Introduction
Vitiligo is an acquired multifactorial depigmenting
disorder characterized by the appearance of white macules in the
skin in response to the progressive loss of functional melanocytes
within the epidermis (1). A number
of types of vitiligo are distinguished according to the
distribution patterns of the lesions. Generalized vitiligo (GV) is
the predominant type and is characterized by multiple scattered
lesions that form a symmetrical distribution pattern. GV is often
progressive and patients exhibit phases of stabilized
depigmentation, an extending vitiligo with enlarging lesions, or
the development of new lesions, which is defined as active vitiligo
(2). Three major hypotheses have
been proposed to explain the pathogenesis of vitiligo: Autoimmune
injury, oxidative stress and the neural hypothesis. GV is commonly
hypothesized to arise in response to autoimmune injury and is the
type of vitiligo that was focused on in the present study. Two
additional dysfunctions of the immune system have been proposed to
result in the development of autoimmune-associated vitiligo, a T
cell-based pathomechanism in cases of localized disease and T
cells, NK cells and antibodies have been implicated in the
pathogenesis of diffuse vitiligo (3).
Hydroxychloroquine (HCQ) is an antimalarial compound
that has been demonstrated as an effective immunosuppressive
therapeutic agent, it is widely applied in the treatment of
systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA)
(4). Considering the high
protein-binding capacity of HCQ (4), the present study aimed to determine
whether HCQ may directly affect the formation of antigen-antibody
complexes and whether disturbed autoantibodies mediate either
complement-dependent cytotoxicity (CDC) and/or antibody-dependent
cell-mediated cytotoxicity (ADCC) activity. The present study
measured the effects of self-reactive antibodies in progressive GV
patients and investigated the protection offered by HCQ in reducing
antigen-antibody complex binding.
Materials and methods
Ethics statement
The present study was approved by the ethics
committee of The General Hospital of Air Force (Beijing, China).
All participants provided written informed consent prior to
participation in the study.
Patients
The present study included 32 progressive GV
patients who were recruited based on the following criteria: i) A
clinical diagnosis of GV in accordance with associated criteria
(5); ii) no use of any systemic
therapy for ≥4 weeks prior to enrollment in the study; and iii) the
development of new lesions within the 3 months preceding study
enrollment. Unaffected individuals (n=27) were recruited as
controls. The clinical characteristics of the patients and controls
are summarized in Table I.
 | Table IClinical characteristic of vitiligo
patients and healthy controls. |
Table I
Clinical characteristic of vitiligo
patients and healthy controls.
| Characteristic | Healthy controls | GV patients |
|---|
| Sex
(male/female) | 12/15 | 16/16 |
| Age, median
(range) | 30 (24–63) | 25 (9–61) |
| Months since
diagnosis median (range) | ND | 54 (4–360) |
| Intensity %, median
(range) | ND | 15 (0.1–80) |
Peripheral blood samples
For serological studies, 10 ml of peripheral blood
was drawn from each subject and stored at −80°C. Human peripheral
blood mononuclear cells (PBMCs) were isolated from the heparinized
blood of healthy volunteer donors via centrifugation over a
Ficoll-Hypaque density gradient (TBD Science, Tianjin, China).
Cell culture and propagation
Human primary melanocytes (HMCs) and human
keratinocytes (HKCs) were obtained from the unaffected skin of each
patient. Fresh skin biopsies were rinsed 3 times in
phosphate-buffered saline (PBS; pH 7.4) containing antibiotics
(5,000 U/ml penicillin and 5 mg/ml streptomycin), cut into small
pieces (5×5 mm), and incubated for 14 h at 4°C in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing dispase (2 mg/ml; Roche Diagnostics,
Basel, Switzerland). The epidermis was separated from the dermis
using sterile forceps. The cells of the epidermis were then
isolated using 0.05% trypsin with 2 mM EDTA (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). The protocols used to culture HMCs and
HKCs were slightly modified from our previously described method
(6). Briefly, cells were adjusted
to 2.5×104 cells/cm2 following washing in PBS
and then cultured in either M-254 medium (Gibco; Thermo Fisher
Scientific, Inc.) for HMCs or EpiLife medium (Gibco; Thermo Fisher
Scientific, Inc.) for HKCs. The cell cultures were maintained at
37°C in a humidified 5% CO2 atmosphere and the medium
was changed at 3–4 day intervals. When cells reached 70–90%
confluence they were split using trypsin-EDTA (Nanjing KeyGen
Biotech Co., Ltd.) treatment for 5 min at room temperature and
inactivated with fetal bovine serum (TBD Science). HMC and HKC
populations were considered pure following 3–4 passages.
Cell-based ELISA
HMCs were detached and seeded on 96-well cell
culture plates at a density of 40,000 cells/well. Following
incubation for 24 h, cells were fixed in 4% paraformaldehyde (PFA)
for 15 min at room temperature. Fixed cells were then washed 3
times with PBS and blocked with 100 µl 5% bovine serum
albumin (BSA; Solarbio Science & Technology Co., Ltd., Beijing,
China) in PBS for 1 h at 37°C. Following this, supernatants were
removed and the cells were incubated with 100 µl diluted
serum obtained from healthy volunteers or from vitiligo patients
(diluted 1:10 in 5% BSA in PBS) at 37°C for 1 h. The plates were
washed 3 times with PBS and incubated with horseradish peroxidase
(HRP)-conjugated goat anti-human immunoglobulin G (IgG) heavy and
light chains (H+L) antibody (cat. no. ZB-2304; ZSGB-BIO, Beijing,
China; dilution 1:10,000 in PBS with 5% BSA) for 1 h at 37°C.
Following washing three times in PBS, the results were visualized
using tetramethylbenzidin substrate solution (Beyotime Institute of
Biotechnology, Haimen, China). The enzymatic reaction was then
arrested with sulfuric acid and the intensity of the resulting
color was determined by measuring absorbance at a wavelength of 450
nm (A450) using microplate reader (RT-600; Rayto,
Shenzhen, China). The cutoff value for a positive reaction was
based on the A450 values obtained from 12 healthy
controls and calculated as the mean +2.0x standard deviation.
Indirect immunofluorescence assay
HMCs and HMKs were seeded onto glass coverslips in
6-well culture plates and fixed with 4% PFA for 10 min at room
temperature. The cells were then washed with PBS and blocked with
5% BSA in PBS for 30 min at 37°C. Following blocking, the cells
were stained with either patient or control serum (diluted 1:10 in
5% BSA in PBS) or the mouse anti-CK (pan) monoclonal antibody (cat.
no. ZN-0069; diluted 1:100 in 5% BSA in PBS; ZSGB-BIO) for 30 min
at 37°C. Cells were then washed and incubated with either
fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG
(H+L) antibody (cat. no. ZF-0308; 1:200; ZSGB-BIO) or
tetramethylrhodamine-conjugated goat anti-mouse IgG (H+L) antibody
(cat. no. ZF-0313; 1:200; ZSGB-BIO) for 30 min at 37°C. Following
three washes in PBS, the cells were observed by fluorescence
microscopy.
Detecting autoantibodies using western
blotting
HMCs and HKCs were grown in 100-mm culture dishes
and protein extraction was extracted using RIPA lysis buffer on ice
for 10 min [150 mM NaCl, 1% Triton X-100, 50 mM Tris-HCl (pH 7.4),
0.5% sodium dezoxycholate, 0.1% SDS and sodium orthovanadate,
sodium fluoride, EDTA (pH 7.4); Applygen Technologies, Inc.,
Beijing, China] containing protease inhibitors (Applygen
Technology, Inc.). The total protein concentration of the lysate
was estimated using a BCA protein assay kit (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). The extracts
were mixed with loading buffer (Applygen Technologies, Inc.) and
incubated at 100°C for 10 min, following which they were
electrophoresed on a 10% SDS-polyacrylamide gel. A 20 µg
aliquot of each sample was loaded in each lane. The separated
proteins were electrotransferred onto a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA), which was then
blocked using 5% BSA in Tris-buffered saline and Tween 20 (TBST; pH
7.4). The membrane was probed with either control or patient serum
(diluted 1:25 in 5% BSA in TBST) or a mouse anti-β-actin monoclonal
antibody (cat. no. TA-09; diluted 1:1,000 in 5% BSA in TBST;
ZSGB-BIO), overnight at 4°C. The blot was then incubated with
either an HRP-conjugated goat anti-human IgG monoclonal antibody
(ZSGB-BIO) or an HRP-conjugated goat anti-mouse IgG monoclonal
antibody (ZSGB-BIO) at a dilution of 1:20,000 (in 5% BSA in TBST)
for 1 h at room temperature. The signals were detected using
EnlightTM Western Blot Detection Reagents (Engreen Biosystem New
Zealand Ltd., Auckland, New Zealand) followed by exposure to X-ray
film (Kodak, Rochester, NY USA). The results were analyzed using
Quantity One 4.62 software.
Separation and purification of IgG
Positive serum samples were identified based on the
results obtained from the cell-based ELISA. Serum IgG fractions
(isolated from 12 positive patients and normal controls) were
enriched by repeated ammonium sulfate precipitation and further
purified by diethylaminoethyl cellulose-52 exchange chromatography
(Whatman; GE Healthcare Life Sciences, Little Chalfont, UK).
Following centrifugal ultrafiltration at 4,000 × g, for 15 min at
4°C, IgG fractions were concentrated ~40 mg/ml and stored at
−80°C.
Cytotoxicity of HCQ
HCQ-induced cytotoxicity was detected using the MTT
assay (Sigma-Aldrich, St. Louis, MO, USA). HMCs were seeded into
96-well culture plates at a density of 2,000 cells/well and then
incubated at 37°C in a humidified atmosphere with 5% CO2
for 24 h. The HMCs were treated with HCQ at concentrations of 0,
0.5, 1, 2.5, and 5 µg/ml. Following incubation for 0, 24,
48, and 72 h, MTT solution (5 mg/ml; Sigma-Aldrich) was added to
each well and the cells were incubated for a further 4 h. The
culture medium was then removed and 100 µl of dimethyl
sulfoxide was added to each well to dissolve the formazan crystals.
The absorbance was measured at wavelength of 490 nm
(A490). Cell viability was associated with
A490 measurements.
Flow cytometry (FCM)
The effects of HCQ on IgG binding to HMCs were
determined using flow cytometry. IgG was obtained from patient
serum samples as described above. HMCs were fixed in 4% PFA and
incubated with purified serum IgG at 800 µg/ml for 1 h at
37°C, with or without the addition of HCQ (1 µg/ml). Cells
were then washed and stained with FITC-labeled goat anti-human IgG
(H+L) antibody (1:800 dilution; ZSGB-BIO). Subsequently, the cells
were washed with PBS and the samples were subjected to FCM. BD
CellQuest Pro software was used to analyze the results (BD
Biosciences, Franklin Lakes, NJ, USA)
CDC assay
HMCs were used to probe the CDC activity of purified
immunoglobulin fractions from healthy controls or vitiligo
patients. Briefly, the HMCs were seeded into 96-well culture plates
at a density of 5,000 cells/well and incubated at 37°C for 24 h.
Subsequently, a 50 µl aliquot of M-254 medium that was
premixed with various concentrations of IgG (from vitiligo patients
or unaffected individuals), with or without the addition of HCQ (1
µg/ml), was added to each well in replicates of six and
incubated at 37°C for 1 h. The serum was discarded following the
incubation and 50 µl of normal human serum was incubated
with the cells for 8 h at 37°C. The MTT assay was then used to
quantify the number of viable cells. Inhibition ratio (%) =
(A490 of control group − A490 of test group)
/ A490 of control group ×100.
ADCC assay
The ADCC activity of purified IgG was measured using
a lactate dehydrogenase (LDH)-releasing assay via the CytoTox96
Non-Radioactive Cytotoxicity Assay kit (Promega Corporation,
Madison, WI, USA). HMCs were incubated with normal IgG (400
µg/ml), patient IgG (400 µg/ml), patient IgG (400
µg/ml) + HCQ (1 µg/ml), or media only for 1 h at
37°C. Human PBMCs were then added to the HMCs to serve as effector
cells [effector cell:target (E:T) ratio =5:1, 10:1, 20:1].
Following an additional incubation at 37°C for 8 h, the degree of
cell lysis was determined by measuring the quantity of released
LDH. This value is positively associated with the optical density
at a wavelength of 490 nm. Controls included an LDH max control
(target cells treated with 9% Triton X-100), an effector cell
control (only effector cells) and a target cell control (only
target cells). Inhibition ratio (%) = (A490 of test
group − A490 of effector cells control − A490
of target cells control) / (A490 of LDH max control −
A490 of target cells control) ×100.
Statistical analyses
Statistical tests were conducted using SPSS 16.0
software (IBM SPSS, Armonk, NY, USA). Relative anti-HMC
autoantibody levels from patient and control groups were analyzed
by one-way analysis of variance, and the LSD t-test was used to
compare the CDC and ADCC activity of each group. Graphs were
produced with GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Serum autoantibodies against melanocytes
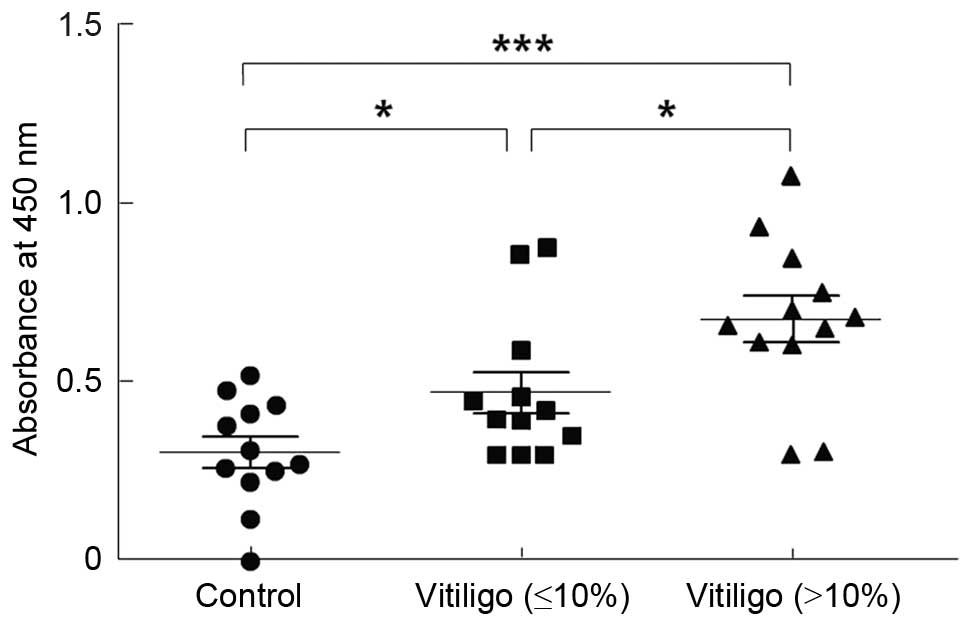
were significantly increased in patients with GV
Patients were divided into two groups depending on
the bodily area affected by vitiligo; the first group had an
intensity of vitiligo of <10%, and the second group had an
intensity of >10%. The two groups demonstrated significantly
higher levels of IgG anti-HMCs autoantibodies compared with the
controls (P<0.05 and P<0.01, respectively; Table II and Fig. 1).
 | Table IIMelanocyte-specific autoantibodies in
the sera of vitiligo patients and healthy controls. |
Table II
Melanocyte-specific autoantibodies in
the sera of vitiligo patients and healthy controls.
| Groups | n | Positive (%) | Absorbance at 450
nm |
|---|
| Control | 12 | 0 (0.00) | 0.30±0.15 |
| Vitiligo | | | |
| Intensity
(≤10%) | 12 | 2 (16.67) | 0.47±0.20a |
| Intensity
(>10%) | 12 | 10 (83.33) | 0.68±0.23b |
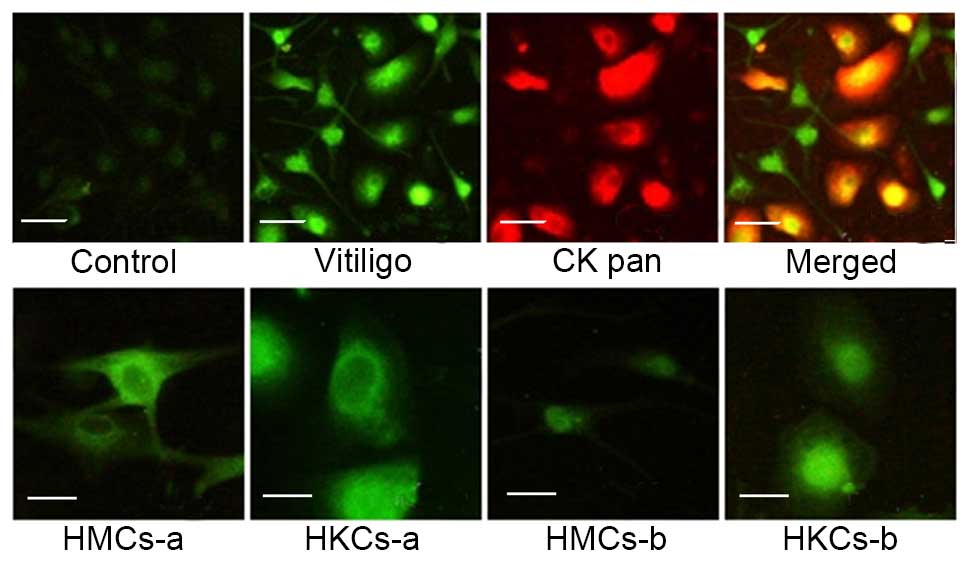
The autoantigens in melanocytes and
keratinocytes were localized by immunofluorescence
The present study investigated the subcellular
distribution and appearance of autoantigens in HMCs and HKCs using
a indirect immunofluorescence assay. Serum-positive patients (n=6)
and negative controls (n=6) were observed. HMCs and HKMs that were
incubated with patient serum samples produced brighter signals than
healthy controls (Fig. 2).
Furthermore, a signal was observed in the cytoplasm and cell
surface of the HMCs and HKMs and reduced in the nucleus (Fig. 2; HMCs-a and HKCs-a) in 5/6 patients
and 1/6 patients produced a higher nuclear signal (Fig. 2; HMCs-b and HKCs-b).
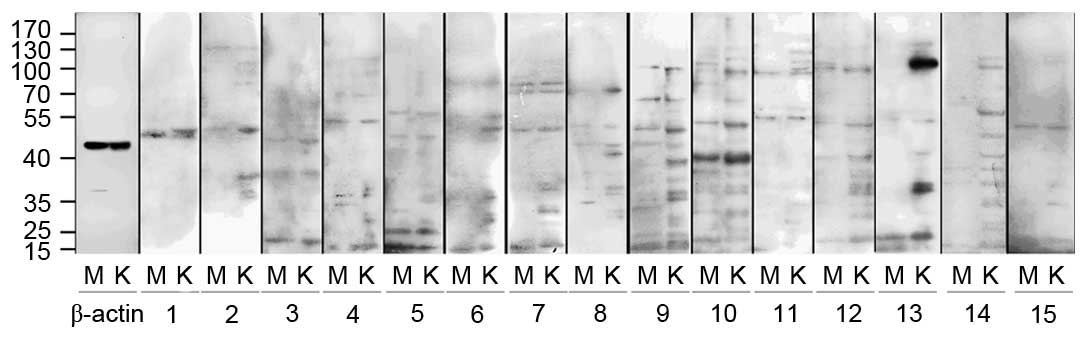
Antigens with molecular weight of 30 kDa
were preferentially expressed in HMCs
Western blotting indicated that HMCs and HKCs
contained proteins that reacted with serum antibodies (Fig. 3). Compared with healthy controls,
the antigens were predominantly observed to have a molecular weight
of 30, 37–39, 42, 53, 75, 90 and 100 kDa, and occasionally of 60,
65, 68, 85, 110 or 126 kDa. However, only the 30 kDa antigens were
preferentially expressed in HMCs, all other antigens were expressed
in HMCs and HKCs. These results are summarized in Tables III and IV.
 | Table IIIPositive reactions between vitiligo
patient and control serum samples and a collection of
electrophoretic fractions obtained from an extract of human
melanocytes. |
Table III
Positive reactions between vitiligo
patient and control serum samples and a collection of
electrophoretic fractions obtained from an extract of human
melanocytes.
| Sample |
Electrophoretic fractions, kDa
|
|---|
| 26–28 | 30 | 33–35 | 37–39 | 42 | 50 | 53 | 60 | 65 | 68 | 75 | 85 | 90 | 100 | 110 | 126 |
|---|
| Patients | | | | | | | | | | | | | | | | |
| 1 | | | | | | + | | | | | | | + | | | |
| 2 | | | | | | + | | | | | | | | | | + |
| 3 | | | | + | + | + | + | + | | | | | | | | |
| 4 | | + | + | | | + | | | + | | | + | | + | | |
| 5 | | + | | | | + | | + | | | | + | | + | | |
| 6 | | | | | | | + | | | | + | | | | | |
| 7 | | + | + | + | + | + | | | | + | | | | | | |
| 8 | | | | | | + | | | | | + | + | | | | |
| 9 | + | + | + | + | + | + | | | | + | | | | + | | |
| 10 | + | | + | + | + | + | + | | + | | + | | + | + | + | |
| 11 | | + | | | | | + | | | | + | | | | | |
| 12 | | + | | + | + | + | | | | | | | | | | + |
| 13 | | | | | | + | + | | | | | | + | + | | |
| 14 | | | | + | + | | | + | | | + | | | | | |
| 15 | | | | | | + | | | | | | | | | | |
| Controls | | | | | | | | | | | | | | | | |
| 1 | | | | | | + | | | | | | | | | | |
| 2 | + | | + | | | + | | | | | | | | | | |
| 3 | | | | | | | | | | | | | | | | |
| 4 | | | | | | | | | | | | | | | | |
| 5 | | | | | | + | | | | | | | | | | |
| 6 | + | | + | + | | + | | | | | + | | | | | |
| 7 | | | | | | | | | | | | | | | | |
| 8 | + | | | | | + | | | | | | | | | | |
| 9 | | | | | | | | | | | | | | | | |
| 10 | | | | | | + | | | | | | | | | | |
| 11 | | | | | | | | | | | | | | | | |
| 12 | | | + | | | + | | | | | | | | | | |
| 13 | | | | | | | | | | | | | | | | |
| 14 | | | | | | + | | | | | | | | | | |
| 15 | | | | | | | | | | | | | | | | |
 | Table IVPositive reactions between vitiligo
patient and control serum samples and a collection of
electrophoretic fractions obtained from an extract of human
keratinocytes. |
Table IV
Positive reactions between vitiligo
patient and control serum samples and a collection of
electrophoretic fractions obtained from an extract of human
keratinocytes.
| Sample |
Electrophoretic fractions, kDa
|
|---|
| 26–28 | 30 | 33–35 | 37–39 | 42 | 50 | 53 | 60 | 65 | 68 | 75 | 85 | 90 | 100 | 110 | 126 |
|---|
| Patients | | | | | | | | | | | | | | | | |
| 1 | | | | + | + | + | + | | | | | | + | | | |
| 2 | | | + | + | | + | | | | | + | | + | + | | + |
| 3 | + | | + | + | + | + | + | | + | | | | | | | |
| 4 | + | | + | | | + | | | + | | | + | | + | | |
| 5 | + | | + | | | + | | + | | | | | | + | | |
| 6 | + | | + | | | + | + | | | + | + | | | | | |
| 7 | + | | + | + | + | + | | | | | | | | | | |
| 8 | + | | + | | | + | | | | | + | | | | | |
| 9 | + | | + | + | + | + | | + | + | | | | | + | | |
| 10 | + | | + | + | + | + | | | + | | | | + | | + | + |
| 11 | + | | + | | | + | + | | | | + | + | + | | + | + |
| 12 | + | | + | + | + | + | | | | | | | + | | | + |
| 13 | + | | + | + | | + | | | | | | | + | | + | + |
| 14 | | | | + | + | + | + | + | | | + | | | | + | |
| 15 | | | + | + | | + | | | | | | | | | | |
| Controls | | | | | | | | | | | | | | | | |
| 1 | + | | + | + | | + | | | | | | | | | | |
| 2 | | | | | | + | | | | | + | | | | | |
| 3 | | | | | | | | | | | | | | | | |
| 4 | | | | | | | | | | | | | | | | |
| 5 | | | | | | | | | | | | | | | | |
| 6 | + | | + | + | | + | | | | | + | | | + | | + |
| 7 | | | | | | | | | | | | | | | | |
| 8 | + | | + | | | + | | | | | | | | | | |
| 9 | | | | | | | | | | | | | | | | |
| 10 | | | | | | | | | | | | | | | | |
| 11 | | | | | | | | | | | | | | | | |
| 12 | | | | | | + | | | | | | | | + | | |
| 13 | | | | | | | | | | | | | | | | + |
| 14 | + | | + | | | + | | | | | | | | | | |
| 15 | | | | | | + | | | | | | | | | | |
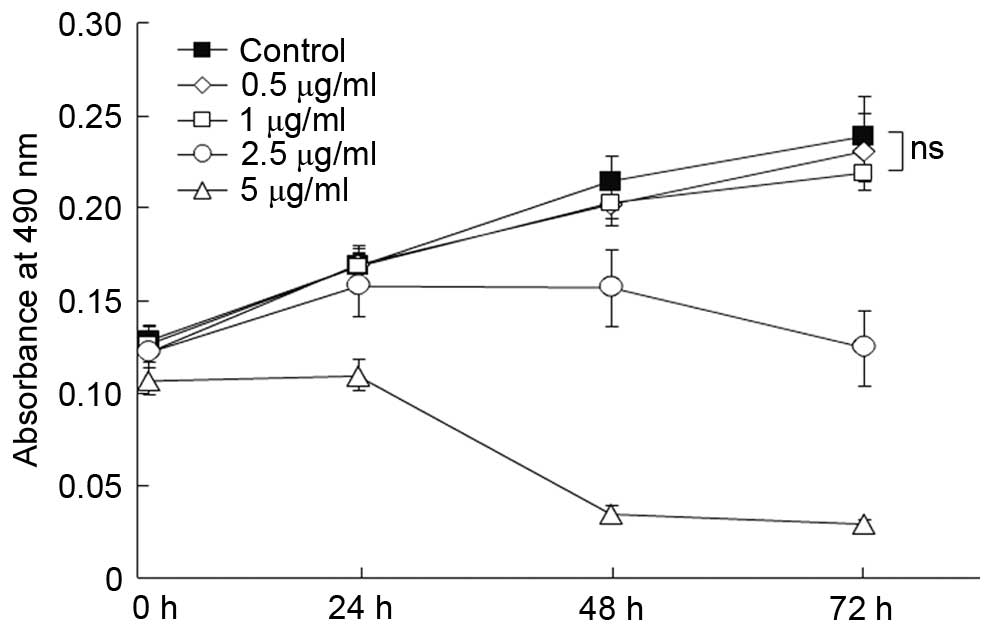
The cytotoxicity of HCQ in melanocytes in
concentration-dependent
The cytotoxicity of HCQ was monitored in HMCs using
the MTT assay. As presented in Fig.
4, concentration-dependent cytotoxicity was observed when the
concentration of HCQ was >2.5 µg/ml. The cytotoxic effect
was not observed when the concentration of HCQ was <1
µg/ml when compared with the control (P>0.1).
HCQ reduced autoantibody binding to
melanocytes
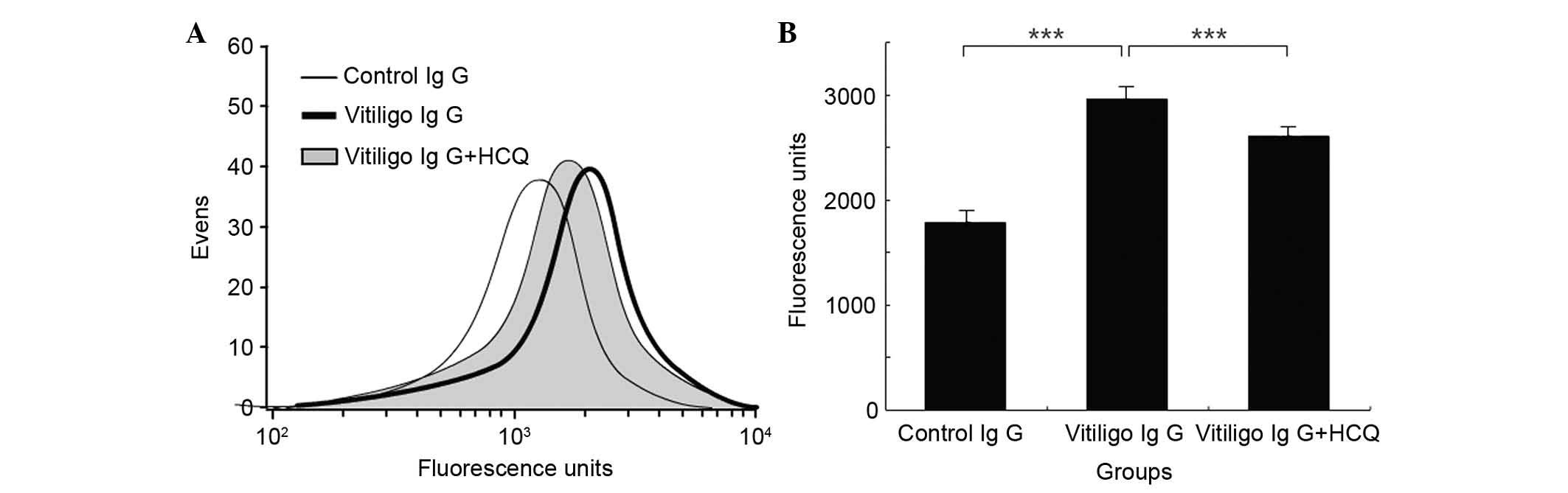
The effects of HCQ on IgG binding to HMCs were
determined using the FCM assay. Incubation of HMCs with IgG samples
obtained from vitiligo patients resulted in significantly greater
binding of IgG compared with incubation with IgG obtained from
controls (2,962.00±35.68 vs. 1,793.67±12.67; P<0.001; Fig. 5). In addition, the presence of HCQ
at concentrations of 1 µg/ml significantly reduced the
binding of IgG from patients with vitiligo compared with IgG that
was not exposed to HCQ (2,602.5±14.56 vs. 2,962.00±35.68;
P<0.001; Fig. 5).
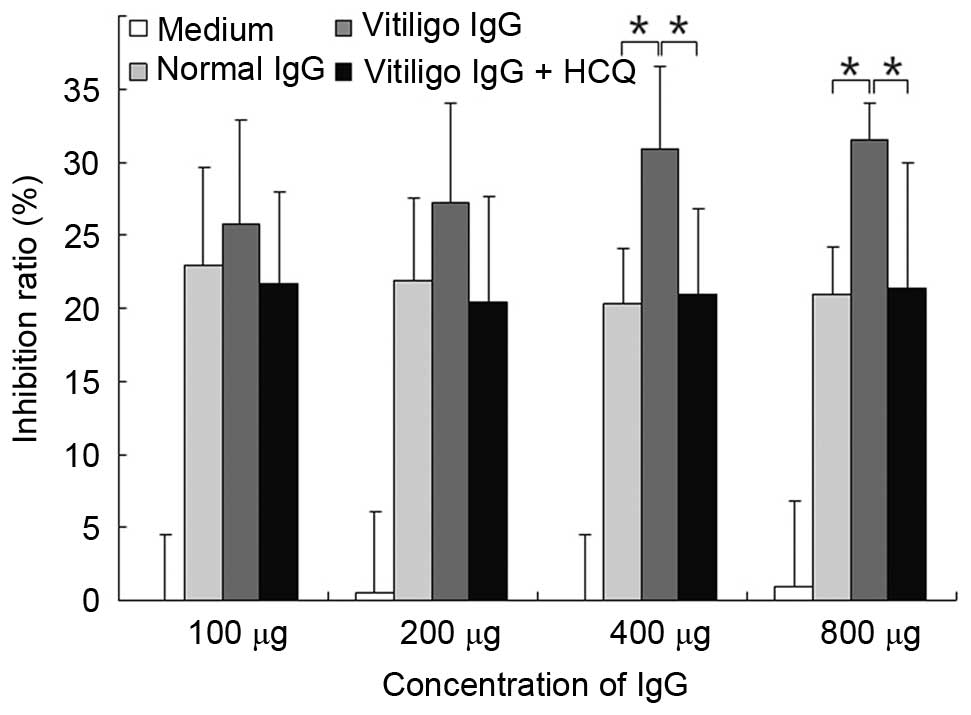
HCQ reduced the CDC activity of IgG
obtained from vitiligo patients
The CDC activity was measured using the MTT assay.
IgG (at concentration of 400 or 800 µg) obtained from
patients with vitiligo significantly increased CDC activity
compared with IgG obtained from controls (P<0.05; Fig. 6). The addition of HCQ at 1
µg/ml significantly reduced the CDC activity when the serum
IgG concentration was >400 µg/ml (P<0.05; Fig. 6).
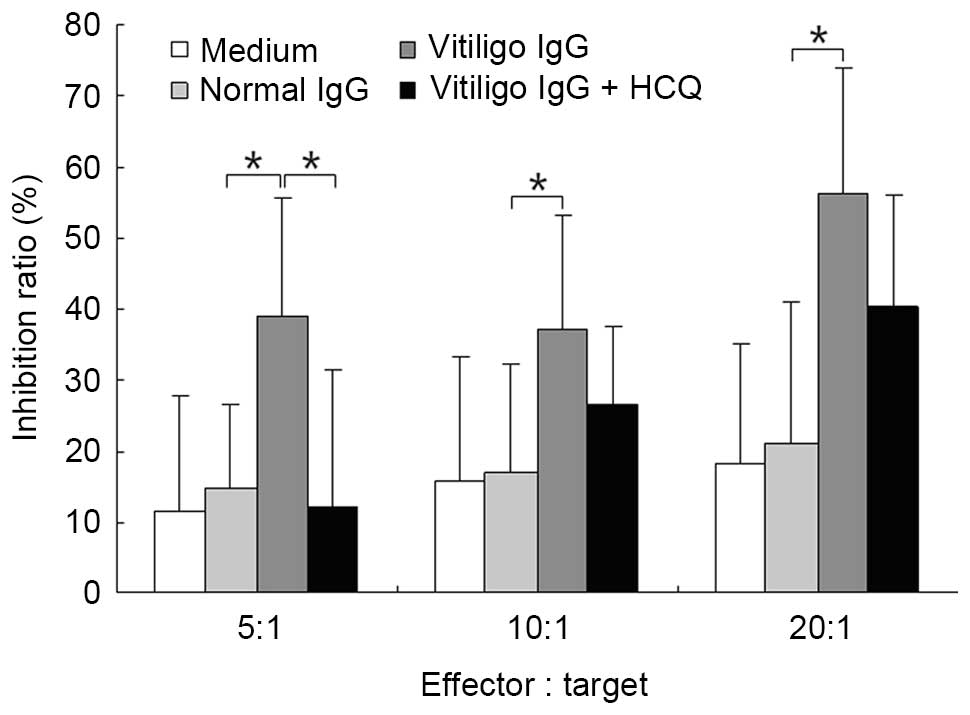
HCQ reduced the ADCC activity of IgG
obtained from vitiligo patients
The ADCC activity of purified IgG was measured using
a lactate dehydrogenase-releasing assay. IgG obtained from patients
with vitiligo significantly increased ADCC activity compared with
IgG obtained from controls at different E:T ratios (P<0.05;
Fig. 6). The addition of HCQ at 1
µg/ml significantly reduced ADCC activity at an E:T ratio of
5:1 (P<0.05; Fig. 7).
Discussion
Previous studies support that autoimmunity is
important in vitiligo pathogenesis (7,8).
However, the exact mechanism underlying this observation remains to
be elucidated. Although cellular immunity has more frequently been
the subject of investigation in recent years, humoral immunity has
also been demonstrated to participate in the destruction of
melanocytes, particularly in the process of extension (7,8). In
the present study, it was observed that serum concentrations of
autoantibodies against melanocytes were higher in the majority of
GV patients compared with controls, particularly in patients whose
GV involved >10% of the body surface. Notably, fluorescence
staining for IgG indicated that IgG obtained from patient serum
samples primarily bound to HMCs and HKCs and was often localized to
the cytoplasm and membrane of the cells, only occasionally
appearing in the nuclear region. In addition to exerting an effect
on CDC and ADCC, these antibodies are likely to also have a role in
increasing antigen uptake and presentation by dendritic cells. The
present study used serum samples obtained from vitiligo patients,
the auto-antibody antigens were determined to have molecular
weights of 30, 37–39, 42, 53, 60–75, 90, 100, 110 and 126 kDa,
whereas the corresponding protein bands were rare in the control
group. In addition, the majority of antigens were observed to be
present in HMCs and HKCs, although the 30 kDa antigen was observed
only in the extracts of HMCs. These results are in agreement with
those obtained in the immunofluorescence study. Candidate antigens
for these reactions include tyrosinase, proto-oncogene C-kit,
lysosomal-associated membrane protein-2, vitiligo (Vit)-40, Vit-75,
and Vit-90 (9). These results
support the hypothesis that melanocyte-specific antibodies and
autoantibodies are important in the development of GV. However, it
remains unknown why there appears to be selective destruction of
HMCs in vitiligo instead of the destruction of HMCs and HKCs, as
the same autoantigens are present in the two cell types.
Chloroquine (CQ) and HCQ are antimalarial
therapeutic agents that are widely used in the immunosuppressive
treatment of SLE and RA. The two molecules have similar structures
and chemical properties, however, HCQ is safer and results in fewer
side effects during treatment. In addition to their antimalarial
action, the immunosuppressive effects of CQ and HCQ have been
extensively investigated. Fox et al (10) reported that HCQ reduces proteolysis
and antigen presentation by elevating lysosomal pH. van den Borne
et al (11) demonstrated
that CQ and HCQ equally inhibit phytohemagglutinin-induced tumor
necrosis factor-α (TNF-α) and interferon-γ production and
lipopolysaccharide-induced TNF-α and interleukin-6 production.
Notably, previous studies have also demonstrated that CQ (200
mg/ml) could dissociate antigen-antibody complexes from red blood
cells without leading to protein degeneration (12,13).
Rand et al (12) reported
that HCQ (1 µg/ml) directly reduced the binding of
antiphospholipid antibody-β2-glycoprotein I complexes in
vitro. These results implied that HCQ may alter
antibody-antigen interactions and exert a beneficial effect on
antibody-antigen reaction-induced autoimmune disease. The MTT assay
was used to measure the cytotoxicity of HCQ. The results of the
present study demonstrated that 1 µg/ml HCQ produced no
cytotoxicity in HMCs. It is notable that 1 µg/ml is the mean
blood concentration of HCQ in SLE patients undergoing HCQ treatment
(14). The current study clearly
demonstrates that HCQ reduced the binding of autoantibodies to HMCs
in vitiligo patients and effectively decreased CDC and ADCC
activity in response to the application of patient IgG. These
results support the conclusion that HCQ may represent a promising
therapeutic strategy for patients presenting with GV. However,
further studies are required to examine whether HCQ produces
clinical efficacy in GV patients.
In conclusion, the present study has provided
preliminary evidence that HCQ dissociates antibody-antigen
complexes and reverses the activities of ADCC and CDC in
vitro. Additional studies are required to further elucidate the
molecular mechanisms underlying these observations and the possible
role of HCQ in the treatment of patients with progressive GV.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81271745 and
81271774).
References
|
1
|
Guerra L, Dellambra E, Brescia S and
Raskovic D: Vitiligo: Pathogenetic hypotheses and targets for
current therapies. Curr Drug Metab. 11:451–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Njoo MD and Westerhof W: Vitiligo:
Pathogenesis and treatment. Am J Clin Dermatol. 2:167–181. 2001.
View Article : Google Scholar
|
|
3
|
Michelsen D: The double strike hypothesis
of the vitiligo pathomechanism: New approaches to vitiligo and
melanoma. Med Hypotheses. 74:67–70. 2010. View Article : Google Scholar
|
|
4
|
Furst DE: Pharmacokinetics of
hydroxychloroquine and chloroquine during treatment of rheumatic
diseases. Lupus. 5(Suppl 1): S11–S15. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taïeb A and Picardo M; VETF Members: The
definition and assessment of vitiligo: A consensus report of the
Vitiligo European Task Force. Pigment Cell Res. 20:27–35. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma HJ, Yue XZ, Wang DG, Li CR and Zhu WY:
A modified method for purifying amelanotic melanocytes from human
hair follicles. J Dermatol. 33:239–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui J and Bystryn JC: Melanoma and
vitiligo are associated with antibody responses to similar antigens
on pigment cells. Arch Dermatol. 131:314–318. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu HS, Kao CH and Yu CL: Coexistence and
relationship of antikeratinocyte and antimelanocyte antibodies in
patients with non-segmental-type vitiligo. J Invest Dermatol.
100:823–828. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruiz-Argüelles A, Brito GJ,
Reyes-Izquierdo P, Pérez-Romano B and Sánchez-Sosa S: Apoptosis of
melanocytes in vitiligo results from antibody penetration. J
Autoimmun. 29:281–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fox RI: Mechanism of action of
hydroxychloroquine as an anti-rheumatic drug. Semin Arthritis
Rheum. 23(2 Suppl 1): S82–S91. 1993. View Article : Google Scholar
|
|
11
|
van den Borne BE, Dijkmans BA, de Rooij
HH, le Cessie S and Verweij CL: Chloroquine and hydroxychloroquine
equally affect tumor necrosis factor-alpha, interleukin 6, and
interferon-gamma production by peripheral blood mononuclear cells.
J Rheumatol. 24:55–60. 1997.PubMed/NCBI
|
|
12
|
Rand JH, Wu XX, Quinn AS, Chen PP,
Hathcock JJ and Taatjes DJ: Hydroxychloroquine directly reduces the
binding of antiphospholipid antibody-beta2-glycoprotein I complexes
to phospholipid bilayers. Blood. 112:1687–1695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holtz G, Mantel W and Buck W: The
inhibition of antigen-antibody reactions by chloroquine and its
mechanism of action. Z Immunitatsforsch Exp Klin Immunol.
146:145–157. 1973.PubMed/NCBI
|
|
14
|
Costedoat-Chalumeau N, Amoura Z, Hulot JS,
Aymard G, Leroux G, Marra D, Lechat P and Piette JC: Very low blood
hydroxychloroquine concentration as an objective marker of poor
adherence to treatment of systemic lupus erythematosus. Ann Rheum
Dis. 66:821–824. 2007. View Article : Google Scholar : PubMed/NCBI
|