Introduction
Abdominal aortic aneurysm (AAA) is a degenerative
disease characterized by structural damage to the wall of the
abdominal aorta and the gradual expansion into a pulsating mass
(1). It is generally accepted that
AAA is the expansion of the abdominal aortic artery diameter to
greater than 1.5 times the neighboring normal artery (2). To the best of our knowledge, an
epidemiological survey remains to be conducted in China thus far.
In western countries, the incidence of AAA is increasing; a
previous study reported incidence of 12% in male hypertensive
patients between the ages of 60 and 74 and 20–29% in male siblings
of the patients (3). The natural
rupture rate of two years for symptomatic AAA without treatment is
up to 50%. The overall AAA mortality rate is has been reported to
be 80–90% (4). Therefore, the aim
of the present study is to investigate the pathogenesis of AAA to
aid the development of early drug treatment programs.
The interaction of various factors, including
genetic, environmental and biochemical factors, is the etiology of
AAA (5). Human AAA is
predominantly a result of hypertension, atherosclerosis and other
diseases and conditions, including congenital aortic hypoplasia,
syphilis, trauma, Takayasu arteritis, Marfan syndrome, infection
and Bechet syndrome (6). Previous
studies demonstrated that the pathological process of the aneurysm
derives from the abnormal degradation of the artery wall, and this
alteration is often present in atherosclerotic lesions (7–9).
These studies suggest that atherosclerotic plaques may weaken the
arterial wall structure or lead to a significant reduction in the
mechanical strength of the abdominal aorta, resulting in focal
protrusion to form a tumor, as the lipid infiltration directly
destroys the normal structure of the arterial wall and the
compression of the plaque results in the reduction of blood flow,
thus causing ischemia in the film (10). Numerous clinical studies have
demonstrated that there is widespread inflammation in the majority
of AAA walls (11–13).
There are 12 types of isoflavones in soy, divided
into three categories, daidzin, genistin and glycitin (14). The soy isoflavones exist in free
form, glucoside, acetyl-glucoside or malonyl-glucoside (14). Daidzein has been reported to
exhibit anti-oxidative and anti-inflammatory activity, reduce
estrogenic activity, and prevent menopause, osteoporosis and
cardiovascular diseases (15).
Thus, this may suggest that daidzein attenuates AAA, and that the
NF-κB, p38MAPK and TGF-β1 pathways may be critical in the action of
daidzein against AAA.
Materials and methods
Reagents
Angiotensin II and daidzein were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Tumor necrosis factor α (TNF-α)
and interleukin 1β (IL-1β) mouse enzyme-linked immunosorbent assay
(ELISA) antibody set was obtained from eBioscience Inc. (San Diego,
CA, USA). Bradford reagent and SDS-PAGE were obtained from Beyotime
Institute of Biotechnology (Jiangsu, China).
Angiotensin II-induced AAA mice model and
treatment
Male BALB/C male mice (n=30; age, 8 weeks; weight,
20–22 g) were purchased from the Experimental Center of The First
Affiliated Hospital of Dalian Medical University (Liaoning, China).
This is a prospective interventional animal study. All mice were
housed at 22±2°C with relative humidity of 55±5% and a 12-h
dark:light cycle. All mice were fed with normal chow ad
libitum, housed in a pathogen-free barrier facility and bred as
littermate controls. Mice were randomly divided into three groups
(n=10) as follows: Control, mice were infused subcutaneously (i.s.)
with saline vehicle for 4 weeks as previously described (16); angiotensin II-treated, mice were
i.s. with 1,000 ng/kg/min angiotensin II, then received an
intraperitoneal injection (i.p.) of saline vehicle for 4 weeks as
previously described (17);
daidzein, angiotensin II-induced mice were treated with 0.2 mg/kg
daidzein i.p. for 4 weeks. Mice was sacrificed by an excess of
anesthetic (500 µl 3% pentobarbital sodium;
Sigma-Aldrich)
Quantification of angiotensin II-induced
AAA mice
The perfusion aorta was fixated with 4% cold
paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd, Shanghai,
China), and exposed under a dissecting microscope (Olympus SZX7;
Olympus Corporation, Tokyo, Japan) to remove the periadventitial
tissue from the aortic wall. The gross appearances of the aorta
were then observed to measure the maximal external diameter of the
suprarenal aorta using the MultiGauge 3000 imaging processing
software (Fujifilm Holdings Co., Tokyo, Japan). The increase in the
aortic outer diameter was defined as >50% and used to describe
the development of the aortic aneurysm.
Measurement of inflammation
Serum was obtained from the peripheral vessel and
centrifuged at 1,200 x g for 10 min at room temperature. TNF-α and
IL-1β serum levels were determined using the eBioscience mouse
ELISA antibody set. Absorbance was determined at 450 nm wavelength
using an ELISA reader (Spectramax Plus, Molecular Devices, LLC,
Sunnyvale, CA, USA).
Western blot analysis of nuclear factor
κB (NF-κB), inducible nitric oxide synthase (iNOS) and p38
mitogen-activated protein kinase (MAPK)
The whole aorta was harvested and homogenated with
phosphatase inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland) in radioimmunoprecipitation assay lysis buffer (EMD
Millipore, Billerica, MA, USA). The mixed solution was centrifuged
at 1,200 × g for 10 min at 4°C, and protein concentration was
determined using Bradford's reagent (Beyotime Institute of
Biotechnology, Jiangsu, China) according to the manufacturer's
instructions. Equal amounts of protein were subjected to 8–10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE; Sigma-Aldrich) for 50 min at 60 V and transferred to
nitrocellulose membranes (110 V for 75 min; EMD Millipore). The
membranes were blocked with 5% skimmed milk in tris-buffered saline
with 0.1% Tween 20 (Beyotime Institute of Biotechnology) for 1 h at
4°C. The membranes were then incubated with rabbit anti-mouse NF-κB
(cat no. sc-372; 1:1,000), rabbit anti-mouse iNOS (cat no. sc-650;
1:1,500), rabbit anti-mouse p38MAPK (cat no. sc-101427; 1:500)
p-p38MAPK (cat no. sc-7973; 1:500) and β-actin (cat no. sc-130656
1:2,000) primary antibodies at 4°C overnight, all Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). Subsequently, the membranes
were incubated with anti-mouse IgG horseradish
peroxidase-conjugated secondary antibodies (cat no. 7074, 1:5000;
Cell Signaling Technology, Inc., Danvers, MA, USA), and proteins
were detected with the SuperSignal West Femto chemiluminescent
Substrate (Thermo Fisher Scientific, Inc., Rockford, IL, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of cyclooxygenase
(COX)-2, matrix metalloproteinase 2 (MMP-2), tissue inhibitor of
metalloproteinase 1 (TIMP-1) and transforming growth factor β1
(TGF-β1)
Total RNA was isolated from the aorta samples using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions.
Total RNA (500 ng) was utilized to synthesize cDNA using TaqMan
Gold RT-PCR kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. cDNA (1
µg) was used to compound DNA. RT-qPCR was performed using
the SYBR green JumpStart Taq ReadyMix (Sigma-Aldrich), SYBR Green
PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and the iCycler iQ Real-Time PCR detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). RT-qPCR was performed using
the SYBR green JumpStart Taq ReadyMix (Sigma-Aldrich), SYBR Green
PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and the iCycler iQ Real-Time PCR detection system (Bio-Rad
Laboratories, Inc.). The reactions were performed at 95°C for 10
min, followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec,
72°C for 45 sec and 4°C for saving. The sequences for gene-specific
primers are demonstrated in Table
I.
 | Table ISequences for gene-specific
primers. |
Table I
Sequences for gene-specific
primers.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| COX-2 |
CACTCAGTTTGTTGAGTCATTC |
GATTAGTACTGTAGGGTTAATG |
| MMP-2 |
ACACTGGGACCTGTCACTCC |
TGTCACTGTCCGCCAAATAA |
| TIMP-1 |
GCAACTCGGACCTGGTCATAA |
CGGCCCGTGATGAGAAACT |
| TGF-β1 |
TGCTTCAGCTCCACAGAGAA |
TGGTTGTAGAGGGCAAGGAC |
| GAPDH |
TGTACCGTCTAGCATATCTCCGAC |
ATGATGTGCTCTAGCTCTGGGTG |
Statistical analysis
Data are presented as means ± standard error
Statistical analysis was performed using Student's t-test for
paired or unpaired data, and data were assessed using SPSS
software, version 13 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Daidzein affects angiotensin II-induced
AAA mice
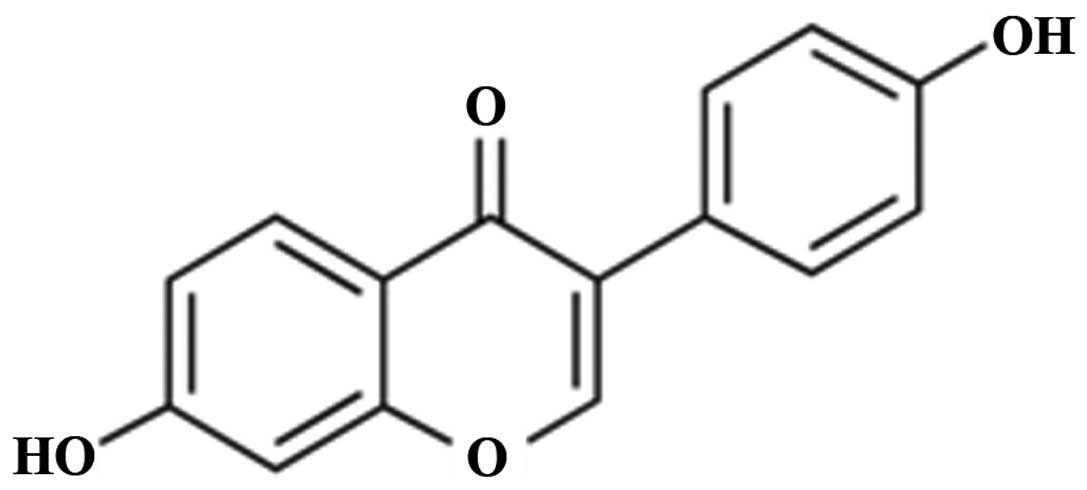
The chemical structure of daidzein is presented in
Fig. 1. To evaluate the possible
effect of daidzein on AAA mice, AAA was induced by continuous
angiotensin II treatment. Mice were treated with normal saline and
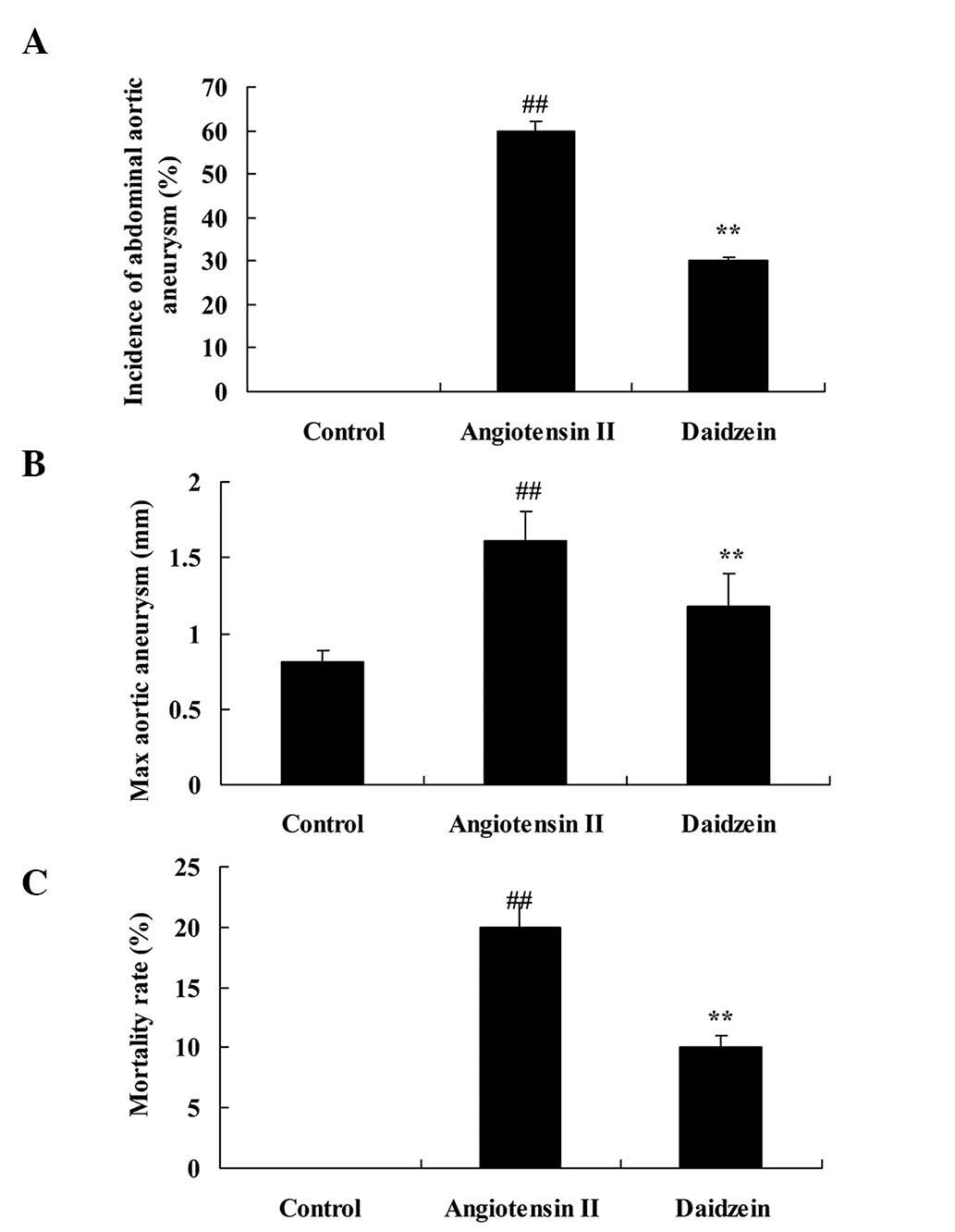
0.2 mg/kg daidzein per day. Incidence of AAA, max aortic aneurysm
and mortality rate of the angiotensin II-induced AAA mice were
observed to be higher compared with those of the control mice
(Fig. 2; P<0.01). In addition,
administration of daidzein significantly attenuated these indexes
in the angiotensin II-induced AAA mice compared with the
angiotensin II model mice (Fig. 2;
P<0.01).
Daidzein affects inflammation in
angiotensin II-induced mice
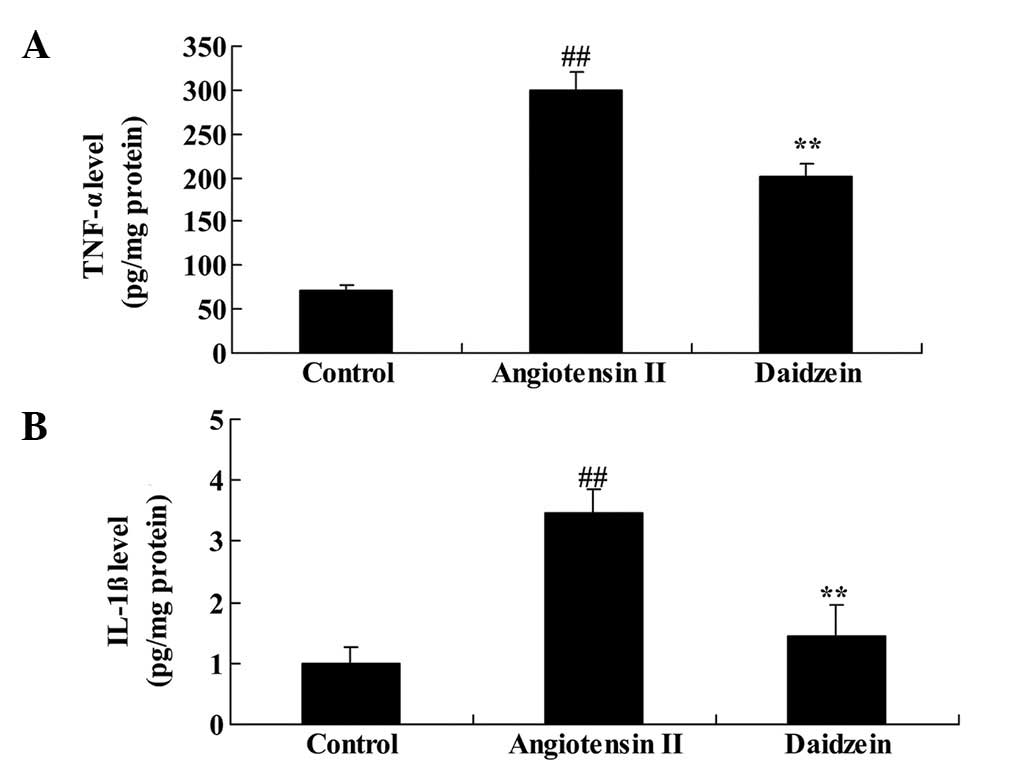
To determine the effect of daidzein on inflammation
in the angiotensin II-induced mice, TNF-α and IL-1β serum levels
were measured. As demonstrated in Fig.
3, angiotensin II infusion significantly increased the serum
levels of TNF-α and IL-1β in AAA mice, compared with the control
mice (P<0.01). However, a significant reduction in the TNF-α and
IL-1β serum levels was demonstrated in the daidzein-treated group,
compared with the angiotensin II model mice (Fig. 3; P<0.01).
Daidzein affects NF-κB protein expression
in angiotensin II-induced mice
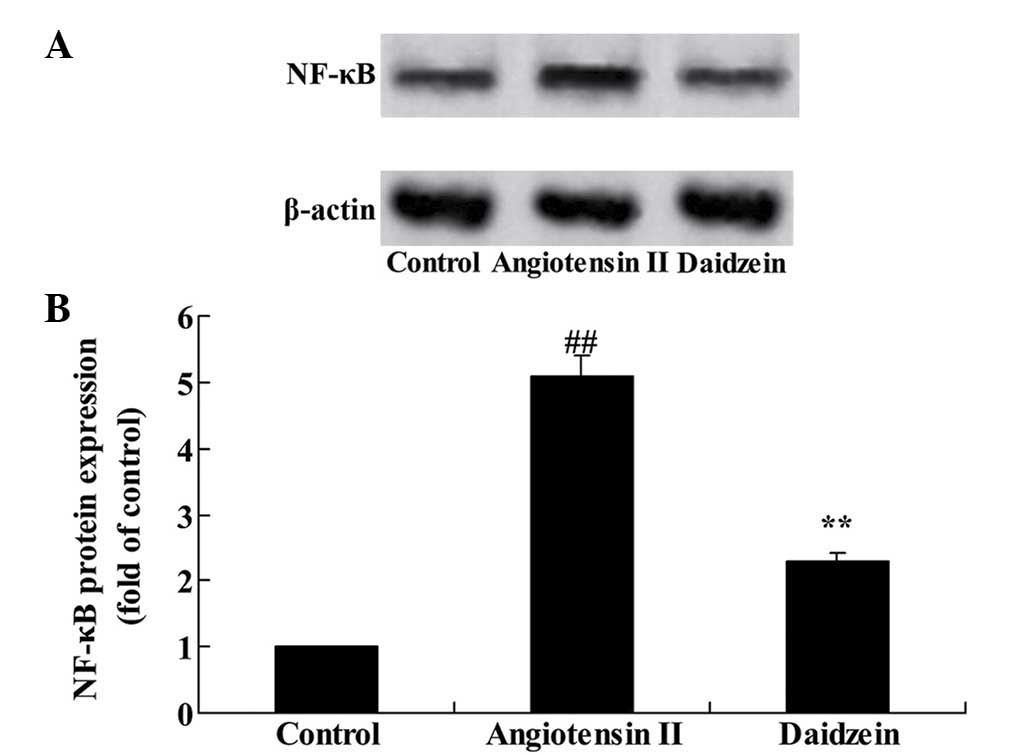
To confirm the mechanism of daidzein on inflammation
in the angiotensin II-induced mice, NF-κB protein expression was
assessed. Western blot analysis results demonstrated that
angiotensin II significantly increased the NF-κB protein expression
in the AAA mice, compared with the control group (Fig. 4; P<0.01). Additional treatment
with daidzein significantly inhibited the activation of NF-κB
protein expression in the AAA mice compared with the angiotensin II
model mice (Fig. 4;
P<0.01).
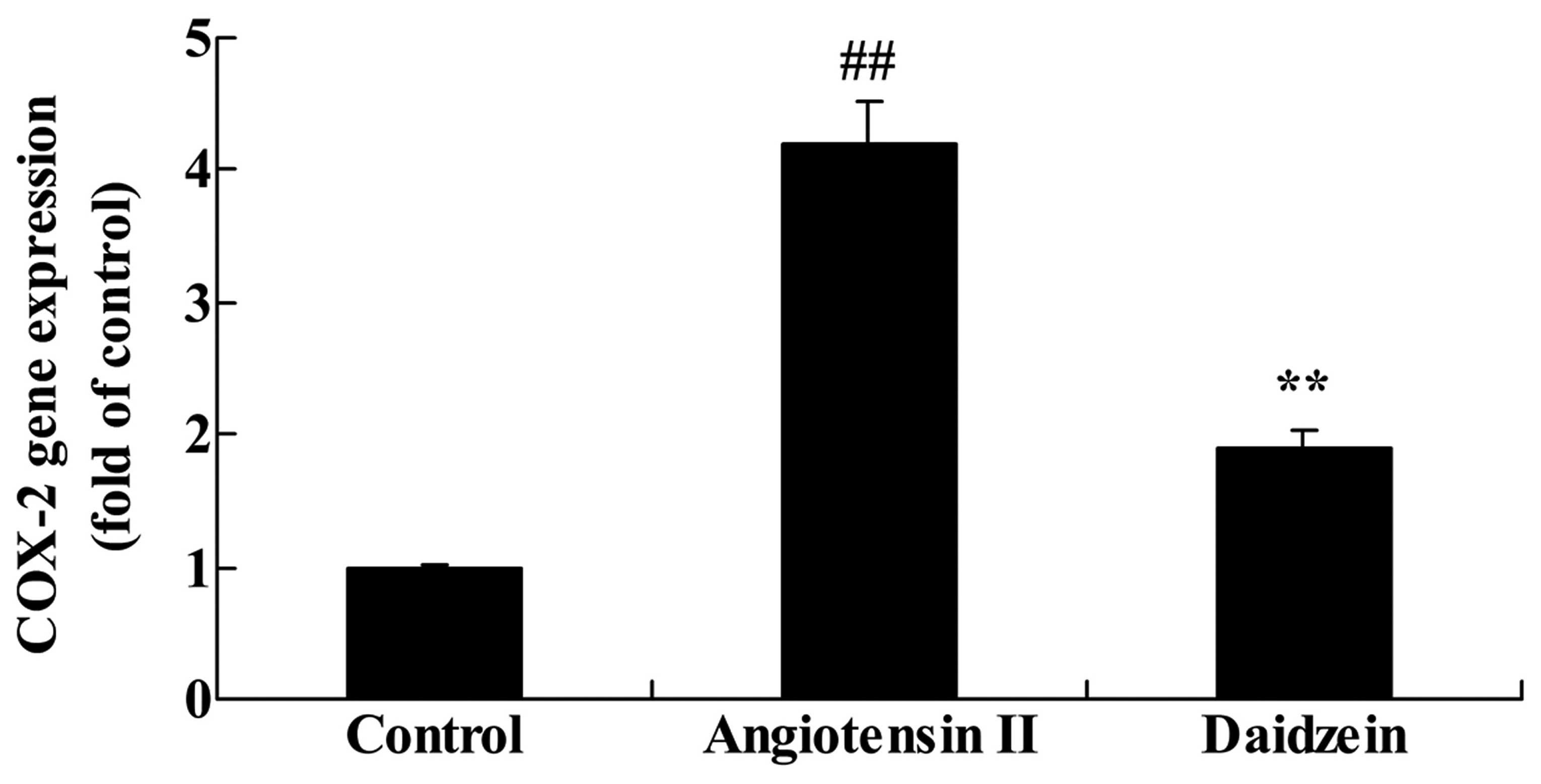
Daidzein affects COX-2 gene expression in
angiotensin II-induced mice
A previous study demonstrated that the suppression
of COX-2 expression has an effect on angiotensin II-induced AAA
mice (18). Therefore, the effect
of daidzein on COX-2 gene expression in angiotensin II-induced mice
was determined. As demonstrated in Fig. 5, COX-2 gene expression was
significantly increased in the angiotensin II-induced AAA mice
compared with the control mice (P<0.01). COX-2 gene expression
in the daidzein-treated mice was significantly suppressed compared
with the angiotensin II model mice (Fig. 5; P<0.01).
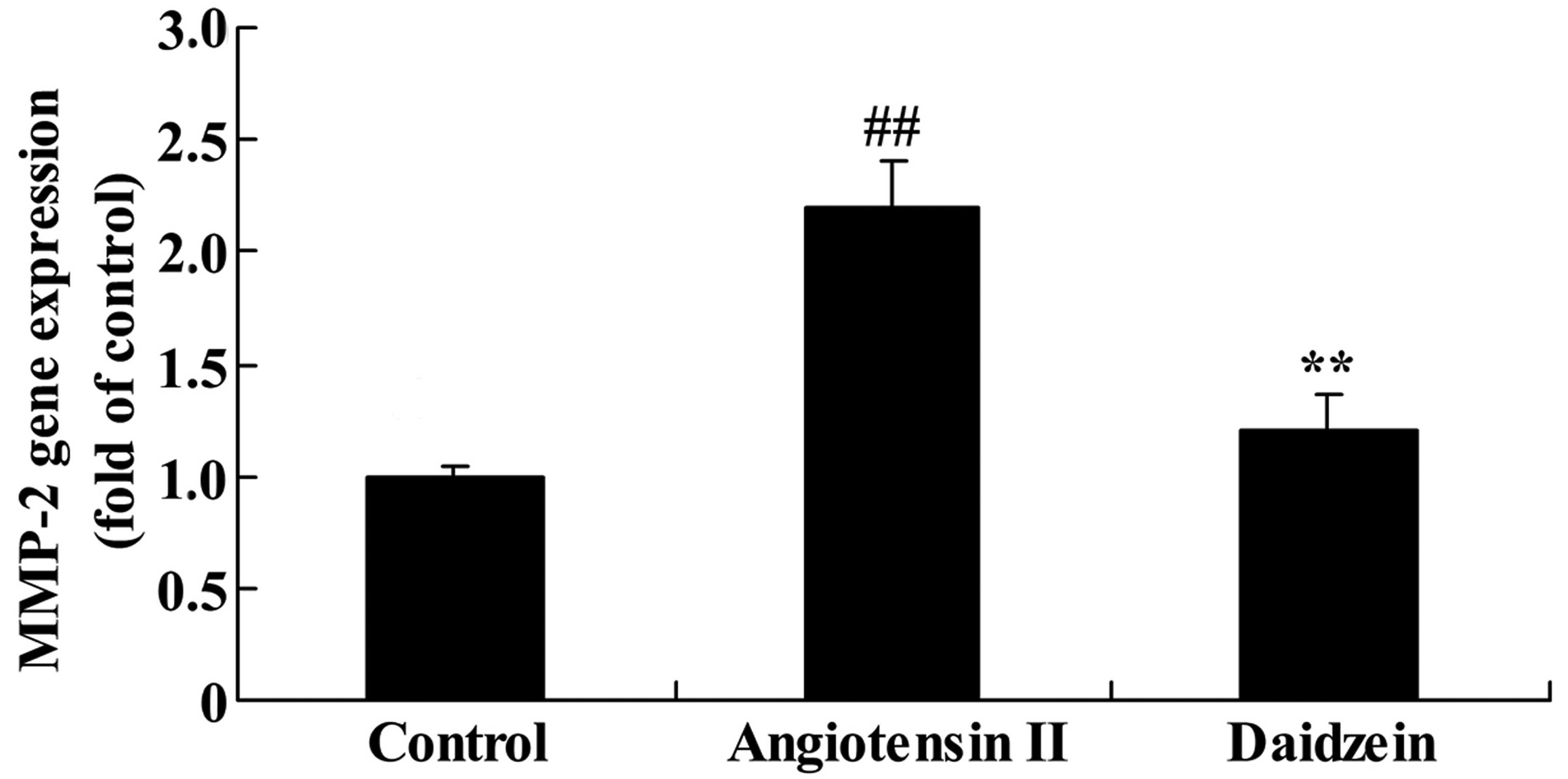
Daidzein affects MMP-2 gene expression in
angiotensin II-induced mice
The mediated inflammation in angiotensin II-induced
mice was assessed and the MMP-2 gene expression was measured using
RT-qPCR analysis. A significant increase was observed in the MMP-2
gene expression in the angiotensin II-induced AAA mice compared
with the control mice (Fig. 6;
P<0.01). Additional treatment with daidzein significantly
suppressed the promotion of MMP-2 gene expression in the
angiotensin II-induced AAA mice compared with the angiotensin II
model mice (Fig. 6;
P<0.01).
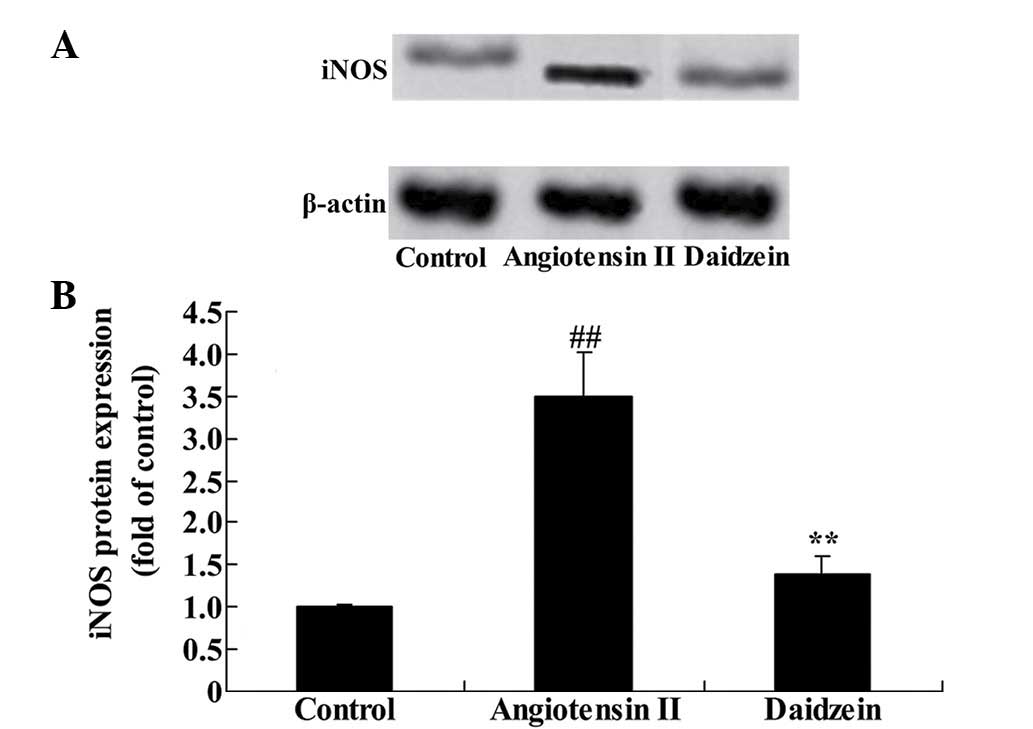
Daidzein affects iNOS protein expression
in angiotensin II-induced mice
Western blot analysis was performed to determine
iNOS protein expression levels in the aortic aneurysmal tissue. A
significant elevation of iNOS protein expression levels was
observed in the angiotensin II-induced AAA mice, compared with the
control mice (Fig. 7; P<0.01).
Administration of daidzein significantly inhibited the increase of
iNOS protein expression compared with the angiotensin II model mice
(Fig. 7; P<0.01).
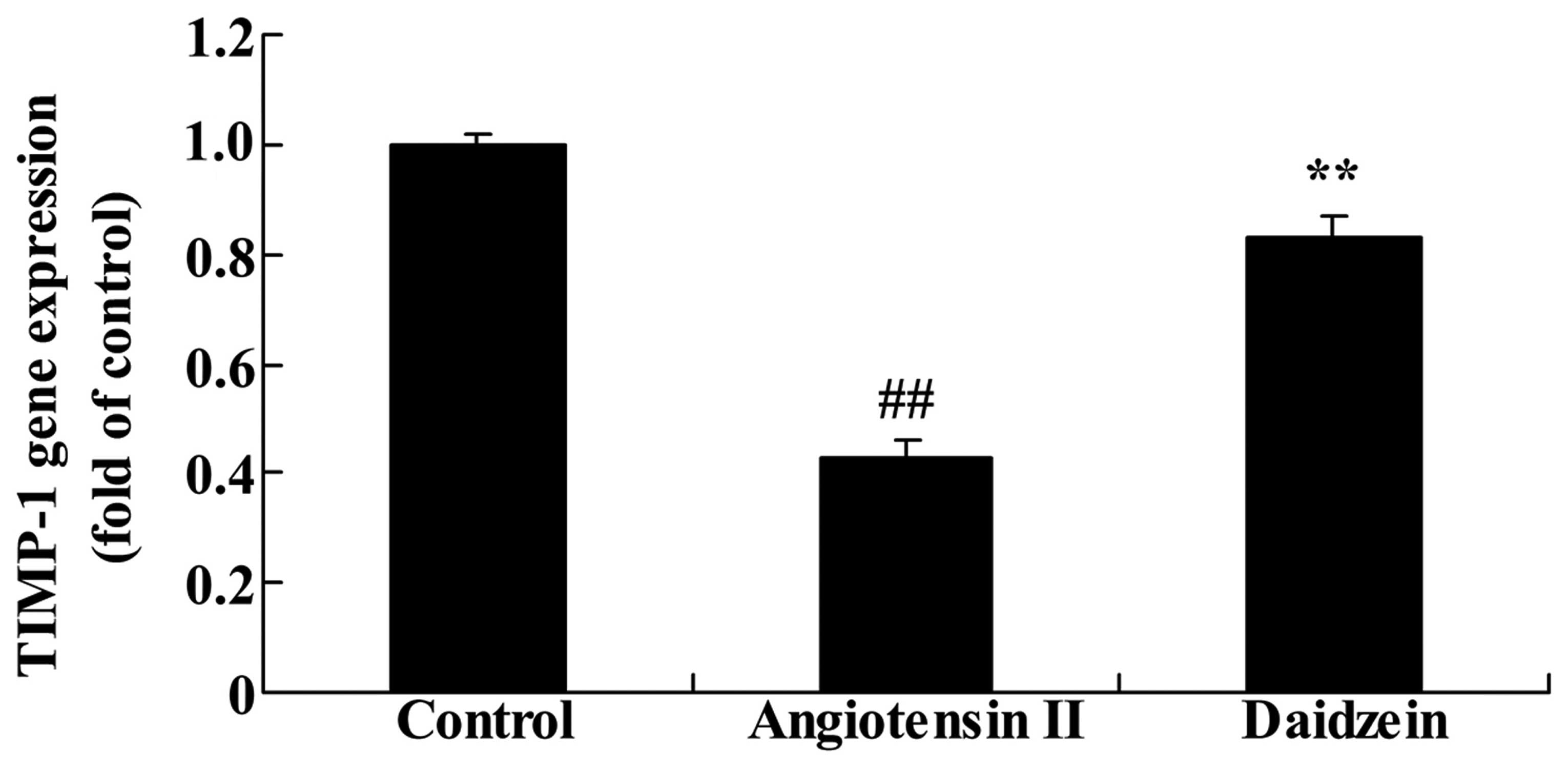
Daidzein affects TIMP-1 gene expression
in angiotensin II-induced mice
RT-qPCR was utilized to assess the effect of
daidzein on TIMP-1 gene expression. As demonstrated in Fig. 8, TIMP-1 gene expression in the
angiotensin II-induced AAA mice was lower than that of the control
mice (P<0.01). Compared with the angiotensin II model mice,
additional treatment with daidzein significantly increased the
suppression of TIMP-1 gene expression in the angiotensin II-induced
AAA mice (Fig. 8; P<0.01).
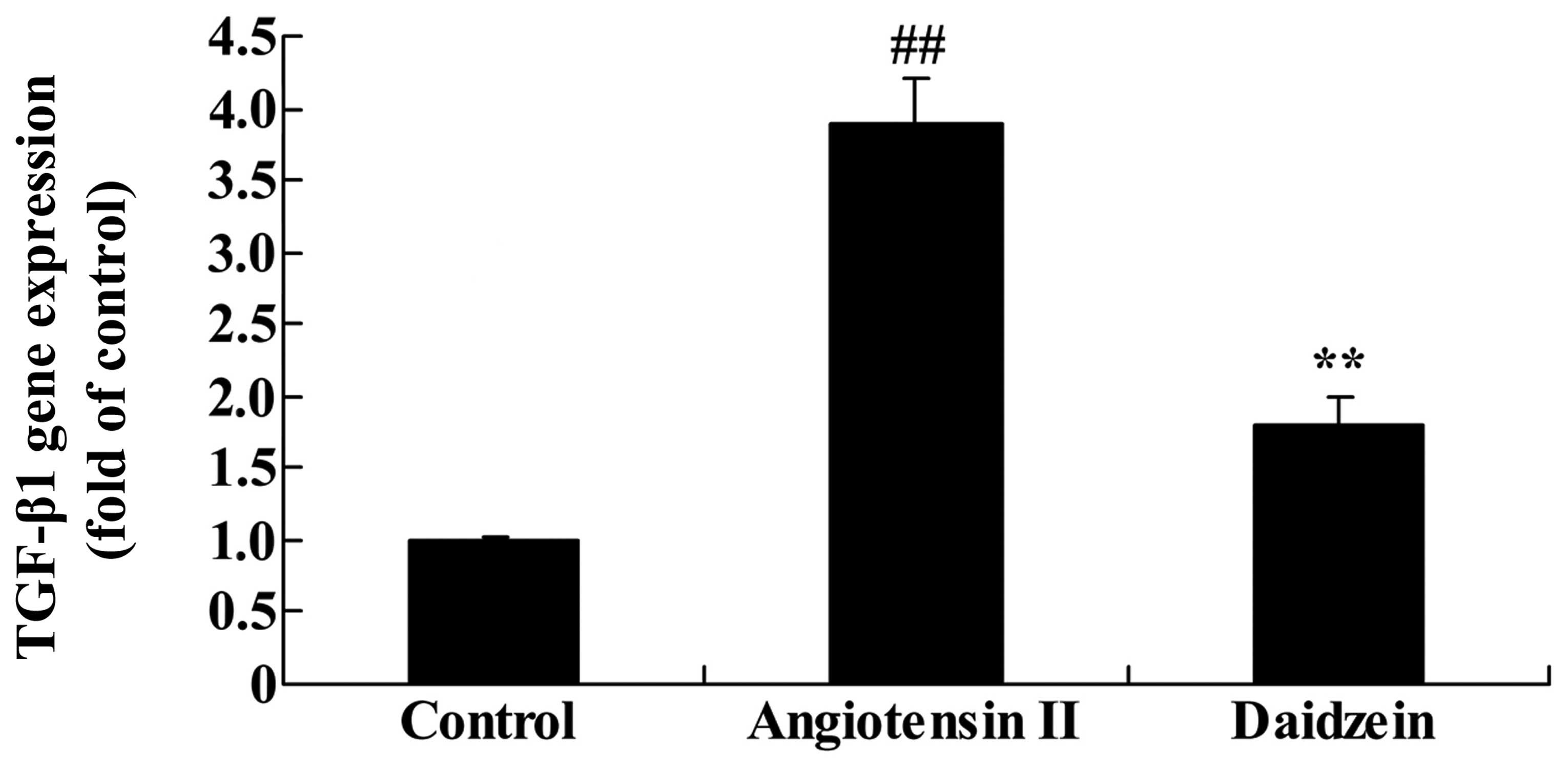
Daidzein affects TGF-β1 gene expression
in angiotensin II-induced mice
RT-qPCR was utilized to assess the effect of
daidzein on TGF-β1 gene expression in the angiotensin II-induced
mice. As demonstrated in Fig. 9,
TGF-β1 gene expression significantly increased in the angiotensin
II-induced AAA mice compared with the control mice. However,
administration of daidzein significantly inhibited the activation
of TGF-β1 gene expression in the angiotensin II-induced AAA mice
compared with the angiotensin II model mice (Fig. 9; P<0.01).
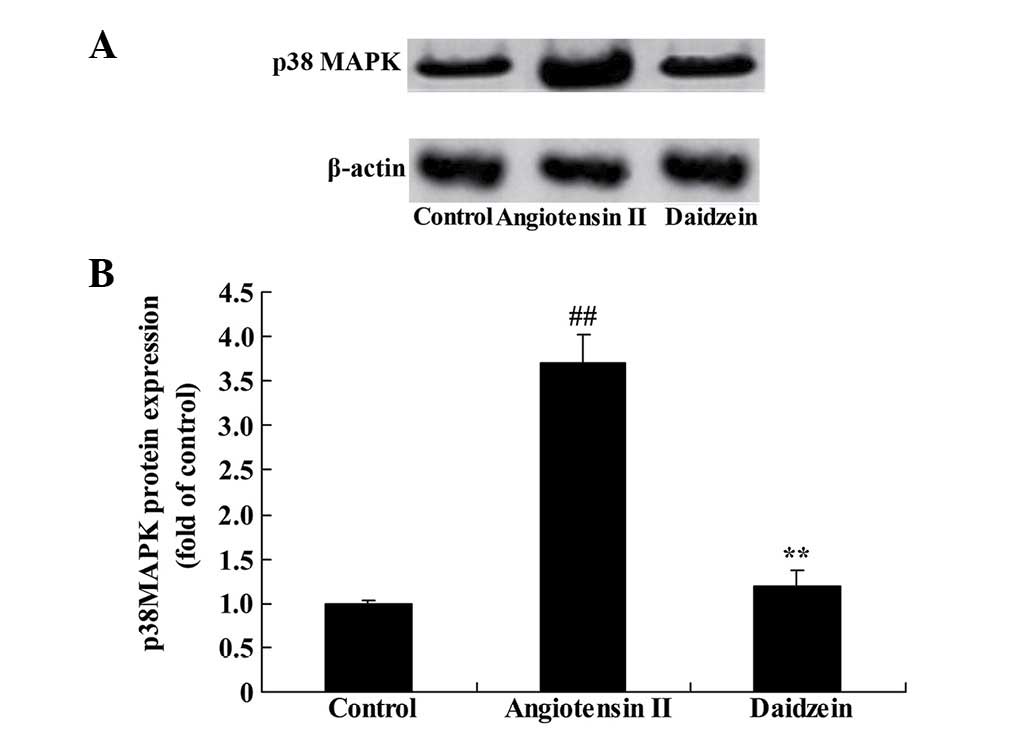
Daidzein affects p38MAPK protein
expression in angiotensin II-induced mice
The expression of p38MAPK protein was assessed to
investigate the role of p38MAPK signaling in the effect of daidzein
on angiotensin II-induced AAA mice. Western blot analysis
demonstrated that the expression of p38MAPK protein in the
angiotensin II-induced AAA mice was significantly higher compared
with the control mice (Fig. 10;
P<0.01). Following daidzein treatment, the activation of p38MAPK
signaling was suppressed in the angiotensin II-induced AAA mice
compared with the angiotensin II model mice (Fig. 10; P<0.01).
Discussion
AAA results from the interaction of various factors,
such as anatomical, genetic, environmental and biochemical factors
(1). Compared with the structure
of suprarenal area of the abdominal aorta, the infrarenal aortic
wall is weak and the partial load is higher, short of nourishing
blood vessels and without adequate blood supply in cases of AAA
(19). The anatomical defect is
one of the basic pathological causes of the AAA disease (20). Previous studies demonstrated that
immune inflammation is a common mechanism of AAA pathogenesis,
which likely promotes the formation of AAA by infiltration of
inflammatory cells, inducing inflammatory cytokines and the
initiation of apoptosis by the MMP family (20–22).
The present study demonstrated that administration of daidzein
significantly attenuated these indexes in the angiotensin
II-induced AAA mice compared with those of model mice. These
results indicate that daidzein may be a novel therapeutic drug for
the treatment and prevention of AAA.
Previous studies have demonstrated that inherent
inflammation and antigen-induced immune responses during chronic
inflammatory response in AAA tissues (21–23).
Monocytes accumulate in the outer membrane of the cell to be
activated and differentiate into macrophages, an important step for
the degradation of the extracellular matrix (5). As a part of a non-specific immune
response, lymphocytes may serve a regulatory role in the activity
of macrophages, and the accumulation of inflammatory cells is a
manifestation of autoimmunity (5).
A previous study conducted cellular localization of MMPs by
staining anti-MMP-2, -3 and -9 in human AAA specimens, and the
results demonstrated that MMP-2 and -9 were located in macrophages,
which have strong matrix solubility (24). Previous studies indicated similar
results, that MMP-2 and MMP-9 serve important roles in the
pathogenesis of AAA, and the positive cells of MMPs are mainly
localized in macrophages (25,26).
These data suggest that the infiltration of macrophages is vital
for the early activity (elastin damage to the arterial wall) of AAA
formation (27). As the
infiltration degree of inflammatory cells is gradually increased,
MMP species are increased with the activity, damage of elastic and
collagen fibers, and the dilation extent of the abdominal aorta.
The elastin degradation products produced by the degrading
extracellular matrix due to the MMPs, may lead to further
chemotaxis of mononuclear macrophages that are involved in
inflammation (6). The present
study demonstrated that daidzein treatment inhibited the promotion
of TNF-α and IL-1β serum levels, and activation of NF-κB protein
expression in the angiotensin II-induced AAA mice. These results
are consistent with Khan et al (28), who suggested that daidzein
inhibited the 12-O-tetradecanoylphorbol-13-acetate-induced
cutaneous inflammation through the NF-κB and COX-2 expression.
MMPs are predominantly produced by macrophages, T or
B lymphocytes, fibroblasts and smooth muscle cells. Macrophages
generate MMPs depending on the role of prostaglandin E2 (PGE2),
that is synthesized by the arachidonic acid under the effect of a
series of enzymes, in which COX-2 is the rate-limiting enzyme. PGE2
inhibits COX-2, thus inhibiting the generation of MMPs (6,29).
Previous studies have demonstrated that inflammatory cytokines are
associated with the vascular response to injury and
atherosclerosis. Numerous inflammatory mediators, such as PGE2,
IL-1, IL-6, IL-8, TNF-α and nitric oxide, have direct or indirect
angiogenic activity (18,30–32).
The results of the present study demonstrated that administration
of daidzein significantly suppressed COX-2 and MMP-2 relative gene
expression levels, and inhibited iNOS protein expression levels in
the angiotensin II-induced AAA mice. Choi et al (15) demonstrated that daidzein inhibited
inflammation by suppressing iNOS protein expression.
TIMPs are endogenous low-molecular-weight proteis,
hydrolytic enzymes in the degradation of the extracellular matrix
and endogenous inhibitors of MMPs, serving a role in various
pathophysiological processes of the body (33). TIMPs serve a role in cardiac
remodeling, myocardial ischemia-reperfusion injury and mediating
the remodeling of external cardiac extracellular matrix (34). TIMPs are protease inhibitors
secreted by the cells in the body, which may be expressed in the
sarcomere of myocardial cells, thin filaments and stromal cells,
and may be additionally expressed in tumor cells (35). TIMPs are a family of
multi-functional factors, that regulate the activity of MMPs in
vivo, as TIMP-1 may be bound to numerous MMPs, including MMP-1,
3 and 9 (10). The present study
demonstrated that daidzein significantly increased the suppression
of the TIMP-1 gene expression in the angiotensin II-induced AAA
mice. Soumyakrishnan et al (17) indicated that daidzein exhibits an
anti-fibrotic effect by reducing the expression of TIMP-1 and
TGF-β1 in Bleomycin-induced experimental pulmonary fibrosis. These
results demonstrate that the TIMP signaling pathway serves an
important role in the effect of daidzein on AAA.
TGF exists widely in the body, regulating cell
growth and differentiation. In the cardiovascular system,
predominantly TGF-β1 promotes matrix synthesis and secretion
(24). Although previous studies
suggested that overexpression of TGF-β1 and apoptosis of smooth
muscle are increased simultaneously, TGF-β1 has been demonstrated
to exhibit different effects in smooth muscle cells. TGF-β1
inhibited apoptosis in cultured bovine aortic smooth muscle cells,
however triggered apoptosis in the cultured rat aortic smooth
muscle cells (36–38). This effect may be due to the TGF-β1
regulation of the apoptosis of smooth muscle cells dependent on the
local microenvironment and the concentration of TGF-β1 (39). In addition, the cytotoxic medium
generated by the infiltrated T lymphocytes in the aortic aneurysm
results in the apoptosis of smooth muscle cells, thus TGF-β1 may
regulate apoptosis by influencing the degree of inflammatory
infiltration in the wall of the aneurysm (38,39).
Similar to the results of previous studies, the results of the
current study demonstrated that daidzein significantly inhibited
the activation of TGF-β1 gene expression in the angiotensin
II-induced AAA mice (40,41). The results of the present study
demonstrated that the TGF-β1 signaling pathway induced by daidzein
serves an important role in the treatment of AAA.
In the p38/MAPK signaling pathway, the phosphokinase
p38MAPK mediates the proliferation and migration of vascular smooth
muscle cells (VSMCs), and the hypertrophy of VSMCs and the
deposition of extracellular matrix may be the required signaling
pathway for hypertensive vascular remodeling (42). Protein kinase 1 activated by
TGF-β1, combined with protein, may lead to the autophosphorylation
of p38MAPK, to activate the p38/MAPK signaling pathway (9). Therefore, TGF-β1 and p38MAPK serve
important roles in the occurrence and development of hypertension
(9,42). The results of the current study
demonstrated that daidzein significantly suppressed the activation
of p38MAPK signaling in the angiotensin II-induced AAA mice. Lim
et al (14) demonstrated
that daidzein is a novel inhibitor of protein kinase α by
suppressing the solar ultraviolet-induced MMP-1 through p38. Thus,
these data suggest that p38 signaling associates with the effect of
daidzein on AAA.
In conclusion, the results of the current study
demonstrated that the anti-inflammatory effects and inhibitory
mechanism of daidzein attenuate AAA in the angiotensin II-induced
mice. The anti-inflammatory effect of daidzein was associated with
NF-κB, COX-2, MMP-2 and iNOS expression. In addition, the results
suggested that suppression of TIMP, TGF-β1 and p38MAPK signaling
promotes the effect of daidzein in AAA. Daidzein had a strong
anti-inflammatory activity and affects various mechanism pathways,
including NF-κB, p38MAPK and TGF-β1, suggesting it may be a novel
therapeutic target for the treatment of AAA formation.
References
|
1
|
Gyoten T, Doi T, Yamashita A, Fukahara K,
Kotoh K and Yoshimura N: Ruptured abdominal aortic aneurysm and
aortoiliac vein fistula. Asian Cardiovasc Thorac Ann. 23:449–451.
2015. View Article : Google Scholar
|
|
2
|
Knops AM, Goossens A, Ubbink DT, Balm R,
Koelemay MJ, Vahl AC, de Nie AJ, van den Akker PJ, Willems MC,
Koedam NA, et al: A decision aid regarding treatment options for
patients with an asymptomatic abdominal aortic aneurysm: A
randomised clinical trial. Eur J Vasc Endovasc Surg. 48:276–283.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonfreschi V, Giuliani E, Malagnino FC,
Navi A, Coppi G, Silingardi R, D'Amico R and Barbieri A: Analgesia
during abdominal aortic aneurysm endovascular repair: Remifentanil
vs. fentanyl-midazolam - a randomized controlled trial. Eur J
Anaesthesiol. 26:782–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Candell L, Tucker LY, Goodney P, Walker J,
Okuhn S, Hill B and Chang R: Early and delayed rupture after
endovascular abdominal aortic aneurysm repair in a 10-year
multicenter registry. J Vasc Surg. 60:1146–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choke E, Cockerill GW, Laing K, Dawson J,
Wilson WR, Loftus IM and Thompson MM: Whole genome-expression
profiling reveals a role for immune and inflammatory response in
abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg.
37:305–310. 2009. View Article : Google Scholar
|
|
6
|
Watanabe A, Ichiki T, Sankoda C, Takahara
Y, Ikeda J, Inoue E, Tokunou T, Kitamoto S and Sunagawa K:
Suppression of abdominal aortic aneurysm formation by inhibition of
prolyl hydroxylase domain protein through attenuation of
inflammation and extracellular matrix disruption. Clin Sci (Lond).
126:671–678. 2014. View Article : Google Scholar
|
|
7
|
Duellman T, Warren CL, Peissig P, Wynn M
and Yang J: Matrix metalloproteinase-9 genotype as a potential
genetic marker for abdominal aortic aneurysm. Circ Cardiovasc
Genet. 5:529–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henriksen NA, Sorensen LT, Jorgensen LN
and Lindholt JS: Lack of association between inguinal hernia and
abdominal aortic aneurysm in a population-based male cohort. Br J
Surg. 100:1478–1482. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi Y, Maki T, Liang AC, Itoh K, Lok
J, Osumi N and Arai K: p38 MAP kinase mediates transforming-growth
factor-β1-induced upregulation of matrix metalloproteinase-9 but
not -2 in human brain pericytes. Brain Res. 1593:1–8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yurube T, Takada T, Suzuki T, Kakutani K,
Maeno K, Doita M, Kurosaka M and Nishida K: Rat tail static
compression model mimics extracellular matrix metabolic imbalances
of matrix metalloproteinases, aggrecanases, and tissue inhibitors
of metalloproteinases in intervertebral disc degeneration.
Arthritis Res Ther. 14:R512012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown LC, Thompson SG, Greenhalgh RM and
Powell JT: Endovascular Aneurysm Repair trial participants:
Incidence of cardiovascular events and death after open or
endovascular repair of abdominal aortic aneurysm in the randomized
EVAR trial 1. Br J Surg. 98:935–942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walsh SR, Sadat U, Boyle JR, Tang TY,
Lapsley M, Norden AG and Gaunt ME: Remote ischemic preconditioning
for renal protection during elective open infrarenal abdominal
aortic aneurysm repair: Randomized controlled trial. Vasc
Endovascular Surg. 44:334–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morbelli S, Ghigliotti G, Spinella G,
Marini C, Bossert I, Cimmino M, Pane B, Rousas N, Cittadini G,
Massollo M, et al: Systemic vascular inflammation in abdominal
aortic aneurysm patients: A contrast-enhanced PET/CT study. Q J
Nucl Med Mol Imaging. 58:299–309. 2014.PubMed/NCBI
|
|
14
|
Lim TG, Kim JE, Lee SY, Park JS, Yeom MH,
Chen H, Bode AM, Dong Z and Lee KW: The daidzein metabolite,
6,7,4′-Trihydroxyisoflavone, is a novel inhibitor of PKCα in
suppressing solar UV-induced matrix metalloproteinase 1. Int J Mol
Sci. 15:21419–21432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi EY, Jin JY, Lee JY, Choi JI, Choi IS
and Kim SJ: Anti-inflammatory effects and the underlying mechanisms
of action of daidzein in murine macrophages stimulated with
Prevotella intermedia lipopolysaccharide. J Periodontal Res.
47:204–211. 2012. View Article : Google Scholar
|
|
16
|
Zheng YH, Li FD, Tian C, Ren HL, Du J and
Li HH: Notch γ-secretase inhibitor dibenzazepine attenuates
angiotensin II-induced abdominal aortic aneurysm in ApoE knockout
mice by multiple mechanisms. PLoS One. 8:e833102013. View Article : Google Scholar
|
|
17
|
Soumyakrishnan S, Divya T, Kalayarasan S,
Sriram N and Sudhandiran G: Daidzein exhibits anti-fibrotic effect
by reducing the expressions of Proteinase activated receptor 2 and
TGFβ1/smad mediated inflammation and apoptosis in Bleomycin-induced
experimental pulmonary fibrosis. Biochimie. 103:23–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
King VL, Trivedi DB, Gitlin JM and Loftin
CD: Selective cyclooxygenase-2 inhibition with celecoxib decreases
angiotensin II-induced abdominal aortic aneurysm formation in mice.
Arterioscler Thromb Vasc Biol. 26:1137–1143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iezzi R, Cotroneo AR, Filippone A, Di
Fabio F, Santoro M and Storto ML: MDCT angiography in abdominal
aortic aneurysm treated with endovascular repair: Diagnostic impact
of slice thickness on detection of endoleaks. AJR Am J Roentgenol.
189:1414–1420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dawson JA, Choke E, Loftus IM, Cockerill
GW and Thompson MM: A randomised placebo-controlled double-blind
trial to evaluate lipid-lowering pharmacotherapy on proteolysis and
inflammation in abdominal aortic aneurysms. Eur J Vasc Endovasc
Surg. 41:28–35. 2011. View Article : Google Scholar
|
|
21
|
Peshkova IO, Schaefer G and Koltsova EK:
Atherosclerosis and Aortic Aneurysm: Is inflammation a common
denominator? FEBS J. Dec 24–2015.ePub ahead of print.
|
|
22
|
Fu XM, Yamawaki-Ogata A, Oshima H, Ueda Y,
Usui A and Narita Y: Intravenous administration of mesenchymal stem
cells prevents angiotensin II-induced aortic aneurysm formation in
apolipoprotein E-deficient mouse. J Transl Med. 11:1752013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arnaoutoglou E, Kouvelos G, Papa N,
Koulouras V, Milionis H and Matsagkas M: Regarding 'the impact of
endograft type on inflammatory response after endovascular
treatment of abdominal aortic aneurysm'. J Vasc Surg. 58:5702013.
View Article : Google Scholar
|
|
24
|
Gao F, Chambon P, Offermanns S, Tellides
G, Kong W, Zhang X and Li W: Disruption of TGF-β signaling in
smooth muscle cell prevents elastase-induced abdominal aortic
aneurysm. Biochem Biophys Res Commun. 454:137–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciavarella C, Alviano F, Gallitto E, Ricci
F, Buzzi M, Velati C, Stella A, Freyrie A and Pasquinelli G: Human
Vascular Wall Mesenchymal Stromal Cells Contribute to Abdominal
Aortic Aneurysm Pathogenesis Through an Impaired Immunomodulatory
Activity and Increased Levels of Matrix Metalloproteinase-9. Circ
J. 79:1460–1469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dilmé JF, Bellmunt S, Camacho M,
Solà-Villà D, Romero JM, Escudero JR and Vila L: Influence of
cardiovascular risk factors on levels of matrix metalloproteinases
2 and 9 in human abdominal aortic aneurysms. Eur J Vasc Endovasc
Surg. 48:374–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pay S, Abbasov T, Erdem H, Musabak U,
Simsek I, Pekel A, Akdogan A, Sengul A and Dinc A: Serum MMP-2 and
MMP-9 in patients with Behçet's disease: Do their higher levels
correlate to vasculo-Behçet's disease associated with aneurysm
formation? Clin Exp Rheumatol. 25:S70–75. 2007.PubMed/NCBI
|
|
28
|
Khan AQ, Khan R, Rehman MU, Lateef A,
Tahir M, Ali F and Sultana S: Soy isoflavones (daidzein &
genistein) inhibit 12-O-tetradecanoylphorbol-13-acetate
(TPA)-induced cutaneous inflammation via modulation of COX-2 and
NF-κB in Swiss albino mice. Toxicology. 302:266–274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Armstrong PJ, Franklin DP, Carey DJ and
Elmore JR: Suppression of experimental aortic aneurysms: Comparison
of inducible nitric oxide synthase and cyclooxygenase inhibitors.
Ann Vasc Surg. 19:248–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luehmann HP, Detering L, Fors BP, Pressly
ED, Woodard PK, Randolph GJ, Gropler RJ, Hawker C and Liu Y: PET/CT
Imaging of Chemokine Receptors in Inflammatory Atherosclerosis
Using Targeted Nanoparticles. J Nucl Med. Jan 21–2016.ePub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rohm I, Atiskova Y, Drobnik S,
Fritzenwanger M, Kretzschmar D, Pistulli R, Zanow J, Krönert T,
Mall G, Figulla HR and Yilmaz A: Decreased regulatory T cells in
vulnerable atherosclerotic lesions: Imbalance between pro- and
anti-inflammatory cells in atherosclerosis. Mediators Inflamm.
2015:3647102015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eo HS, Lee KB, Kim AK, Kim MH, Kim DH and
Kim DI: Association with inflammatory cells and apolipoproteins to
the progression of atherosclerosis. J Korean Surg Soc. 80:289–296.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khan JA, Abdul Rahman MN, Mazari FA,
Shahin Y, Smith G, Madden L, Fagan MJ, Greenman J, McCollum PT and
Chetter IC: Intraluminal thrombus has a selective influence on
matrix metalloproteinases and their inhibitors (tissue inhibitors
of matrix metalloproteinases) in the wall of abdominal aortic
aneurysms. Ann Vasc Surg. 26
|
|
34
|
Takawale A, Fan D, Basu R, Shen M,
Parajuli N, Wang W, Wang X, Oudit GY and Kassiri Z: Myocardial
recovery from ischemia-reperfusion is compromised in the absence of
tissue inhibitor of metalloproteinase 4. Circ Heart Fail.
7:652–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang W, Han Y, Meng G, Bai W, Xie L, Lu
H, Shao Y, Wei L, Pan S, Zhou S, et al: Direct renin inhibition
with aliskiren protects against myocardial ischemia/reperfusion
injury by activating nitric oxide synthase signaling in
spontaneously hypertensive rats. J Am Heart Assoc. 3:e0006062014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang W, Yang J, Zhang F, Guo H, Peng F and
Wang X: Activation of extracellular signal-regulated kinase 1/2 and
Sp1 may contribute to the expression of tissue inhibitor of
metal-loproteinases-1 induced by transforming growth factor-β1 in
human pulmonary arterial smooth muscle cells. Cytotherapy.
16:225–233. 2014. View Article : Google Scholar
|
|
37
|
Gao YD, Zheng JW, Li P, Cheng M and Yang
J: Store-operated Ca2+ entry is involved in transforming growth
factor-β1 facilitated proliferation of rat airway smooth muscle
cells. J Asthma. 50:439–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Golledge J, Clancy P, Jones GT, Cooper M,
Palmer LJ, van Rij AM and Norman PE: Possible association between
genetic polymorphisms in transforming growth factor beta receptors,
serum transforming growth factor beta1 concentration and abdominal
aortic aneurysm. Br J Surg. 96:628–632. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dai J, Losy F, Guinault AM, Pages C,
Anegon I, Desgranges P, Becquemin JP and Allaire E: Overexpression
of transforming growth factor-beta1 stabilizes already-formed
aortic aneurysms: A first approach to induction of functional
healing by endovascular gene therapy. Circulation. 112:1008–1015.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang CK, Luo J, Lai KP, Wang R, Pang H,
Chang E, Yan C, Sparks J, Lee SO, Cho J and Chang C: Androgen
receptor promotes abdominal aortic aneurysm development via
modulating inflammatory interleukin-1α and transforming growth
factor-β1 expression. Hypertension. 66:881–891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thompson AR, Cooper JA, Jones GT, Drenos
F, van Bockxmeer FM, Biros E, Walker PJ, van Rij AM, Golledge J,
Norman PE, et al: Assessment of the association between genetic
polymorphisms in transforming growth factor beta, and its binding
protein (LTBP), and the presence, and expansion, of Abdominal
Aortic Aneurysm. Atherosclerosis. 209:367–373. 2010. View Article : Google Scholar
|
|
42
|
Yang CQ, Li W, Li SQ, Li J, Li YW, Kong
SX, Liu RM, Wang SM and Lv WM: MCP-1 stimulates MMP-9 expression
via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth
muscle cells. Cell Physiol Biochem. 34:266–276. 2014. View Article : Google Scholar : PubMed/NCBI
|