Introduction
Disabled homolog 2 interactive protein (DAB2IP) is a
novel member of the Ras-GTPase-activating protein family, involved
in cell proliferation and apoptosis (1). Downregulation of DAB2IP is often
detected in high-grade and metastatic prostate cancer (PCa)
specimens (2). Kong et al
(3) determined that PCa cells with
DAB2IP deficiency were also resistant to γ radiation and exhibited
increased clonogenic survival, robust G2-M checkpoint
control and resistance to ionizing radiation (IR)-induced
apoptosis. Wu et al (4)
reported that loss of DAB2IP expression in PCa indicated
chemoresistance via increased expression of the secretory form of
clusterin. Recently, Yun et al (5) observed that DAB2IP was important in
modulating cancer stem cell properties via the CD117-mediated zinc
finger E-box binding homeobox 1 signaling pathway. All these
previous studies suggest that loss of DAB2IP may complicate PCa
treatment as the tumor cells become resistant to conventional
radiotherapy and chemotherapy.
α-particles are heavy particulate emissions that
travel a short linear distance but deposit a large quantity of
energy. This high linear energy transfer (LET) radiation is
characterized by enhanced ability to induce cell death, ability to
overcome the radioresistance to hypoxia, low-LET radiotherapies and
not relying on dose rate (6). The
applications of α-particle therapy, also termed targeted α-particle
therapy, in anticancer therapeutic strategies have been widely
investigated (7,8). The US Food and Drug Administration
approved α-particle therapy against PCa in 2013 (9). Novel methods have combined
α-particles and effective radiosensitizers in order to target
malignant cells without harming normal cells (10,11).
In the present study, the radiation strengths of α-particles were
utilized to overcome the radioresistance induced by the
downregulation of DAB2IP in PCa cells. In addition, the methods of
enhancing cellular sensitivity to irradiation were also
investigated.
Materials and methods
Cell culture
PC3 human PCa cell line-derivative lines [PC3 short
hairpin (sh)DAB2IP and PC3 shVector] were generated using an
shRNA-lentiviral system as described previously (1) and maintained in T medium supplemented
with 5% fetal calf serum (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin, 100 µg/ml
streptomycin, 900 µg/ml G418 and 700 ng/ml puromycin
(Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) in a
humidified atmosphere with 5% CO2 at 37°C.
Cell irradiation
Cells were irradiated at room temperature in ambient
air using a 137Cs source (γ-rays; Nordion, Inc.,
Toronto, Canada) at a dose rate of 0.79 Gy/min (12) or a 241Am plate source
(α-particle; Atom High Tech Co. Ltd., Beijing, China) at a dose
rate of 0.25 Gy/min (13). For the
fractionated irradiation study, PC3 cells were plated in T25 tissue
culture flasks on day 0 and exposed to γ-irradiation with daily
dose of 2 Gy from day 1 to day 3. Following a 2 day recovery, cells
were exposed to 2 Gy γ-rays for an additional 3 days as indicated
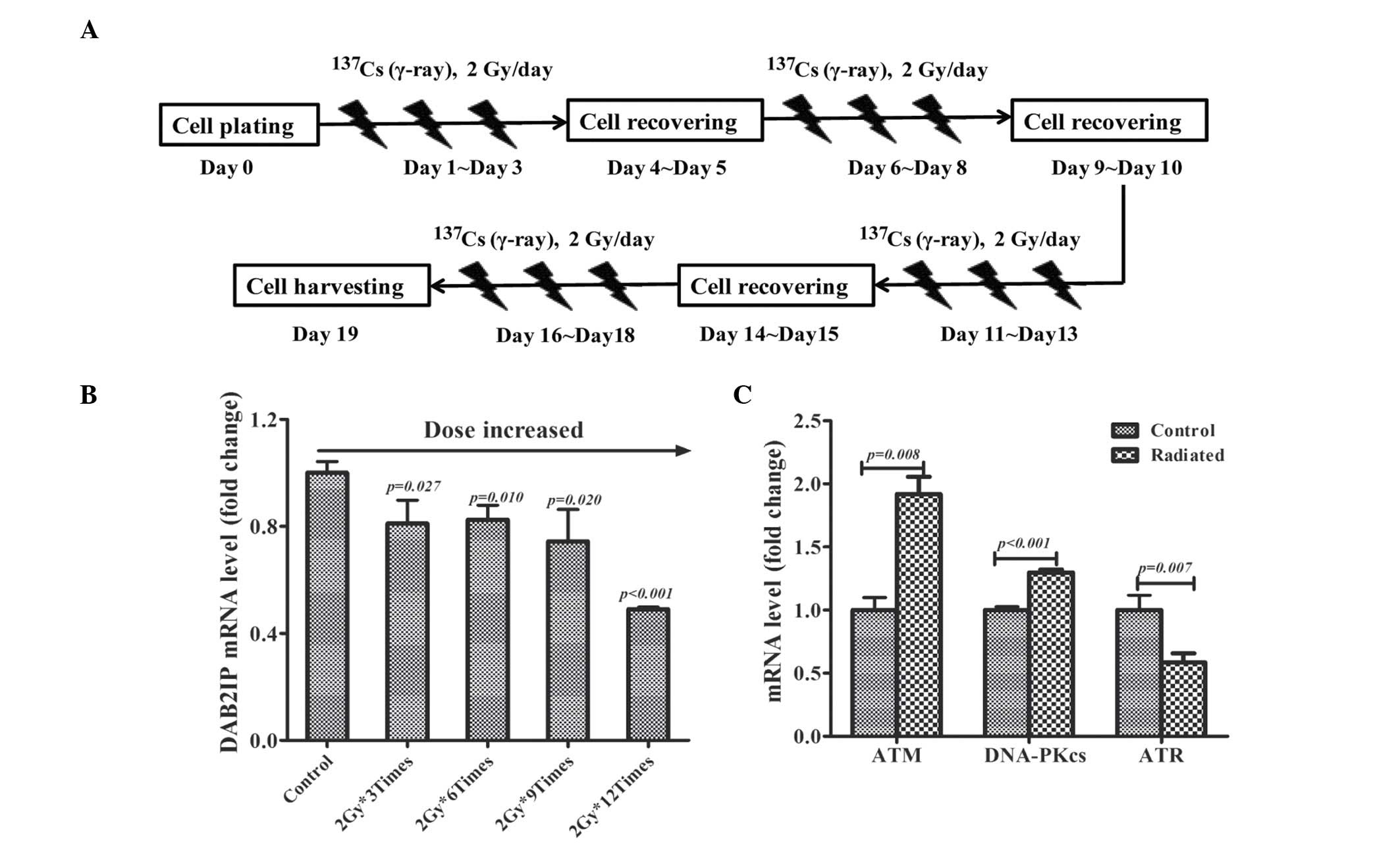
in Fig. 1A. The total RNA was
harvested for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) assay on days 0, 4, 9, 14 and 19.
Clonogenic survival
The radiosensitivity of cells was determined using a
colony formation assay (CFA). KU55933 or NU7026 (Tocris Bioscience,
Ellisville, MO, USA) was added to the medium 1 h prior to IR to a
final concentration of 10 µM. The logarithmic-phase cells
were treated with increasing doses of γ-rays (0, 2, 4, 6, 8 Gy) or
α-radiation (0, 0.2, 0.4, 0.6, 0.8, 2 Gy) and cultured in 60 mm
dishes. Subsequent to a 14 day incubation, the colonies were rinsed
twice with phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology, Haimen, China), then fixed with methanol (Sinopharm
Chemical Reagent Co. Ltd., Shanghai, China) for 30 min. Next, 0.1%
crystal violet solution (Sangon Biotech Co., Ltd., Shanghai, China)
was used to stain the colonies. Finally, colonies containing a
minimum of 50 viable cells were counted using an inverted
microscope (37XAE; Shanghai Optical Instrument Factory Co., Ltd.,
Shanghai, China). The surviving fraction curve
S=e−(αD+βD2) was fitted to the experimental data with a
least square fit algorithm using SigmaPlot 11.0 (Systat Software,
Inc., San Jose, CA, USA). The surviving fraction at 2 Gy
(SF2) was calculated to compare the sensitivity of cells
to IR.
RT-qPCR analysis
Cells were subjected to 0.2 Gy α-particles or 2 Gy
γ-rays, respectively. Total RNA was extracted from irradiated-cells
or control cells using RNAsimple Total RNA kit (cat. no. DP419;
Tiangen Biotech Co. Ltd., Beijing, China) 24 h after IR according
to the manufacturer's protocol. The RNA was incubated with DNase I
(2.5 µl; cat. no. RT411; Tiangen Biotech Co. Ltd.) in order
to eliminate any genomic DNA contamination. The total RNA was then
reverse-transcribed using the ReverTra Ace qPCR RT kit (cat. no.
FSQ-101; Toyobo Co. Ltd., Osaka, Japan). The temperature protocol
for the RT was as follows: 65°C for 5 min, followed by 42°C for 60
min and 70°C for 5 min. cDNA was analyzed by qPCR using 2.5 ng cDNA
in a 20 µl reaction volume containing primers and Accupower
2X Greenstar Master mix (cat. no. K6251; Bioneer Corporation,
Daejeon, Korea). The program conditions included 95°C for 5 min,
and 40 cycles of 95°C for 30 sec and 60°C for 45 sec. 18s rRNA
served as the housekeeping control gene. The primers were designed
by GenScript (Nanjing, China), as follows: Sense,
5′-TCGTGGAAGGACTCATGACC-3′ and antisense,
5′-TCCACCACCCTGTTGCTGTA-3′ for DAB2IP; sense,
5′-TTAAGGTGGACCACACAGGA-3′ and antisense,
5′-GGCCCTTAACAAGCTGTCTC-3′ for ataxia-telangiectasia mutated (ATM);
sense, 5′-GTACAAGCCCTGAGGCTTTC-3′ and antisense,
5′-GCTGATGCATATCAGAGCGT-3′ for DNA-dependent protein kinase
catalytic subunit (DNA-PKcs); sense, 5′-AATGTGAGTGGAAGCCATGA-3′ and
antisense, 5′-TCCGCAGAAGTCTCGTTATG-3′ for ataxia-telangiectasia and
Rad3 related protein (ATR);sense, 5′-GGAATTGACGGAAGGGCACCACC-3′ and
antisense, 5′-GTGCAGCCCCGGACATCTAAGG-3′ for 18s RNA. The qPCR was
performed using MyGo Pro RealTime PCR system (IT-IS International,
Ltd., Middlesbrough, UK). Each sample was examined in triplicate
and the product quantity was normalized relative to 18s rRNA. The
2−ΔΔCq method was applied to calculate gene expression
as described previously (12).
Western blot assay
Lysates from cells irradiated with γ-rays and
α-particles were extracted with radioimmunoprecipitation assay
lysis buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
mixed with 10 mM phenylmethylsulfonyl fluoride (cat. no. ST506;
Beyotime Institute of Biotechnology) 30 min after IR, and the
supernatant was collected following centrifugation at 12,000 × g
for 10 min at 4°C. The protein concentration was quantified by
Micro BCA Protein assay kit (cat. no. SK3061, Sangon Biotech Co.,
Ltd.) according to the manufacturer's protocol. An aliquot of total
protein (40 µg) was subjected to 6% (for the
high-molecular-weight proteins, p-ATM and P-DNA PKcs) or 8% [for
phospho-checkpoint kinase 2 (p-CHK2), DAB2IP and β-actin] sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (100 V for 2 h)
and transferred onto polyvinylidene difluoride membranes (0.45
µm, EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% bovine serum albumin (cat. no. AR2440, Sangon
Biotech Co. Ltd.) for 1 h at room temperature. The membranes were
then incubated with primary antibodies at 4°C overnight. Primary
antibodies are listed as follows: Polyclonal rabbit anti-DAB2IP
(1:650; received from Professor Hsieh, UT Southwestern Medical
Center, Dallas, TX, USA) (1);
monoclonal rabbit anti-phospho-ATM (pS1981; 1:1,000; cat. no.
2152-1; Epitomics; Abcam, Cambridge, MA, USA); monoclonal rabbit
anti-phospho-DNA-PKcs (pS2056; 1:1,000; cat. no. 3892-1, Epitomics;
Abcam); monoclonal rabbit anti-p-CHK2 (pT68; 1:1,000; cat. no.
1538-1, Epitomics; Abcam); monoclonal mouse anti-β-actin as an
internal loading control (1:1,000, cat. no. AA128, Beyotime
Institute of Biotechnology). The membranes were washed with PBS (pH
7.4) three times and then incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies goat anti-rabbit (1:1,000,
cat. no. A0208, Beyotime Institute of Biotechnology) and goat
anti-mouse (1:1,000, cat. no. A0216, Beyotime Institute of
Biotechnology) for 1 h at room temperature. The membranes were
washed with PBS (pH 7.4) three times prior to visualization of
protein bands by chemiluminescence (BeyoECL Plus, cat. no. P0018,
Beyotime Institute of Biotechnology) and detected with the ChemiDoc
XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data are presented as the mean ± standard error
of the mean of at least three independent experiments. The
different groups were compared using the unpaired Student's t-test.
Statistical analyses were performed with SPSS 18.0 statistics
software (SPSS, Inc., Chicago, IL, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
Fractionated irradiation decreases DAB2IP
mRNA expression levels
PC3 cells were fractionally irradiated with 2 Gy/day
and the expression of DAB2IP mRNA was monitored by RT-qPCR assay.
Compared with non-irradiated cells, the expression of DAB2IP gene
was gradually decreased in irradiated cells with the accumulative
dose increase (Fig. 1B). It was
also determined that the gene expression of three established DNA
damage response (DDR) molecules: ATM, DNA-PKcs and ATR in cells
irradiated 12 times with 2 Gy. It was observed that fractionated
irradiation induced the overexpression of ATM (P=0.008) and
DNA-PKcs (P<0.001) mRNA levels, whereas ATR (P=0.007) was
downregulated in IR-treated cells (Fig. 1C).
DAB2IP modulates the radiosensitivity of
PC3 cells
In the current study, endogenous DAB2IP expression
in PC3 was knocked down with DAB2IP shRNA plasmid transfection.
Western blot and RT-qPCR analysis indicated that DAB2IP mRNA levels
were significantly decreased in PC3 shDAB2IP cells compared with
PC3 shVector (P<0.05; Fig. 2A and
B). The sensitivity of cells to γ-irradiation was evaluated by
CFA. DAB2IP-negative PCa cells exhibited higher levels of
clonogenic survival than DAB2IP-positive cells (Fig. 2C). In order to overcome cellular
radioresistance of DAB2IP-negative cells, PC3 shVector and PC3
shDAB2IP cells were exposed to a 241Am α-particle plate
source and the cell lines exhibited higher sensitivity to
α-particles than that to γ-rays, as indicated by the lower level of
survival (Fig. 2C). However,
DAB2IP-negative cells retained a significantly higher
SF2 value compared with DAB2IP-positive cells
(P<0.001; Fig. 2D). Thus,
downregulation of DAB2IP produced cells resistant to γ-ray and
α-particle irradiation.
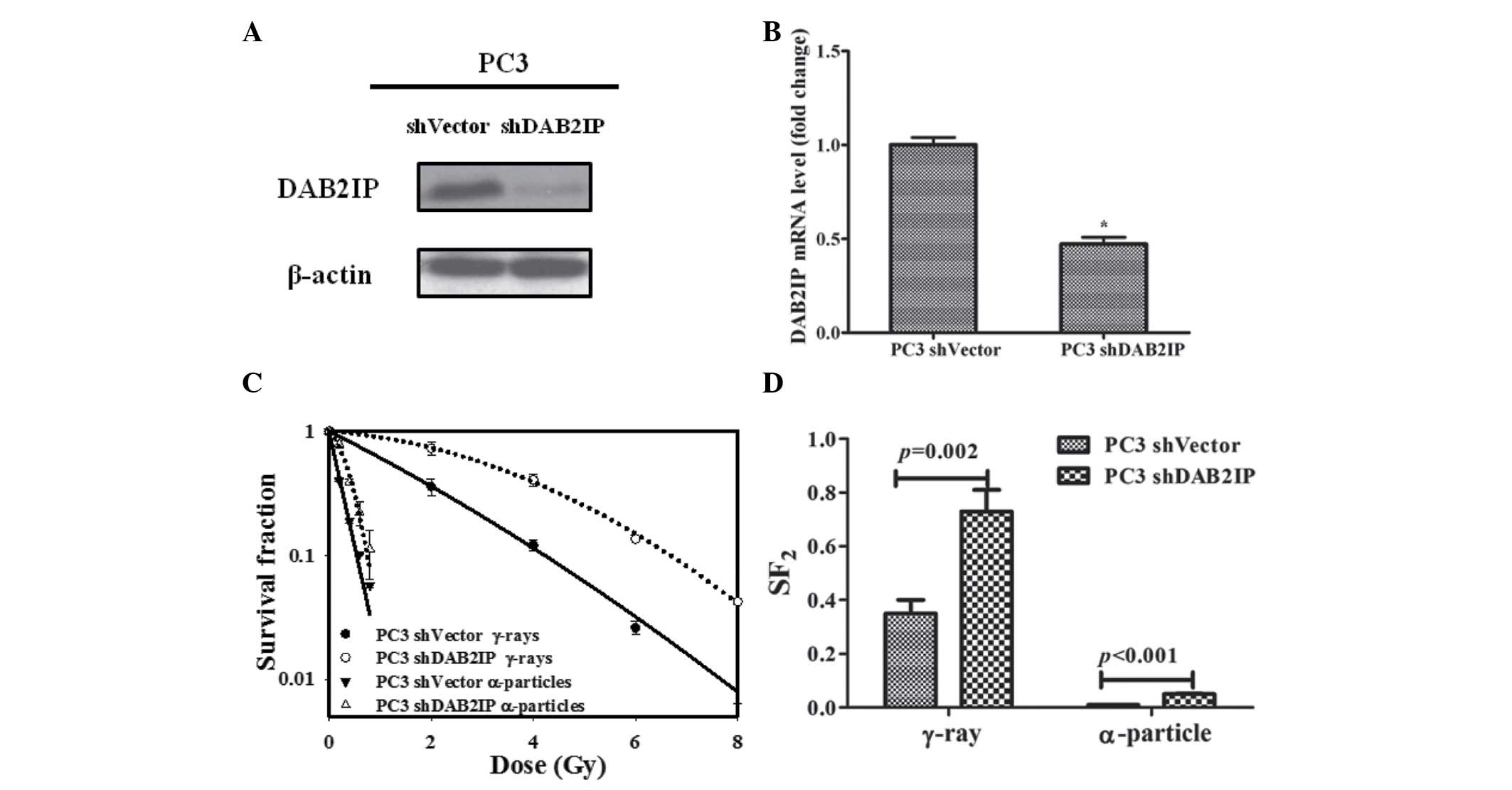 | Figure 2DAB2IP knockdown decreased sensitivity
of PC3 cells to γ-rays and α-particles. DAB2IP (A) protein and (B)
mRNA expression levels of PC3 shVector and PC3 shDAB2IP cells was
determined by western blotting and reverse
transcription-quantitative polymerase chain reaction assay,
respectively. β-actin served as an internal loading control.
*P<0.05 vs. the PC3 shVector cells. (C) Sensitivity
of PC3 shVector and PC3 shDAB2IP cells to γ-ray (0, 2, 4, 6, 8 Gy)
and α-particle (0, 0.2, 0.4, 0.6, 0.8 Gy) irradiation were detected
by a colony formation assay. (D) SF2 was calculated to
compare the sensitivity of cells to γ-rays and α-particles.
P-values are presented above the error bars vs. the control group.
The results are presented as the mean of three experiments ±
standard error of the mean. shDAB2IP, short haripin disabled
homolog 2 interactive protein; shVector, short hairpin vector;
SF2, surviving fraction at 2 Gy. |
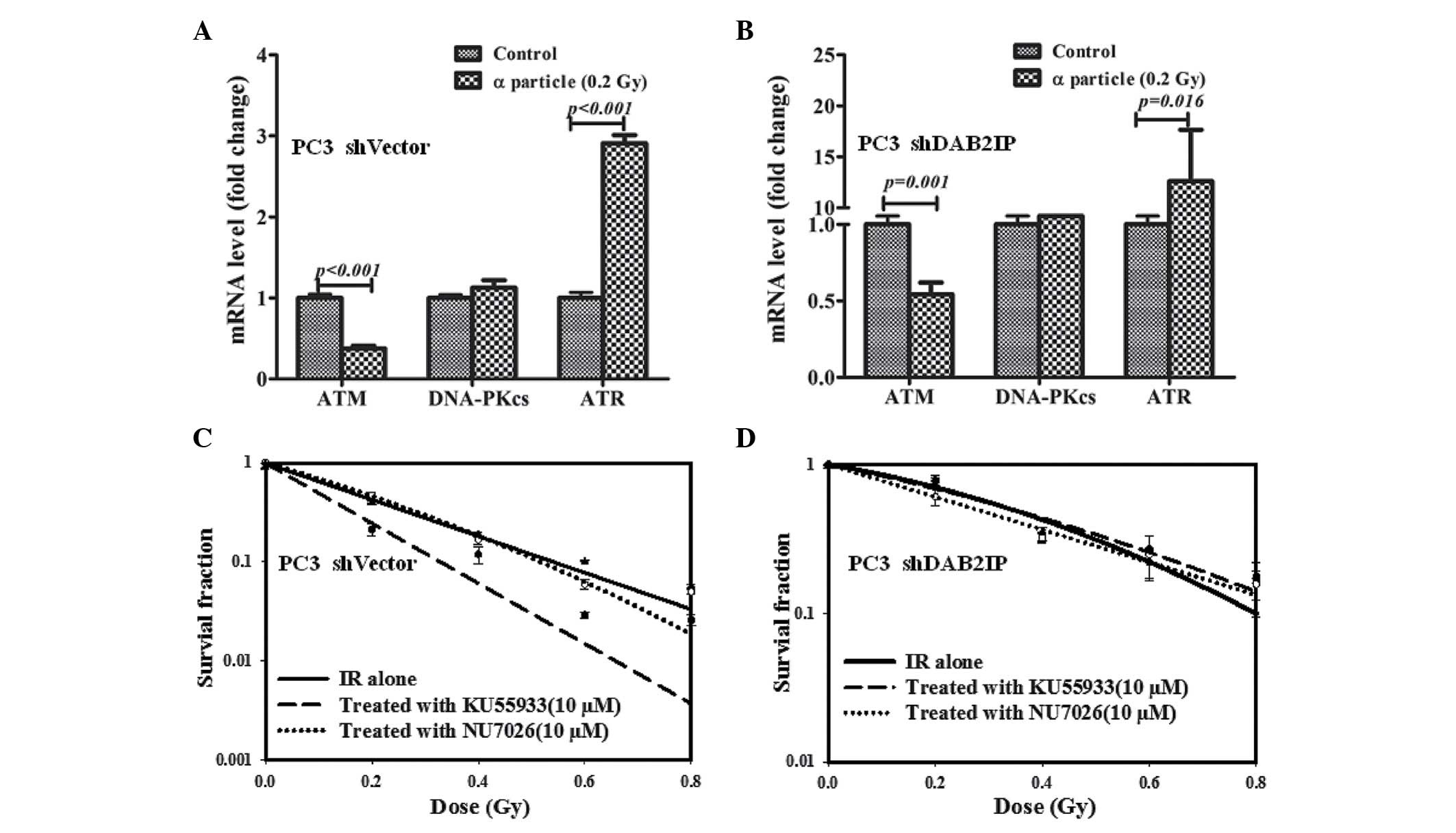
DAB2IP influences DDR signaling pathways
in response to γ-irradiation
To elucidate the underlying mechanism of DAB2IP loss
resulting in radioresistance, the effect of IR on the expression of
relevant genes, including ATM, DNA-PKcs and ATR, was investigated
and compared. Our previous studies demonstrated that downregulation
of DAB2IP gene may induce elevated expression of ATM (12,14).
There was no difference between the mRNA expression levels of ATM,
DNA-PKcs and ATR in PC3 shVector cells regardless of IR exposure
(Fig. 3A). However, significantly
higher mRNA expression levels of ATM were detected in PC3 shDAB2IP
cells exposed to 2 Gy of γ-rays (P=0.001; Fig. 3B). When ATM phosphorylation was
inhibited with 10 µM KU55933 (Fig. 3C), shVector and shDAB2IP cells
exhibited enhanced sensitivity to IR, particularly when treated
with higher dose (Fig. 3D and E).
On the other hand, NU7026 (12),
which limits the activation of DNA-PKcs and CHK2 phosphorylation in
response to IR (Fig. 3C), did not
significantly affect the survival of the PC3 shVector and PC3
shDAB2IP cells when exposed to γ-rays (Fig. 3D and E).
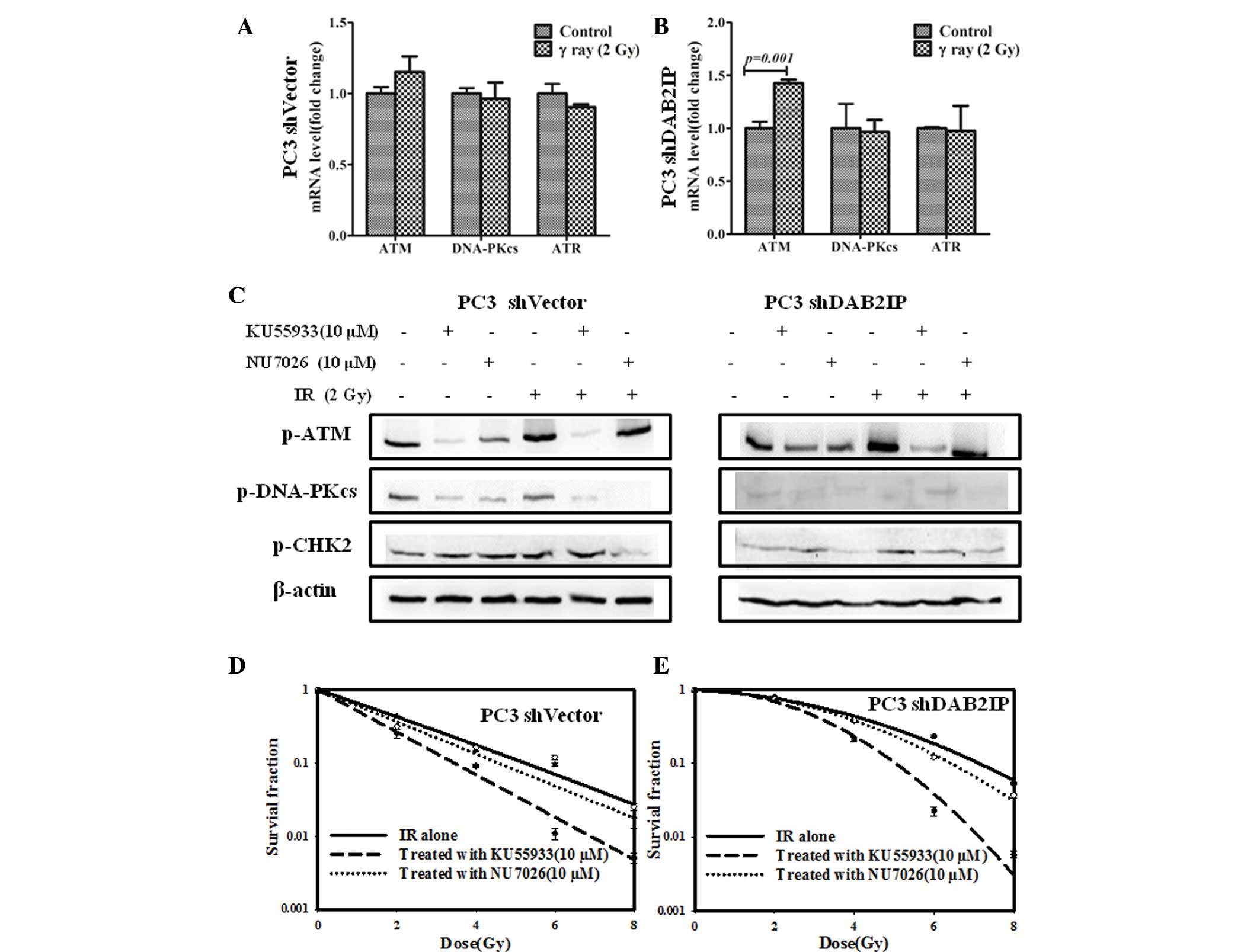 | Figure 3Increased ATM mRNA expression levels
were observed in PC3 shDAB2IP cells in response to 2 Gy γ-ray
irradiation. Relative mRNA levels of ATM, DNA-PKcs and ATR in (A)
PC3 shVector cells and (B) PC3 shDAB2IP cells 24 h after 2 Gy γ-ray
irradiation were determined by reverse transcription-quantitative
polymerase chain reaction vs. the control. (C) Whole cell lysates
were collected 30 min after irradiation and analyzed by western
blotting for ATM, DNA-PKcs, CHK2 proteins. Radiosensitivity of (D)
PC3 shVector cells and (E) PC3 shDAB2IP cells to irradiation
combined with KU55933 or NU7026 were measured by colony formation
assay. KU55933 or NU7026 were added to the medium 1 h prior to
irradiation to a final concentration of 10 µM. P-values are
presented above the error bars vs. control group. The results are
presented as the mean of three experiments ± standard error of the
mean. shDAB2IP, short haripin disabled homolog 2 interactive
protein; shVector, short hairpin vector; IR, ionizing radiation;
p-ATM, phospho-ataxia-telangiectasia mutated; p-DNA-PKcs,
phospho-DNA-dependent protein kinase catalytic subunit; ATR,
ataxia-telangiectasia and Rad3 related protein; p-CHK2, checkpoint
kinase. |
DAB2IP-deficient cells become resistant
to the combined treatment of KU55933 and α-particles
When 137Cs sourced γ-rays were used as
reference radiation, the RBE of α-particles was estimated at 9.2
and 11 for 10% survival of PC3 shVector and PC3 shDAB2IP cells,
respectively. Compared with the control, PC3 shVector and shDAB2IP
cells subjected to 0.2 Gy of α-particle exhibited lower ATM
(P<0.001; Fig. 4A and P=0.001;
Fig, 4B) and higher ATR
(P<0.001; Fig. 4A and P=0.016;
Fig. 4B) mRNA expression levels.
In addition, there was no significant difference in DNA-PKcs mRNA
level detected between untreated and irradiated cells (Fig. 4A and B). The effect of KU55933 and
NU7026 on cellular sensitivity to α-particles was determined using
CFA. PC3 shVector cells were radiosensitized by KU55933 (Fig. 4C); however, the survival curve of
α-irradiated PC3 shDAB2IP cells was not affected by NU7026 and
KU55933 (Fig. 4C and D).
Therefore, neither the ATM nor the DNA-PKcs inhibitor increased
α-particle-induced lethality in DAB2IP-deficient cells.
Discussion
PCa is one of the leading causes of
cancer-associated mortality in males. External beam radiation
therapy alone, or combined with androgen deprivation, with daily
fraction of 1.8~2 Gy over a 7~9-week period is a standard treatment
option for organ-confined and regionally advanced PCa patients
(15). However, for a significant
proportion of high-risk patients with PCa this therapy will fail
and metastasis will develop, for which, currently, no curative
treatment exists (16). Tumor
cells evading IR-induced cell death may lead to disease
progression, cancer relapse and acquired radioresistance. A
previous study by Ghisolfi et al (17) partly elucidated why IR-treated
patients with tumor recurrence are often resistant to conventional
radiotherapy: Irradiation may induce and accumulate tumor stem
cells, which are resistant to antineoplastic therapeutic agents and
IR. Currently, numerous studies have presented that DAB2IP is
important in associating PCa cells stemness (5), radio/chemosensitivity and the DDR
signaling pathway (3–5,12).
Previous clinical data indicated that loss of DAB2IP function may
be a potential biomarker indicating an unfavorable outcome despite
the use of radiotherapy in high-risk patients with PCa (18).
In the present study, clinical fractioned
irradiation was mimicked and the effect of IR on mRNA expression
levels of DAB2IP, ATM, DNA-PKcs and ATR was investigated by
RT-qPCR. ATM, DNA-PKcs and ATR have been established as important
signal proteins mediating DDR by homologous recombination and
non-homologous end joining (19).
Elevated expression of those DDR signal molecules is often
associated with enhanced cell viability from irradiation (20). As the IR dose was increased, a
reduction of DAB2IP mRNA expression levels was detected in
irradiated cells accompanied with elevated mRNA expression of ATM
and DNA-PKcs. ATR, which usually initiates IR-induced DDR in ATM
deficient cells (19), was
downregulated in IR-treated PC3 cells. This negative association
between DAB2IP and ATM expression was also observed in our previous
studies (12,14). DAB2IP is normally distributed in
the cytoplasm, whereas ATM is located in the nucleus. The
association between DAB2IP and ATM remains to be elucidated. Di
Minin et al (21) reported
that the tumor necrosis factor-dependent transcriptional profile
via the nuclear factor-κB and mitogen-activated protein kinase 8
signaling pathway may be induced by the combination of DAB2IP and
mutant p53 in the cytoplasm. It is also suggested that DAB2IP may
impact ATM expression by feedback regulation, including depleting
the substrates of ATM (p53 for example). In addition, tumor cells
surviving from fractionated irradiation are usually resistant to IR
(22). Therefore, it is possible
that DAB2IP deficiency due to long-term exposure to IR may be one
of the reasons for the occurrence of acquired radioresistance. It
is of note, that at the beginning of the current study, the
clinical radiotherapy plan was followed and cells were exposed to 2
Gy for 5 days at 2 day intervals. It was observed that when the
accumulated dose >12 Gy was applied, PC3 cells were severely
damaged and >10 days were required for cells to recover and
continue to proliferate. However, when an IR period of 3 days was
selected, along with a 2 day recovery period described in Fig. 1A, cells required a shorter recovery
time. Therefore, the fractionated-irradiation schedule does not
match the clinical plan.
In order to demonstrate the effect of DAB2IP on the
DDR signaling pathway, endogenous DAB2IP of PC3 cells was knocked
down using shRNA-lentiviral system and it was observed that low
expression of DAB2IP resulted in cells resistance to irradiation by
γ-rays and α-particles. Compared with sparely IR of γ-rays,
high-LET particles (α-particle) have a higher relative biological
effectiveness (RBE) as they lead to more severe and complex damage
to DNA, which is more difficult to repair (23). When 137Cs sourced γ-rays
were used as reference radiation, the RBE of α-particles was
estimated at 9.2 and 11 for 10% survival of PC3 shVector and PC3
shDAB2IP cells, respectively. When comparing the cellular response
at biologically equivalent doses, it was observed that the change
of cells DDR signaling pathway upon γ-rays at 2 Gy with α-particles
at 0.2 Gy, which demonstrate similar cell death rates in the
present study. It was determined that ATM mRNA expression in PC3
shDAB2IP cells was significantly upregulated in γ-ray-treated
cells; however, it was significantly reduced in cells exposed to
α-particles. It is of note that a significant increase in ATR mRNA
expression was observed in α-particles-irradiated DAB2IP-deficient
cells (>15 fold vs. the control group). This trend of ATM and
ATR activation following IR was also reported by Xue et al
(24). Their work also supported
that ATR is important for checkpoint regulation following heavy ion
beams compared with low-LET radiation. It suggested that ATR may be
more important in conferring cellular response to high-LET
irradiation. In addition, ATR inhibitors, including VE281 and
VE822, may be used to enhance the sensitivity of DAB2IP-deficient
cells to high-LET radiation.
ATM phosphorylation is a conserved response to IR
across multiple tumor types. KU55933, an ATM inhibitor, may reduce
ATM activation in response to low and high-LET radiation (12,25),
it attenuated the survival of PC3 shDAB2IP and PC3 shVector cells
in response to γ-rays in the current study. However, pretreatment
with KU55933 prior to IR did not impact the sensitivity of shDAB2IP
cells to α-particles. In addition, NU7026, a DNA-PKcs inhibitor,
was used as a negative control for the current study as DNA-PKcs
mRNA expression was not affected by γ-rays or α-particles. As
expected, inhibition of DNA-PKcs did not influence the
radio-sensitivity of cells to the two types of radiation.
In conclusion, the results of the present study
indicate that DAB2IP may be involved in forming acquired
radioresistance in PC3 cells. DAB2IP-deficient cells are resistant
to low and high-LET radiation using different mechanisms.
DAB2IP-deficient cells are resistant to both γ-rays and
α-particles. ATM could be the key molecule mediating the cells'
response to low-LET irradiation, whereas the ATR signaling pathway
is involved in the resistance to high-LET radiation. Inhibited ATM
activation did not enhance the sensitivity of DAB2IP-negative cells
to high-LET radiation, which may be due to the increased ATR mRNA
expression in the cells.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (grant no. 31270896), the
Shanghai Nature Science Foundation (grant no. 11ZR1402100), the
Scientific Research Foundation for the Returned Overseas Chinese
Scholars, State Education Ministry (grant no. 44-8) and the Zhuoxue
Project of Fudan University to Ms. Zhaolu Kong.
Abbreviations:
|
DAB2IP
|
disabled homolog 2 interactive
protein
|
|
PCa
|
prostate cancer
|
|
LET
|
linear energy transfer
|
|
SF2
|
surviving fraction at 2 Gy
|
|
IR
|
ionizing radiation
|
|
ATM
|
ataxia-telangiectasia mutated
|
|
DNA-PKcs
|
DNA-dependent protein kinase catalytic
subunit
|
|
ATR
|
ataxia-telangiectasia and Rad3 related
protein
|
|
CFA
|
colony formation assay
|
|
DDR
|
DNA damage response
|
References
|
1
|
Xie D, Gore C, Zhou J, Ponga RC, Zhang HF,
Yu LY, Vessellad RL, Min W and Hsieh JT: DAB2IP coordinates both
PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc
Natl Acad Sci USA. 106:19878–19883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang X, Li N, Li X, Zhao W, Qiao Y, Liang
L and Ding Y: Low expression of DAB2IP contributes to malignant
development and poor prognosis in hepatocellular carcinoma. J
Gastroenterol Hepatol. 27:1117–1125. 2012. View Article : Google Scholar
|
|
3
|
Kong Z, Xie D, Boike T, Raghavan P, Burma
S, Chen DJ, Habib AA, Chakraborty A, Hsieh JT and Saha D:
Downregulation of human DAB2IP gene expression in prostate cancer
cells results in resistance to ionizing radiation. Cancer Res.
70:2829–2839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu K, Xie D, Zou Y, Zhang T, Pong RC, Xiao
G, Fazli L, Gleave M, He D, Boothman DA and Hsieh JT: The mechanism
of DAB2IP in chemoresistance of prostate cancer cells. Clin Cancer
Res. 19:4740–4749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yun EJ, Beak ST, Xie D, Tseng SF, Dobin T,
Hernandez E, Zhou J, Zhang L, Yang J, Sun H, et al: DAB2IP
regulates cancer stem cell phenotypes through modulating stem cell
factor receptor and ZEB1. Oncogene. 34:2741–2752. 2015. View Article : Google Scholar
|
|
6
|
Wadas TJ, Pandya DN, Solingapuram Sai KK
and Mintz A: Molecular targeted α-particle therapy for oncologic
applications. Am J Roentgenol. 203:253–260. 2014. View Article : Google Scholar
|
|
7
|
Baidoo KE, Yong K and Brechbiel MW:
Molecular pathways: Targeted α-particle radiation therapy. Clin
Cancer Res. 19:530–537. 2013. View Article : Google Scholar
|
|
8
|
El-Amm J and Aragon-Ching JB: Radium-223
for the treatment of castration-resistant prostate cancer. Onco
Targets Ther. 8:1103–1109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wissing MD, van Leeuwen FW, van der Pluijm
G and Gelderblom H: Radium-223 chloride: Extending life in prostate
cancer patients by treating bone metastases. Clin Cancer Res.
19:5822–5827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbas N, Heyerdahl H, Bruland OS, Borrebak
J, Nesland J and Dahle J: Experimental α-particle
radioimmunotherapy of breast cancer using 227Th-labeled
p-benzyl-DOTA-trastuzumab. EJNMMI Res. 1:182011. View Article : Google Scholar
|
|
11
|
Borchardt PE, Yuan RR, Miederer M,
McDevitt MR and Scheinberg DA: Targeted Actinium-225 in vivo
generators for therapy of ovarian cancer. Cancer Res. 63:5084–5090.
2003.PubMed/NCBI
|
|
12
|
Zhang T, Shen Y, Chen Y, Hsieh JT and Kong
Z: The ATM inhibitor KU55933 sensitizes radioresistant bladder
cancer cells with DAB2IP gene defect. Int J Radiat Biol.
91:368–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren R, He M, Dong C, Xie Y, Ye S, Yuan D
and Shao C: Dose response of micronuclei induced by combination of
α-particles and γ-rays in human lymphoblast cells. Mutat Res.
741–742:51–56. 2013. View Article : Google Scholar
|
|
14
|
Yang C, Zhang TT, Chen Y and Kong ZL:
Relationship between expression level of ATM and DAB2IP-knockdown
induced radio-resistance in prostate cancer cells. J Radiat Res
Radiat Process. 33:0202032015.In Chinese.
|
|
15
|
Alberti C: Prostate cancer:
Radio-resistance molecular target-related markers and foreseeable
modalities of radiosensitization. Eur Rev Med Pharmacol Sci.
18:2275–2282. 2014.PubMed/NCBI
|
|
16
|
Thompson IM, Tangen CM, Paradelo J, Lucia
MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, et
al: Adjuvant radiotherapy for pathological T3N0M0 prostate cancer
significantly reduces risk of metastases and improves survival:
Long-term followup of a randomized clinical trial. J Urol.
181:956–962. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghisolfi L, Keates AC, Hu X, Lee DK and Li
CJ: Ionizing radiation induces stemness in cancer cells. PLoS One.
7:e436282012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacobs C, Tumati V, Kapur P, Yan J, Hong
D, Bhuiyan M, Xie XJ, Pistenmaa D, Yu L, Hsieh JT, et al:
DOC-2/DAB2 interacting protein status in high-risk prostate cancer
correlates with outcome for patients treated with radiation
therapy. Int J Radiat Oncol Biol Phys. 89:729–735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomimatsu N, Mukherjee B and Burma S:
Distinct roles of ATR and DNA-PKcs in triggering DNA damage
responses in ATM-deficient cells. EMBO Rep. 10:629–635. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaudhary MW and Al-Baradie RS:
Ataxia-telangiectasia: Future prospects. Appl Clin Genet.
7:159–167. 2014.PubMed/NCBI
|
|
21
|
Di Minin G, Bellazzo A, Dal Ferro M,
Chiaruttini G, Nuzzo S, Bicciato S, Piazza S, Rami D, Bulla R,
Sommaggio R, et al: Mutant p53 reprograms TNF signaling in cancer
cells through interaction with the tumor suppressor DAB2IP. Mol
Cell. 56:617–629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu H, Yang W, Gao H, Jiang T, Gu B, Dong
Q, Xu W, Wu S and Sun X: Nimotuzumab abrogates acquired
radio-resistance of KYSE-150R esophageal cancer cells by inhibiting
EGFR signaling and cellular DNA repair. Onco Targets Ther.
8:509–518. 2015. View Article : Google Scholar :
|
|
23
|
Hada M and Georgakilas AG: Formation of
clustered DNA damage after high-LET irradiation: A review. J Radiat
Res. 49:203–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue L, Furusawa Y, Okayasu R, Miura M, Cui
X, Liu C, Hirayama R, Matsumoto Y, Yajima H and Yu D: The
complexity of DNA double stand breaks is a curcial factor for
activating ATR signaling pathway for G2/M checkpoint regulation
regardless of ATM function. DNA Repair (Amst). 25:72–83. 2015.
View Article : Google Scholar
|
|
25
|
Xue L, Yu D, Furusawa Y, Okayasu R, Tong
J, Cao J and Fan S: Regulation of ATM in DNA double strand break
repair accounts for the radiosensitivity in human cells exposed to
high linear energy transfer ionizing radiation. Mutat Res.
670:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|