Introduction
Spinal cord injury (SCI) is a serious central
nervous system disorder. Sociological changes, and infrastructural
and transport system development have resulted in an increased
incidence of SCI (1). Patients
with SCI experience irreversible sensory and motor dysfunction. In
modern society, the length of time that patients with paralysis and
SCI survive has increased, which results in a heavy financial
burden for the families and society.
SCI commonly leads to muscle atrophy (2). Previous research has demonstrated
that within the initial 6–18 months following SCI, 27 to 56% of the
muscle fibers have atrophied (3).
Currently, there is no clear consensus regarding classification of
the type of atrophy that occurs following SCI, certain reports
suggest disuse (4–7) and denervated atrophy (8,9).
However, the atrophy induced by SCI is different from the
aforementioned types of atrophy. Disuse models are based on
variable degrees of unloading and inactivity, including ankle
immobilization and hindlimb unloading. Disuse-induced muscle
atrophy occurs when the central nervous system is intact. Another
scenario is denervated atrophy, which is often induced by the model
of peripheral axotomy (10,11).
The central nervous system is not damaged in this type of muscle
atrophy, as the injury is restricted to the axon of the dorsal root
ganglion. The difference between SCI-induced muscle loss and the
aforementioned types of atrophy is that in SCI the lower motor
neurons remain intact and upper neurons cannot transmit information
to the lower neurons.
Due to the complexity of the central nervous system
and the observed cases of spontaneous functional recovery (12,13),
the present study hypothesizes that the SCI-induced muscle atrophy
(SIMA) may be the result of a special pathological mechanism. To
develop novel treatment strategies or find novel diagnostic
markers, it is required to investigate the molecular mechanisms and
signaling pathways that mediate SCI-induced muscle atrophy.
Previous studies have demonstrated the involvement of certain
molecules and genes during this pathological process (14–16).
However, the pathological mechanisms of SIMA are complex, thus,
only investigating certain molecules is insufficient. Microarray
data has provided some indication of the changes to mRNA expression
levels that occur within the whole genome following SCI. However,
the mRNA expression level does not always correlate with the
protein expression level. Post-translational modifications and
microRNAs may affect the protein expression levels (17). Thus, adopting a proteomics approach
to analyze the global protein profile in SIMA may provide more
accurate information that cannot be obtained from microarray
analysis.
To the best of our knowledge, there have been no
systematic studies aimed at identifying the proteins associated
with SIMA on a proteome scale. The present study compared normal
soleus muscle samples with post-SCI soleus muscle samples by
two-dimensional (2D) gel electrophoresis, followed by matrix
assisted laser desorption/ionization-time of flight mass
spectrometry (MALDI-TOF MS) analysis and identified a number of
specific proteins that demonstrated differential expression levels.
It is important to accumulate basic data, and the results of the
present study may improve diagnostic procedures and the design of
future strategies to treat SIMA.
Materials and methods
Animals and treatments
In the present study, female Wistar rats (n=30;
weight, 220–250 g, age, 8 weeks; Tianjin Medical University,
Tianjin, China) were used. Rats were single-housed in standard
plastic cages in a room temperature (24±2°C), relative humidity
(50±10%), and light/dark conditions (12/12-h light/dark cycle) room
with access to food and water ad libitum. The study was
approved by the ethics committee of Tianjin Medical University
General Hospital. All procedures in the current study, including
the use of experimental animals, followed the guidelines of the
Animal Care and Research Committee of Tianjin Medical University.
The rats were divided into a sham group and two injury groups (7
and 14 days post-SCI; n=10/group). The contusion injury models were
induced by the standard New York University (NYU) impactor, as
described previously (18).
Animals were anesthetized (3 ml/kg 10% chloral hydrate,
intraperitoneally) and laminectomized to expose the T10 spinal
cord. A moderate injury was generated by dropping a metal rod (10
g, 12.5 mm) onto the back side of the spinal cord. The compression
force and velocity were controlled by a surveillance system to
maintain uniformity between animals. These rats were manually
treated with bladder emptying. Sham subjects underwent a T10
laminectomy without contusion. Locomotive behavior was assessed at
0, 2, 4, 6, 8, 10, 12 and 14 days following injury using Basso,
Beattie, and Bresnahan (BBB) score (19) to ensure that all subjects exhibited
a normal curve of functional changes following SCI.
After 7 and 14 days, rats were euthanized with
pentobarbital anesthesia (100 mg/kg, intraperitoneally), and then
perfused with phosphate buffered-saline (PBS). Following perfusion,
the posterior hind limbs were sliced to retrieve soleus muscle
samples. Initially, the gastrocnemius muscle was lifted by cutting
the terminus of the Achilles tendon to expose the soleus muscle.
The soleus muscle was carefully excised from the posterior limbs
and all connective tissue was removed to reserve muscles. Prior to
each operation, the body weight of the rats was measured and then
the isolated soleus muscle was weighed following bilateral
harvesting. Following the surgery, the ratio of muscle weight to
body weight was calculated, then muscle samples were frozen in
liquid nitrogen and stored at −80°C. Control group rats were
sacrificed following the same protocol at 14 days post-injury.
Histological examination
Samples from each group (n=10) were fixed in 10%
phosphate-buffered formaldehyde solution. Following the process of
decalcification and dehydration, the muscles were embedded in
paraffin, cut into 5-µm sections and stained using
hematoxylin and eosin. Muscle fibers (≥100 in each group) were
observed in bright-field using Nikon ECLIPSE TS100 light microscope
(Tokyo, Japan) and Nikon D7200 image acquisition system. Their
diameters were measured using Image J software (National Institutes
of Health, Bethesda, MD, USA).
Sample preparation and 2D gel
electrophoresis
Muscles samples were washed twice with PBS, once
with distilled water and then ground to a fine powder with a pestle
in liquid nitrogen. Lysis buffer [8 M urea, 2 M thiourea, 2% (w:v)
CHAPS, 1% dithiothreitol (DTT), 1 mM phenylmethanesulfonyl fluoride
(PMSF); 0.17 ml], 50 µg/ml DNase I and 1 mM PMSF was added
to each sample. The samples were lysed by exposure to ultrasound
for 30 sec (time interval 0.5 sec) repeated four times. The lysates
were centrifuged at 12,000 × g for 30 min at 4°C to obtain the
supernatants. Protein concentration was determined using the
Bradford method and samples were stored at −80°C.
For first-dimension isoelectric focusing, pH 3-10
non-linear range immobilized pH gradient (IPG) strips (18 cm) were
rehydrated with solubilized protein samples (800 µg) for 12
h. Isoelectric focusing was performed Isoelectric focusing was
performed using the Protean II Xi cell electrophoresis system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) under the following
voltage time program: 0–500 V for 1 h, 500 V for 5 h, 500–3500 V
for 3.5 h, 3500 V for 14 h and finally 3500–5000 V for 4 h.
Immediately following focusing, the IPG strips were
equilibrated for 15 min in equilibration buffer I [50 mM Tris-HCl
pH 6.8, 6 M urea, 30% glycerol, 2% sodium dodecyl sulfate (SDS), 2%
DTT, 0.02% bromophenol blue], and then for 15 min in equilibration
buffer II (50 mM Tris-HCl pH 6.8, 6 M urea, 30% glycerol, 2% SDS,
2.5% iodoacetamide, 0.02% bromophenol blue). The second dimension
separation was performed at 15°C using a vertical electrophoresis
system (Multiphor II; GE Healthcare Life Sciences, Chalfont, UK)
with 1-mm 12.5% acrylamide gels run at 20 mA/gel until the tracking
dye reached the bottom of the gel. The 2D PAGE gel was then stained
with Coomassie Blue.
Visualization of proteins and image
analysis
Following electrophoresis, the 2D gels were
subjected to staining with a solution of 10% ammonium sulfate, 10%
phosphoric acid, 0.12% G250 and 20% methanol overnight.
The gels were washed with a destaining solution (3%
glacial acetic acid) in horizontal rotators. Finally, the gels were
washed with Milli-Q water for 30 min. Images were obtained by
scanning the 2D gels with a PowerLook 2100XL scanner (UMAX
Technologies, Inc., Dallas, TX, USA).
PDQuest v 8.0 (Bio-Rad Laboratories, Inc.) software
was used to calculate the intensities of protein spots to identify
differentially expressed proteins. To correct quantitative
variations in the intensity of protein spots, spot volumes were
normalized as a percentage of the total volume of all the spots
present in a gel. Only spots that were consistently present in the
gels from at least three rats in each group were analyzed to avoid
spot differences that were due to gel-to-gel or biological
variation inherent among the rats. The protein molecular weight,
which ranged from 10 to 100 kDa, and the isoelectric point (pI),
which ranged from 3 to 10, of each protein spot were calculated by
the software using the distributions of standard markers and pI
positions, respectively. Analysis of variance (AVOVA) with Tukey's
post-hoc multiple comparison tests were performed to analyze
statistically different intensities among groups. P<0.05 was
considered to indicate a statistically significant difference.
In-gel tryptic digestion and MALDI-TOF-MS
analysis
Selected protein spots were excised from the gel and
cut into ~1 mm2 pieces. Samples were destained using 50%
(vv) acetonitrile (ACN) and 25 mM ammonium bicarbonate (100
µl, pH 8.0) for 15 min, this was repeated three times until
the destaining process was complete. The gel samples were dipped
into 100% ACN (30 µl, pH 8.0) for 5 min until they turned
white. They were dried at room temperature and the samples were
then digested using trypsin (8 µl, 0.005 mg/ml) at 37°C for
16 h. The digested proteins (0.3 µl) coupled with 0.3
µl matrix were subjected to MALDI-TOF MS analysis using a
4700 Proteomics Analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) to obtain the peptide mass
fingerprint (PMF) with 4,600 laser intensity. The peak list was
generated by GPS Explorer v 3.5 software (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and searched using Mascot (version
2.1.0; Matrix Science Ltd., London, UK). The PMFs were processed by
using the NCBI databases (www.ncbi.nlm.nih.gov) and the fingerprinting method
was applied allowing a maximum of one missed tryptic cleavage per
protein. The protein score confidence calculated using the Mascot
algorithm. Confidence score ≥95% and P<0.05 was considered to
indicate a statistically significant difference.
Classification of proteins
Identified proteins were submitted to the Protein
Analysis Through Evolutionary Relationships (PANTHER)
classification system (www.pantherdb.org) to determine their function via
high-throughput analysis (20).
Proteins were classified according to family and subfamily,
molecular function, biological process and pathway.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Significant differences were determined by ANOVA testing.
SPSS software version 22.0 was used for data analysis (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
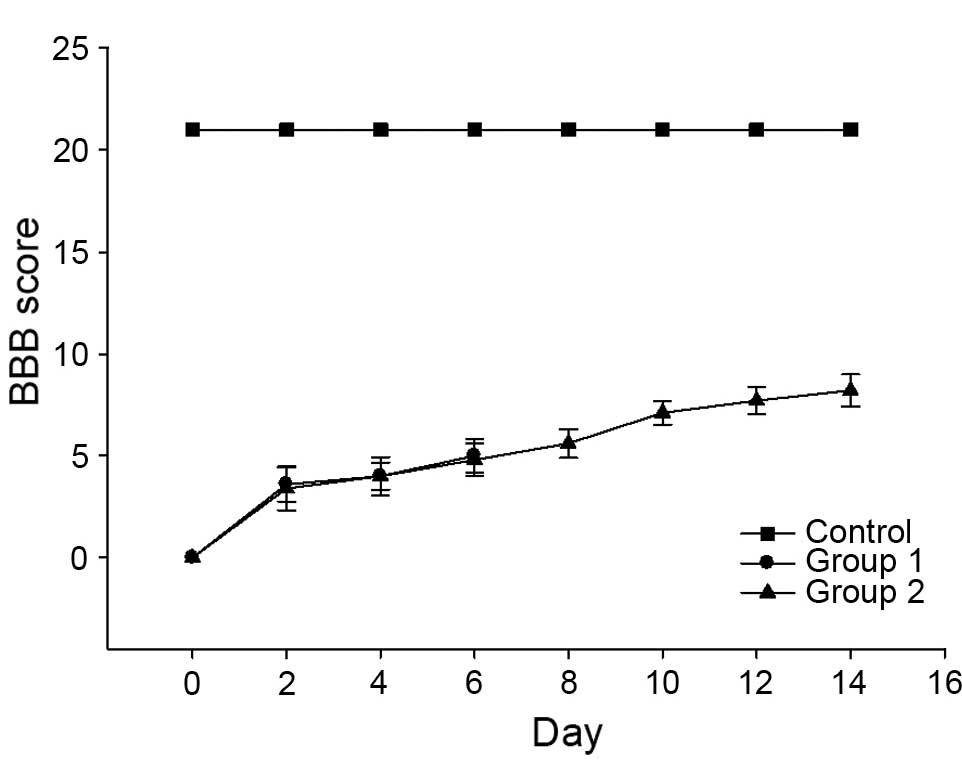
BBB score in rats post-SCI increased with
recovery time
In the control group, all rats exhibited a BBB score
of 21. In the injured groups, immediately following SCI, the motor
function of rats' hind limbs was severely impaired leading to BBB
scores of ~0. Subsequently, the score gradually increased and all
injured subjects demonstrated a similar recovery process. SCI rats
recovered to exhibit BBB scores of ~9 by 7 days post-injury. Scores
reached 10 after 14 days. Rats in the control group exhibited BBB
scores of 21 throughout the experiment (Fig. 1).
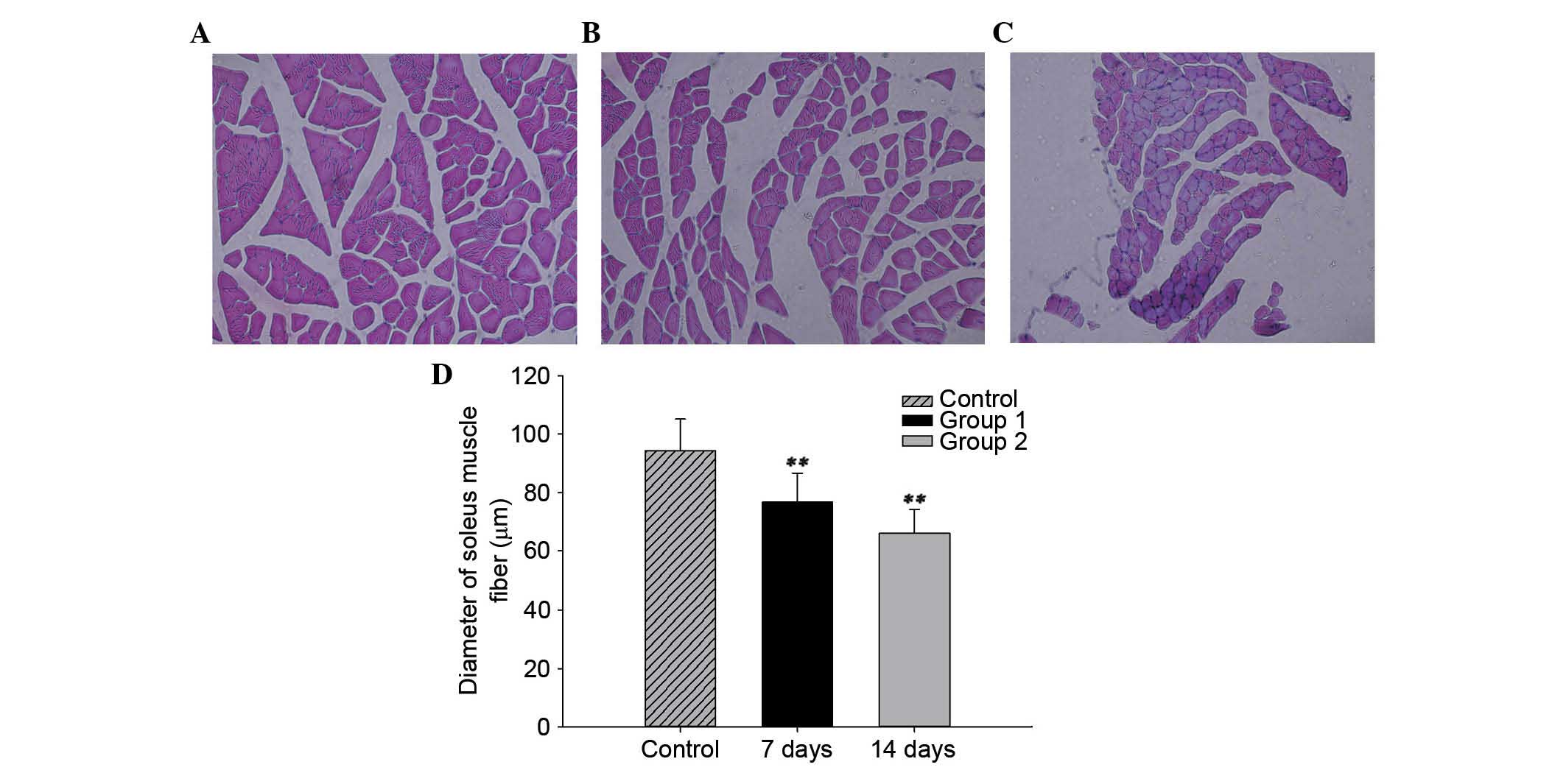
SCI reduces soleus muscle fiber
diameter
The mean diameter of the soleus muscle fiber was
94.4±10.8 µm in the control group. In the SCI groups, the
mean diameters of the soleus muscle fiber were 76.7±10.0 µm
and 66.2±8.2 µm at 7 and 14 days post-injury, respectively.
The diameters in the SCI groups were significantly decreased
compared with the control group (P<0.01). The mean diameter of
the soleus muscle fiber decreased gradually at different time
points (Fig. 2).
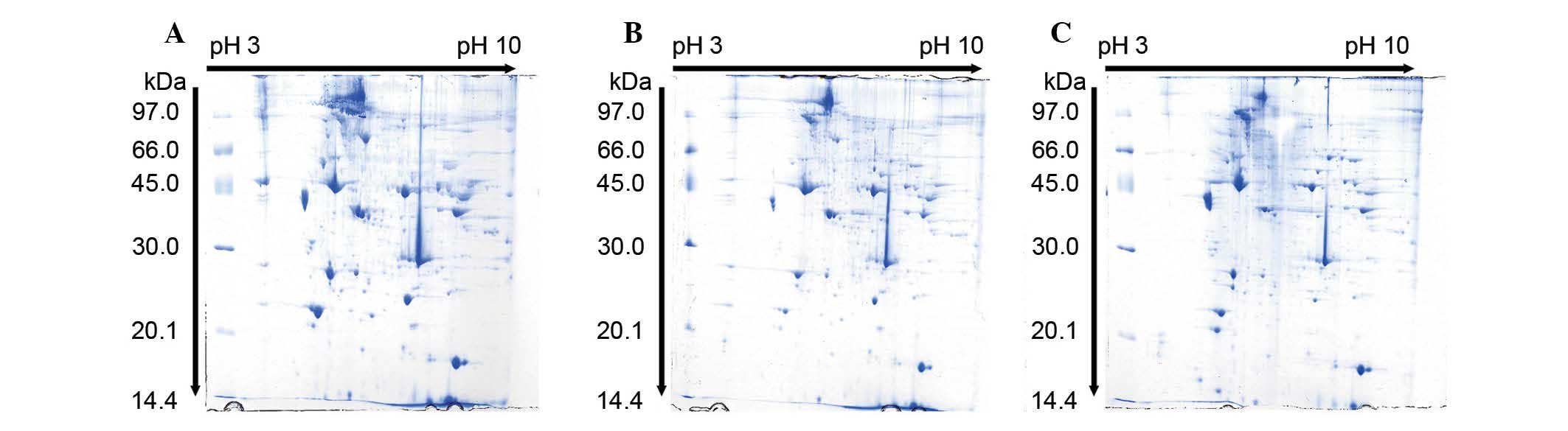
SCI induced differential protein
expression in soleus muscle samples
Differentially expressed proteins in the soleus
muscle of injured and control rats were identified and analyzed
using 2D PAGE and MALDI TOF. Gel images of proteins isolated from
the muscle of control, 7 and 14 days post-SCI groups were compared
(Fig. 3). Per gel, >500 protein
spots were detected. The protein expression profile in the soleus
muscle of SCI rats indicated that the expression levels of >50
proteins were changed compared with the control group. Among
altered proteins, 23 protein spots exhibited significant changes
(Table I) and were quantitatively
high enough to be identified by MALDI-TOF. MS data were analyzed
using the Mascot search engine. The Mascot search identified 20
spots with high confidence (Table
II). The confidence score of proteins was >95%.
 | Table IIdentification of differentially
expressed proteins by MALDI-TOFMS in rat soleus muscle at different
time points following SCI. |
Table I
Identification of differentially
expressed proteins by MALDI-TOFMS in rat soleus muscle at different
time points following SCI.
| Protein name | Fold-change protein
expression
|
|---|
| 7 days
post-SCI/control | 14 days
post-SCI/control | 14 days post-SCI/7
days post-SCI |
|---|
| Myosin regulatory
light chain 2 | 0.157911 | 0.242524 | 0.651114 |
| 14-3-3 Protein
ε | 2.198649 | 1.282510 | 1.714334 |
| Probable
C>U-editing enzyme APOBEC-2 | 0.742312 | 2.545599 | 0.291606 |
| Tropomyosin β
chain | 2.230306 | 0.752411 | 2.964213 |
| ATP synthase β
subunit | 1.201723 | 3.443378 | 0.348995 |
| Myosin light
chain | 0.247498 | 0.156377 | 1.582694 |
| Myosin light chain
3 | 0.247498 | 0.156377 | 1.582694 |
| α-Actinin-2 | 0.253011 | 0.070115 | 3.608539 |
| Chain A, crystal
structure of the 70-kDa heat shock cognate protein | 0.455156 | 1.406490 | 0.323611 |
| Heat shock protein
β 6 | 0 | 1.292586 | 0 |
| Serum albumin
precursor | 2.528853 | 2.490987 | 1.015201 |
| α B-crystallin | +∞a | 00b | 0 |
| Troponin T class
IIIb β | +∞a | +∞a | 0.326424 |
| β-Enolase | +∞a | +∞a | 0.117352 |
| Creatine kinase
M-type | +∞a | +∞a | 2.717467 |
| Fibrinogen β chain
precursor | 0.401764 | 0.946549 | 0.424452 |
| Fibrinogen α
chain | 0.139896 | 1.026677 | 0.136261 |
| MMSDH | 0.127811 | 0.816477 | 0.156540 |
| Phosphoglycerate
kinase 1 | 0.081919 | 0.458707 | 0.178586 |
 | Table IIIdentification of differentially
expressed proteins by MALDI-TOF MS in rat soleus muscle samples at
different time points following SCI. |
Table II
Identification of differentially
expressed proteins by MALDI-TOF MS in rat soleus muscle samples at
different time points following SCI.
| SSP | Accession
number | Protein name | MWpIa | Peptide count | Scoreb | Potential
participation of signaling pathways |
|---|
| 1108 | 386869343 | Myosin regulatory
light chain 2 | 18868.394.86 | 13 | 100 | Focal adhesion,
regulation of actin cytoskeleton |
| 1306 | 13928824 | 14-3-3 Protein
ε | 29326.484.63 | 8 | 99.39 | Hippo, PI3K-Akt,
p53 signaling |
| 1407 | 9507245 | 14-3-3 Protein
ε | 28455.984.8 | 11 | 100 | Hippo, PI3K-Akt,
p53 signaling |
| 2102 | 157822795 | Probable
C>U-editing enzyme APOBEC-2 | 25871.964.75 | 12 | 100 | Not annotated |
| 2209 | 11875203 | Tropomyosin β
chain | 32930.594.66 | 12 | 99.95 | Cardiac muscle
contraction, adrenergic signaling in cardiomyocytes |
| 2617 | 1374715 | ATP synthase β
subunit | 51170.654.92 | 22 | 100 | Not annotated |
| 3501 | 205474 | Myosin light
chain | 20948.594.99 | 13 | 100 | Focal adhesion,
regulation of actin cytoskeleton |
| 4108 | 6981240 | Myosin light chain
3 | 22256.135.03 | 15 | 100 | Focal adhesion,
regulation of actin cytoskeleton |
| 4406 | 157951643 | α-Actinin-2 | 104338.545.31 | 41 | 100 | Not annotated |
| 5112 | 178847300 | Chain A, crystal
structure of the 70-kDa | 59894.935.91 | 23 | 100 | MAPK signaling
pathway, heat shock cognate protein, endocytosis, protein
processing in endoplasmic reticulum |
| 5310 | 20302069 | Heat shock protein
β 6 | 17551.126.05 | 7 | 99.97 | Nicotinate and
nicotinamide metabolism |
| 5406 | 158138568 | Serum albumin
precursor | 70709.866.09 | 17 | 100 | Metabolic pathways,
endocrine and other factor-regulated calcium reabsorption, Ras and
Rap1 signaling |
| 5525 | 30387800 | α B-crystallin | 20155.446.84 | 16 | 100 | Protein processing
in endoplasmic reticulum |
| 6517 | 207391 | Troponin T class
IIIb β | 28301.929.36 | 15 | 100 | Not annotated |
| 6518 | 126723393 | β-Enolase | 47326.457.08 | 16 | 100 | Not annotated |
| 6520 | 6978661 | Creatine kinase
M-type | 43219.876.58 | 31 | 100 | Not annotated |
| 7303 | 158186678 | Fibrinogen β chain
precursor | 54827.927.9 | 24 | 100 | Complement and
coagulation cascades, platelet activation |
| 1202 | 144922622 | Fibrinogen α
chain | 60980.867.11 | 23 | 100 | Endocytosis,
complement and coagulation cascades, platelet activation |
| 1205 | 400269 | MMSDH | 58226.748.47 | 12 | 100 | Not annotated |
| 1206 | 40254752 | Phosphoglycerate
kinase 1 | 44909.148.02 | 20 | 100 |
Glycolysisgluconeogenesis, HIF-1
signaling |
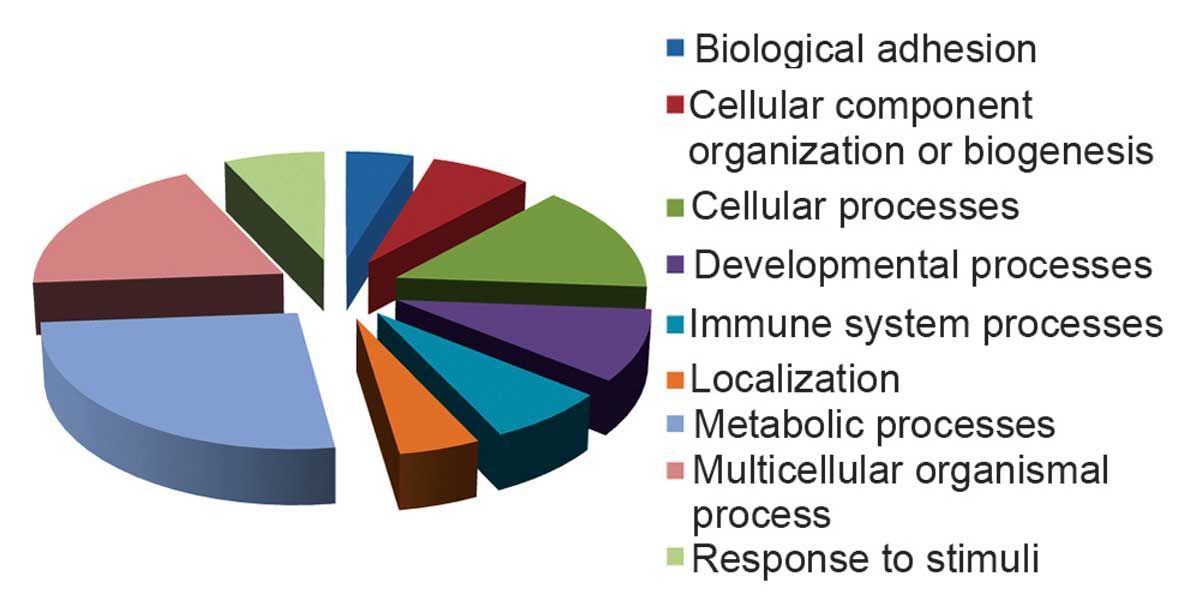
To investigate the dynamic molecular mechanisms
involved in SCI, the identified altered proteins were classified
into 9 functional categories using the PANTHER database, including
biological adhesion, cellular component organization or biogenesis,
cellular processes, developmental processes, immune system
processes, localization, metabolic processes, multicellular
organism processes and response to stimulus (Fig. 4).
Discussion
Repeatable injuries are essential for assessing the
severity of muscle atrophy in SCI animal models in original and
replicative studies. The current study used an NYU impactor to
generate the SCI and verified that SCI animals shared similar
recovery curves, which were in accordance with the results of Basso
et al (21). Furthermore,
the BBB recovery curves were identical for the experimental groups
at 14 days post-injury. This indicates that the present study
produced a consistent and reproducible contusion SCI model.
Using 2D gels and MALDI-TOF MS analysis, the current
study identified differentially expressed proteins in soleus muscle
fibers of control and SCI model rats. In present study, it was
demonstrated that this method is efficient for the identification
of multiple proteins, a number of them at low abundance. Previous
studies investigating muscle atrophy typically focus on the disuse
and denervated animal models to simulate their corresponding
clinical phenomenon. SIMA is often classified as one of these
models of muscle atrophy or a combination of these models. However,
the pathological process of SIMA involves multiple factors,
including signal transduction, immunization, electrical conduction,
stimuli and metabolism (22),
which results in a complex condition that cannot be attributed to
one molecular mechanisms or a simpler combination of a small
number. Thus, the present study investigated the pathological
changes that occur during SIMA. Using 2D gels, numerous proteins
were identified. Certain proteins exhibited a similar expression
pattern as previously described (23), but others exhibited different
curves or inconsistent ones (24).
The potential importance and function of these proteins in SIMA is
discussed further below.
The 14-3-3 proteins, as conserved regulatory
molecules, have been demonstrated to be involved in numerous
intracellular processes, including cell cycle regulation, metabolic
control, apoptosis, and the control of gene transcription in almost
all eukaryotic cells. Previous research demonstrated that 14-3-3
proteins are upregulated in the denervated tibialis anterior muscle
of rats (25). However, other
studies have observed that 14-3-3 was not increased in the skeletal
muscles of patients with chronic obstructive pulmonary disease,
which is a hypoxemic disease with similar characteristics to the
early changes observed in the microenvironment during SCI (26). As a prognostic indicator, early
accumulation of the 14-3-3 protein in the cerebrospinal fluid of
patients with acute transverse myelitis was perceived to have been
associated with little or no recovery of neurological function
(27). However, deficiencies have
been noted regarding certain aspects of these previous studies. The
limited number of subjects (n=4 per group) may influence the
accuracy of results. Furthermore, a previous study (28) observed that 14-3-3 protein was only
expressed in ~10% of the patients and concluded that 14-3-3
expression was, thus, not associated with a poor patient outcome.
Therefore, the present study hypothesizes that 14-3-3 may be
important for the prediction of recovery from muscle atrophy
following SCI. Certain studies support this hypothesis. In the
cerebrospinal fluid of SCI rats, severe injury induced a slight
reduction in 14-3-3 protein expression compared with moderate
injury (29). Another study with a
contusion model demonstrated that the expression levels of three
isoforms of the 14-3-3 protein family increased at the mid-stage of
the recovery following the restoration of bladder function
(30). Despite the possibility
that elevated levels of 14-3-3 protein following SCI may reflect
the process of neuronal damage (31), in the central system (32) or peripheral tissues (33), the present results demonstrate that
14-3-3 protein, coupled with functional recovery, was increased
despite an early decrease, which may be attributed to the slow
reaction of the protein. Upregulation of 14-3-3 protein expression
levels can activate downstream reaction of the
phosphatidylinositol-4,5-bispho-sphate 3-kinase-AKT signaling
pathway, which may suppress apoptosis. An aim of future research
will be to investigate that underlying mechanism that results in
changes to 14-3-3 expression levels following SCI. Different
severities of SCI and more time points will be useful to elucidate
the biochemical and pathophysiological function of proteins
differentially expressed in SIMA.
αB-crystallin (CryAB) is a small heat shock protein
involved in preventing protein aggregation (34). Dysfunction of CryAB may result in
skeletal muscle disorders and enhancing the function of this
protein is accepted as a potential therapeutic strategy (35). Previous studies indicate that CryAB
is a protective protein that maintains the capability of satellite
cells to regenerate skeletal muscle (34) and that it is important for muscle
homeostasis (36). Denervated
tibialis anterior muscle exhibits increased expression of CryAB.
CryAB can prevent apoptosis induced by a variety of stimuli, which
may explain why fast-twitch anterior muscle exhibits reduced
atrophy (25). In the present
study, CryAB protein expression was upregulated at 7 days post-SCI
and then reduced to the level as that of the control by day 14.
This notable finding is contrary to previous research that
demonstrated that denervation decreased the expression levels of
CryAB in slow muscle, particularly in the soleus (37). However, this discrepancy suggests
that SIMA, which differs from denervation, has the potential for
recovery at an early stage and that the upregulation of CryAB may
be a marker of protection. This hypothesis is supported by indirect
evidence demonstrating that expression of CryAB returned to the
level observed in the normal controls following successful
reinnervation (38). Together, the
results of the present study indicate that, following SCI, there is
a transient time window for the self-restoration of skeletal
muscle, but ultimately the positive effect is suppressed by
persistent disadvantageous pathogenic factors. Thus, confirming the
accurate time window and the upregulation of CryAB may be
investigated as future therapeutic strategies.
β-enolase is widely distributed in cells and
involved in the glycolysis pathway (39). Increasing evidence has demonstrated
that β-enolase may assist DNA transcription, replication and
repair. Previous studies demonstrated that the expression of
β-enolase is dependent on regular nerve activity (40) and its upregulation indicates
regeneration of skeletal muscle (41). Research indicates that the
expression of β-enolase following denervation remains at a low
level in slow-twitch muscle, however, continues to decrease in the
fast-twitch muscle (40,42). However, the present study
demonstrated that the expression levels of β-enolase in SCI muscle
increased steadily, which indicates that SIMA has the reverse
effect on this protein. Previous evidence suggested that following
reinnervation, the expression level of β-enolase in soleus muscle
increased, which may support the hypothesis that the increase of
β-enolase expression in the soleus muscle depends on the integrity
of peripheral nerves. However, the functional recovery of SCI is
limited and muscle atrophy is inevitable. In sum, the present study
hypothesized that the role of β-enolase is altering the muscle
types, as demonstrated in a previous study (43) that supports the current
observations. In addition, a longer period of observation is
required in future research.
The bio-functions of apolipoprotein B mRNA editing
enzyme catalytic polypeptide like 2 (APOBEC2) have been
hypothesized to include mediation of the cytidine-to-uridine
transcriptional editing of mRNA (44). Due to exclusive expression in
skeletal and cardiac muscles, APOBEC2 was suggested to be important
in the physiological function of intramuscular development.
However, APOBEC2 knockout mice did not exhibit a change in
phenotype (45). This may indicate
that APOBEC2 subtly regulates certain aspects of muscle function,
including the response to damage stimuli. The results of the
present study demonstrated that the protein expression levels of
APOBEC2 in the soleus increased gradually following SCI, in
contrast to the change in expression levels during denervation
atrophy (24). This finding may be
attributed to intact neurons that give priority to compensation of
muscle function by switching the muscle types. A previous study
demonstrated that APOBEC2-deficient mice exhibited a marked shift
in muscle fiber type, from fast to slow (46). It is hypothesized that the increase
of APOBEC2 expression levels may result in enhanced conversion of
slow fibers to fast ones. Further evidence indicates that the ratio
of slow to fast fibers in soleus muscle was decreased gradually
following spinal cord transection (47).
α-actinin-2 is a major Z-disk component, which is
crucial for the crosslinking of actin and titin filaments (48,49).
It is understood that α-actinin-2 is expressed in all types of
muscle fibers, including slow and fast fibers (50). Previous studies demonstrated that
α-actinin-2 can bond with calsarcin-2, a key inhibitor of
calcineurin activation, to release calcineurin from calsarcin-2 and
change the fast fibers to a slow phenotype (51–53).
The present study demonstrated that following SCI, the expression
of α-actinin-2 in the soleus dropped sharply over time. This
decrease indicates that downregulation of α-actinin-2 may result in
enhancement of calsarcin-2, which inhibits the activation of
calcineurin signaling, and a shift in fiber type from slow to fast.
The results of the current study are consistent with previous
research and it is hypothesized that the shift of metabolic
phenotype of muscle fibers following SCI preserves the energy
metabolism and function of the muscle.
Heat shock protein family A member 4 (Hsp70) is a
highly conserved molecular chaperone protein that has been reported
to regulate various processes associated with stress, damage or
degeneration (54). The protective
effects of Hsp70 in muscle atrophy have also been demonstrated in a
number of previous studies (55–57).
Upregulation of Hsp70 may prevent muscle atrophy as a result of
denervation, aging or disuse (58–60).
The present study observed that the expression of Hsp70 in the
soleus muscle was slightly reduced at 7 days post-SCI and then
increased sharply at 14 days to a level markedly higher than that
of the control. This indicates that the muscle exhibited the
potential for restoration following central nervous system injury.
The slight reduction observed at the early stage of SCI may be
attributed to a temporary disorder of energy metabolism (61) or inhibition of glucocorticoid due
to the stress response (62).
However, it remains necessary to investigate why the protective
effect of Hsp70 is too limited to prevent atrophy and whether the
increased expression of this protein is also a transient
phenomenon.
Another notable finding from the present study is
that the protein levels of certain myosin regulatory light chain
(MLC) isoforms and enzymes involved in cellular and metabolic
processes were decreased in the soleus muscle at early stages (7
days) post-SCI. Subsequently, the expression was increased at 14
days post-SCI, with certain isoforms almost at similar levels to
the control. The function of MLCs is associated with contractile
force and velocity in muscle fibers (63). Multiple reports have demonstrated
that the changes to MLCs were associated with fiber-type shift
(8,64–66).
Certain enzymes key in cellular and metabolic processes, including
fibrinogen α and β chain, aldehyde dehydrogenase 6 family member A1
and phosphoglycerate kinase 1, participate in various biological
processes and their normal function is for homeostasis of
physiological and pathological metabolism (67–69).
Fluctuations in the expression levels of these proteins may
indicate that skeletal muscle has been damaged by central nervous
system injury, but with relatively integrated peripheral nervous
control, will exhibit reduced function of multiple molecular
processes at an early stage and subsequently adapt during the
middle stage. The results of the current study are in accordance
with a previous study that demonstrated that muscle appears to have
plasticity and can adapt following moderate spinal cord contusion,
which was similar to the present animal model (70).
The data of the current study indicated that
SCI-induced atrophic muscles exhibited limited molecular
improvements, which may be associated with functional recovery.
However these improvements cannot reverse the changes to the size
of muscle fibers of the soleus muscles of SCI rats. It is accepted
that following moderate contusion, rats will exhibit spontaneous
recovery of motor function following a fixed pattern (71,72).
Furthermore, previous findings indicated that the growth effects of
muscle fibers may result in improvement of motor function (73–75),
however, this does not mean that the size of the fibers is directly
associated with BBB score. Notably, the results of the present
study demonstrated that the extent of variation in the second week
was reduced compared with the change in the first week, which may
be attributed to the temporary molecular compensatory mechanisms.
These mechanisms may be used as therapeutic targets to induce
anti-atrophic effects or reverse the effects on skeletal muscle,
potentially coupled with more observable improvement of motor
function (34,62). However, in the current study, due
to specific molecular improvements, the process of muscle atrophy
slowed at a later stage, however, it may not be able to be stopped
or reversed. In addition, the increases to BBB scores also slowed
down at this stage. Concerning the delayed effects of molecules
prior to phenotypic changes, it is concluded that the decreased
extent of atrophic response was consistent with the sharp increase
to motor function recovery at the early stage. Future studies are
required to investigate prolonged observation over increased time
points.
In conclusion, the current study used a proteomics
approach to identify differential expression of proteins in the
soleus muscle at different time points following SCI. The
identified proteins were associated with biological adhesion,
cellular processes, developmental processes, immune system
processes, localization and metabolic processes. The results of the
present study demonstrated that SIMA has characteristics different
to disuse and denervated atrophy, or a combination of them. The
present study may improve the understanding of the association
between central nervous system injury and skeletal muscle atrophy.
Furthermore, the results may provide insight into potential
mechanisms or cellular pathways that may be adopted as biomarkers
and targets for the treatment of SIMA.
Acknowledgments
This study was supported by the State Program of the
National Natural Science Foundation of China (grant nos. 81330042
and 81371957); the Special Program for Sino-Russian Joint Research
Sponsored by the Ministry of Science and Technology, China (grant
no. 2014DFR31210); the Key Program Sponsored by the Tianjin Science
and Technology Committee, China (grant no/13RCGFSY19000); and the
Program for Research Sponsored by the Health Authority of Binhai
New Area of Tianjin, China (grant no. 2012BWKY028).
References
|
1
|
van den Berg ME, Castellote JM,
Mahillo-Fernandez I and de Pedro-Cuesta J: Incidence of spinal cord
injury worldwide: A systematic review. Neuroepidemiology.
34:184–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taillandier D, Aurousseau E, Meynial-Denis
D, Bechet D, Ferrara M, Cottin P, Ducastaing A, Bigard X, Guezennec
CY, Schmid HP and Attaix D: Coordinate activation of lysosomal,
Ca2+-activated and ATP-ubiquitin-dependent proteinases
in the unweighted rat soleus muscle. Biochem J. 316:65–72. 1996.
View Article : Google Scholar
|
|
3
|
Castro MJ, Apple DF Jr, Rogers S and
Dudley GA: Influence of complete spinal cord injury on skeletal
muscle mechanics within the first 6 months of injury. Eur J Appl
Physiol. 81:128–131. 2000. View Article : Google Scholar
|
|
4
|
Bodine SC: Disuse-induced muscle wasting.
Int J Biochem Cell Biol. 45:2200–2208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Booth FW and Gollnick PD: Effects of
disuse on the structure and function of skeletal muscle. Med Sci
Sports Exerc. 15:415–420. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohira Y, Yoshinaga T, Nomura T, Kawano F,
Ishihara A, Nonaka I, Roy RR and Edgerton VR: Gravitational
unloading effects on muscle fiber size, phenotype and myonuclear
number. Adv Space Res. 30:777–781. 2002. View Article : Google Scholar
|
|
7
|
Roy RR, Baldwin KM and Edgerton VR: The
plasticity of skeletal muscle: Effects of neuromuscular activity.
Exerc Sport Sci Rev. 19:269–312. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furuno K, Goodman MN and Goldberg AL: Role
of different proteolytic systems in the degradation of muscle
proteins during denervation atrophy. J Biol Chem. 265:8550–8557.
1990.PubMed/NCBI
|
|
9
|
Midrio M, Danieli-Betto D, Megighian A,
Velussi C, Catani C and Carraro U: Slow-to-fast transformation of
denervated soleus muscle of the rat, in the presence of an
antifibrillatory drug. Pflugers Arch. 420:446–450. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldberg AL: Protein turnover in skeletal
muscle. II. Effects of denervation and cortisone on protein
catabolism in skeletal muscle. J Biol Chem. 244:3223–3229.
1969.PubMed/NCBI
|
|
11
|
Goldspink DF: The effects of denervation
on protein turnover of rat skeletal muscle. Biochem J. 156:71–80.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gonzalez-Rothi EJ, Rombola AM, Rousseau
CA, Mercier LM, Fitzpatrick GM, Reier PJ, Fuller DD and Lane MA:
Spinal inter-neurons and forelimb plasticity after incomplete
cervical spinal cord injury in adult rats. J Neurotrauma.
32:893–907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaegi S, Schwab ME, Dietz V and Fouad K:
Electromyographic activity associated with spontaneous functional
recovery after spinal cord injury in rats. Eur J Neurosci.
16:249–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kostovski E, Boon H, Hjeltnes N, Lundell
LS, Ahlsén M, Chibalin AV, Krook A, Iversen PO and Widegren U:
Altered content of AMP-activated protein kinase isoforms in
skeletal muscle from spinal cord injured subjects. Am J Physiol
Endocrinol Metab. 305:E1071–E1080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park S and Hong Y, Lee Y, Won J, Chang KT
and Hong Y: Differential expression of caveolins and myosin heavy
chains in response to forced exercise in rats. Lab Anim Res.
28:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin W, Bauman WA and Cardozo C: Bone and
muscle loss after spinal cord injury: Organ interactions. Ann NY
Acad Sci. 1211:66–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Collier L, Qin W, Creasey G, Bauman
WA, Jarvis J and Cardozo C: Electrical stimulation modulates Wnt
signaling and regulates genes for the motor endplate and calcium
binding in muscle of rats with spinal cord transection. BMC
Neurosci. 14:812013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hill CE, Beattie MS and Bresnahan JC:
Degeneration and sprouting of identified descending supraspinal
axons after contusive spinal cord injury in the rat. Exp Neurol.
171:153–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mi H, Lazareva-Ulitsky B, Loo R, Kejariwal
A, Vandergriff J, Rabkin S, Guo N, Muruganujan A, Doremieux O,
Campbell MJ, Kitano H and Thomas PD: The PANTHER database of
protein families, subfamilies, functions and pathways. Nucleic
Acids Res. 33:D284–D288. 2005. View Article : Google Scholar :
|
|
21
|
Basso DM, Beattie MS, Bresnahan JC,
Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ,
Nockels R, et al: MASCIS evaluation of open field locomotor scores:
Effects of experience and teamwork on reliability. Multicenter
Animal Spinal Cord Injury Study. J Neurotrauma. 13:343–359. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bickel CS, Slade JM, Haddad F, Adams GR
and Dudley GA: Acute molecular responses of skeletal muscle to
resistance exercise in able-bodied and spinal cord-injured
subjects. J Appl Physiol (1985). 94:2255–2262. 2003. View Article : Google Scholar
|
|
23
|
Johnston TE, Modlesky CM, Betz RR and
Lauer RT: Muscle changes following cycling andor electrical
stimulation in pediatric spinal cord injury. Arch Phys Med Rehabil.
92:1937–1943. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato Y, Shimizu M, Mizunoya W, Wariishi H,
Tatsumi R, Buchman VL and Ikeuchi Y: Differential expression of
sarco-plasmic and myofibrillar proteins of rat soleus muscle during
denervation atrophy. Biosci Biotechnol Biochem. 73:1748–1756. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun H, Li M, Gong L, Liu M, Ding F and Gu
X: iTRAQ-coupled 2D LC-MSMS analysis on differentially expressed
proteins in denervated tibialis anterior muscle of Rattus
norvegicus. Mol Cell Biochem. 364:193–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Favier FB, Costes F, Defour A, Bonnefoy R,
Lefai E, Baugé S, Peinnequin A, Benoit H and Freyssenet D:
Downregulation of Aktmammalian target of rapamycin pathway in
skeletal muscle is associated with increased REDD1 expression in
response to chronic hypoxia. Am J Physiol Regul Integr Comp
Physiol. 298:R1659–R1666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Irani DN and Kerr DA: 14-3-3 protein in
the cerebrospinal fluid of patients with acute transverse myelitis.
Lancet. 355:9012000. View Article : Google Scholar
|
|
28
|
de Seze J, Peoc'h K, Ferriby D, Stojkovic
T, Laplanche JL and Vermersch P: 14-3-3 Protein in the
cerebrospinal fluid of patients with acute transverse myelitis and
multiple sclerosis. J Neurol. 249:626–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lubieniecka JM, Streijger F, Lee JH,
Stoynov N, Liu J, Mottus R, Pfeifer T, Kwon BK, Coorssen JR, Foster
LJ, et al: Biomarkers for severity of spinal cord injury in the
cerebrospinal fluid of rats. PLoS One. 6:e192472011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JY, Kim BJ, Sim G, Kim GT, Kang D,
Jung JH, Hwa JS, Kwak YJ, Choi YJ, Park YS, et al: Spinal cord
injury markedly altered protein expression patterns in the affected
rat urinary bladder during healing stages. J Korean Med Sci.
26:814–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cuadrado-Corrales N, Jiménez-Huete A, Albo
C, Hortigüela R, Vega L, Cerrato L, Sierra-Moros M, Rábano A, de
Pedro-Cuesta J and Calero M: Impact of the clinical context on the
14-3-3 test for the diagnosis of sporadic CJD. BMC Neurol.
6:252006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Namikawa K, Su Q, Kiryu-Seo S and Kiyama
H: Enhanced expression of 14-3-3 family members in injured
motoneurons. Res Mol Brain Res. 55:315–320. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peoc'h K, Beaudry P, Lauprêtre N and
Laplanche JL: CSF detection of the 14-3-3 protein in unselected
patients with dementia. Neurology. 58:509–510. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neppl RL, Kataoka M and Wang DZ:
Crystallin-αB regulates skeletal muscle homeostasis via modulation
of argonaute2 activity. J Biol Chem. 289:17240–17248. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sanbe A: Molecular mechanisms of
α-crystallinopathy and its therapeutic strategy. Biol Pharm Bull.
34:1653–1658. 2011. View Article : Google Scholar
|
|
36
|
Singh BN, Rao KS and Rao ChM:
Ubiq-uitin-proteasome-mediated degradation and synthesis of MyoD is
modulated by alphaB-crystallin, a small heat shock protein, during
muscle differentiation. Biochim Biophys Acta. 1803:288–299. 2010.
View Article : Google Scholar
|
|
37
|
Atomi Y, Yamada S and Nishida T: Early
changes of alpha B-crystallin mRNA in rat skeletal muscle to
mechanical tension and denervation. Biochem Biophys Res Commun.
181:1323–1330. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tews DS, Goebel HH, Schneider I, Gunkel A,
Stennert E and Neiss WF: Expression profile of stress proteins,
intermediate filaments, and adhesion molecules in experimentally
denervated and reinnervated rat facial muscle. Exp Neurol.
146:125–134. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gomes RA, Vicente Miranda H, Silva MS,
Graça G, Coelho AV, Ferreira AE, Cordeiro C and Freire AP: Yeast
protein glycation in vivo by methylglyoxal. Molecular modification
of glycolytic enzymes and heat shock proteins. FEBS J.
273:5273–5287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nozais M, Merkulova T, Keller A, Janmot C,
Lompré AM, D'Albis A and Lucas M: Denervation of rabbit
gastrocnemius and soleus muscles: Effect on muscle-specific
enolase. Eur J Biochem. 263:195–201. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Merkulova T, Dehaupas M, Nevers MC,
Creminon C, Alam-eddine H and Keller A: Differential modulation of
alpha, beta and gamma enolase isoforms in regenerating mouse
skeletal muscle. Eur J Biochem. 267:3735–3743. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kato K, Shimizu A, Semba R and Satoh T:
Tissue distribution, developmental profiles and effect of
denervation of enolase isozymes in rat muscles. Biochim Biophys
Acta. 841:50–58. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsushita H, Yamada S, Satoh T, Kato K
and Adachi M: Muscle-specific beta-enolase concentrations after
cross- and random innervation of soleus and extensor digitorum
longus in rats. Exp Neurol. 93:84–91. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liao W, Hong SH, Chan BH, Rudolph FB,
Clark SC and Chan L: APOBEC-2, a cardiac- and skeletal
muscle-specific member of the cytidine deaminase supergene family.
Biochem Biophys Res Commun. 260:398–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mikl MC, Watt IN, Lu M, Reik W, Davies SL,
Neuberger MS and Rada C: Mice deficient in APOBEC2 and APOBEC3. Mol
Cell Biol. 25:7270–7277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sato Y, Probst HC, Tatsumi R, Ikeuchi Y,
Neuberger MS and Rada C: Deficiency in APOBEC2 leads to a shift in
muscle fiber type, diminished body mass, and myopathy. J Biol Chem.
285:7111–7118. 2010. View Article : Google Scholar :
|
|
47
|
Dupont-Versteegden EE, Houlé JD, Gurley CM
and Peterson CA: Early changes in muscle fiber size and gene
expression in response to spinal cord transection and exercise. Am
J Physiol. 275:C1124–C1133. 1998.PubMed/NCBI
|
|
48
|
Ribeiro EA Jr, Pinotsis N, Ghisleni A,
Salmazo A, Konarev PV, Kostan J, Sjöblom B, Schreiner C, Polyansky
AA, Gkougkoulia EA, et al: The structure and regulation of human
muscle α-actinin. Cell. 159:1447–1460. 2014. View Article : Google Scholar :
|
|
49
|
Takada F, Vander Woude DL, Tong HQ,
Thompson TG, Watkins SC, Kunkel LM and Beggs AH: Myozenin: An
alpha-actinin- and gamma-filamin-binding protein of skeletal muscle
Z lines. Proc Natl Acad Sci USA. 98:1595–1600. 2001.PubMed/NCBI
|
|
50
|
Ichinoseki-Sekine N, Yoshihara T, Kakigi
R, Ogura Y, Sugiura T and Naito H: Fiber-type specific expression
of α-actinin isoforms in rat skeletal muscle. Biochem Biophys Res
Commun. 419:401–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chin ER, Olson EN, Richardson JA, Yang Q,
Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R and Williams
RS: A calcineurin-dependent transcriptional pathway controls
skeletal muscle fiber type. Genes Dev. 12:2499–2509. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Frey N, Richardson JA and Olson EN:
Calsarcins, a novel family of sarcomeric calcineurin-binding
proteins. Proc Natl Acad Sci USA. 97:14632–14637. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Seto JT, Quinlan KG, Lek M, Zheng XF,
Garton F, MacArthur DG, Hogarth MW, Houweling PJ, Gregorevic P,
Turner N, et al: ACTN3 genotype influences muscle performance
through the regulation of calcineurin signaling. J Clin Invest.
123:4255–4263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Gampert L, Nething K and Steinacker
JM: Response and function of skeletal muscle heat shock protein 70.
Front Biosci. 11:2802–2827. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
55
|
Krawiec BJ, Frost RA, Vary TC, Jefferson
LS and Lang CH: Hindlimb casting decreases muscle mass in part by
proteasome-dependent proteolysis but independent of protein
synthesis. Am J Physiol Endocrinol Metab. 289:E969–E980. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Milne KJ and Noble EG: Exercise-induced
elevation of HSP70 is intensity dependent. J Appl Phsiol (1985).
93:561–568. 2002. View Article : Google Scholar
|
|
57
|
Thompson HS, Scordilis SP, Clarkson PM and
Lohrer WA: A single bout of eccentric exercise increases HSP27 and
HSCHSP70 in human skeletal muscle. Physiol Scand. 171:187–193.
2001. View Article : Google Scholar
|
|
58
|
Evertsson K, Fjällström AK, Norrby M and
Tågerud S: p38 mitogen-activated protein kinase and
mitogen-activated protein kinase-activated protein kinase 2 (MK2)
signaling in atrophic and hypertrophic denervated mouse skeletal
muscle. J Mol Signal. 9:22014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Senf SM, Dodd SL and Judge AR: FOXO
signaling is required for disuse muscle atrophy and is directly
regulated by Hsp70. Am J Physiol Cell Physiol. 298:C38–C45. 2010.
View Article : Google Scholar :
|
|
60
|
Senf SM, Dodd SL, McClung JM and Judge AR:
Hsp70 over-expression inhibits NF-kappaB and Foxo3a transcriptional
activities and prevents skeletal muscle atrophy. FASEB J.
22:3836–3845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Macario AJ and Conway de Macario E:
Molecular chaperones: Multiple functions, pathologies, and
potential applications. Front Biosci. 12:2588–2600. 2007.
View Article : Google Scholar
|
|
62
|
Kukreti H, Amuthavalli K, Harikumar A,
Sathiyamoorthy S, Feng PZ, Anantharaj R, Tan SL, Lokireddy S,
Bonala S, Sriram S, et al: Muscle-specific microRNA1 (miR1) targets
heat shock protein 70 (HSP70) during dexamethasone-mediated
atrophy. J Biol Chem. 288:6663–6678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Stevens L, Firinga C, Gohlsch B, Bastide
B, Mounier Y and Pette D: Effects of unweighting and clenbuterol on
myosin light and heavy chains in fast and slow muscles of rat. Am J
Physiol Cell Physiol. 279:C1558–C1563. 2000.PubMed/NCBI
|
|
64
|
Gosker HR, Zeegers MP, Wouters EF and
Schols AM: Muscle fibre type shifting in the vastus lateralis of
patients with COPD is associated with disease severity: A
systematic review and meta-analysis. Thorax. 62:944–949. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nwoye L, Mommaerts WF, Simpson DR,
Seraydarian K and Marusich M: Evidence for a direct action of
thyroid hormone in specifying muscle properties. Am J Physiol.
242:R401–R408. 1982.PubMed/NCBI
|
|
66
|
Bozzo C, Stevens L, Toniolo L, Mounier Y
and Reggiani C: Increased phosphorylation of myosin light chain
associated with slow-to-fast transition in rat soleus. Am J Physiol
Cell Physiol. 285:C575–C583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang YH, Tsai MM and Lin KH: Thyroid
hormone dependent regulation of target genes and their
physiological significance. Chang Gung Med J. 31:325–334.
2008.PubMed/NCBI
|
|
68
|
Stines-Chaumeil C, Talfournier F and
Branlant G: Mechanistic characterization of the MSDH
(methylmalonate semialdehyde dehydrogenase) from Bacillus subtilis.
Biochem J. 395:107–115. 2006. View Article : Google Scholar :
|
|
69
|
Ahmad SS, Glatzle J, Bajaeifer K, Bühler
S, Lehmann T, Königsrainer I, Vollmer JP, Sipos B, Ahmad SS,
Northoff H, et al: Phosphoglycerate kinase 1 as a promoter of
metastasis in colon cancer. Int J Oncol. 43:586–590.
2013.PubMed/NCBI
|
|
70
|
Hutchinson KJ, Linderman JK and Basso DM:
Skeletal muscle adaptations following spinal cord contusion injury
in rat and the relationship to locomotor function: A time course
study. J Neurotrauma. 18:1075–1089. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hill CE, Brodak DM and Bartlett Bunge M:
Dissociated predegenerated peripheral nerve transplants for spinal
cord injury repair: A comprehensive assessment of their effects on
regeneration and functional recovery compared to Schwann cell
transplants. J Neurotrauma. 29:2226–2243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fouad K, Hurd C and Magnuson DS:
Functional testing in animal models of spinal cord injury: Not as
straight forward as one would think. Front Integr Neurosci.
7(85)2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jayaraman A, Liu M, Ye F, Walter GA and
Vandenborne K: Regenerative responses in slow- and fast-twitch
muscles following moderate contusion spinal cord injury and
locomotor training. Eur J Appl Phsiol. 113:191–200. 2013.
View Article : Google Scholar
|
|
74
|
Park S, Lee SK, Park K, Lee Y and Hong Y,
Lee S, Jeon JC, Kim JH, Lee SR, Chang KT and Hong Y: Beneficial
effects of endogenous and exogenous melatonin on neural
reconstruction and functional recovery in an animal model of spinal
cord injury. J Pineal Res. 52:107–119. 2012. View Article : Google Scholar
|
|
75
|
Stevens JE, Liu M, Bose P, O'Steen WA,
Thompson FJ, Anderson DK and Vandenborne K: Changes in soleus
muscle function and fiber morphology with one week of locomotor
training in spinal cord contusion injured rats. J Neurotrauma.
23:1671–1681. 2006. View Article : Google Scholar : PubMed/NCBI
|