Introduction
Solar ultraviolet radiation (UVR) has been
demonstrated to have systemic suppressive effects on the immune
system. UVR can suppress immune reactions at a local and systemic
level (1,2). One of the major molecular mediators
of photoimmunosuppression is UVR-induced DNA damage (3). It has also been shown that chronic
UVR can lead to the induction of skin cancer, which is the most
serious adverse health effect of UVR (4). Excess sun exposure increases the risk
of non-melanoma and melanoma skin cancer (5,6). It
has been estimated by the United Nations Environment Panel that, in
the last few years, >2,000,000 cases of non-melanoma and 200,000
cases of malignant melanoma have occurred annually worldwide
(7). UVR inhibits antigen
presentation and induces the release of immunosuppressive
cytokines, and this specific immunosuppression is mediated by
antigen-specific suppressor/regulatory T cells. Langerhans cells
are considered to be the exclusive antigen-presenting cells in the
skin, and Langerhans cells have been found to be depleted upon UVR
exposure (8,9). However, the precise mechanism
underlying the induced immunosuppression remains to be elucidated
(10).
The use of sunscreen has been shown to have a
protective effect against sun exposure, and several studies have
indicated that sunscreens afford a level of protection against
UVR-induced immunosuppression (11,12).
Thus, in addition to sunburn protection factor (SPF), the
determination of the immune protection factor (IPF) has been
suggested as an alternative measurement (13,14),
which may reflect immune protection effect more accurately. The
majority of previous mouse models evaluating the immunoprotective
effects of sunscreen have been directly based on SPF parameters,
indicating the contact hypersensitivity (15,16).
The present study aimed to investigate the immunoprotection offered
by Anthelios sunscreen in a Candida albicans-induced
delayed-type hypersensitivity mouse model, which demonstrates a
protective localized cell-mediated immune response and has been
widely used in investigations to assess immune responses (17,18).
The present study aimed to clarify the effect of Anthelios
sunscreen on UVR-induced immunosuppression and provide evidence to
support the application of sunscreen to prevent UVR-induced skin
injury. The present study provided evidence to support the
application of sunscreen in the prevention UVR-induced skin
injury.
Materials and methods
Animals
The current study was approved by approved by the
animal ethics committee of Sun Yat-sen University (Guangzhou,
China). Pathogen-free BALB/c mice (6-week-old; body weight, 15±1 g)
were purchased from the Animal Center of Sun Yat-sen University
(Guangzhou, China). The animals were housed in a specific
pathogen-free grade, forced air laboratory under standard natural
lighting conditions (12 h light/12 h dark) at 20–24°C, and were
provided with food and water. During the entire experimental
process, all efforts were made to minimize the suffering of the
animals, in accordance with the National Institute of Health
Guidelines for the Care and Use of Laboratory Animals (19).
Reagents and equipment
The UVA, UVB and solar-simulated (ss)UVR spectra
were produced using an SUV-1000 Solar UV simulator (Sigma-Aldrich,
St. Louis, MO, USA). The intensity (mJ/cm2) of the UV
output was measured using a PMA2100 radiometer (Solar Light
Company, Glenside, PA, USA), and the timing of the UV exposure was
adjusted using a timing device. The spectral output of the solar
simulator was measured using an OL-754 spectroradiometer (Optronic
Laboratories, Orlando, FL, USA). Formalin-fixed Candida
albicans was prepared by the Biochemistry Laboratory of Sun
Yat-sen University. Sunscreen Anthelios XL (SPF 50; PPD 28) was
provided by L'Oreal (Paris, France). Antibodies were purchased from
commercial suppliers, as indicated below.
Dose-response to UVR
The present study first investigated the minimal
erythema dose (MED) of UVA and UVB exposure to the mice by exposing
the animals to various doses of ssUVR. A total of 120 mice were
randomly divided into a sunscreen group and a non-sunscreen group.
These groups were each divided into six subgroups (n=10),
which were treated with different doses of UVR. An area measuring
~8 cm2 of the dorsal skin of the mice was shaved using
hair clippers 1 day prior to UV irradiation, and the skin was
covered with or without sunscreen (2 mg/cm2). The other
body areas were protected from UV exposure. The dorsal skin was
then exposed to the sunlight system with various doses of ssUVR
(UVA, 1,000-3,500 mJ/cm2; UVB: 30-1,200
mJ/cm2) for 60 sec. The pre-UV and post-UV skin
thickness was measured using hand-held Episcan-1-200 high frequency
ultrasound (Longport International, Silchester, UK). The changes in
skin thickness were plotted to obtain the response curves against
various UVR doses under a constant sunscreen dose. The minimum dose
of ssUVR required to cause a significant increase in skin thickness
in the sunscreen group, compared with the non-sunscreen group, was
used for further experiments, with a factor of 0.7.
Immunization with Candida albicans
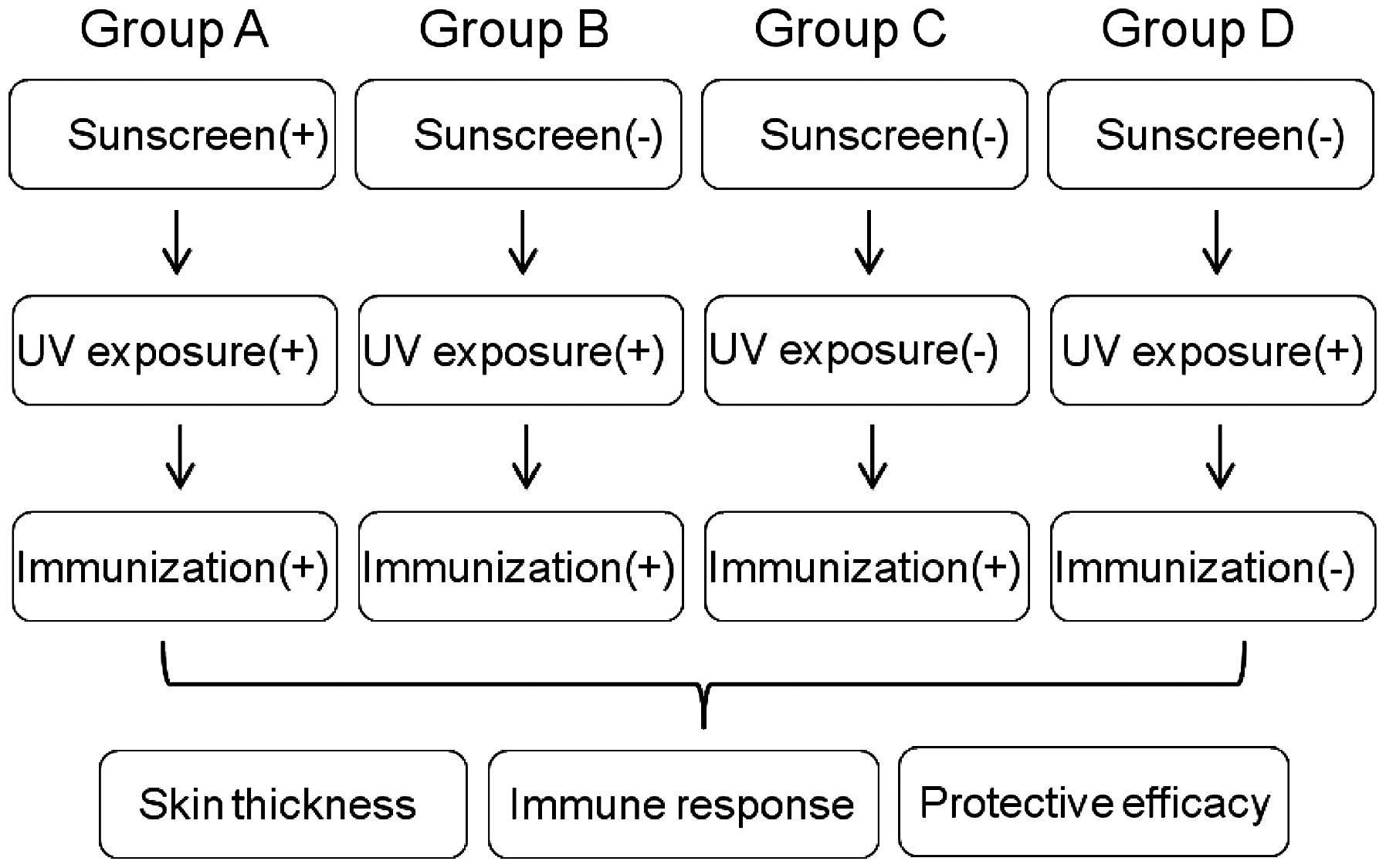
The experimental design is shown in Fig. 1. A total of 40 mice were randomly
divided into four groups (n=10): Group A
(Sunscreen+UV+immunization), Group B (UV+immunization), Group C
(positive control) and Group D (negative control). The mice were
anesthetized with 10% chloral hydrate (Sinopharm Chemical Reagent
Co., Ltd, Shanghai, China) by intraperitoneal injection at a dose
of 280–350 mg/kg. For the UV groups, 0.7X MED was provided to
individual animals, and this procedure was repeated for 5 days. On
the fifth day, the mice were immunized by subcutaneous injection of
107 formalin-fixed Candida albicans to each foot
pad as previously reported (20).
After 24 h, the thickness of each foot was measured with vernier
calipers and the mean footpad swelling in each mouse was calculated
as follows: Mean swelling = (left foot swelling + right foot
swelling) / 2, according to a previous study (20). Additional mice, which received UV
treatment only and immunization only were used as a negative
control and positive control, respectively. The increase in skin
thickness, compared with the negative control was used to normalize
data. Immunosuppression rates were calculated as: Immunosuppression
rate = (1 - experimental accurate edema value / positive control of
accurate edema value) × 100%.
Determining expression levels of CD207,
CD80 and CD86 using western blot analysis
Following measurements of the thickness of each
foot, the present study investigated the expression levels of
CD207, CD80 and CD86, which indicate immune responses from
Langerhans cells. Biopsy specimens (2 g) from the UV-exposed dorsal
skin were dissected to extract total protein and the mice were then
sacrificed by decapitation. The skin tissue from each group was
homogenized in radioimmunoprecipitation assay buffer with protease
inhibitor cocktail (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). Total tissue extracts were separated by centrifugation at
12,000 × g and 4°C for 30 min. Subsequently, 5 µg of the
total tissue extract was resolved by 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and electrotransferred onto a
polyvinylidene difluoride membrane (Whatman; GE Healthcare Life
Sciences, Chalfont, UK) at 100 V for 1 h. The membranes were
blocked in 5% non-fat milk (Amresco, LLC, Solon, OH, USA) with
shaking at room temperature for 1 h, then incubated with monoclonal
rat anti-mouse CD207/Langerin (1:2,000; cat. no. 14-2073;
eBioscience, Inc., San Diego, CA, USA), polyclonal rabbit
anti-mouse CD80 (1:2,000; cat. no. bs-2211R; BIOSS, Beijing,
China), polyclonal rabbit anti-mouse CD86 (1:2,000; cat. no.
bs-1035R; BIOSS) and monoclonal rabbit anti-actin (1:2,000; cat.
no. 04-1040; EMD Millipore, Billerica, MA, USA) antibodies at room
temperature for 2 h. Following 3 washes in 1X Tris-buffered saline
with 1% Tween 20 (TBS-T; Guangzhou Whiga Technology Co., Ltd.,
Guangzhou, China) for 10 min, the PVDF membranes were then
incubated with polyclonal anti-rat horseradish peroxidase
(HRP)-conjugated IgG (1:10,000; cat. no. AP136P; EMD Millipore) and
anti-rabbit HRP-conjugated IgG (1:10,000; cat. no. 12-348; EMD
Millipore) at room temperature for 30 min. Antibodies were diluted
in TBS-T. The Western blotting experiments were performed using
biological replicates. The immunoblot bands were visualized using
an enhanced chemiluminescence method (Thermo Fisher Scientific,
Waltham, MA, USA) and quantified using Image J software (version
1.49; National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
The biopsy specimens obtained from the UV-exposed
back skin were dissected immediately following the measurement of
footpad thicknesses. The tissues were fixed in 4% formaldehyde
(Guangzhou Chemical Reagent Factory, Guangzhou, China) solution in
phosphate-buffered saline (PBS) and cut using a Leica RM2235
microtome (Leica Microsystems GmbH- Wetzlar, Germany) into sections
of 4 µm. The sections were then deparaffinized in xylene
(Guangzhou Chemical Reagent Factory), and hydrated in a series of
ethanol and distilled water. Endogenous peroxidase was eliminated
by incubating the sectioned tissues in 5%
H2O2 in methanol for 30 min. Non-specific
staining was blocked by incubation in 5% normal mouse serum in PBS
for 30 min at 37°C. The sections were then incubated overnight at
4°C with primary antibodies, including mouse anti-CD207/Langerin,
anti-CD80 and anti-CD86 antibodies (1:200). Following rinsing three
times for 5 min with PBS, the sections were incubated with
HRP-conjugated anti-mouse IgG (1:2,000) for 1 h. The sections were
rinsed with PBS twice for 10 min, following which the slides were
developed using 3,3′-diaminobenzidine (Beijing Biosynthesis
Biotechnology). The sections were examined using a Nikon Eclipse
TE2000-U light microscope (Nikon Corporation, Tokyo, Japan), and
images captured with a digital camera.
Statistical analysis
SPSS version 13.0 software (SPSS, Inc., Chicago, IL,
USA) was used in the present study for all statistical analyses.
The results are presented as the mean ± standard deviation. The
increases in skin thickness in the groups were compared using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Dose-response to UVR
To determine the MED value of the UVR, preliminary
experiments were performed, in which animals were exposed to
various doses of ssUVR. Response curves were drawn by plotting the
increases in skin thickness against various ssUVR doses under a
constant sunscreen dose. The dose response curves from the
sunscreen group and non-sunscreen group are shown in Fig. 2. The dose of ssUVR varied between
1,030 and 4700 mJ/cm2. The increase in skin thickness
started to differentiate from 1,570 mJ/cm2, and a
significant difference was found at 2,145 mJ/cm2
(P<0.05), at which UVA and UVB were 2,000 and 145
mJ/cm2, respectively. In consideration of animal ethics,
a 0.7X MED was set as the ssUVR dose, of which UVA and UVB were
1,400 and 101.5 mJ/cm2, respectively.
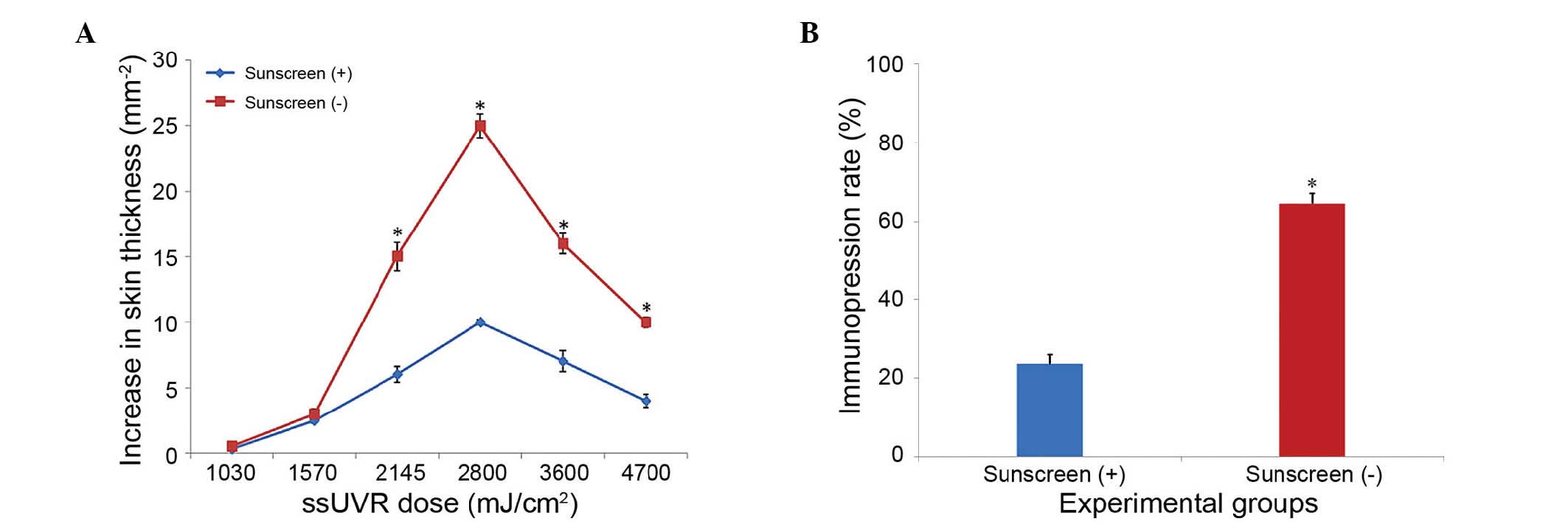 | Figure 2Dose-response curve showing the
increase in skin thickness against UVR. Mice were randomly divided
into a sunscreen group and a non-sunscreen group. Each group was
divided into six subgroups (n=10), each treated with a
different dose of UVR. Prior to UVR, an area of ~8 cm2
of the dorsal skin of the mice was covered with, or without,
sunscreen (2 mg/cm2). The dorsal skin was then exposed
to a sunlight system at various ssUVR doses (UVA, 1,000–3,500
mJ/cm2; UVB, 30–1,200 mJ/cm2) for 60 sec. (A)
The increases in skin thickness were plotted to obtain the response
curves against various ssUVR doses under a constant sunscreen dose.
The minimum dose of ssUVR required to cause a significant increase
(*P<0.05) in skin thickness in the sunscreen group,
compared with the non-sunscreen group, was used for further
experiments, (factor of 0.7). (B) The immunosuppresion rate of the
sunscreen and non-sunscreen groups were 24.39 and 65.85%,
respectively. *P<0.05 vs. sunscreen (+). The data are
presented as the mean ± standard deviation. UVR, ultraviolet
radiation. ssUVR, solar-stimulated UVR. |
Immunosuppression
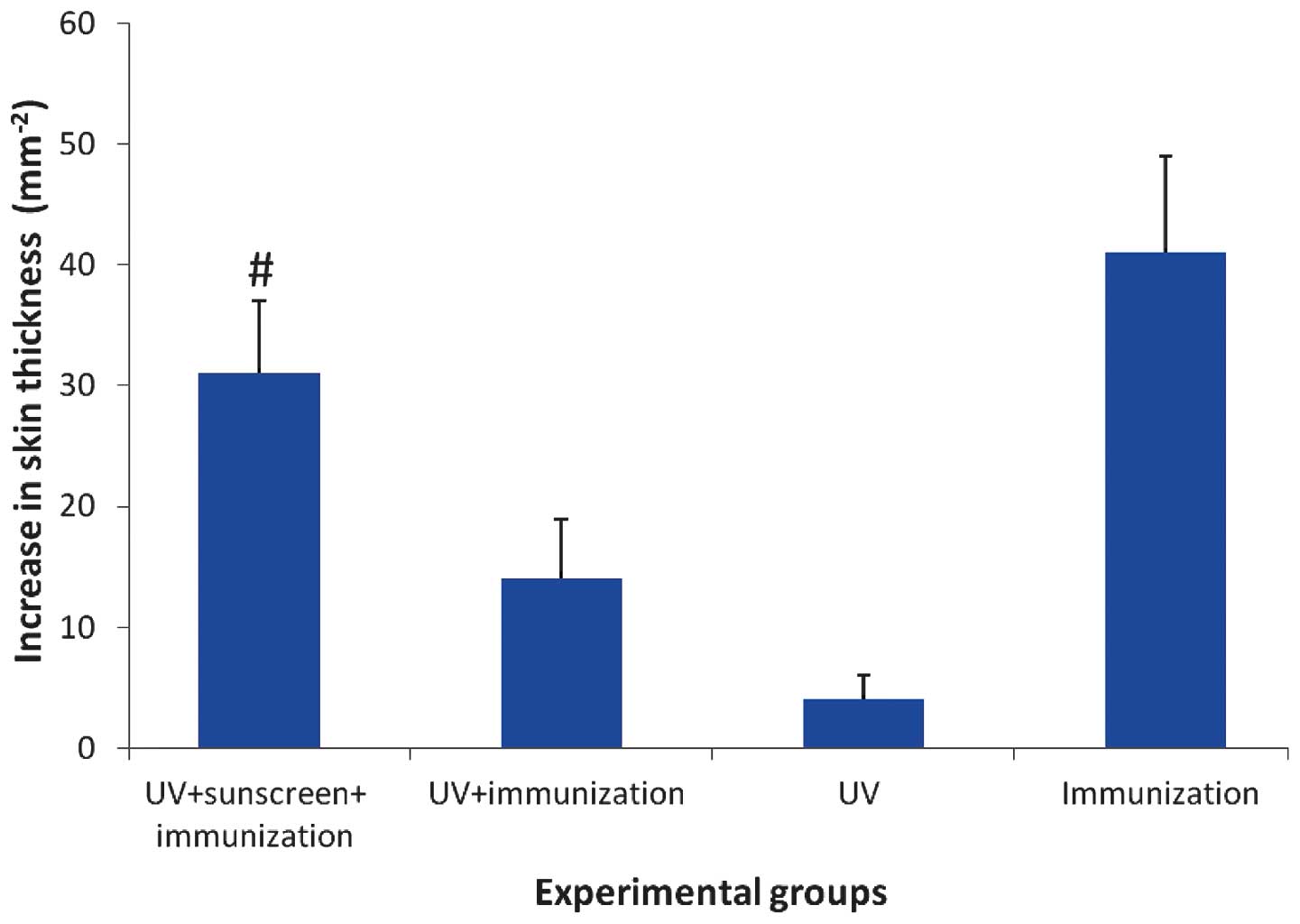
Overall, the increases in skin thickness of Group A
(sunscreen+UV+immunization) and Group C (positive control) were
higher, compared with Group B (UV+immunization) and Group D
(negative control), indicating UVR-induced immunosuppression in the
Candida albicans-induced delayed-type hypersensitivity mouse
model was successfully established (Fig. 3). The severity of foot pad swelling
in the sunscreen group was significantly higher, compared with that
in the non-sunscreen group (P<0.01). The immunosuppresion rate
of the sunscreen group and non-sunscreen group were 24.39 and
65.85%, respectively.
Western blot analysis
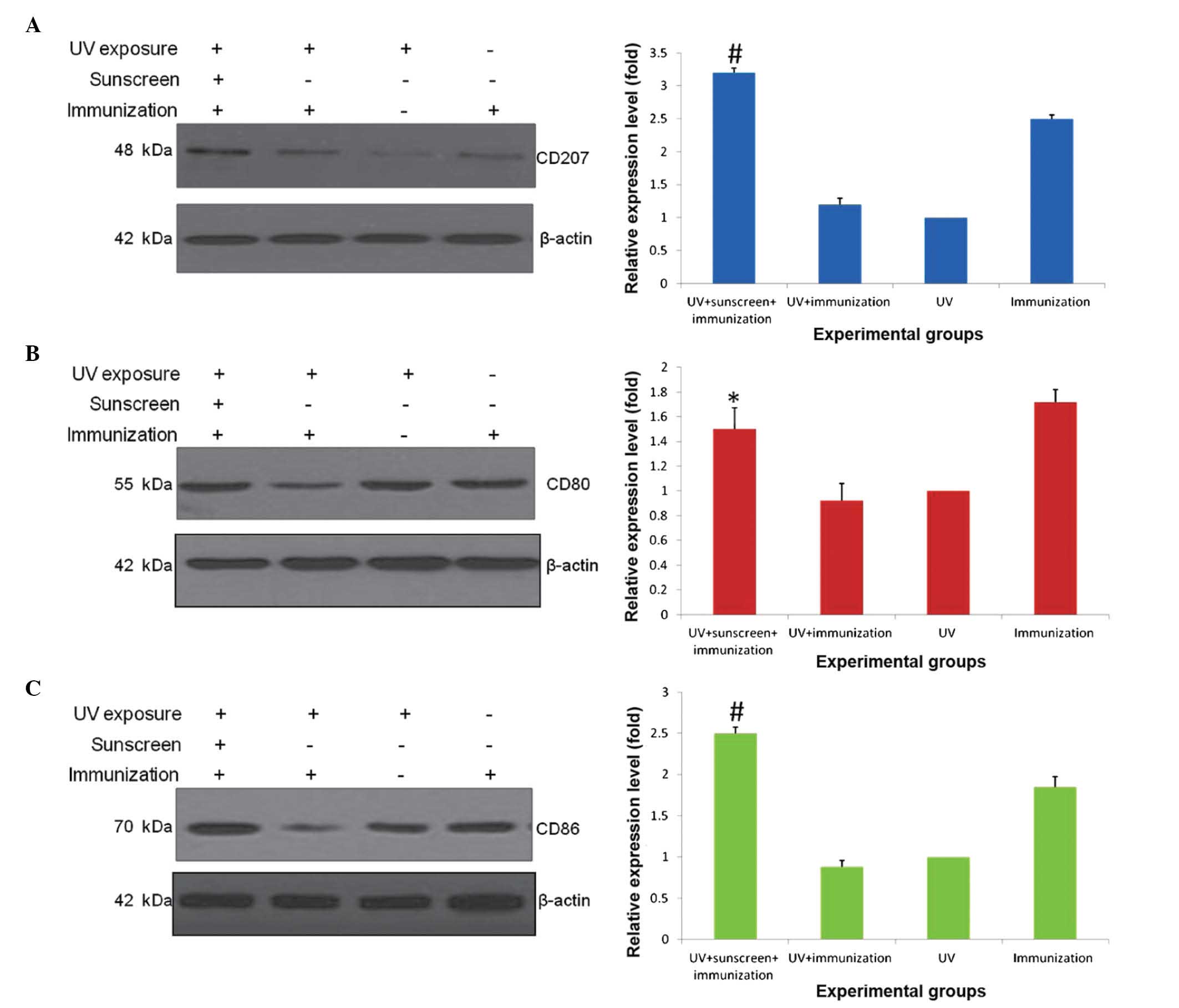
As shown in Fig. 4,
the expression level of CD207 was measured in biopsy specimens from
UV-exposed dorsal skin. Compared with the positive control, the
expression level of CD207 in the non-sunscreen group was
significantly decreased, whereas the use of sunscreen upregulated
the expression of CD207 (P<0.01; Fig. 4A and B). Accordingly, the present
study further measured the expression levels of CD80 and CD86,
which also represent immune responses of Langerhans cells. The
results of the Western blotting showed that, compared with the
non-sunscreen group, the expression levels of CD80 (P<0.05;
Fig. 4C and D) and CD86
(P<0.01; Fig. 4E and F) were
recovered by the sunscreen treatment.
Immunohistochemistry results
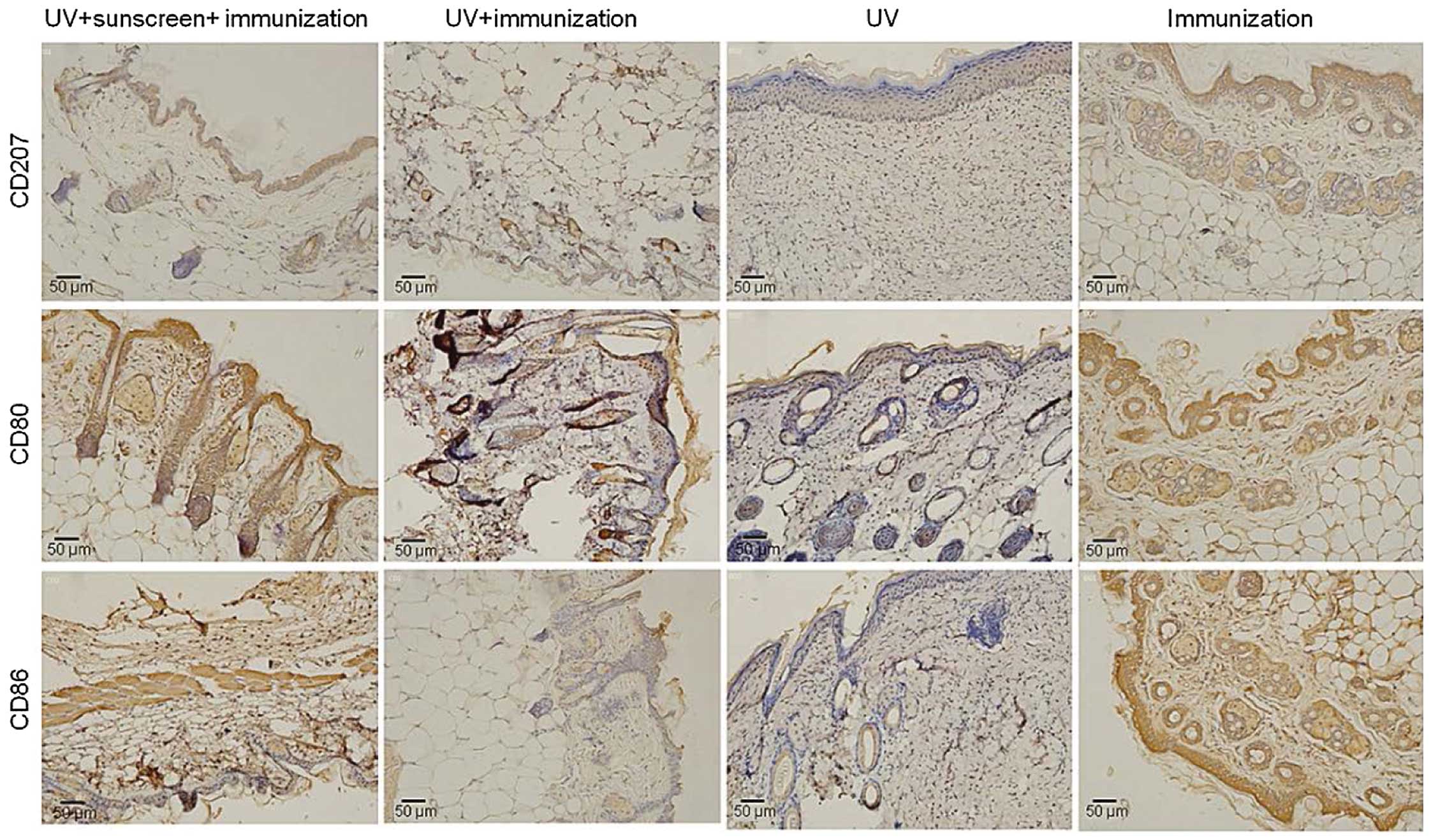
To confirm the effect of sunscreen on the immune
responses against Candida albicans-induced delayed-type
hypersensitivity, the present study performed an
immunohistochemical assay to measure the expression levels of
CD207, CD80 and CD86 in the treatment groups. The results
demonstrated that these molecules were expressed at high levels in
the positive control (immunization group; Fig. 5), whereas the expression levels
were markedly suppressed by UVR treatment. Compared with the
non-sunscreen group (UV+immunization group), the expression levels
of these immune molecules were maintained in the sunscreen group
(UV+sunscreen+immunization; Fig.
5), which was consistent with the results of the western blot
analysis.
Discussion
The association between over-exposure to UVR and
increasing incidence of skin cancer has been investigated in
previous years. Photoimmunosupression of adaptive immune responses
is important, however, the pathways involved are complex and remain
to be fully elucidated (21).
Sunscreen is an effective and available agent for protection
against light damage in dermatological practice. The majority of
evaluation methods for sunscreen protection is an SPF-based measure
of UVB-induced erythema response, however, this fails to provide an
appropriate evaluation of immune protection. IPF has been suggested
for years as an immune protection indicator in sunscreen (6). However, the lack of appropriate
measurements to evaluate immunosuppression remains a challenge in
studies in photodermatology. Kim et al examined the
UVB-induced erythema response in hairless mice for 5 days following
the injection of Candida albicans, and found that the
Candida albicans-induced delayed-type hypersensitivity
response in the mouse model is similar to the response to sunlight
in humans (19). In the present
study, BALB/c mice were irradiated using ssUVR, and showed that a
sub-erythema dose of ssUVR exposure caused immune suppression. To
determine the MED value of UVR, preliminary experiments were
peroformed, in which animals to various dose of ssUVR. The ssUVR
dose was set at 0.7X MED for the further experiments. It was found
that the immunosuppression rate of the sunscreen group (24.39%) was
significantly lower, compared with that of the non-sunscreen group
(65.85%).
The present study investigated the potential
mechanism involved by measuring the expression levels of CD207,
CD80 and CD86 in biopsy specimens of the UV-exposed dorsal skin.
The results showed that the expression levels of CD207, CD80 and
CD86 were higher in the sunscreen group, compared with the
non-sunscreen group. CD207/langerin is a type II
membrane-associated C-type lectin, known to be expressed
exclusively by Langerhans cells (22). When it is activated, it expresses
membrane-associated antigens CD80 and CD86, which are T cell
co-stimulatory molecules. These can be combined with the excitatory
CD28 receptor and inhibitory CTLA-4 receptor (23,24).
It is generally considered that a large quantity of CD86 is
expressed rapidly following antigen activation of Langerhans cells.
CD86 and CD80 are important in the immune response in early and
late stages, respectively (25).
Therefore, upon antigen injection, the expression levels of CD207,
CD86 and CD80 may reflect immune responses conferred by Langerhans
cells. In the present study, the results of Western blot analysis
and immunohistochemistry coincidently supported the hypothesis that
Anthelios sunscreen can protect the skin from immunosuppression
through activating epidermal Langerhans cells.
In the present study, a Candida
albicans-induced delayed-type hypersensitivity mouse model was
successfully established, which was indicated by the positive
control. Footpad swelling was marked in the positive control group,
compared with the other groups. In addition, Langerhans
cell-activating molecules were detected upon injection of
Candida albicans antigen 24 h following challenge.
Considering the value of the Candida albicans-induced
delayed-type hypersensitivity mouse model in the immune response,
this model may be used to evaluate the protective efficacy of other
commercial sunscreens. Taken together, the present study
demonstrated that Anthelios sunscreen prevented UVR-induced
immunosuppression through activating Langerhans cells. The results
of the present study provide evidence to support the application of
sunscreen in the prevention of UVR-induced skin injury.
Acknowledgments
This study was supported by grants from the Natural
Science Foundation of Guangdong province (grant no. 2014A030313782)
and the Science and Technology Planning Project of Guangdong
Province (grant. no. 2013B021800044).
References
|
1
|
Goswami S and Haldar C: Melatonin as a
possible antidote to UV radiation induced cutaneous damages and
immune-suppression: An overview. J Photochem Photobiol B.
153:281–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo B, Naish S, Hu W and Tong S: The
potential impact of climate change and ultraviolet radiation on
vaccine-preventable infectious diseases and immunization service
delivery system. Expert Rev Vaccines. 14:561–577. 2015. View Article : Google Scholar
|
|
3
|
Schwarz T and Schwarz A: Molecular
mechanisms of ultraviolet radiation-induced immunosuppression. Eur
J Cell Biol. 90:560–564. 2011. View Article : Google Scholar
|
|
4
|
Fernandes TR, Santos I, Korinsfky JP, Lima
e Silva BS, Carvalho LO and Plapler H: Cutaneous changes in rats
induced by chronic skin exposure to ultraviolet radiation and
organophosphate pesticide. Acta Cir Bras. 29:7–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Granstein RD and Matsui MS: UV
radiation-induced immunosuppression and skin cancer. Cutis. 74(5
Suppl): 4–9. 2004.PubMed/NCBI
|
|
6
|
Poon TS, Barnetson RS and Halliday GM:
Sunlight-induced immunosuppression in humans is initially because
of UVB, then UVA, followed by interactive effects. J Invest
Dermatol. 125:840–846. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim Y and He YY: Ultraviolet
radiation-induced non-melanoma skin cancer: Regulation of DNA
damage repair and inflammation. Genes Dis. 1:188–198. 2014.
View Article : Google Scholar
|
|
8
|
Schwarz A, Noordegraaf M, Maeda A, Torii
K, Clausen BE and Schwarz T: Langerhans cells are required for
UVR-induced immunosuppression. J Invest Dermatol. 130:1419–1427.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee CH, Wu SB, Hong CH, Yu HS and Wei YH:
Molecular mechanisms of UV-induced apoptosis and its effects on
skin residential cells: The implication in UV-based phototherapy.
Int J Mol Sci. 14:6414–6435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwarz T: Mechanisms of UV-induced
immunosuppression. Keio J Med. 54:165–171. 2005. View Article : Google Scholar
|
|
11
|
Moyal DD and Fourtanier AM: Broad-spectrum
sunscreens provide better protection from solar
ultraviolet-simulated radiation and natural sunlight-induced
immunosuppression in human beings. J Am Acad Dermatol. 58(5 Suppl
2): S149–S154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serre I, Cano JP, Picot MC, Meynadier J
and Meunier L: Immunosuppression induced by acute solar-simulated
ultraviolet exposure in humans: Prevention by a sunscreen with a
sun protection factor of 15 and high UVA protection. J Am Acad
Dermatol. 37:187–194. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fourtanier A, Moyal D, Maccario J, Compan
D, Wolf P, Quehenberger F, Cooper K, Baron E, Halliday G, Poon T,
et al: Measurement of sunscreen immune protection factors in
humans: A consensus paper. J Invest Dermatol. 125:403–409. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooper KD, Baron ED, LeVee G and Stevens
SR: Protection against UV-induced suppression of contact
hypersensitivity responses by sunscreens in humans. Exp Dermatol.
11(Suppl 1): 20–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roberts LK and Beasley DG: Commercial
sunscreen lotions prevent ultraviolet-radiation-induced immune
suppression of contact hypersensitivity. J Invest Dermatol.
105:339–344. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walker SL and Young AR: Sunscreens offer
the same UVB protection factors for inflammation and
immunosuppression in the mouse. J Invest Dermatol. 108:133–138.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heriazon A, Yager JA, Sears W and Mallard
BA: Induction of delayed-type hypersensitivity and interferon-gamma
to Candida albicans and anti-hen-egg white lysozyme antibody as
phenotypic markers of enhanced bovine immune response. Vet Immunol
Immunopathol. 129:93–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ullrich SE, Kripke ML and Ananthaswamy HN:
Mechanisms underlying UV-induced immune suppression: Implications
for sunscreen design. Exp Dermatol. 11(Suppl 1): 13–16. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council (US): Guide for
the Care and Use of Laboratory Animals. National Academies Press;
Washington DC: 1996
|
|
20
|
Kim TH, Ananthaswamy HN, Kripke ML and
Ullrich SE: Advantages of using hairless mice versus haired mice to
test sunscreen efficacy against photoimmune suppressions. Photochem
Photobiol. 78:37–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gibbs NK and Norval M:
Photoimmunosuppression: A brief overview. Photodermatol
Photoimmunol Photomed. 29:57–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hunger RE, Sieling PA, Ochoa MT, Sugaya M,
Burdick AE, Rea TH, Brennan PJ, Belisle JT, Blauvelt A, Porcelli SA
and Modlin RL: Langerhans cells utilize CD1a and langerin to
efficiently present nonpeptide antigens to T cells. J Clin Invest.
113:701–708. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Slavik JM, Hutchcroft JE and Bierer BE:
CD28/CTLA-4 and CD80/CD86 families: Signaling and function. Immunol
Res. 19:1–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laihia JK and Jansen CT: Up-regulation of
human epidermal Langerhans' cell B7-1 and B7-2 co-stimulatory
molecules in vivo by solar-simulating irradiation. Eur J Immunol.
27:984–989. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujihara M, Takahashi TA, Azuma M, Ogiso
C, Maekawa TL, Yagita H, Okumura K and Sekiguchi S: Decreased
inducible expression of CD80 and CD86 in human monocytes after
ultraviolet-B irradiation: Its involvement in inactivation of
allogenecity. Blood. 87:2386–2393. 1996.PubMed/NCBI
|