Introduction
Septicaemia is a systemic bacterial infection
resulting from the spread of microorganisms and their toxins in the
blood (1). Although modern medical
technology has improved greatly in recent years, septicaemia often
leads to morbidity and mortality in children in developing
countries (2). Therefore, it is
necessary to explore the risk factors, which influence the
prognosis of children with septicaemia.
NOD-like receptor (NLR) family, CA R D
domain-containing protein 4 (NLRC4) was initially described in
2001, and demonstrated to detect cytosolic flagellin (3–5).
NLRC4 is a key component of the inflammasome response to a variety
of microbial stimuli and endogenous danger signals via caspase-1
activation, cytokine production and macrophage pyroptosis (6–9).
NLRC4 is involved in the control of infections. A number of
bacteria have been demonstrated to prompt caspase-1 activation and
the inflammatory cytokines interleukin (IL)-1β and IL-18 maturation
via the activation of NLRC4 (7,10–13).
However, the molecular mechanisms underlying the importance of
NLRC4 in the immune response of macrophages have not been
thoroughly investigated.
The present study detected the expression of NLRC4
for, to the best of our knowledge, the first time in the blood
samples of children with septicaemia. Furthermore, an in
vitro investigation was performed on lipopolysaccharide
(LPS)-stimulated RAW264.7 macrophage cells in order to investigate
the effect of NLRC4 in cytokine production and the signaling
pathways that regulate NLRC4 expression.
Materials and methods
Sample collection
The Ethics Committee of Soochow University
Affiliated Children's Hospital (Suzhou, China) approved the
protocols for the present study. A total of 42 children aged
between 1–6 years with confirmed bacterial septicaemia diagnosis
were recruited in the current study. A total of 40 healthy children
(age, 1–6 years), who underwent routine physical examination,
served as the control group. Written informed consent was obtained
from the parents of all children prior to enrollment in the current
study. Blood samples were collected from each child and centrifuged
at 1,000 × g for 10 min to obtain the blood serum.
Cell culture
RAW264.7 macrophage cells were purchased from
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The medium was
supplemented with 2 mM glutamine and 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.). Cells were maintained
at 37°C in a humidified atmosphere with 5% CO2. LPS, and
the inhibitors U0126, SP600125 and SB203580 were obtained from
Sigma-Aldrich (St. Louis, MO, USA). LPS was dissolved in
phosphate-buffered saline to concentrations of 100, 500 and 1,000
ng/ml. U0126, SP600125, and SB203580 were dissolved in dimethyl
sulphoxide (Sigma-Aldrich) to a final concentration of 20
µM. U0126, SP600125 and SB203580 were used to treat cells
for 24 h at 5 µM.
Transfection
Transfection of scramble small interfering RNA
(siRNA) and NLRC4 siRNA (Sangon Biotech Co., Ltd., Shanghai, China)
was performed on RAW264.7 cells using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Briefly, 0.1 nmol siRNA and 5 µl
Lipofectamine 2000 reagent were separately diluted in 250 µl
Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.), and
incubated at room temperature for 5 min. The two dilutions were
then mixed and incubated at room temperature for an additional 20
min. The DNA-Lipofectamine 2000 complex that was produced was added
to 5×105 cells/well for 6 h. The media was then replaced
with fresh DMEM supplemented with 2 mM glutamine and 10% FBS.
Enzyme-linked immunosorbent assay
(ELISA)
Cytokine levels in the culture media of RAW264.7
cells were determined using an ELISA assay. Mouse IL-1β/IL-1F2
Quantikine ELISA kit and Mouse IL-18/IL-1F4 ELISA kit were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Briefly, the samples to be measured or ELISA standards were
pipetted into the wells of the plate and incubated at room
temperature for 2 h. Following washing, mouse IL-1β conjugate or
mouse IL-18 conjugate was added to each well and incubated at room
temperature for another 2 h. Following washing, a substrate
solution was added to each well, and the reaction was terminated by
the addition of the stop solution from the kit. The optical density
was measured at a wavelength of 450 nm using an AMR-100 microplate
reader (Hangzhou Allsheng Instruments Co., Ltd., Hangzhou,
China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
was used for the extraction of RNA from the blood serum. The RNA
was purified using the Transcript RNA CleanUp kit (Takara
Biotechnology Co., Ltd., Dalian, China) and DNase I (Beyotime
Institute of Biotechnology, Haimen, China) was used to cleave DNA.
Reverse transcription-quantitative PCR was performed using One Step
SYBR PrimeScript RT-PCR kit II (Takara Biotechnology Co, Ltd.)
according to the manufacturer's protocol. Primer sequences were as
follows: forward, 5′-AGCTCAAAGGTTCAAGCCAA-3′ and reverse,
5′-TGCGAGGTGCTTCATAACAG-3′ for NLRC4; and forward,
5′-GTCAGTGGTGGACCTGACCT-3′ and reverse, 5′-GGGTCTTACTCCTTGGAGGC-3′
for GAPDH. PCR was performed on a ABI 7500 Fast Real Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The RT
reaction was performed at 42°C for 5 min. The PCR reaction
conditions were: 95°C for 10 min; followed by 40 cycles of 95°C for
30 sec, 60°C for 30 sec; and 72°C for 20 sec. GAPDH served as an
internal standard. The PCR was conducted at least three times and
quantified using the 2−∆∆Cq method (14)
Western blotting
Total protein was extracted from the serum and the
cells using radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology). The protein concentration was
determined using the Bradford protein assay kit from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). The samples (50 µg)
were resolved on 10% SDS-PAGE at 200 V for 80 min, and the proteins
were transferred onto a polyvinylidene difluoride membranes
(Bio-Rad Laboratories) by electroblotting. Following blocking with
5% non-fat milk, the membranes were further incubated with the
primary antibodies, including rabbit polyclonal anti-NLRC4 (1:800;
cat. no. ab189593, Abcam, Cambridge, MA, USA), rabbit monoclonal
anti-phospho-extracellular regulated protein kinase (ERK) 1/2
(1:1,000; cat. no. 4377; Cell Signaling Technology, Inc., Danvers,
MA, USA), rabbit polyclonal anti-phospho-c-Jun N-terminal kinase
(JNK; 1:1,000; cat. no. 9251; Cell Signaling Technology, Inc.),
rabbit monoclonal anti-phospho-p38 (1:400; cat. no. 4631; Cell
Signaling Technology, Inc.) and rabbit polyclonal anti-GAPDH
(1:2,000; cat. no. bs-2188R; BIOSS, Beijing, China). The membranes
were then washed with Tris-buffered saline containing 0.05% Tween
20 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) three
times prior to incubation with the goat anti-rabbit IgG horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no.
bs-0295G; BIOSS). GAPDH served as a loading control. ECL Plus
chemiluminescence detection kit from GE Healthcare Life Sciences
(Chalfont, UK) was used for protein detection. The band intensity
was quantified using Image J (imagej.nih.gov/ij/) and the experiments were
independently performed three times.
Statistical analysis
All data are expressed as the mean ± standard
deviation and were analyzed using a Student's t-test for the
comparison of two groups or analysis of variance followed by least
significant difference test for comparison of multiple groups. The
statistical analysis was conducted with SPSS, version 19.0 (IBM
SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
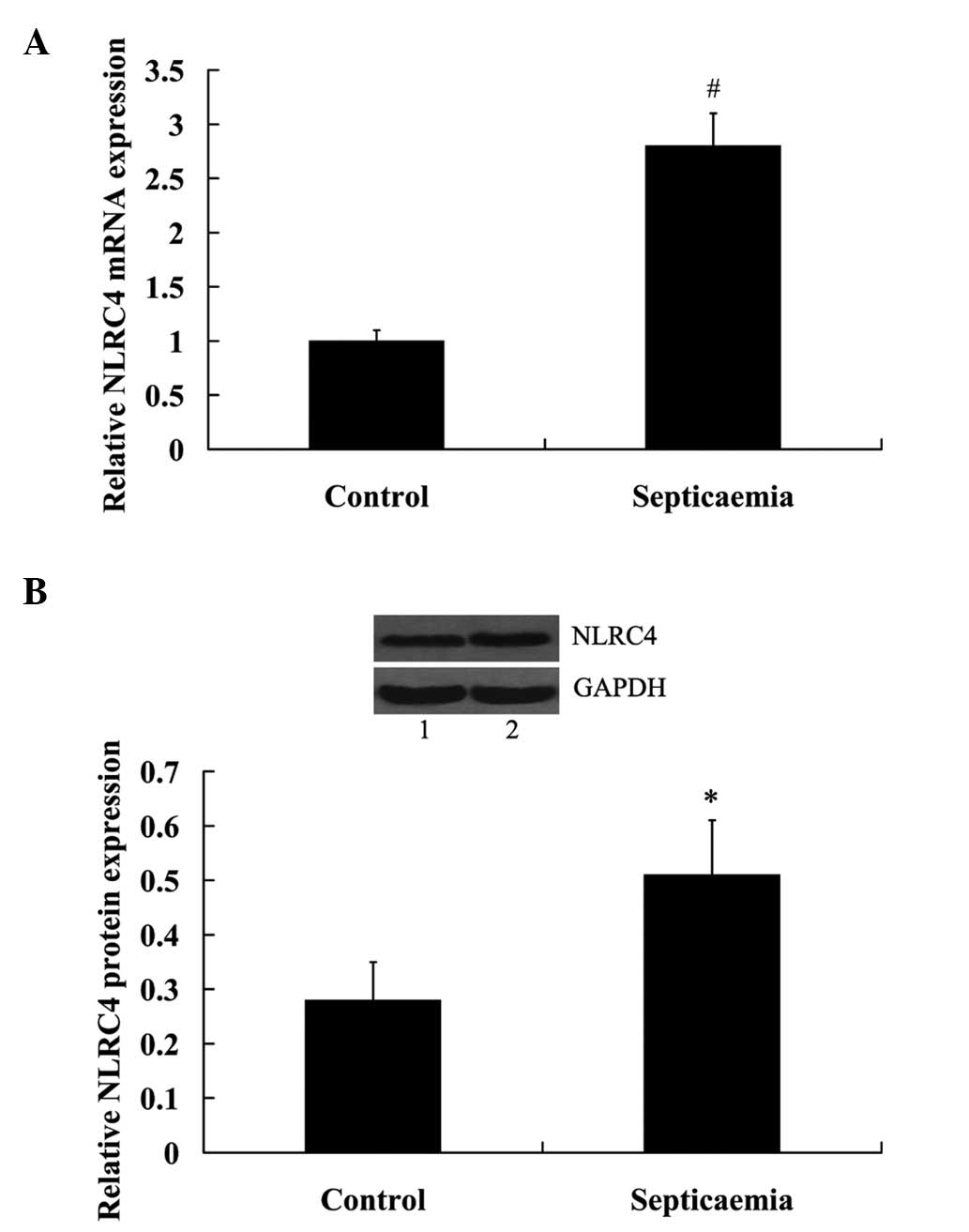
NLRC4 expression increased in the blood
serum of children with septicaemia
mRNA and protein expression levels of NLRC4 in blood
serum were determined. The results from the RT-qPCR indicated that
NLRC4 mRNA expression levels were significantly increased in the
septicaemia group compared with the control group (P<0.01;
Fig. 1A). Consistently, the
expression levels of NLRC4 protein were significantly upregulated
in the blood serum of children with septicaemia (P<0.05;
Fig. 1B).
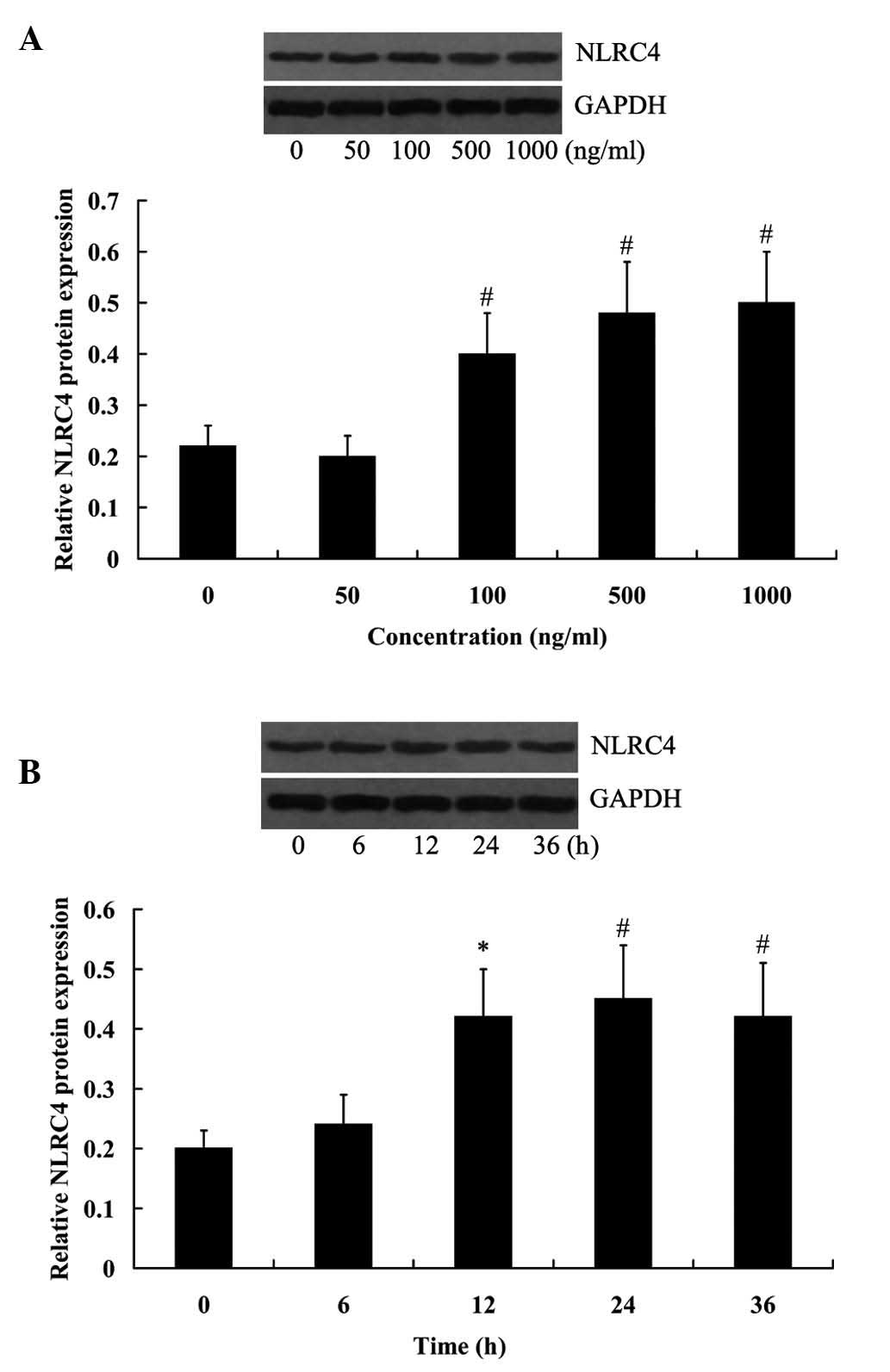
LPS-induced NLRC4 expression in RAW264.7
cells
RAW264.7 cells were treated with various
concentrations of LPS for 24 h, and then collected to investigate
the effect of LPS on NLRC4 expression. As presented in Fig. 2A, LPS at the concentrations of 100,
500 and 1,000 ng/ml significantly increased the expression of NLRC4
and reaching a peak value at 1,000 ng/ml (P<0.01). RAW264.7
cells were treated with 500 ng/ml LPS for 6, 12, 24 and 36 h,
respectively, it was determined that NLRC4 expression levels were
significantly increased with prolonged cell incubation (Fig. 2B; P<0.05 for 12 h, P<0.01 for
24 and 36 h).
NLRC4 knockdown enhances LPS-induced
cytokine production
To determine the importance of NLRC4 in mediating
the effect of LPS on cytokine production, RAW264.7 cells were
transfected with the NLRC4 siRNA and incubated with 500 ng/ml LPS
for 24 h. As indicated in Fig. 3A,
NLRC4 expression was significantly decreased in the cells following
transfection with NLRC4 siRNA (P<0.01).
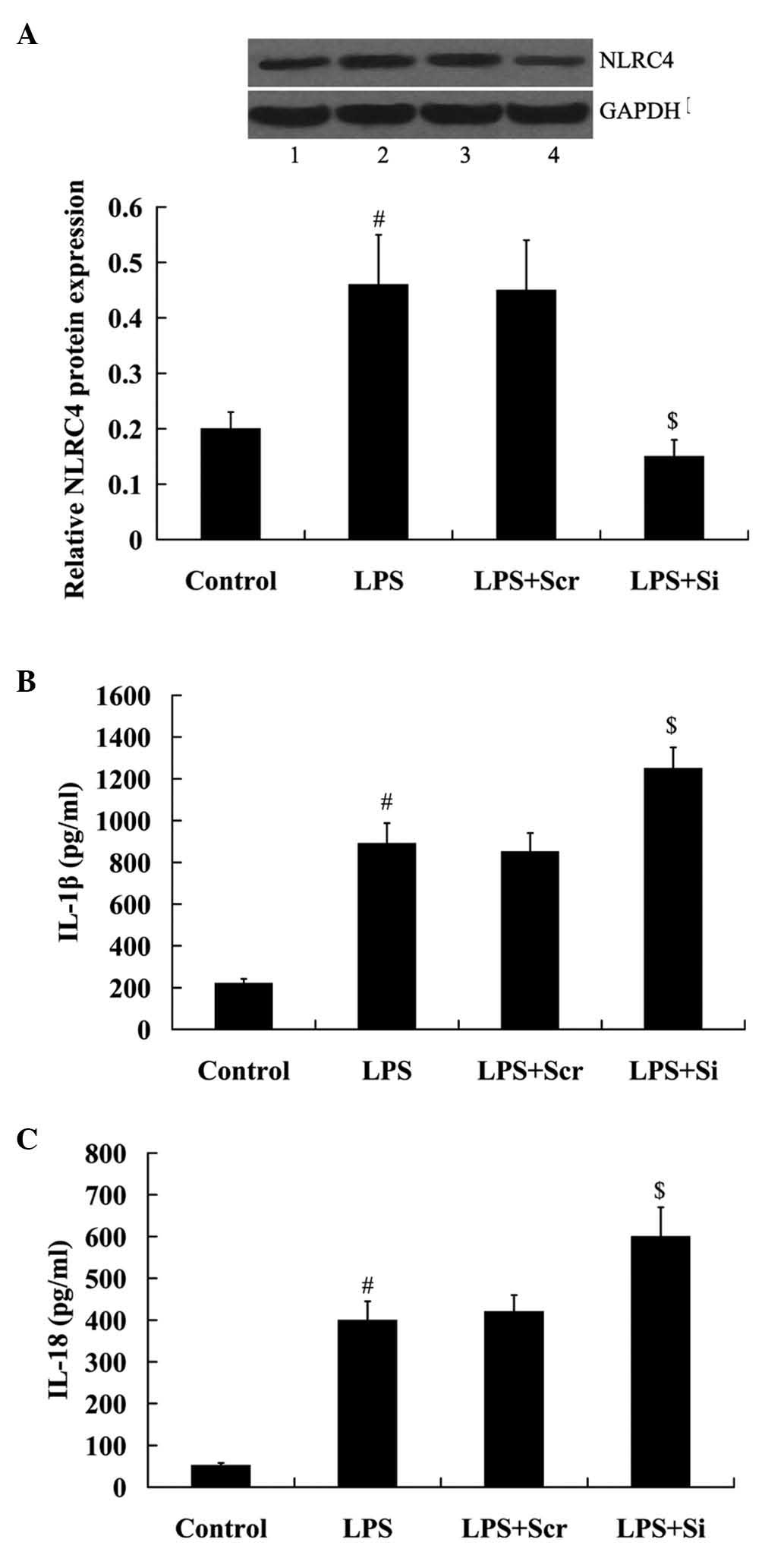 | Figure 3Effect of NLRC4 knockdown on
LPS-induced cytokine production. Expression levels of NLRC4 in
Raw264.7 cells following transfection with (A) NLRC4 siRNA and/or
LPS treatment. (B) IL-1β and (C) IL-18 production in RAW264.7 cells
following transfection with the NLRC4 siRNA and/or LPS treatment.
Data are presented as the mean ± standard deviation.
#P<0.01 vs. the control group; $P<0.01
vs. the LPS + Scr group. Lane 1, control; lane 2, LPS; lane 3, LPS
+ Scr; lane 4, LPS + Si. LPS, lipopoly-saccharide; NLRC4, NOD-like
receptor family, CARD domain-containing protein 4; IL, interleukin;
Scr, scramble; Si, siRNA. |
Notably, IL-1β and IL-18 production was
significantly induced in Raw264.7 cells incubated with LPS
(P<0.01). Furthermore, it was determined that this effect was
enhanced by the transfection of NLRC4 siRNA (Fig. 3B and C).
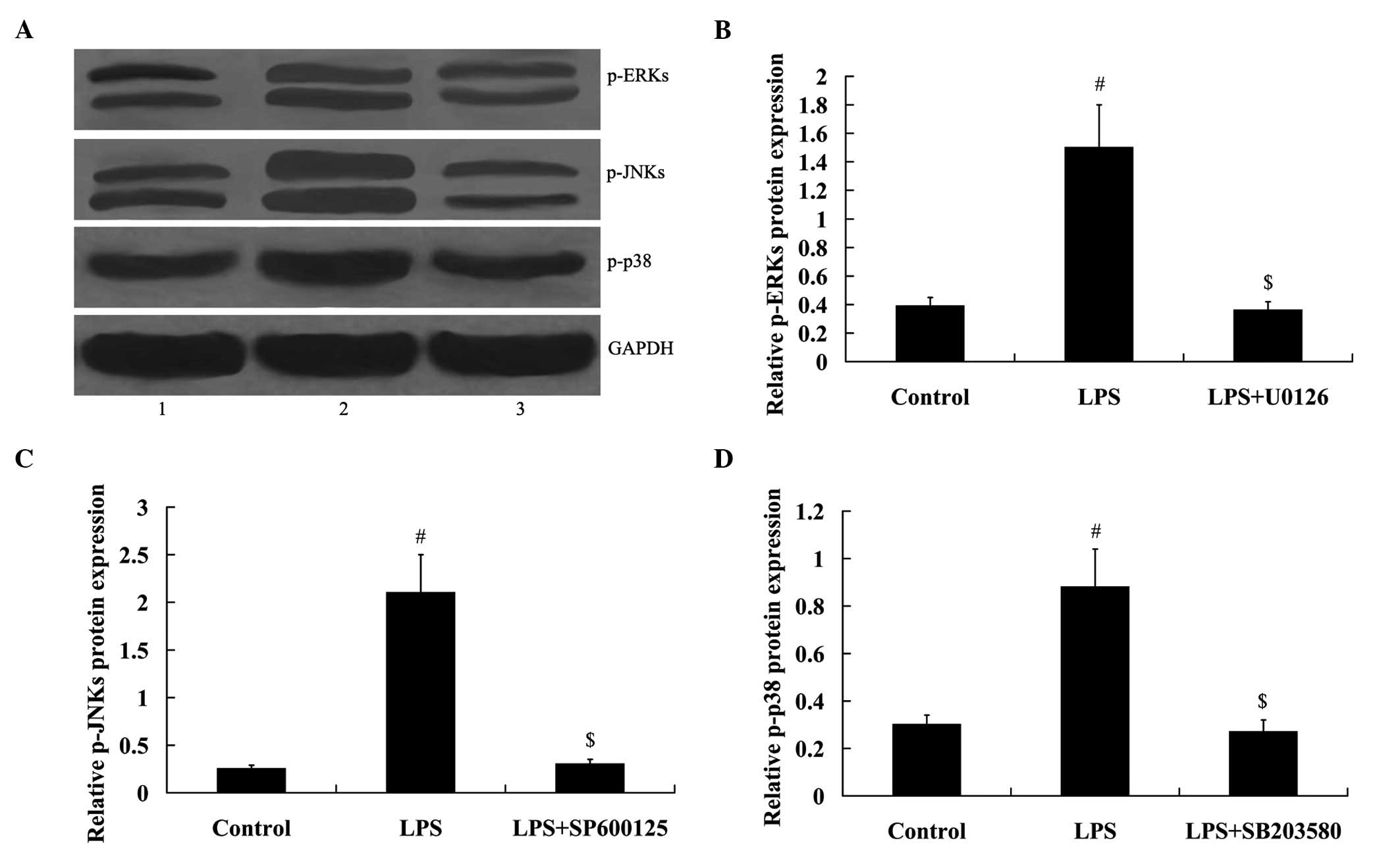
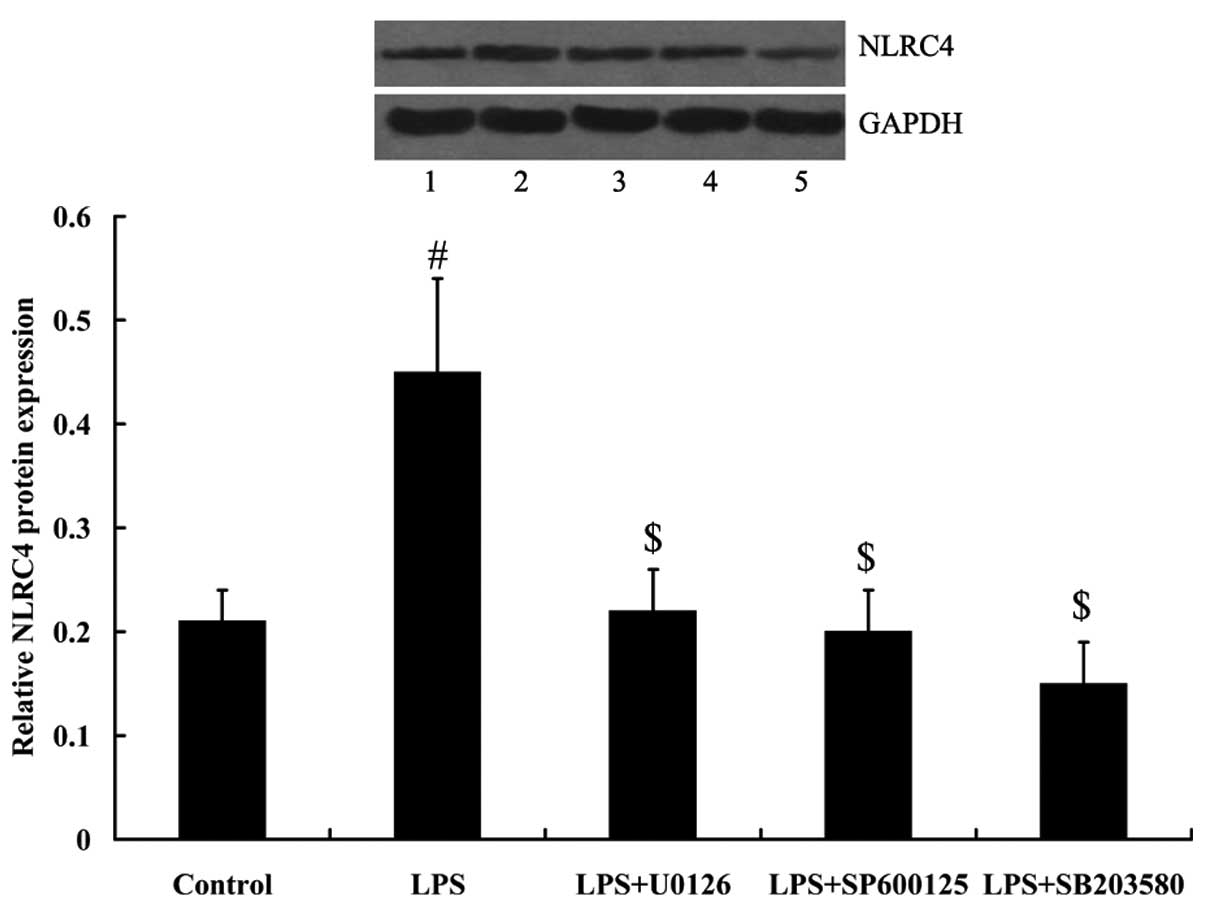
Mitogen activated protein kinases (MAPKs)
mediated the effect of LPS on NLRC4 expression
To determine whether MAPKs were involved in the
effect of LPS on NLRC4 expression, ERK inhibitor U0126, JNKs
inhibitor SP600125, and p38 inhibitor SB203580 were added to treat
RAW264.7 cells. As demonstrated in Fig. 4, MAPK signaling was activated in
RAW264.7 cells under LPS treatment, as demonstrated by the
increased expression of phosphorylated (p)-ERKs, p-JNKs and p-p38.
However, MAPK signaling was inhibited in RAW264.7 cells following
treatment with inhibitors. Furthermore, LPS-induced NLRC4
expression was attenuated by the treatment of MAPK inhibitors (all
P<0.01; Fig. 5).
Discussion
The present study, to the best of our knowledge, is
the first to report that the expression of NLRC4 was significantly
upregulated in the blood serum of children with septicaemia. This
finding suggested that NLRC4 may exert an important function in
infectious diseases. In vitro experiments were performed to
investigate the importance of NLRC4 in the immune responses of
macrophages, and the associated molecular mechanisms.
Bacteria, particularly Gram-negative bacteria, are
the predominant causes of infection (15). LPS is the representative endotoxin
on the outer membrane of Gram-negative bacteria (16). It has been established that LPS
activates the inflammatory response and innate immune system in
infection, and induces overproduction of pro-inflammatory cytokines
(17).
In the present study, LPS-stimulated RAW264.7 cells
were used to investigate the effect of NLRC4 on the production of
IL-1β and IL-18. They are cytokines produced by macrophages and
other cells under various stimuli. They are important mediators of
the inflammatory response (18–21).
In a previous study by Ceballos-Olvera et al (22), it was reported that
NLRC4−/− mice produced IL-1β and IL-18 in higher
quantities than wild type mice. By contrast, DeSantis et al
(23) reported that IL-18 was
reduced in NLRC4−/− mice. Consistent with the reports by
Ceballos-Olvera et al (22), the current study determined that
knockdown of NLRC4 enhanced the effect of LPS on IL-1β and IL-18
production. This may indicate that NLRC4 suppresses LPS-induced
overproduction of inflammatory cytokines.
The MAPK signaling pathway is involved in a variety
of physiological processes, including cellular growth, development,
differentiation, stress and cell death (24–26).
In addition, MAPKs are also associated with numerous innate immune
responses (27–29). LPS may activate the MAPK signaling
pathway and downstream transcription factors may be induced to
regulate the release of large quantities of pro-inflammatory
cytokines (30). The present study
initially reported that LPS induces NLRC4 expression in a time- and
dose-dependent manner. As MAPKs are important in inflammatory and
immune responses, it was further investigated whether LPS regulates
the expression of NLRC4 via the MAPK signaling pathway. ERK, JNK,
and the p38 MAPK are three widely studied conventional MAPK
signaling pathways and inhibitors of ERK, JNK and p38 were used in
the present study to block the MAPK signaling pathway. The results
demonstrated that LPS-induced NLRC4 expression was reversed by the
suppression of the MAPK signaling pathway.
These data support the hypothesis that LPS activates
the MAPK signaling pathway in macrophages, thus resulting in the
upregulation of NLRC4. However, NLRC4 inhibits IL-1β and IL-18
production, contributing to the anti-inflammatory response. To the
best of our knowledge, the present study is the first to
investigate the expression of NLRC4 in children with septicaemia.
Furthermore, a novel molecular mechanism for NLRC4 regulation in
LPS-induced RAW264.7 cells was elucidated. NLRC4 requires further
investigation as a potential therapeutic strategy against
infectious diseases.
Acknowledgments
The present study was supported by the Science and
Technology Project of Jiangsu Province Department of Health (grant
no. Z201406).
References
|
1
|
Misallati A, el-Bargathy S and Shembesh N:
Blood-culture-proven neonatal septicaemia: A review of 36 cases.
East Mediterr Health J. 6:483–486. 2000.
|
|
2
|
Meremikwu MM, Nwachukwu CE, Asuquo AE,
Okebe JU and Utsalo SJ: Bacterial isolates from blood cultures of
children with suspected septicaemia in Calabar, Nigeria. BMC Infect
Dis. 5:1102005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geddes BJ, Wang L, Huang WJ, Lavellee M,
Manji GA, Brown M, Jurman M, Cao J, Morgenstern J, Merriam S, et
al: Human CARD12 is a novel CED4/Apaf-1 family member that induces
apoptosis. Biochem Biophys Res Commun. 284:77–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franchi L, Amer A, Body-Malapel M,
Kanneganti TD, Ozören N, Jagirdar R, Inohara N, Vandenabeele P,
Bertin J, Coyle A, et al: Cytosolic flagellin requires Ipaf for
activation of caspase-1 and interleukin 1beta in
salmonella-infected macrophages. Nat Immunol. 7:576–582. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miao EA, Alpuche-Aranda CM, Dors M, Clark
AE, Bader MW, Miller SI and Aderem A: Cytoplasmic flagellin
activates caspase-1 and secretion of interleukin 1beta via Ipaf.
Nat Immunol. 7:569–575. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lightfield KL, Persson J, Trinidad NJ,
Brubaker SW, Kofoed EM, Sauer JD, Dunipace EA, Warren SE, Miao EA
and Vance RE: Differential requirements for NAIP5 in activation of
the NLRC4 inflammasome. Infect Immun. 79:1606–1614. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu
H, Liu L and Shao F: The NLRC4 inflammasome receptors for bacterial
flagellin and type III secretion apparatus. Nature. 477:596–600.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kofoed EM and Vance RE: NAIPs: Building an
innate immune barrier against bacterial pathogens. NAIPs function
as sensors that initiate innate immunity by detection of bacterial
proteins in the host cell cytosol. Bioessays. 34:589–598. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poyet JL, Srinivasula SM, Tnani M, Razmara
M, Fernandes-Alnemri T and Alnemri ES: Identification of Ipaf, a
human caspase-1-activating protein related to Apaf-1. J Biol Chem.
276:28309–28313. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miao EA, Ernst RK, Dors M, Mao DP and
Aderem A: Pseudomonas aeruginosa activates caspase 1 through Ipaf.
Proc Natl Acad Sci USA. 105:2562–2567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miao EA, Mao DP, Yudkovsky N, Bonneau R,
Lorang CG, Warren SE, Leaf IA and Aderem A: Innate immune detection
of the type III secretion apparatus through the NLRC4 inflammasome.
Proc Natl Acad Sci USA. 107:3076–3080. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brodsky IE, Palm NW, Sadanand S, Ryndak
MB, Sutterwala FS, Flavell RA, Bliska JB and Medzhitov R: A
yersinia effector protein promotes virulence by preventing
inflammasome recognition of the type III secretion system. Cell
Host Microbe. 7:376–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Warren SE, Mao DP, Rodriguez AE, Miao EA
and Aderem A: Multiple nod-like receptors activate caspase 1 during
listeria monocytogenes infection. J Immunol. 180:7558–7564. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Luna CM, Rodriguez-Noriega E, Bavestrello
L and Guzmán-Blanco M: Gram-negative infections in adult intensive
care units of latin america and the Caribbean. Crit Care Res Pract.
2014:4804632014.PubMed/NCBI
|
|
16
|
Moran AP, Prendergast MM and Appelmelk BJ:
Molecular mimicry of host structures by bacterial
lipopolysaccharides and its contribution to disease. FEMS Immunol
Med Microbiol. 16:105–115. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeuchi O, Hemmi H and Akira S:
Interferon response induced by toll-like receptor signaling. J
Endotoxin Res. 10:252–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dennis VA, Jefferson A, Singh SR, Ganapamo
F and Philipp MT: Interleukin-10 anti-inflammatory response to
borrelia burgdorferi, the agent of lyme disease: A possible role
for suppressors of cytokine signaling 1 and 3. Infect Immun.
74:5780–5789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zediak VP and Hunter CA: IL-10 fails to
inhibit the production of IL-18 in response to inflammatory
stimuli. Cytokine. 21:84–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasama T, Miwa Y, Isozaki T, Odai T,
Adachi M and Kunkel SL: Neutrophil-derived cytokines: Potential
therapeutic targets in inflammation. Curr Drug Targets Inflamm
Allergy. 4:273–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brodsky IE and Monack D: NLR-mediated
control of inflammasome assembly in the host response against
bacterial pathogens. Semin Immunol. 21:199–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ceballos-Olvera I, Sahoo M, Miller MA, Del
Barrio L and Re F: Inflammasome-dependent pyroptosis and IL-18
protect against burkholderia pseudomallei lung infection while
IL-1β is deleterious. PLoS Pathog. 7:e10024522011. View Article : Google Scholar
|
|
23
|
DeSantis DA, Ko CW, Liu Y, Liu X, Hise AG,
Nunez G and Croniger CM: Alcohol-induced liver injury is modulated
by Nlrp3 and Nlrc4 inflammasomes in mice. Mediators Inflamm.
2013:7513742013. View Article : Google Scholar
|
|
24
|
Plotnikov A, Zehorai E, Procaccia S and
Seger R: The MAPK cascades: Signaling components, nuclear roles and
mechanisms of nuclear translocation. Biochim Biophys Acta.
1813:1619–1633. 2011. View Article : Google Scholar
|
|
25
|
Runchel C, Matsuzawa A and Ichijo H:
Mitogen-activated protein kinases in mammalian oxidative stress
responses. Antioxid Redox Signal. 15:205–218. 2011. View Article : Google Scholar
|
|
26
|
Keshet Y and Seger R: The MAP kinase
signaling cascades: A system of hundreds of components regulates a
diverse array of physiological functions. Methods Mol Biol.
661:3–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung
GH, Yoo BC and Cho JY: Functional roles of p38 mitogen-activated
protein kinase in macrophage-mediated inflammatory responses.
Mediators Inflamm. 2014:3523712014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tiedje C, Holtmann H and Gaestel M: The
role of mammalian MAPK signaling in regulation of cytokine mRNA
stability and translation. J Interferon Cytokine Res. 34:220–232.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|