filtrate was dried into serum powder sing a pressure
blowing concentrator (TTL-DCI model; Beijing Tongtailian Science
and Technology Development Co., Ltd., Beijing, China).
Subsequently, serum samples of the control and SSYX rats were
subjected to high-pressure liquid chromatography (HPLC) analysis.
The analysis was performed with a 5C18-MS-II packed column (model
38020–41; 250×4.6 mm; 5 μm; Cosmosil, Tokyo, Japan)
maintained at 30°C under isocratic flow conditions. The mobile
phase was 80% methanol, and the flow rate was 1 ml/min. SSYX serum
was monitored at 254 nm and identified using the LC-20A HPLC system
of Shimadzu (Shimadzu, Tokyo, Japan).
Separation and isolation of
cardiomyocytes
Cardiomyocytes were isolated from the rat hearts by
enzymatic dissociation (19).
Hearts were taken out from the chest of the rats following
anesthetization with sodium pentobarbital, and reversely perfused
through the aorta using a modified Langendorff perfusion system
with Ca2+ (−) Tyrode's solution containing (in mM):
NaCl, 126.0; KCl, 5.4; MgCl2, 1;
NaH2PO4, 0.33; glucose, 10 and Hepes, 10, at
pH 7.4 for 5 min. Ca2+ (−) Tyrode's solution was
oxygenated and maintained at 37°C prior to the experiment.
Subsequently, perfusate containing 160 mg/l collagenase II (Wako,
Osaka, Japan) and 160 mg/l bovine serum albumin (Sigma-Aldrich) was
used to digest myocardium continuously for ~20 min. After the heart
had become soft and light, the left ventricular tissues were cut up
and agitated gently in a test tube. Isolated single cardiomyocytes
were stored in preservation solution containing (in mM): glutamic
acid, 70; taurine, 15; KCl, 30; KH2PO4, 10;
MgCl2, 0.5; ethylene
glycol-O-O′-bis(2-amino-ethyl)-N,N,N′, N′-tetraacetic acid (EGTA),
0.5; Hepes, 10; and glucose, 10, at pH 7.4 and at room temperature
for later use.
Measurement of the action potential,
transient outward K+ current (Ito) and inward
rectifier K+ current (IK1)
Blank and SSYX sera were dissolved in 20%
Ca2+ Tyrode's solution. The action potential duration
(APD), Ito and IK1 in rat cardiomyocytes were
recorded using the patch-clamp technique (20–22).
Cells were placed in Ca2+ Tyrode's solution in the
chamber at room temperature. Borosilicate glass electrodes (Huaxi
Medical University Instrument Factory, Chengdu, China) with a tip
resistance of 2~4 MΩ were filled with the pipette solution
containing (in mM): KCl, 20; potassium aspartate, 110;
MgCl2, 1.0; Hepes, 5; EGTA, 10; and Na2ATP, 5
at pH 7.4. Action potentials were elicited using the current-clamp
mode. Cardiomyocytes were electrically stimulated by applying an
intracellular depolarizing stimulus (-5 ms duration and 900 pA
amplitude) via a digital pulse generator (DD1550A; Axon
Instruments, Inc., Foster City, CA, USA). Ito and
IK1 were recorded using the voltage-clamp mode.
Ito was evoked by applying 600 ms pulses with the
holding potential of −40 mV, and the test potentials increased from
−40 to +50 mV with a step size of 10 mV. IK1 was evoked
by applying a holding potential of −40 mV and 500 ms
depolarization. The test potentials increased from −120 to +50 mV
with a step size of 10 mV. Transmembrane potentials and ion
currents were recorded in the whole-cell recording mode using an
Axopatch™ 200B amplifier (Molecular Devices, LLC, Sunnyvale, CA,
USA), and processed using pCLAMP 9.0 software (Axon Instruments,
Inc.).
Measurement of
[Ca2+]i
Cardiomyocytes were loaded with 5 μM
Fluo-3/AM and 10 μM Pluronic F-127 (Ambion®;
Thermo Fisher Scientific, Waltham, MA, USA).
[Ca2+]i was determined according to the
changes of fluorescence intensity prior to and following drug
administration (23). The effect
of SSYX on [Ca2+]i was also studied in
cardiomyocytes stimulated with 30 mM KCl. Changes in fluorescence
were monitored using a laser scanning confocal microscope (Olympus
FV-1000; Olympus Optical Co., Ltd, Tokyo, Japan). The excitation
wavelength was set at 488 nm and the emission wavelength was set at
530 nm. Qualitative changes of [Ca2+]i were
indicated as Fmax/F0.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean and analyzed using a two-tailed paired t-test to examine
the individual apparent differences. P<0.05 was used to indicate
a statistically significant difference.
Results
SSYX prevents ischemic arrhythmias
Electrocardiogram and heart rate were continuously
recorded for 30 min following ligating coronary artery.
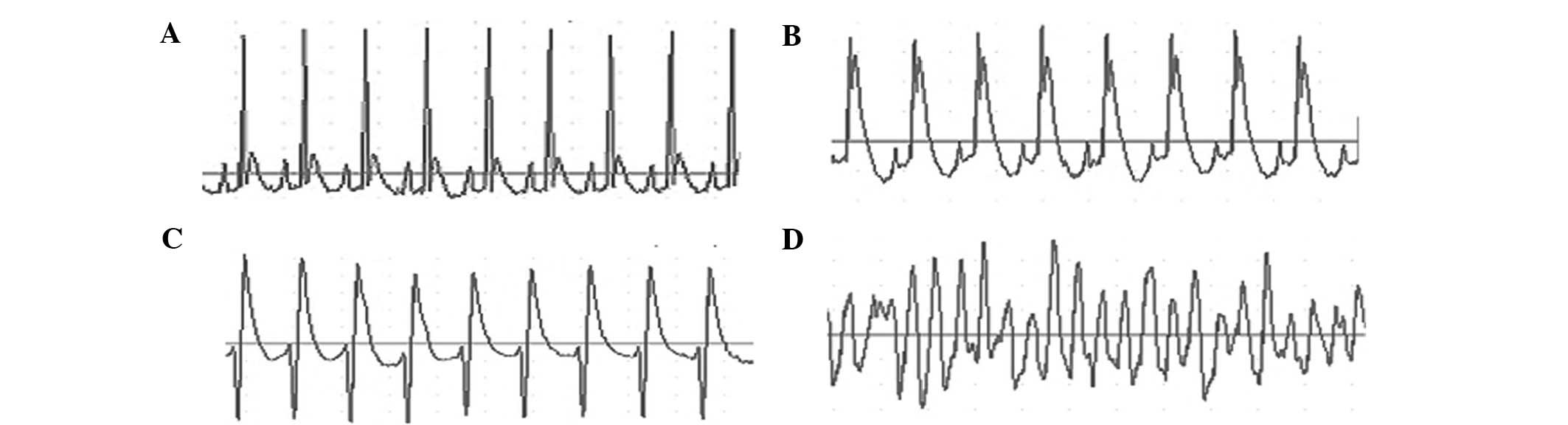
Electrocardiograms of rats prior to and following ligation are
shown in Fig. 1. SSYX and
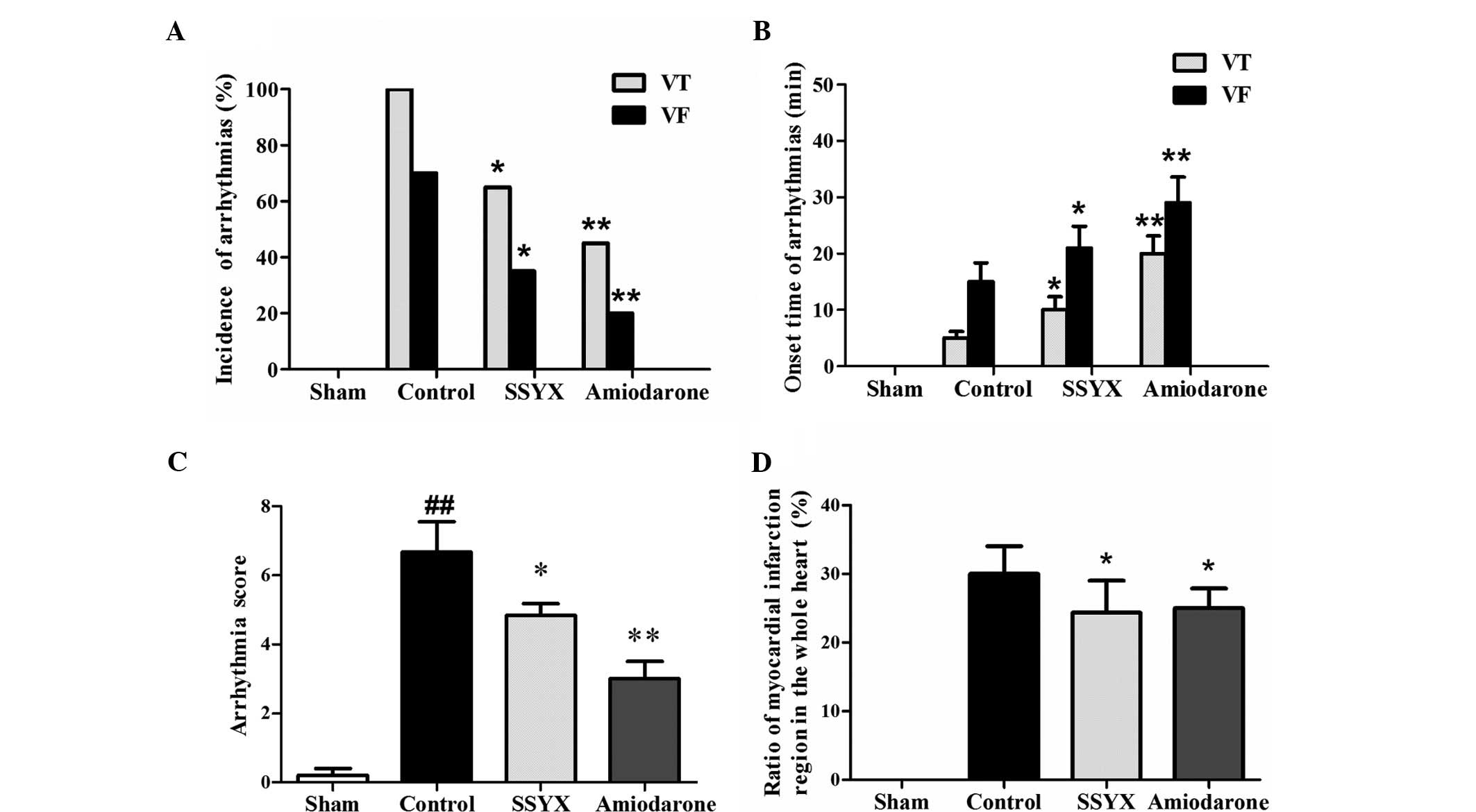
amiodarone significantly reduced the incidence of ventricular
tachycardia (VT) and ventricular fibrillation (VF), and delayed the
onset of arrhythmias compared with the control (Fig. 2A and B). The severity of ischemic
arrhythmias is shown as an arrhythmia score, according to Lambeth
Conventions. The arrhythmia score of the control group was
significantly higher compared with the sham group (P<0.01). SSYX
and amiodarone reduced the arrhythmia scores of AMI rats compared
with the control (Fig. 2C). In
addition, SSYX and amiodarone decreased the ratio of the myocardial
infarction region to the whole heart (Fig. 2D). All these results indicated that
SSYX were able to prevent ischemic arrhythmias.
SSYX prolongs the action potential of rat
ventricular myocytes
Myocardial action potentials reflect the process of
cardiac depolarization and repolarization. The effect of SSYX on
myocardial action potentials was evaluated in rat cardiomyocytes
using a serum pharmacological method and the patch-clamp technique.
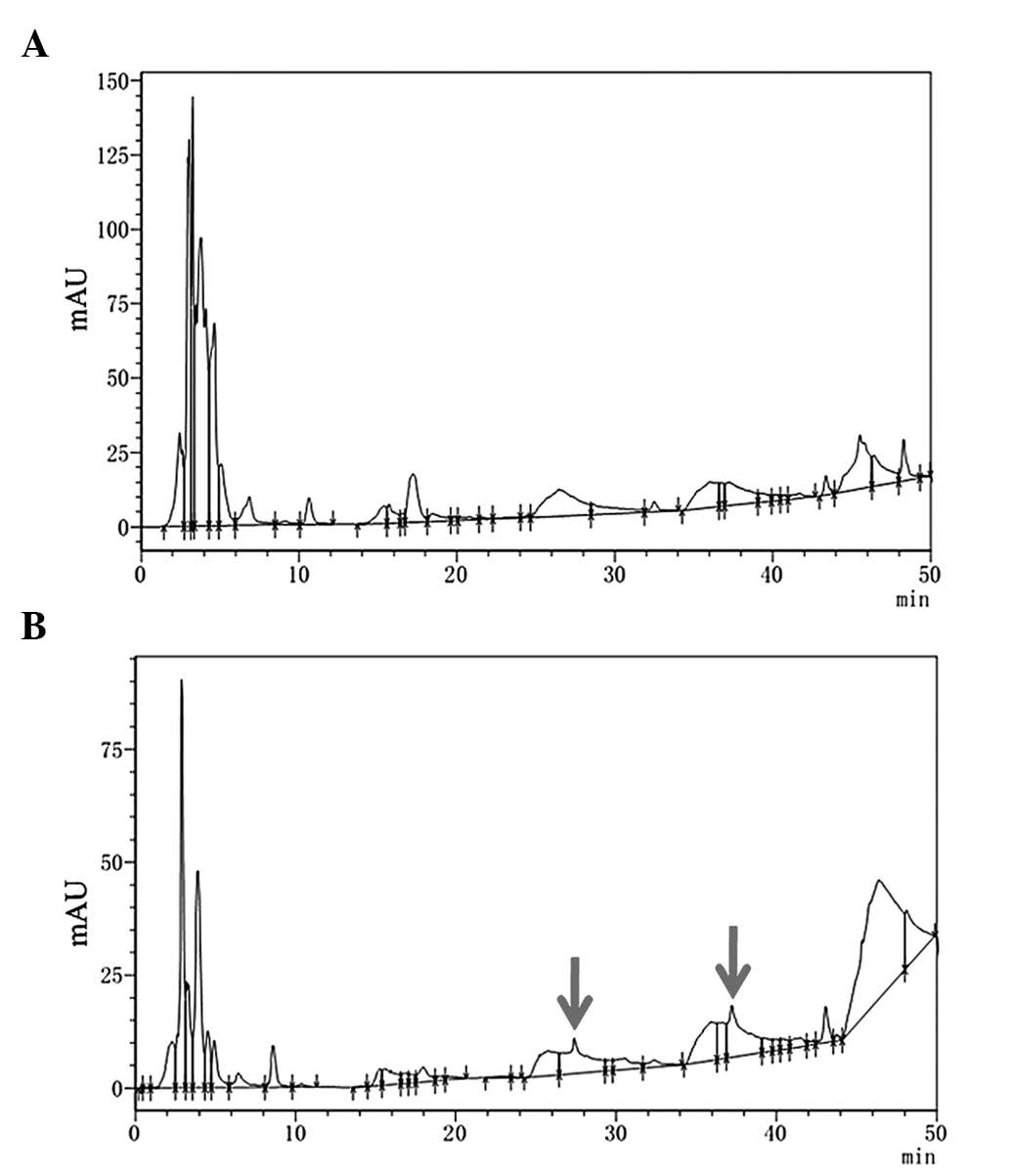
Differences in blank and SSYX serum were then analyzed using HPLC.
The results demonstrated that SSYX serum had two significant
absorption peaks, which were different from blank serum, suggesting
that serum powder containing SSYX had been successfully prepared
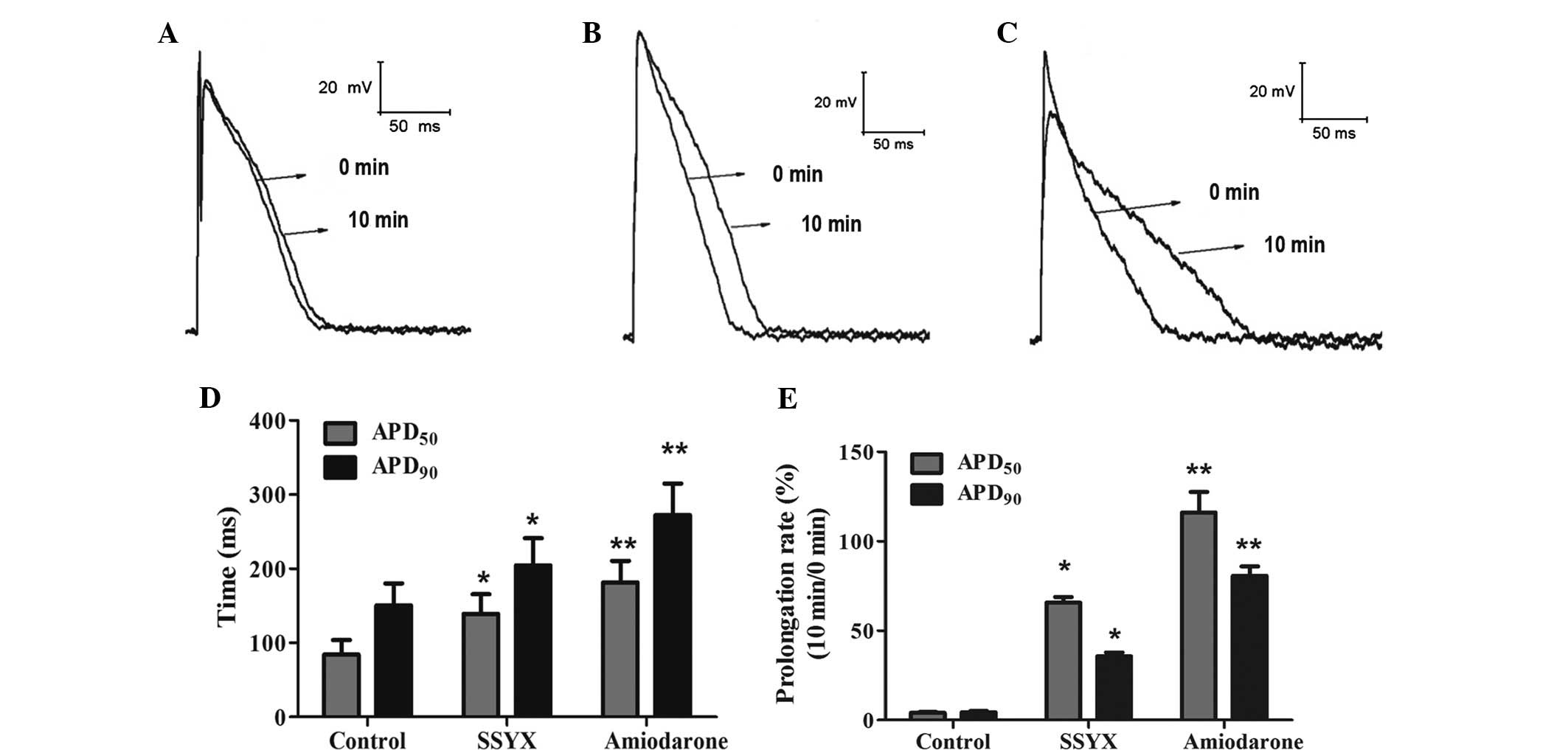
(Fig. 3). SSYX markedly prolonged
the action potential, extending the action potential at 50%
repolarization (APD50; 139.29±45.24 ms) and the action
potential at 90% repolarization (APD90; 204.41±63.66
ms), compared with the control (84.04±34.48 and 150.62±51.25 ms,
respectively). Amiodarone significantly extended APD50
(181.52±50.88 ms) and APD90 (272.14±73.75 ms) compared
with the control (Fig. 4). These
results indicated that SSYX was able to extend the action
potentials and slow down cardiac repolarization in rapid
arrhythmias.
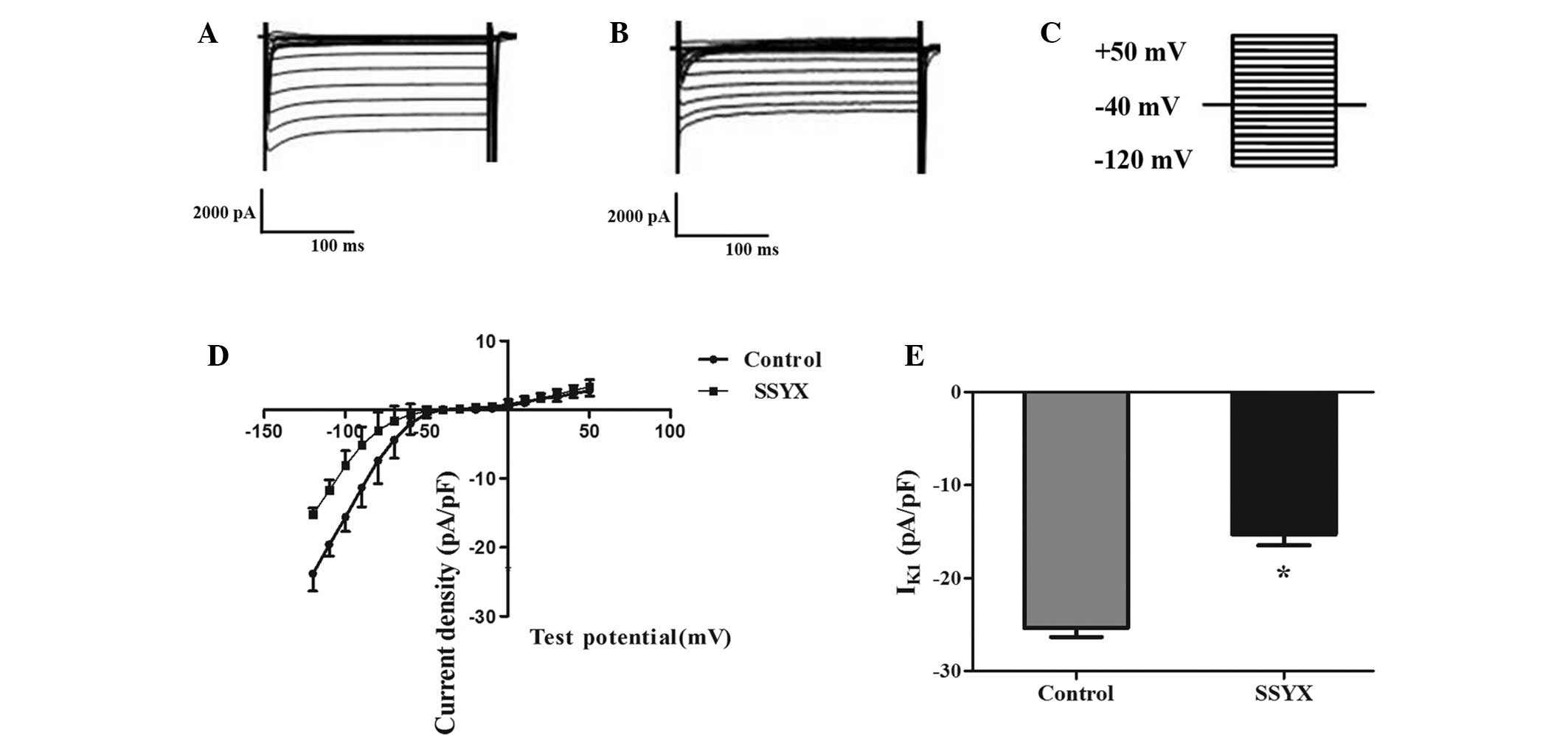
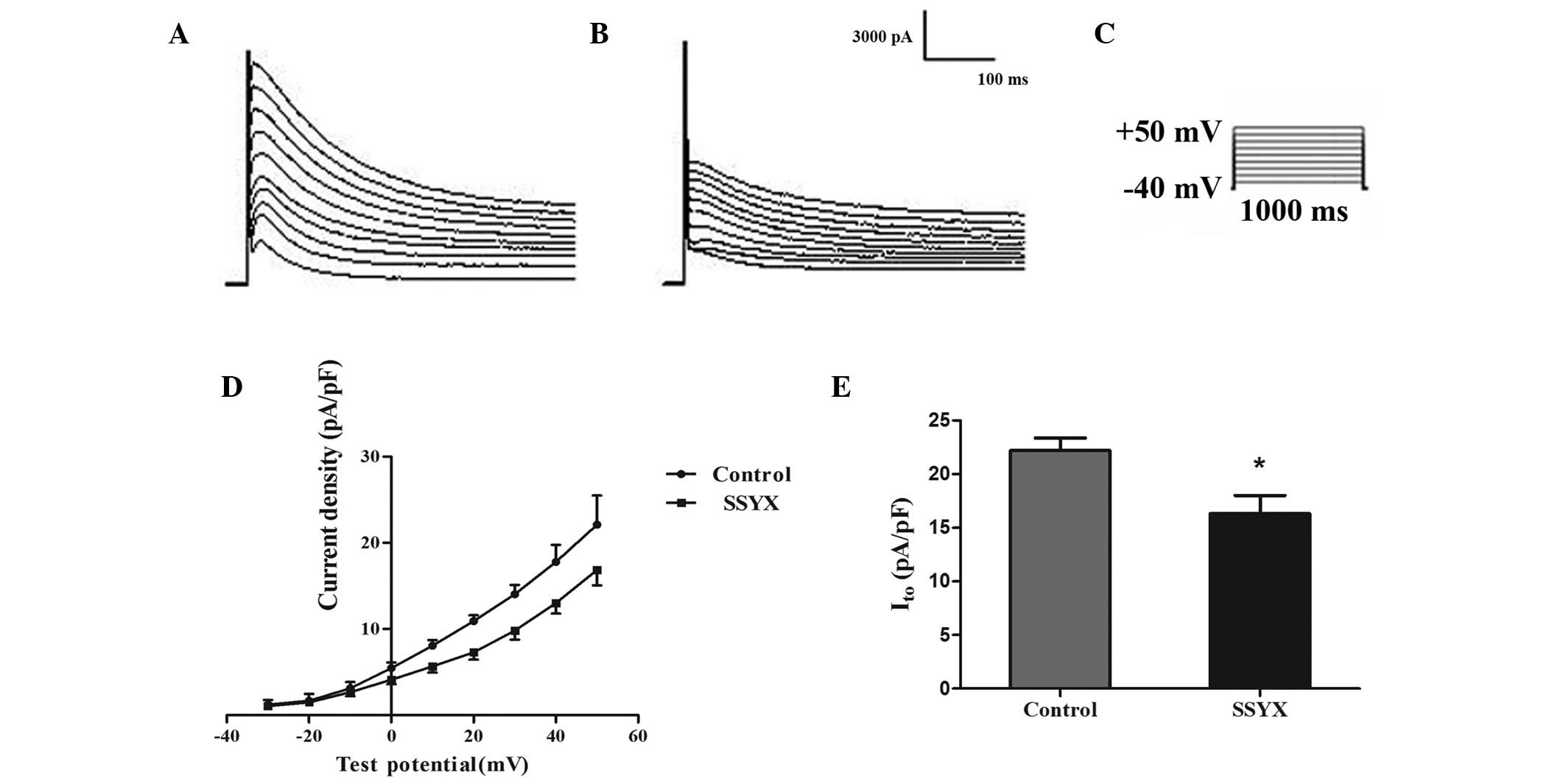
SSYX inhibits IK1 and
Ito in rat ventricular myocytes
Ito and IK1 are involved in
cardiac repolarization, and are associated with cardiac
excitability and arrhythmogenesis (24). In this study, the effects of SSYX
on IK1 and Ito current were recorded.
Fig. 5A and B show the
voltage-dependent IK1 current in the presence of blank
or SSYX serum (holding potential −40 mV, depolarization pulses 500
ms, test potential increased from −120 to +50 mV, step amplitude 10
mV; Fig. 5C). The current-voltage
(I-V) curve revealed that SSYX significantly reduced the density of
IK1. The peak amplitude of IK1 was decreased
from −25.04±2.14 to −15.30±2.05 (P<0.05; Fig. 5D and E). Fig. 6A and B show the voltage-dependent
Ito current in the presence of blank or SSYX serum
(holding potential −40 mV, 600 ms depolarization pulse, test
potential increased from −40 to +50 mV, step amplitude 10 mV;
Fig. 6C). The I–V association for
Ito indicated that SSYX significantly reduced the
density of Ito (Fig.
6D). The peak amplitude of Ito was decreased from
22.21±2.04 to 16.30±3.00 (P<0.05; Fig. 6E).
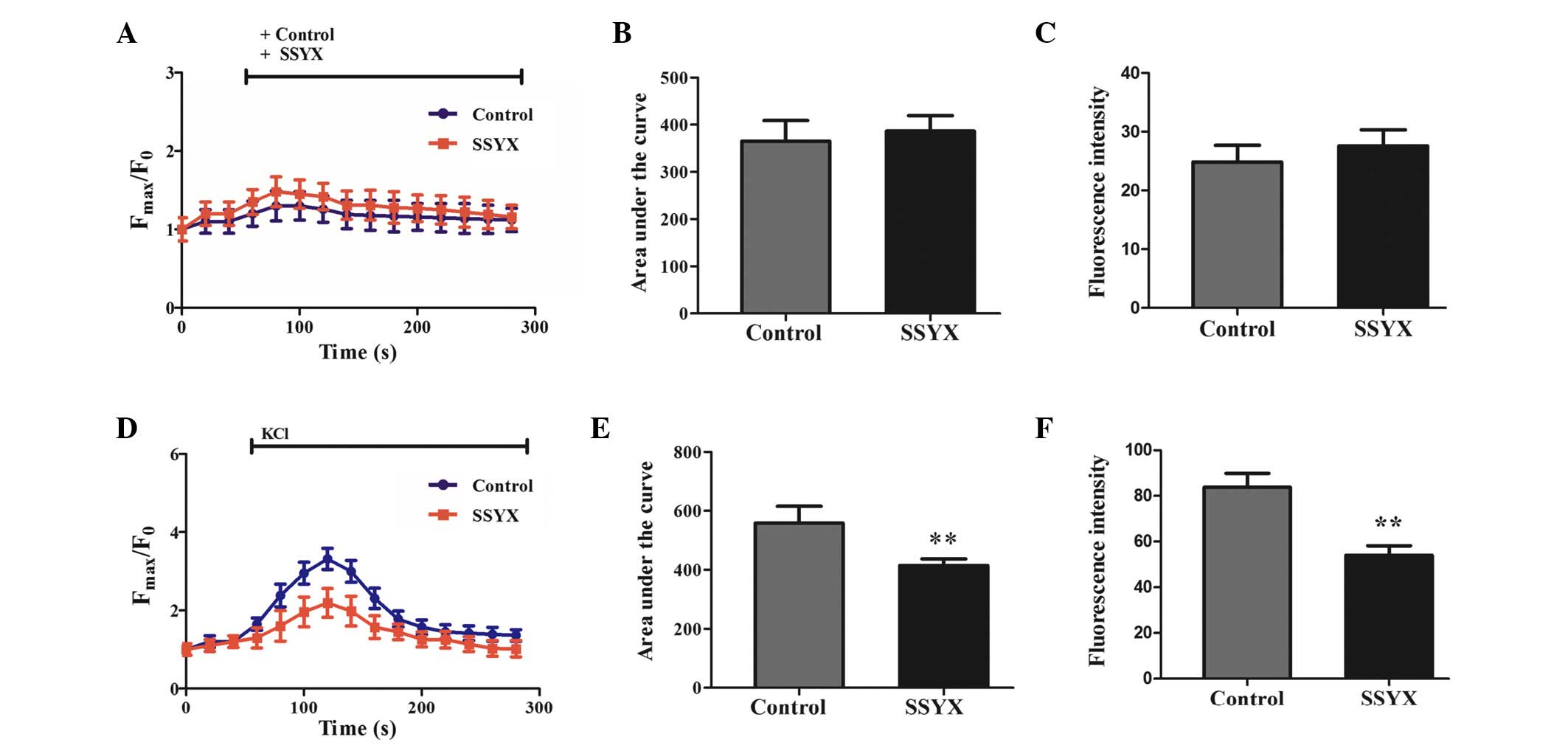
SSYX inhibits Ca2+ overload in
rat cardiomyocytes
The effects of SSYX on Ca2+ regulation
were further investigated in rat cardiomyocytes loaded with the
calcium indicator, Fluo-3/AM. The results demonstrated that the
ratio of Fmax/F0 was increased 1.23±0.19
times following the addition of SSYX serum and 1.16±0.16 times
after adding blank serum (Fig.
7A). SSYX did not increase the area under the fluorescence
intensity curve and the peak fluorescence intensity compared with
the control (Fig. 7B and C).
Following stimulation with 30 mM KCl, the
Fmax/F0 was increased 2.19±0.37 times in
ventricular myocytes pretreated with SSYX serum, and 3.32±0.27
times in the control (Fig. 7D).
SSYX significantly decreased the area under the fluorescence
intensity curve and peak fluorescence intensity compared with the
control (Fig. 7E and F). In
addition, SSYX decreased the baseline fluorescent intensity
compared with the control.
Discussion
Ventricular arrhythmia remains one of the
predominant causes of sudden cardiac death. Prevention and
treatment of ventricular arrhythmia are continuing challenges in
the medical field. With the increasing incidence of cardiovascular
disease, TCMs are becoming more frequently used in China and
Western countries due to their preferable efficacies, multiple
components and therapeutic targets and safety (25). Clinical studies have shown that
SSYX effectively improved heart palpitations, chest tightness,
shortness of breath, insomnia and fatigue; these studies also
indicated that SSYX effectively suppressed paroxysmal atrial
fibrillation and frequent premature ventricular contractions
(26–28). The findings of the present study
have corroborated that SSYX prevents ischemic arrhythmias, and that
this effect was associated with prolonging cardiac action
potentials and reducing the Ca2+ overload.
Myocardial ischemia and anoxia disturb cardiac
conduction, accelerate cardiac repolarization and cause early
afterdepolarization (EAD), which are the primary causes of ischemic
arrhythmias (3). Factors,
including the heart rate, heart size, size of ischemic area and
arrhythmia score, are able to be used to determine the incidence
and severity of ischemic arrhythmias. The results in the present
study demonstrated that SSYX reduced the incidence of VT and VF,
and markedly decreased the arrhythmia scores of AMI rats,
indicating that SSYX suppresses ischemic arrhythmias to a certain
extent. In addition, SSYX decreased the myocardial infarction area,
which contributes towards protection of the myocardium and,
ultimately, the reduction in the onset and severity of arrhythmias.
The effect of SSYX on ameliorating myocardial ischemia damage could
partially reverse left ventricular remodeling, reduce ischemic
injury and prevent myocardial apoptosis.
The heartbeat is automatically initiated by
electrical activity of cardiomyocytes at regular intervals. This
process includes the formation of an action potential in a single
cardiomyocyte and the spread of the action potential through the
myocardium. An action potential reflects the processes of cardiac
depolarization and repolarization; it is based on the collaborative
function of inward and outward currents through the ion channels of
the cell membrane (29). Action
potentials shorten rapidly during the first 2 min of AMI on account
of the combined effects of components of the ischemic environment,
including elevated extracellular K+, hypoxia, a low pH,
high partial pressure of carbon dioxide (pCO2) and the
accumulation of substances, such as catecholamines, resulting from
the deprivation of blood flow. Each may exert an influence on
membrane conductance (30,31). Despite the electrophysiological
mechanisms, cardiac re-entry has been regarded as an important
mechanism causing malignant arrhythmias. In the present study, SSYX
was shown to significantly prolong action potentials and slow
cardiac repolarization. An appropriate extension of the action
potential is conducive to increasing the refractory period and
reducing myocardial excitability and re-entry (32). Therefore, the results of the
present study suggested that SSYX altered the myocardial
refractoriness and conductivity, and exerted a therapeutic effect
on ischemic arrhythmias.
A moderate decrease in the resting potential from
its normal value of −90 to −80 mV increases conduction velocity
during AMI. A predominant cause of the decreased resting potential
of ischemic cells is the elevated extracellular K+ and
intracellular Ca2+, which result from a lack of blood
flow. Ischemia and hypoxia interfere with ion channels, and
predispose individuals to a disturbed cardiac rhythm (33). Cardiac K+ channels
determine the morphology of myocardial action potentials and the
resting potential. IK1 is a strong inward rectification
current, which stabilizes the membrane potential and is involved in
the third period of action potential repolarization. Ito
is the primary outward current of the cardiac rapid repolarization.
Following depolarization, the inward sodium current
(INa) becomes inactivated, and Ito initiates
the rapid repolarization of the action potentials (34–36).
Therefore, IK1 and Ito are both involved in
cardiac repolarization, and are associated with cardiac
excitability and arrhythmogenesis. In the present study, SSYX
significantly decreased the densities of Ito, suppressed
the first period of action potential repolarization and contributed
to a prolongation of the myocardial action potential. Furthermore,
inhibition of Ito reduced the high irregularity of
ischemic myocardial repolarization and the formation of re-entrant
excitability, which helped to suppress re-entry and avoid the
induction of Torsades de Pointes. Similarly, SSYX inhibited the
inward current of IK1 significantly without affecting
its reversal potential and rectification properties. This
inhibitory effect was conductive to automatically eliminating the
fourth period of action potential depolarization and inhibiting
delayed afterdepolarization (DAD).
Ca2+ is involved in
excitation-concentration coupling and Ca2+-dependent
signaling pathways. Ca2+ overload in ischemic myocytes
results in cardiomyocyte death, myocardial ischemia injury and
arrhythmias. Ca2+ overload is commonly associated with
disorders of sarcoplasmic reticulum calcium stores and the L-type
voltage-gated calcium channel, which consequently lead to DADs and
EADs. Therefore, reducing Ca2+ overload would be
beneficial for the treatment of myocardial ischemia and ischemic
arrhythmias. However, prolongation of the action potentials may
increase Ca2+ entry via the L-type calcium current
[ICa(L)] during the long plateau phase, which would cause an
accumulation of Ca2+ in the sarcoplasmic reticulum, and
spontaneous Ca2+ release from the sarcoplasmic
reticulum. In the present study, SSYX significantly prolonged
action potentials by inhibiting Ito and IK1
without affecting the intracellular Ca2+ concentration.
In addition, SSYX alleviated the Ca2+ overload induced
by KCl in cardiomyocytes, which helped to ameliorate the
intracellular Ca2+ dysregulation of ischemic myocytes
and improve cardiac function during ischemic arrhythmias. However,
further studies are required in order to elucidate the precise
mechanism by which SSYX regulates intracellular
Ca2+.
Traditional pharmacology for TCM involves directly
exposing cells, tissues or organs to the crude drug. However, due
to the complexities of drug absorption and metabolism associated
with TCM, the efficacy of the drug in its crude form may not be as
good as the active ingredients after in vivo metabolism of
the drug. Therefore, serum pharmacology for TCM was adopted in the
present study. Serum pharmacology facilitates the direct
application of active ingredients after the in vivo
metabolism of the TCM, and utilizes cytological and molecular
biological methods, thereby providing a conjunction of modern
scientific technology and TCM research. In addition, observing and
analyzing the processes of absorption and metabolism of drugs is
beneficial in terms of eliminating the influence of various
interference factors that are associated with in vitro
experiments. Incubating myocytes with serum containing SSYX also
corroborated previous findings on the efficacy of SSYX identified
by means of in vivo and in vitro experiments. Serum
pharmacology thus provides a novel approach for developing
antiarrhythmic TCMs.
In conclusion, SSYX effectively prevents ischemic
arrhythmias. In addition, SSYX is potent in extending action
potentials and in inhibiting Ito and IK1,
thereby decreasing myocardial autorhythmicity and re-entrant
excitability. Furthermore, SSYX alleviated myocardial
Ca2+ overload, which contributes towards an elimination
of DADs and a reduction in ultrastructural injuries of the
myocardium. Therefore, SSYX may be used as an effective drug to
treat ischemic arrhythmias.
Acknowledgments
This study was funded by the National Key Basic
Research and Development Program (973 Program; grant no.
2012CB518606) and National Natural Science Foundation of China
(grant no. 81100072).
References
|
1
|
Driessen HE, Bourgonje VJ, Van veen TA and
Vos MA: New antiarrhythmic targets to control intracellular calcium
handling. Neth Heart J. 22:198–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao FF, Hao SY, Huang ZQ, Zhang YM, Zhou
YQ, Chen YC, Liu XP and Shi GG: Cardiac electrophysiological and
antiarrhythmic effects of N-n-butyl haloperidol iodide. Cell
Physiol Biochem. 25:433–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies MJ: Pathological view of sudden
cardiac death. Br Heart J. 45:88–96. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marbán E: Cardiac channelopathies. Nature.
415:213–218. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
James RG, Arnold JM, Allen JD, Pantridge
JF and Shanks RG: The effects of heart rate, myocardial ischemia
and vagal stimulation on the threshold for ventricular
fibrillation. Circulation. 55:311–317. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fallavollita JA, Jacob S, Yong RF and
Canty JM Jr: Regional alterations in SR Ca2+-ATPase,
phospholamban and HSP-70 expression in chronic hibernating
myocardium. Am J Physiol. 277:H1418–H1428. 1999.PubMed/NCBI
|
|
7
|
Prunier F, Kawase Y, Gianni D, Scapin C,
Danik SB, Ellinor PT, Hajjar RJ and Del Monte F: Prevention of
ventricular arrhythmias with sarcoplasmic reticulum Ca2+
ATPase pump overexpression in a porcine model of ischemia
reperfusion. Circulation. 118:614–624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang XG, Jia JM and Li YS: Simultaneous
determination of eight bioactive constituents in shensong yangxin
capsule by UPLC. Chinese Herbal Medicines. 5:212–216. 2013.
|
|
9
|
Liu M, Li S, Zhao S, Wang H and Tu P:
Studies on excretion kinetics of ten constituents in rat urine
after oral administration of Shensong Yangxin Capsule by
UPLC-MS/MS. Biomed Chromatogr. 28:525–533. 2014. View Article : Google Scholar
|
|
10
|
Jin ZY, Gong Q and Pu JL: The effect of
Shensong Yangxin capsule on the porcine cardiac electrophysiology.
Chinese Patent Medicine. 31:471–473. 2009.
|
|
11
|
Li N, Ma KJ, Wu XF, Sun Q, Zhang YH and Pu
JL: Effects of Chinese herbs on multiple ion channels in isolated
ventricular myocytes. Chin Med J (Engl). 120:1068–1074. 2007.
|
|
12
|
Sun LP, Li N, Wu YL and Pu JL: Effects of
Shensong Yangxin capsule on pacemaker channels encoded by human
HCN4 gene. Chin Med J (Engl). 123:3148–3150. 2010.
|
|
13
|
Vora A and Kulkami S: Pharmacotherapy to
reduce arrhythmic mortality. Indian Heart J. 66(Suppl 1):
S113–S119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yong SL and Wang QK: Animal models for
cardiac arrhythmias. Methods Mol Med. 129:127–148. 2006.PubMed/NCBI
|
|
15
|
Di Diego JM and Antzelevitch C: Ischemic
ventricular arrhythmias: Experimental models and their clinical
relevance. Heart Rhythm. 8:1963–1968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huggins CE, Bell JR, Pepe S and Delbridge
LM: Benchmarking ventricular arrhythmias in the mouse-revisiting
the 'Lambeth Conventions' 20 years on. Heart Lung Circ. 17:445–450.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu H, Tang Q, Yang J, Ye M and Dong W:
Atorvastatin ameliorates myocardial ischemia/reperfusion injury
through attenuation of endoplasmic reticulum stress-induced
apoptosis. Int J Clin Exp Med. 7:4915–4923. 2014.
|
|
18
|
Bochu W, Liancai Z and Qi C: Primary study
on the application of serum pharmacology in Chinese traditional
medicine. Colloid Surface B Biointerfaces. 43:194–197. 2005.
View Article : Google Scholar
|
|
19
|
Graham EL, Balla C, Franchino H, Melman Y,
del Monte F and Das S: Isolation, culture, and functional
characterization of adult mouse cardiomyoctyes. J Vis Exp.
24:e502892013.
|
|
20
|
Gong DM, Shan HL, Zhou YH, Dong DL and
Yang BF: The ion targets of arrhythmias induced by ouabain and
aconitine in guinea pig and rat ventricular myocytes. Yao Xue Xue
Bao. 39:328–332. 2004.In Chinese. PubMed/NCBI
|
|
21
|
Liang B, Nissen JD, Laursen M, Wang X,
Skibsbye L, Hearing MC, Andersen MN, Rasmussen HB, Wickman K,
Grunnet M, et al: G-protein-coupled inward rectifier potassium
current contributes to ventricular repolarization. Cardiovasc Res.
101:175–184. 2014. View Article : Google Scholar
|
|
22
|
Fang Z, Ren YP, Lu CY, Li Y, Xu Q, Peng L
and Fan YY: Effects of sleep deprivation on action potential and
transient outward potassium current in ventricular myocytes in
rats. Med Sci Monit. 21:542–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun L, Ai J, Wang N, Zhang R, Li J, Zhang
T, Wu W, Hang P, Lu Y and Yang B: Cerebral ischemia elicits
aberration in myocardium contractile function and intracellular
calcium handling. Cell Physiol Biochem. 26:421–530. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Billman GE: Novel Therapeutic targets for
antiarrhythmic drugs. Wiley; Hoboken, New Jersey: 2010
|
|
25
|
Sun J, Tan BK, Huang SH, Whiteman M and
Zhu YZ: Effects of natural products on ischemic heart diseases and
cardiovascular system. Acta Pharmacol Sin. 23:1142–1151.
2002.PubMed/NCBI
|
|
26
|
Wang J, Li J and Feng B: Shen Song Yang
Xin capsule combined with antiarrhythmic drugs, a new integrative
medicine therapy, for the treatment of frequent premature
ventricular contractions (FPVC): A-meta analysis of randomized
controlled trials. Evid Based Complement Alternat Med.
2014:9767132014. View Article : Google Scholar
|
|
27
|
Chai SB, Wang SR, Yao LF and Wu A: A study
of the effect of Shensong Yangxin Capsule on ventricular remodeling
after myocardial infarction and isolated heart action potential in
rats. Beijing J Tradit Chin Med. 12:967–971. 2009.In Chinese.
|
|
28
|
Wang AH, Pu JL, Qi XY, Miao WL, Hou ZS,
Cong HL, Zhou JZ, Liu XF, Li SM, Han QH, et al: Evaluation of
shensongyangxin capsule in the treatment of paroxysmal atrial
fibrillation: A randomized, double-blind and controlled multicenter
trial. Zhonghua Yi Xue Za Zhi. 91:1677–1681. 2011.In Chinese.
PubMed/NCBI
|
|
29
|
Roden DM, Balser JR, George AL Jr and
Anderson ME: Cardiac ion channels. Annu Rev Physiol. 64:431–475.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Isenberg G, Vereecke J, van der Heyden G
and Carmeliet E: The shortening of the action potential by DNP in
guinea-pig ventricular myocytes is mediated by an increase of a
time-independent K conductance. Pflugers Arch. 397:251–259. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vleugels A, Vereecke J and Carmeliet E:
Ionic currents during hypoxia in voltage-clamped cat ventricular
muscle. Circ Res. 47:501–508. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wit AL and Coromilas J: Role of
alterations in refractoriness and conduction in the genesis of
reentrant arrhythmias. Implications for antiarrhythmic effects of
class III drugs. Am J Cardiol. 72:3F–12F. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fox JJ, McHarg JL and Gilmour RF Jr: Ionic
mechanism of electrical alternans. Am J Physiol Heart Circ Physiol.
282:H516–H530. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dhamoon AS and Jalife J: The inward
rectifier current (IKl) controls cardiac excitability and is
involved in arrhythmogenesis. Heart Rhythm. 2:316–324. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anumonwo JM and Lopatin AN: Cardiac strong
inward rectifier potassium channels. J Mol Cell Cardiol. 48:45–54.
2010. View Article : Google Scholar :
|
|
36
|
Li GR and Dong MQ: Pharmacology of cardiac
potassium channels. Adv Pharmacol. 59:93–134. 2010. View Article : Google Scholar : PubMed/NCBI
|