Introduction
Breast cancer is currently the most common cancer
among women in the United States (US), and is the second leading
cause of cancer-associated mortality in women worldwide (1). One of the potential factors that
contributes to breast carcinogenesis is the increased exposure of
epithelial cells to estrogens produced locally by the tissue
microenvironment (2). It is clear
that estrogens increase mammary gland cell proliferation,
predominantly via estrogen receptor (ER)-mediated mechanisms in
estrogen-dependent breast tumors (3,4). The
synthesis of estrogens in breast tissue is catalyzed by the enzyme
aromatase, which is encoded by the gene cytochrome P450 family 19
subfamily A member 1 (CYP19A1). Aromatase is a cytochrome P450,
which synthesizes estrogens by converting C19 androgens to aromatic
C18 estrogenic steroids, thus catalyzing the rate-limiting or final
step of estrogen synthesis (5,6).
Estrogen, and various growth factors in the breast
adipose microenvironment that affect tumor behavior, are
increasingly been recognized. Although the major site of estrogen
production is reproductive tissue, peripheral estrogen synthesis in
adipose or fat tissue is thought to be a major source of estrogen
in postmenopausal women (7).
Estrogen production in adipose tissue has previously been
demonstrated to provide excessive estrogen for the stimulation and
progression of postmenopausal breast cancer cells, and elevated
enzymatic activity of aromatase has been detected in the adjacent
adipose stroma of breast carcinoma (8,9).
Preadipocytes and adipocytes are major cell types present in the
breast adipose tissue microenvironment (10). Cancer-associated adipocytes exhibit
an activated phenotype and contribute to breast cancer invasion
(11). Long-term exposure to
estrogens and non-estrogenic agents with estrogenic actions, such
as endocrine disruptors, may be important in human breast
carcinogenesis. Previous studies have reported that some endocrine
disruptors may interact with in situ steroidogenesis by
altering tissue components, such as increased aromatase expression,
in certain tissues (12,13).
Zeranol is a non-steroidal estrogen agonist that is
approved for use as a growth promoter in livestock, including beef
cattle, in various countries. However, previous studies have
suggested that it may not be as safe as previously demonstrated
(14,15). Exposure to hormonal growth
promoters or endocrine disruptors elevates the probability of
histological alterations in human mammary epithelial cells, which
may lead to the growth of pre-neoplastic cells (16). A previous report demonstrated that
zeranol is comparable to natural 17β-estradiol (E2) and the
synthetic estrogen diethylstilbestrol in its ability to transform
MCF-10A normal human breast epithelial cells to a pre-cancerous
phenotype in vitro (17). A
follow-up study demonstrated that implantation of zeranol in beef
heifers was able to greatly enhance the proliferation of
preadipocytes by elevating cyclin D1 and reducing P53 gene
expression (18). Furthermore, a
previous study suggested that zeranol may increase estrogen
production in breast adipose tissues, which in turn may increase
the proliferation of normal human breast epithelial cells (19).
The long-term goal of the present study is to
investigate the effects of zeranol residues in beef and their
potential adverse effects on human breast health. The present study
hypothesized that aromatase expression and activity may be elevated
in response to low dose zeranol exposure, providing a source of
estrogens, which may promote the proliferation of carcinoma clones
derived from breast cells. The aim of the present study was to
investigate this hypothesis and to improve understanding regarding
the effects of zeranol on breast carcinogenesis.
Materials and methods
Cell culture
Human breast preadipocytes were isolated from normal
adipose tissue during breast reduction surgery (36-year-old female)
by collagenase and hyaluronidase digestion, and cultured as
previously described (19).
Preconfluent human breast preadipocytes were repeatedly subcultured
in Dulbecco's modified Eagle's medium (DMEM)/F12 medium
supplemented with 5% fetal bovine serum (FBS), 100 U/ml penicillin
sodium, 100 µg/ml streptomycin sulfate and 0.25 µg/ml
amphotericin B at 37°C (all from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). All experiments were conducted on primary
cultured preadipocytes up to passage 4. The study was approved by
the ethics committee of Guangdong Ocean University (Zhanjiang,
China) and written informed consent was obtained from the
patient.
Cell proliferation assay
Primary cultured human breast preadipocytes were
cultured in 100 µl DMEM/F12 medium in 96-well culture plates
at an initial density of 4×103 viable cells/well.
Following an overnight culture, the medium was replaced with
DMEM/F12 supplemented with 0.2% bovine serum albumin (BSA;
Sigma-Aldrich, St. Louis, MO, USA) and cultured overnight. The
experimental cells were treated with various doses (0, 2 and 50 nM)
of zeranol (Sigma-Aldrich), and 50 nM zeranol plus 100 nM ICI
182,780 (Sigma-Aldrich), whereas the control group was treated with
0.1% dimethyl sulfoxide at 37°C for 48 h. Subsequently, cell growth
was observed by measuring the optical density at 490 nm according
to the manufacturer's protocol. Briefly,
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2-H-tetrazolium
(MTS) was mixed with phenazine methosulfate (Promega Corporation,
Madison WI, USA), and 20 µl was added to each well and
incubated for 2 h at room temperature. The color density was
determined at 490 nm using a kinetic microplate reader (UV-max;
Molecular Devices, LLC, Sunnyvale, CA, USA), and cell growth was
compared.
Immunocytochemical staining
The cells were grown on slides overnight, and were
then fixed with 4% paraformaldehyde at room temperature for 10 min,
washed in 10 mM phosphate-buffered 150 mM saline (PBS, pH 7.4) with
0.1% Triton X-100 (Sigma-Aldrich) for 10 min, and blocked in 10%
normal donkey serum (Sigma-Aldrich) in 0.1% BSA in PBS for 60 min.
The slides were incubated with monoclonal mouse anti-human
aromatase antibody (MCA2077S; AbD Serotec, Raleigh, NC, USA; 1:50
dilution in 3% BSA) overnight at 4°C. Subsequently, the slides were
incubated with goat anti-mouse Texas Red-labeled antibody (sc-2781;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA; 1:200 dilution in
PBS) for 45 min at room temperature. Prior to mounting, the nuclei
were stained for 5 min with 5 µg/ml
4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc.,
Burlingame, CA, USA), and were then observed under an Olympus
compound microscope (BX51; Olympus Corporation, Tokyo, Japan)
equipped with a Nikon digital camera (E8400; Nikon Corporation,
Tokyo, Japan).
Aromatase activity assay
Preadipocytes were cultured for 24 and 48 h in
DMEM/F12 medium with or without various doses (0, 2 and 50 nM) of
zeranol and 50 nM zeranol plus 1 µM letrozole
(Sigma-Aldrich). The aromatase activity in the culture medium was
examined by tritiated water release assay using [1β-3H]
androst-4-ene-3,17-dione (0.5 µM; Sigma-Aldrich) as a
substrate, as previously described (20). The cells were incubated at 37°C for
5 h in an air/CO2 (5%) atmosphere. The data were
expressed as fmol/h and normalized to mg of protein (fmol/h/mg
protein).
Cell treatment and total RNA
extraction
All experiments were carried out on cells that
exhibited >95% viability, as measured using the trypan blue dye
exclusion method (21).
Preadipocytes were seeded in 6-well plates at 1×105
cells/well in high-calcium DMEM/F12 containing 10% Chelex-100
(Bio-Rad Laboratories, Inc., Hercules, CA, USA)-treated FBS.
Following an overnight culture, the medium was replaced with phenol
red-free high-calcium DMEM/F12 containing 5% dextran-coated
charcoal (DCC; Sigma-Aldrich)-stripped Chelex-100-treated FBS.
After 24 h, the cells were treated with zeranol (2, 10 and 50 nM),
and the control cells were treated with the vehicle, in
phenol-red-free high-calcium DMEM/F12 supplemented with 5%
DCC-treated FBS for 24 h at 37°C. Total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol.
cDNA synthesis
cDNA synthesis was performed as described previously
(19). Briefly, 1 µg total
RNA from the cultured cells was reverse transcribed with 200 U
M-MLV Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C for 50 min, in the presence of 1 µl 10 mM dNTP
mix (dATP, dCTP, dGTP and dTTP), 10 µl 5× first-strand
buffer, 5 µl 0.1 M DDT, 1 µl RNAase inhibitor (all
from Invitrogen; Thermo Fisher Scientific, Inc.) and 1 µM
random hexamers in a total volume of 50 µl. The reaction was
terminated by heating to 95°C for 5 min. Reactions were carried out
using the Eppendorf Mastercycler gradient (Eppendorf, Hamburg,
Germany). RNA concentration was measured using a DU-70
spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
The newly synthesized cDNA was used as a template
for PCR. cDNA (2 µl) was mixed with 1.25 µl
MgCl2 (50 mM), 2.5 µl 10× PCR buffer II, 0.2
µl Taq polymerase (5 U/µl), and 0.3 µl
sense and antisense primers (all from Gibco; Thermo Fisher
Scientific, Inc.) in a total volume of 25 µl. One pair of
primers was for the amplification of human CYP19A1 and the other
was for human ribosomal protein lateral stalk subunit P0 (36B4),
which was used as a positive and loading control. PCR for CYP19A1
was conducted using the T100 Thermal Cycler (Bio-Rad Laboratories,
Inc.) under the following cycling conditions: Initial denaturation
at 94°C for 3 min, 36 cycles of 94°C for 45 sec, 60°C for 45 sec
and 72°C for 60 sec, final extension step of 72°C for 4 min. The
primer sequences used were as follows: CYP19A1, sense
5′-CCTGGCTACTGCATGGGAAT-3′, antisense 3′-GCCTTTCTCATGCATACCGA-5′,
product size 246 bp; and 36B4, sense 5′-AGCTGATCAAGACTGGAGACAAA-3′,
antisense 3′-GGGTAGCCAATCTGCAGACA-5′, product size 220 bp. The
final RT-PCR products (10 µl) were run on a 1.5% agarose gel
containing ethidium bromide (Bio-Rad Laboratories, Inc.). The 100
bp DNA ladder (Promega Corporation) was used as marker. The
specific bands were semi-quantified by Fujifilm MultiGauge
software, version 3.0 (Fuji Life Sciences, Stamford, CT, USA). The
results are presented as the ratio of CYP19A1 to 36B4.
E2 production assay
Cells were seeded in 6-well plates at
1×105 cells/well in 5 ml high-calcium DMEM/F12
containing 10% FBS, and were cultured overnight at 37°C. The medium
was replaced with DMEM/F12 supplemented with 5% DCC-treated FBS for
a further 24 h, and the preadipocytes were then treated with the
indicated doses of zeranol (2, 10 and 30 nM) for 48 h. Following
treatment, 200 µl culture medium was collected, and the
levels of E2 in the culture medium were determined using an
enzyme-linked immunosorbent assay (ELISA) kit (ALPCO, Salam, NH,
USA). The kit was specific for E2 and did not cross-react with
estriol or estrone.
Statistical analysis
Statistical analysis was performed using Minitab 15
(Minitab Inc., State College, PA, USA). Differences between groups
were evaluated by one-way analysis of variance, followed by
Dunnett's test for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
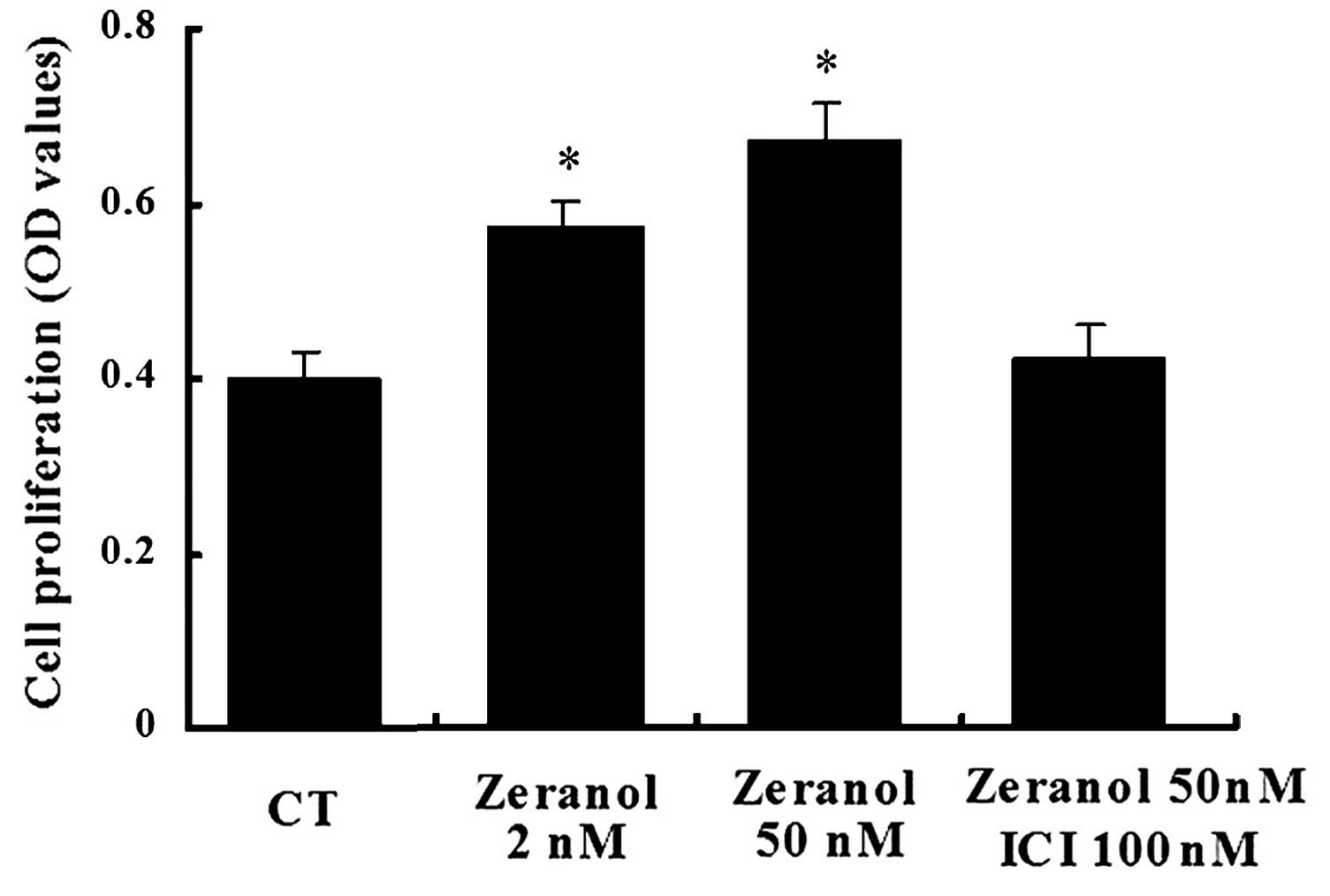
Zeranol promotes the proliferation of
preadipocytes
Following 48 h of treatment, zeranol increased the
proliferation of preadipocytes in a dose-dependent manner, as
determined by an MTS assay (Fig.
1). Compared with the control cells, treatment with 2 and 50 nM
zeranol increased the proliferation of preadipocytes by 30 and 41%
(P=0.036 and 0.023, respectively). Treatment with the ER
antagonist, ICI 182,780 (ICI) abrogated zeranol-induced
proliferation. These results suggest that zeranol may promote cell
proliferation via an effect on ER.
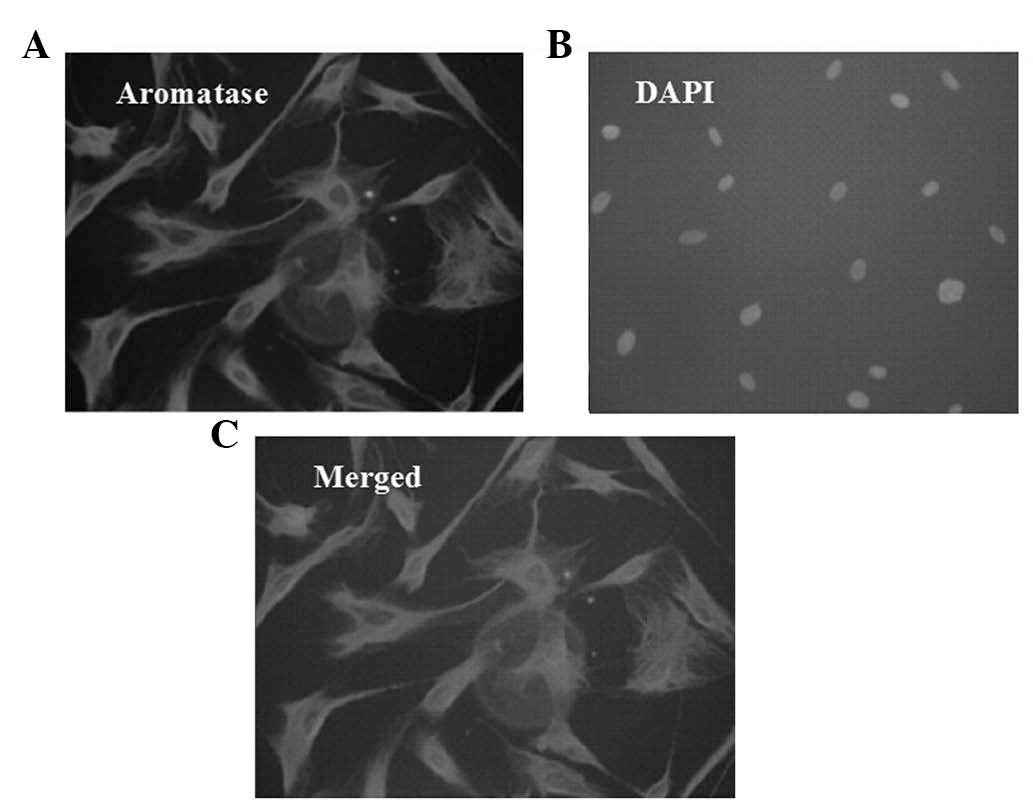
Aromatase-positive staining is observed
in the cytoplasm of preadipocytes
Positive aromatase immunofluorescent staining was
observed in >80% of cells in a random field, whereas DAPI
staining was only observed in the nucleus (Fig. 2).
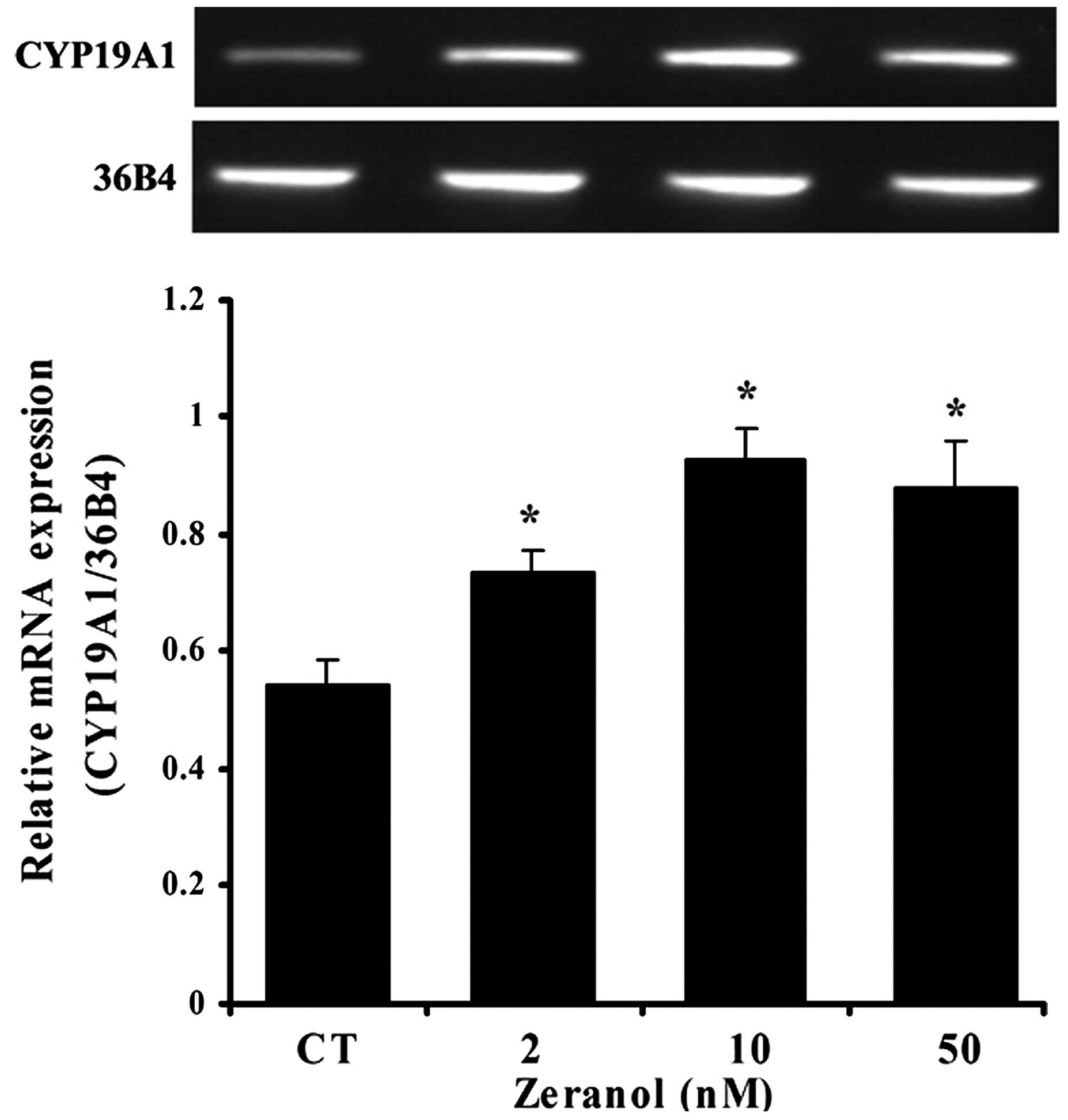
Zeranol enhances aromatase mRNA
expression in preadipocytes
The effects of zeranol on the mRNA expression levels
of aromatase in human breast preadipocytes were investigated by
RT-PCR. The results demonstrated that treatment with 2, 10 and 50
nM zeranol for 48 h induced a significant increase in the mRNA
expression levels of aromatase compared with the control (P=0.039,
0.022 and 0.028, respectively; Fig.
3). The reference gene 36B4 was used for normalization.
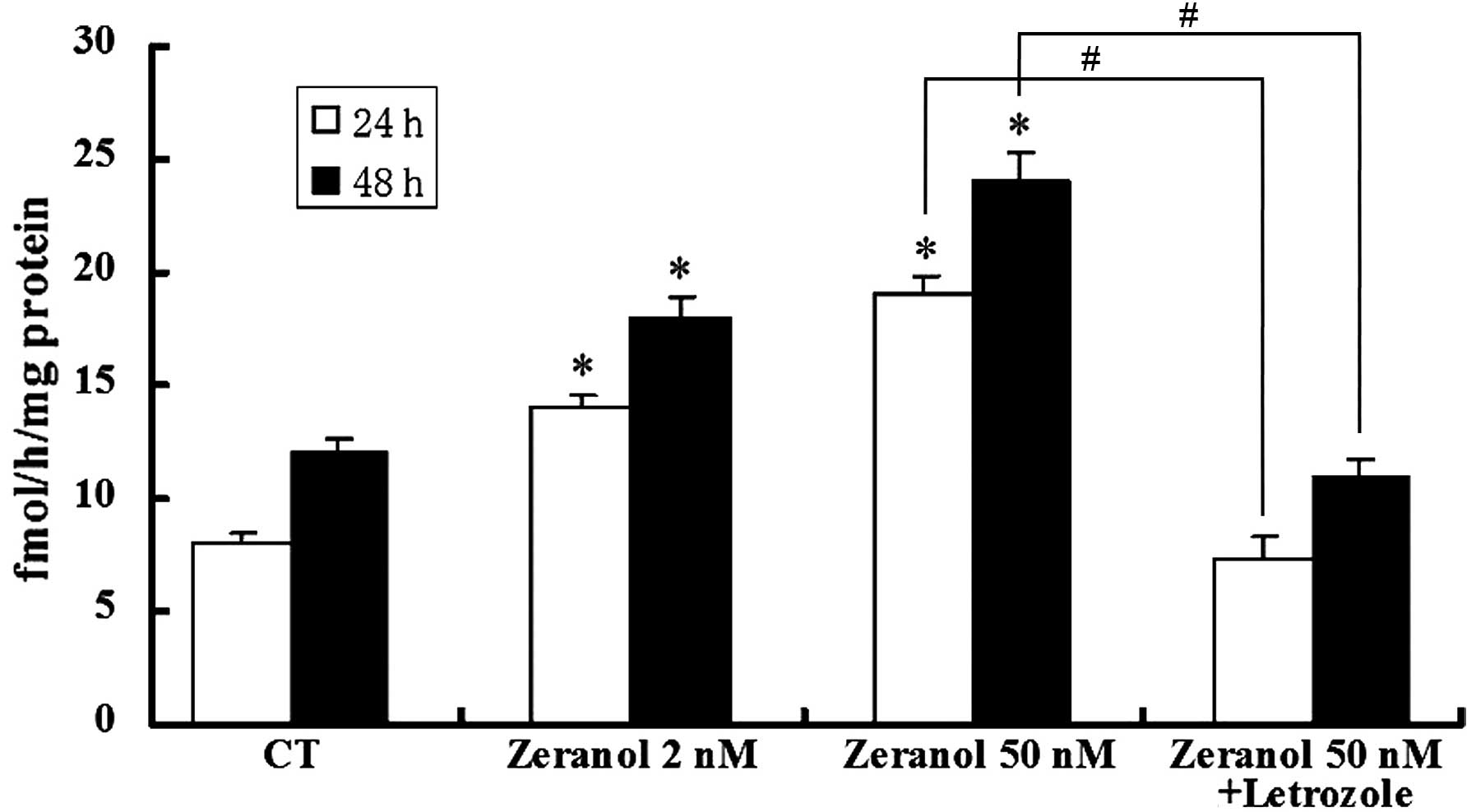
Stimulation of aromatase activity by
zeranol in preadipocytes
Preadipocytes were incubated in the presence of
various concentrations of zeranol (2 and 50 nM) for 24 and 48 h.
Subsequently, the aromatase activity was measured by tritiated
water assay. As demonstrated in Fig.
4, low doses of zeranol (2 and 50 nM) significantly increased
the enzymatic activity of aromatase in preadipocytes compared with
the control cells, following treatment for 24 and 48 h (P=0.031 and
0.033, respectively). However, the promotion of aromatase activity
was completely reversed by co-treatment with the aromatase
inhibitor, letrozole (P=0.025 and 0.023, respectively).
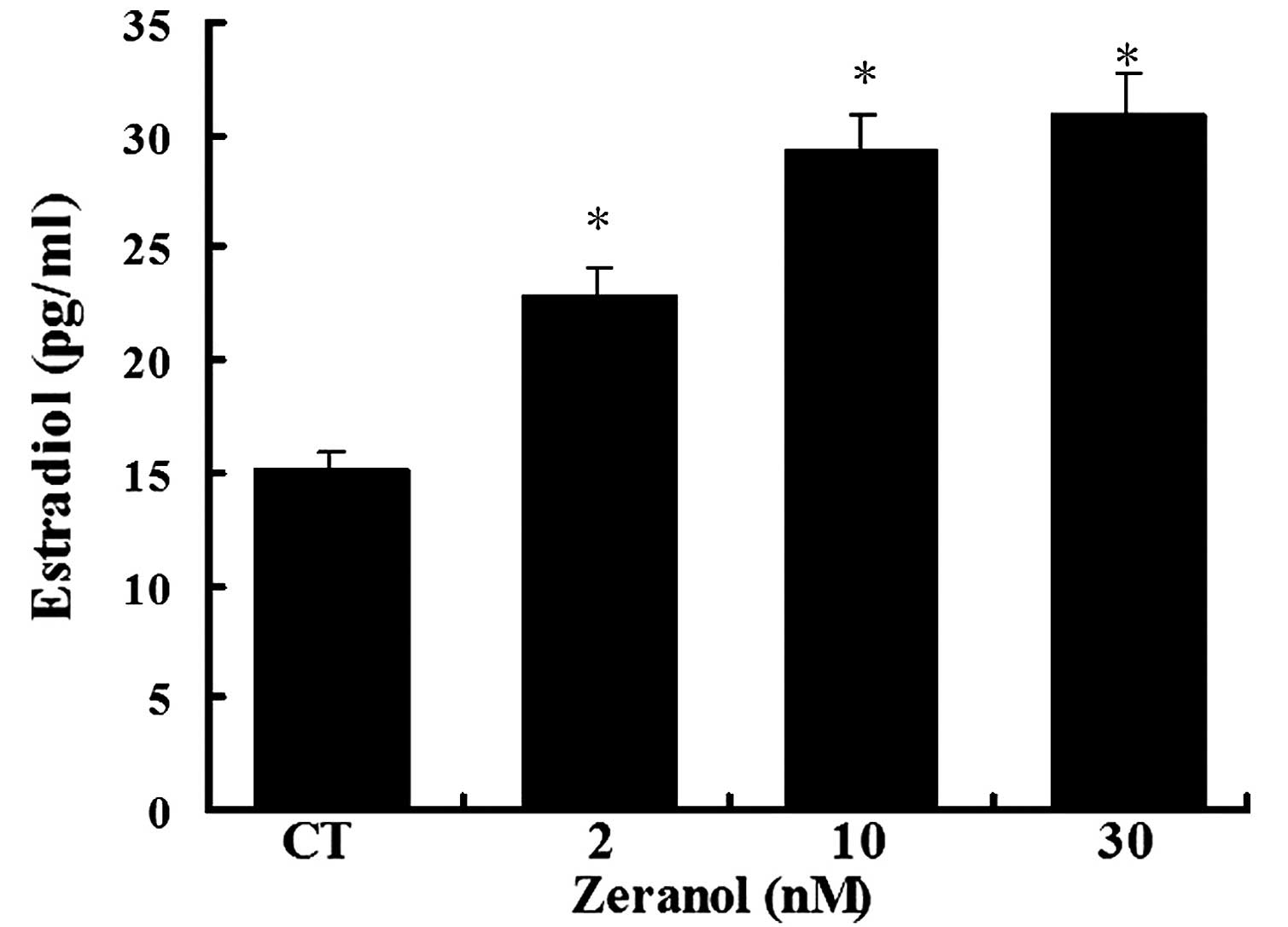
Zeranol significantly increases E2
production
To determine whether the increase in aromatase gene
expression and activity resulted in increased E2 levels, the levels
of E2 were detected in the medium of preadipocytes treated with 2,
10 and 30 nM zeranol for 48 h using a commercially available ELISA
kit. E2 concentrations were significantly increased in the medium
of zeranol-treated cells, as compared with in the control cells in
a dose-dependent manner (P=0.037, 0.026 and 0.024, respectively;
Fig. 5).
Discussion
Cumulative exposure to estrogen is known to be a
risk factor for the development and mitogenic stimulation of breast
cancer (22). The cytochrome P450
enzyme complex, termed aromatase, catalyzes the formation of
estrogens from C19 androgens to aromatic C18 estrogen through three
consecutive hydroxylation reaction steps. Since estrogen is
involved in the development of breast cancer and aromatase is the
final enzyme responsible for estrogen production, high aromatase
expression in breast cancer cells may affect breast cancer
progression and maintenance (23).
It has previously been demonstrated that aromatase is expressed in
breast cancer tissue, and is at a higher level compared with in
non-cancerous breast tissue (24).
Previous studies have demonstrated that aromatase activity in
malignant or surrounding tissues may promote tumor growth by local
estrogen generation (25,26). Furthermore, previous reports have
demonstrated that some endocrine disruptors may increase estrogen
production and aromatase activity (27–29).
Zeranol, which is a nonsteroidal agent with potent
estrogenic activity, is used in the US beef industry as an anabolic
growth promoter. Bioactive zeranol and its metabolites present in
the meat of zeranol-implanted beef cattle may be considered an
endocrine disruptor for human consumers. It has previously been
demonstrated that zeranol enhances cell proliferation and increases
the ERα content of ER-positive breast cancer cells (30,31).
However, the effects of zeranol on aromatase activity and estrogen
production in human breast preadipocytes remain unclear.
In the present study, primary preadipocytes isolated
from human breast adipose tissues were used as a cell model. The
results indicated that zeranol (2–50 nM) increased proliferation of
preadipocytes in a dose-dependent manner, as determined by an MTS
assay, and this effect was blocked by the ER antagonist, ICI
(Fig. 1). This result suggested
that zeranol may increase cell proliferation via an effect on ER.
Furthermore, the present study demonstrated that zeranol induced
the upregulation of aromatase mRNA expression (Fig. 3) and aromatase activity in
preadipocytes (Fig. 4). The
effects on aromatase activity were abrogated by the aromatase
inhibitor, letrozole. In addition, E2 production was increased
following the treatment of preadipocytes with low doses of zeranol
(Fig. 5). The results of the
present study suggested that zeranol may promote breast cancer cell
growth by stimulating aromatase activation and improving estrogen
biosynthesis in the adipose tissue microenvironment.
In conclusion, the results of the present study
indicated that exposure to low doses of zeranol may increase the
risk of breast cancer by increasing estrogen levels in adipose
tissue. Estrogen generated from preadipocytes acts as a functional
signal linking adipose to epithelial tissue, and in vivo
zeranol may stimulate estrogen release, thus inducing the excessive
proliferation of mammary cells. However, more comprehensive studies
are required to confirm these findings.
Acknowledgments
This research project was supported by grants from
the National Natural Science Foundation of China (grant no.
31201424) and the Natural Science Foundation of Guangdong Province
(grant no. S2012040006790), and the Science and Technology
Department of Guangdong Province (grant no. 2015A020209166).
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
2
|
Yager JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. N Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ciocca DR and Fanelli MA: Estrogen
receptors and cell proliferation in breast cancer. Trends
Endocrinol Metab. 8:313–321. 1997. View Article : Google Scholar
|
|
4
|
Boon WC, Chow JD and Simpson ER: The
multiple roles of estrogens and the enzyme aromatase. Prog Brain
Res. 181:209–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang N, Xu Y, Yin Y, Yao G, Tian H, Wang
G, Lian J, Wang Y and Sun F: Steroidogenic factor-1 is required for
TGF-beta3-mediated 17beta-estradiol synthesis in mouse ovarian
granulosa cells. Endocrinology. 152:3213–3225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simpson ER, Michael MD, Agarwal VR,
Hinshelwood MM, Bulun SE and Zhao Y: Cytochromes P450 11:
Expression of the CYP19 (aromatase) gene: An unusual case of
alternative promoter usage. FASEB J. 11:29–36. 1997.PubMed/NCBI
|
|
7
|
Kamat A, Hinshelwood MM, Murry BA and
Mendelson CR: Mechanisms in tissue-specific regulation of estrogen
biosynthesis in humans. Trends Endocrin Metab. 13:122–128. 2002.
View Article : Google Scholar
|
|
8
|
Campbell DR and Kurzer MS: Flavonoid
inhibition of aromatase enzyme activity in human preadipocytes. J
Steroid Biochem Mol Biol. 46:381–388. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bulun SE, Lin Z, Imir G, Amin S, Demura M,
Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, et al:
Regulation of aromatase expression in estrogen-responsive breast
and uterine disease: From bench to treatment. Pharmacol Rev.
57:359–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lorincz AM and Sukumar S: Molecular links
between obesity and breast cancer. Endocr Relat Cancer. 13:279–292.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dirat B, Bochet L, Dabek M, Daviaud D,
Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S,
et al: Cancer-associated adipocytes exhibit an activated phenotype
and contribute to breast cancer invasion. Cancer Res. 71:2455–2465.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arase S, Ishii K, Igarashi K, Aisaki K,
Yoshio Y, Matsushima A, Shimohigashi Y, Arima K, Kanno J and
Sugimura Y: Endocrine disrupter bisphenol A increases in situ
estrogen production in the mouse urogenital sinus. Biolo Reprod.
84:734–742. 2011. View Article : Google Scholar
|
|
13
|
Manna PR, Dyson MT and Stocco DM:
Regulation of the steroidogenic acute regulatory protein gene
expression: Present and future perspectives. Mol Hum Reprod.
15:321–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong S, Ye W, Lin SH, Liu JY, Leong J, Ma
C and Lin YC: Zeranol induces cell proliferation and protein
disulfide isomerase expression in mammary gland of ACI rat.
Anticancer Res. 31:1659–1665. 2011.PubMed/NCBI
|
|
15
|
Zhong S, Ye WP, Feng E, Lin SH, Liu JY,
Leong J, Ma C and Lin YC: Serum derived from zeranol-implanted ACI
rats promotes the growth of human breast cancer cells in vitro.
Anticancer Res. 31:481–486. 2011.PubMed/NCBI
|
|
16
|
Updike MS, Sawdy JC, Wang LS, Liu S, Huang
YW, Ye W, Farrar WB, Lin YC and Wick M: Primary cultured human
breast epithelial cells up-regulate protein disulfide isomerase in
response to zeranol. Anticancer Res. 27:407–410. 2007.PubMed/NCBI
|
|
17
|
Liu S and Lin YC: Transformation of
MCF-10A human breast epithelial cells by zeranol and
estradiol-17beta. Breast J. 10:514–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye W, Xu P, Threlfall WR, Jen R, Li H, Lin
SH, Kuo CT and Lin YC: Zeranol enhances the proliferation of
preadipocytes in beef heifers. Anticancer Res. 29:5045–5052.
2009.
|
|
19
|
Zhong S, Ye W, Xu PP, Feng E, Li H, Lin
SH, Liu JY, Ma C and Lin YC: Aromatase expression in
leptin-pretreated human breast preadipocytes is enhanced by zeranol
and suppressed by (−)-gossypol. Anticancer Res. 30:5077–5084.
2010.PubMed/NCBI
|
|
20
|
Lephart ED and Simpson ER: Assay of
aromatase activity. Methods Enzymol. 206:477–483. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tennant JR: Evaluation of the trypan blue
technique for determination of cell viability. Transplantation.
2:685–694. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Russo J and Russo IH: The role of estrogen
in the initiation of breast cancer. J Steroid Biochem Mol Biol.
102:89–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith IE and Dowsett M: Aromatase
inhibitors in breast cancer. New Engl J Med. 348:2431–2442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Utsumi T, Harada N, Maruta M and Takagi Y:
Presence of alternatively spliced transcripts of aromatase gene in
human breast cancer. J Clin Endocrinol Metab. 81:2344–2349.
1996.PubMed/NCBI
|
|
25
|
Brodie A, Lu Q and Nakamura J: Aromatase
in the normal breast and breast cancer. J Steroid Biochem Mol Biol.
61:281–286. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blankenstein MA, van de Ven J,
Maitimu-Smeele I, Donker GH, de Jong PC, Daroszewski J, Szymczak J,
Milewicz A and Thijssen JH: Intratumoural levels of estrogens in
breast cancer. J Steroid Biochem Mol Biol. 69:293–297. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanderson JT, Seinen W, Giesy JP and van
den Berg M: 2-Chloro-s-triazine herbicides induce aromatase (CYP19)
activity in H295R human adrenocortical carcinoma cells: A novel
mechanism for estrogenicity? Toxicol Sci. 54:121–127. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morinaga H, Yanase T, Nomura M, Okabe T,
Goto K, Harada N and Nawata H: A benzimidazole fungicide, benomyl,
and its metabolite, carbendazim, induce aromatase activity in a
human ovarian granulose-like tumor cell line (KGN). Endocrinology.
145:1860–1869. 2004. View Article : Google Scholar
|
|
29
|
Thibaut R and Porte C: Effects of
endocrine disrupters on sex steroid synthesis and metabolism
pathways in fish. J Steroid Biochem Mol Biol. 92:485–494. 2004.
View Article : Google Scholar
|
|
30
|
Xu P, Ye W, Li H, Lin SH, Kuo CT, Feng E
and Lin YC: Zeranol enhances leptin-induced proliferation in
primary cultured human breast cancer epithelial cells. Mol Med Rep.
3:795–800. 2010.
|
|
31
|
Takemura H, Shim JY, Sayama K, Tsubura A,
Zhu BT and Shimoi K: Characterization of the estrogenic activities
of zearalenone and zeranol in vivo and in vitro. J Steroid Biochem
Mol Biol. 103:170–177. 2007. View Article : Google Scholar
|