Introduction
Ovarian cancer (OC) is an aggressive tumor and the
most lethal gynecologic malignancy (1), and has been shown to be associated
with cell cycle dysregulation (2).
As the result of its sensitivity to chemotherapeutic drugs,
surgical cytoreduction followed by systemic chemotherapy with
platinum and paclitaxel has been the standard treatment for
advanced OC (3). However,
long-term chemotherapy may result in chemoresistance (4) and a high rate of tumor recurrence in
treated patients (5).
The cell cycle normally keeps cells in a balance of
growth and death, and certain key proteins serve essential
regulatory roles, including cyclins, cyclin-dependent kinases
(CDKs) and CDK inhibitors (CDKIs) (6,7).
However, disorder in cell cycle-related proteins may allow cancer
cells to bypass regulatory check-points in the cell cycle and
undergo uncontrolled proliferation (8).
p27/Kip1 is a CDKI that results in cell cycle arrest
in G1 phase by inhibiting CDK2-cyclin E and CDK2-cyclin
A (9). Previous studies have
reported that p27 is downregulated in numerous types of tumor,
including carcinomas of the colon, breast, prostate, lung and ovary
(8,10,11),
and the reduction in p27 expression has been significantly
correlated with tumor grade and cancer prognosis in OC (6). Notably, there is growing evidence
suggesting that loss of p27 can mediate a drug-resistant phenotype.
Chu et al (12)
demonstrated that p27 inhibition is associated with
tamoxifen-resistance. Schmidt and Fan (13) observed that the absence or
cytoplasmic localization of p27 is linked to drug resistance. Xing
et al (14) reported that
p27 serves as a regulator of chemoresistance in ovarian tumors. Le
et al (15) demonstrated
that downregulation of p27 by siRNA can reduce the paclitaxel and
dasatinib sensitivity of OC cells, and overexpression of p27 led to
enhanced sensitivity of OC. Thus, p27 is a candidate biomarker of
chemoresistance in OC. However, the mechanism of p27-associated
chemoresistance in OC is not fully understood.
In the present study, the effect and mechanism of
p27 in the chemoresistance of OC SKOV3 cell lines was investigated,
and this may indicate that p27 is a potential therapeutic target
for the enhancement of sensitivity to chemotherapy in OC.
Materials and methods
Cell culture
SKOV3 human OC cells were purchased from American
Type Culture Collection (Manassas, VA, USA). SKOV3 cells were
cultured as a monolayer in Roswell Park Memorial Institute-1640
medium (GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 2 mM GlutaMAX™. Paclitaxal (TAX), cisplatin
(DDP) and carboplatin (CBP) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). The chemoresistant variants SKOV3/TAX (paclitaxel
resistant), SKOV3/DDP (cisplatin resistant) and SKOV3/CBP
(carboplatin resistant) were derived by step-wise incubation of the
chemotherapeutic agent over a number of weeks, as previously
described (16). In brief, the
SKOV3 cells were initially exposed to a low concentration of each
drug. After 24 h, the drug was replaced with normal medium. Once
cells were 80% confluent, a higher concentration of the drug was
added to the culture dish. SKOV3/TAX, SKOV3/DDP and SKOV3/CBP lines
were maintained at 0.1 µmol/l TAX, 1.0 mg/l DDP and 0.39
mg/l CBP, respectively.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
A total of 5,000 cells were plated in 96-well
plates. Following 24 h, the medium was replaced with a
chemotherapeutic drug at a range of concentrations: TAX, 0, 0.1, 1,
5, 10 and 20 µmol/l; DDP and CBP, 0, 0.01, 0.1, 1, 10 and
100 mg/l. Following 72 h of culture, 20 µl MTT solution (5
mg/ml) was added to each well. After 4 h incubation at room
temperature, the MTT solution was replaced by 150 µl of
dimethyl sufloxide to dissolve the tetrazolium crystals. The
absorption of each well was measured 10 mins later at a wavelength
of 570 nm with a microplate reader (Thermo Fisher Scientific,
Inc.). The cell viability (%) was calculated as follows: Optical
density (OD) of the treated wells/the control wells × 100. The
resistance index (RI) was calculated as follows: IC50 of resistant
SKOV3 vs. SKOV3.
5-aza-2′-deoxyazacytidine demethylation
treatment
For demethylation studies, all SKOV3 cells were
treated with 5 µmol/l 5-aza-2′-deoxyazacytidine
(Sigma-Aldrich) for 72 h. Cells were harvested for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blotting, MTT assay and bisulfite sequencing PCR (BSP).
Clonogenic assay
For colony formation assays, the SKOV3 cells were
seeded in dishes (35 mm; 400 cells/well). TAX (5 µM), DDP
(12.5 mg/l) or CBP (6.25 mg/l) were added into the medium.
Following a 4 h exposure, the medium was replaced and cells were
fixed in 1% glutaraldehyde (BD Biosciences, San Jose, CA, USA) and
stained with 0.5% crystal violet (Sigma-Aldrich) in methanol.
Colonies with greater than 30 cells were counted following 7 days
growth.
Flow cytometric analysis of the cell
cycle
Cells were cultured and treated as described above.
Subsequently, 1×106 cells were collected and fixed with
70% ethanol. Cells were washed twice in phosphate-buffered saline
(PBS), and propidium iodide (PI) was added to the cell suspension
solution and incubated at 4°C for 30 min prior to analysis with a
MoFlo cell sorter (Beckman Coulter, Inc., Brea, CA, USA).
Apoptotic assay
An annexin V apoptosis detection kit (Thermo Fisher
Scientific, Inc.) was used to analyze apoptosis. Following the
indicated treatment, cells were trypsinized, collected and
resuspended. A total of 2×105 cells were harvested and
washed twice with cold PBS, then resuspended in 500 µl
binding buffer. A total of 10 µl annexin V-fluorescein
isothiocyanate and 10 µl PI were added to the solution and
agitated. Following 15 min incubation, the cells were analyzed
using flow cytometric analysis (BD Biosciences, San Jose, USA).
Transfection
SKOV3 cells were cultured as described above for 48
h prior to transfection. A total of 100 pmol of a
p27-overexpression plasmid, pCDNA3.1-P27 (Auragene Bioscience,
Changsha, China), was diluted in 250 µl serum-free medium. A
total of 10 µl Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was added to each sample. Following incubation at
37°C for 4 h, the medium was replaced with complete medium for a
further 48 h of culture, following which the cells were collected
for use.
Western blotting
Whole-cell lysates were harvested and washed with
PBS, and lysed in a buffer containing 150 mM NaCl, 1 mM
phenylmethylsulfonyl fluoride, NaVO4, aprotinin and
leupeptin as protease inhibitors, in 50 mM Tris-HCl pH 8.0, 0.2%
sodium dodecyl sulfate (SDS) and 1% NP-40. The protein
concentrations were determined using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology, Haimen, China). A total of 30
µg of protein per sample was resolved on a 12%
SDS-polyacrylamide gel electrophoresis gel and transfered to a
polyvinylidene difluoride membrane. Membranes were incubated at 4°C
with primary antibody against P27 (1:500; cat. no. BM0447; Abzoom
Biolabs, Inc., Dallas, TX, USA) overnight. Following washing,
membranes were incubated with goat anti-mouse secondary antibody
(1:15,000; cat. no. SA001; Auragene Bioscience) for 1 h at room
temperature, followed by enhanced chemiluminescence (ECL) for
visualization using an ECL Plus Chemiluminescence detection kit
(Auragene Bioscience). For the control, the membrane was stripped
and reprobed using an actin antibody (1:2,300; cat. no. SCA01;
Auragene Bioscience) following the probing of each membrane with
the primary antibody. The radio of gray on the blots was analysed
by Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.,
Rockville, MD, USA).
RT-qPCR analysis of mRNA expression
Total RNA was extracted from SKOV3 cells using
TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. cDNA was reverse transcribed by
incubating oligo dT (1 µl, 0.5 µg/µl), total
RNA (2 µg) and diethylpyrocarbonate water (9 µl) at
65°C for 5 min. Then, 5X reaction buffer (4 µl; Thermo
Fisher Scientific, Inc.), RNase inhibitor (1 µl), dNTPs (2
µl, 10 mM) and RT (1 µl; Thermo Fisher Scientific,
Inc.) were added to the reaction and incubated at 42°C for 1 h and
72°C for 10 min. The cDNA was stored at 4°C proir to use in further
experiments. RT-qPCR was performed using an ABI 7500 Thermocycler
(Thermo Fisher Scientific, Inc.) and SYBR Green Universal PCR
Master Mix (BioRad Laboratories, Inc., Hercules, CA, USA). The
oligonucleotide sequences of the primer sets used were as follows:
Human p27, forward 5′-CAC TGC AGA GAC ATG GAA-3′ and reverse 5′-GCT
TCA TCA AGC AGT GA-3′); and human β-actin, forward 5′-AGG GGC CGG
ACT CGT CAT ACT-3′ and reverse 5′-GGC GGC ACC ACC ATG TAC CCT-3′).
PCR was performed in a total volume of 20 µl, which included
10 µl of 2X SYBR Green qPCR Mix, 1 µl of each forward
and reverse primer (10 µmol/l) and 40 ng of each cDNA sample
(quantified using a Nanodrop). Amplifications were carried out in
triplicate in 96-well microtiter plates. The thermal cycling
conditions were as follows: 95°C for 3 min, followed by 35 cycles
of 95°C for 10 sec, and 58°C for 30 sec, and followed by 95°C for
12 sec, 58°C for 50 sec. The 2−ΔΔCq method was used to
analyze the relative changes in gene expression (17).
DNA methylation analysis using BSP
Genomic DNA from SKOV3 cell lines was isolated with
an Auragene Genomic DNA kit (Auragene Bioscience). Subsequently,
Genomic DNA was subjected to bisulfite conversion and purification
using the EZ DNA Methylation-Gold™ kit (Zymo Research Corporation,
Irvine, CA, USA) according to the manufacturer's instructions. The
BSP primer was designed by Methprimer Express version 1.0 (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
used were as follows: SKOV3, forward 5′-TTT TTT AGG GAT GGT AGA AAT
TTTT-3′ and reverse 5′-CAA CAA ACC TAC TCT AAC TAA CCTC-3′. PCR was
performed in a total volume of 15 µl, which included 1.5
µl of 10X LAmp Buffer (Beijing Kang Century Biotechnology,
Co., Ltd., Beijing, China), 1.2 µl of dNTP Mix, 0.5
µl of forward and reverse primer (10 µM), 0.3
µl of LAmp Taq (Beijing Kang Century Biotechnology, Co.,
Ltd.), 3 µl of 5X C'Solution I (Beijing Kang Century
Biotechnology, Co., Ltd.), 3 µl of template, and 5 µl
of ddH2O. Amplifications were performed in 96-well
microtiter plates. The thermal cycling conditions were as follows:
94°C for 4 min, followed by 35 cycles of 94°C for 30 sec, 65°C for
30 sec, and 72°C for 30 sec, and followed by 72°C for 5 min.
Amplified PCR Products were purified and cloned into pMD19-T
(Takara Biotechnology Co., Ltd., Dalian, China). Each cell line
colony was sent to Beijing Genomics Institute (Shenzhen, China) for
sequencing. The percentage of methylation was calculated
comprehensively and comparatively using a CpG viewer (www.urogene.org/cgi-bin/methprimer/methprimer.cgi).
Statistical analysis
All experiments were repeated three times. All data
are presented as the mean ± standard deviation. Analysis was
performed used SSPS software (version 17; SPSS, Inc., Chicago, IL,
USA). Analysis of variance or General Linear model of Single factor
Variable was used to compare the differences among multiple groups.
Least significant difference and Student-Newman-Keuls methods were
used to compared the differences between two means among multiple
groups P<0.05 was considered to indicate a statistically
significant difference.
Results
Generation of chemoresistant OC cell
lines
To derive cell lines with acquired resistance, SKOV3
cells were cultured in TAX, DDP or CBP by step-wise incubation. The
resistant lines, SKOV3/TAX, SKOV3/DDP and SKOV3/CBP, were obtained
several weeks later.
To confirm chemoresistance in the resistant lines,
the IC50 value was calculated in SKOV3/TAX, SKOV3/DDP,
SKOV3/CBP and the control group using an MTT assay, and the
resulting resistance index observed to be 3.03, 5.989 and 3.32,
respectively (Fig. 1A).
Additionally, a colony formation assay was used to confirm the
proliferation ability of the chemoresistant variants in
chemotherapeutic medium (Fig. 1B).
It was observed that the colony formation efficiency of SKOV3/TAX
(0.48±0.04, P<0.05), SKOV3/DDP (0.77±0.04, P<0.01) and
SKOV3/CBP (0.70±0.02, P<0.05) cells were significantly increased
compared with the SKOV3 cell line (0.30±0.07 in TAX; 0.32±0.05 in
DDP; 0.51±0.02 in CBP) (Fig. 1C),
respectively. Furthermore, the cell cycle was analysed in the
chemoresistant cell lines (Fig.
1D). As presented in Fig. 1E,
the cell population in G1 phase was significantly
increased, while the proportion of cells in S phase were markedly
reduced in all chemoresistant cell lines compared with the SKOV3
group. This indicated that cells were arrested in the G1
phase. Together, these data demonstrate that the SKOV3/TAX,
SKOV3/DDP and SKOV3/CBP cell lines had acquired the resistant
phenotype.
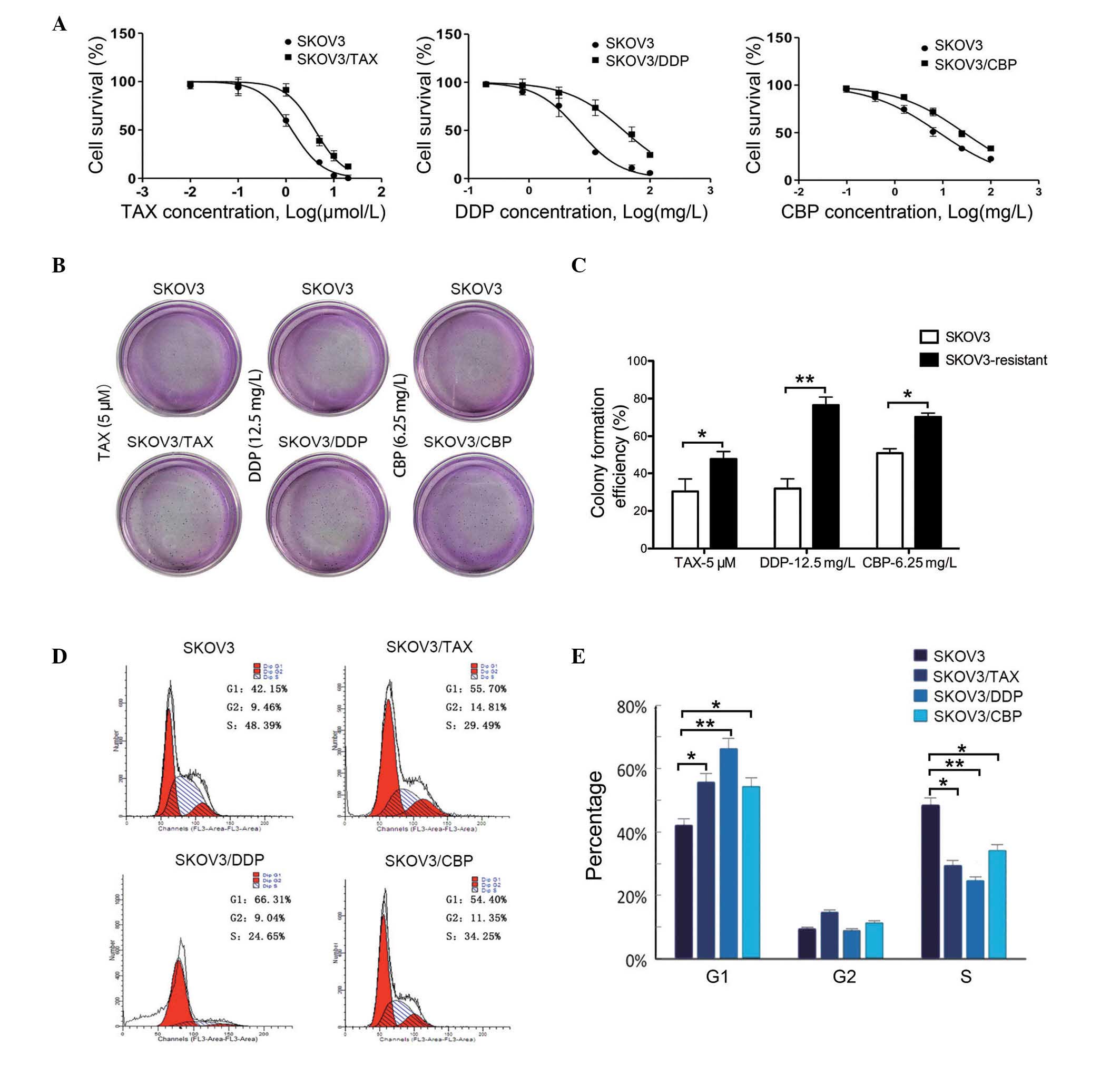 | Figure 1Characteristics of chemoresistant
SKOV3 cell lines. (A) Cell survival of chemoresistant SKOV3 cell
lines measured by
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide
assay. SKOV3 cells were cultured in each drug medium as control and
6 different concentrations of each drug were used in each group.
Data were presented as the mean ± standard deviation, n=3. (B)
Representative images of colonies formed in the different culture
medium. (C) Quantification of colony formation of the different
cell lines. Data are presented as the mean ± standard deviation,
n=3. *P<0.05; **P<0.01. (D) Cell-cycle
analysis for SKOV3 cell lines, using flow cytometry. The red peak
on the left of each histogram represents G1 phase, while
that on the right indicates the cells in G2 phase. The
blue slashed area indicates cells in S phase. (E) Statistical
analysis of cell-cycle distribution in all cell lines. Data are
presented as the mean ± standard deviation, n=3.
*P<0.05, **P<0.01, comparison indicated
by brackets. TAX, paclitaxal; DDP, cisplatin, CBP, carboplatin. |
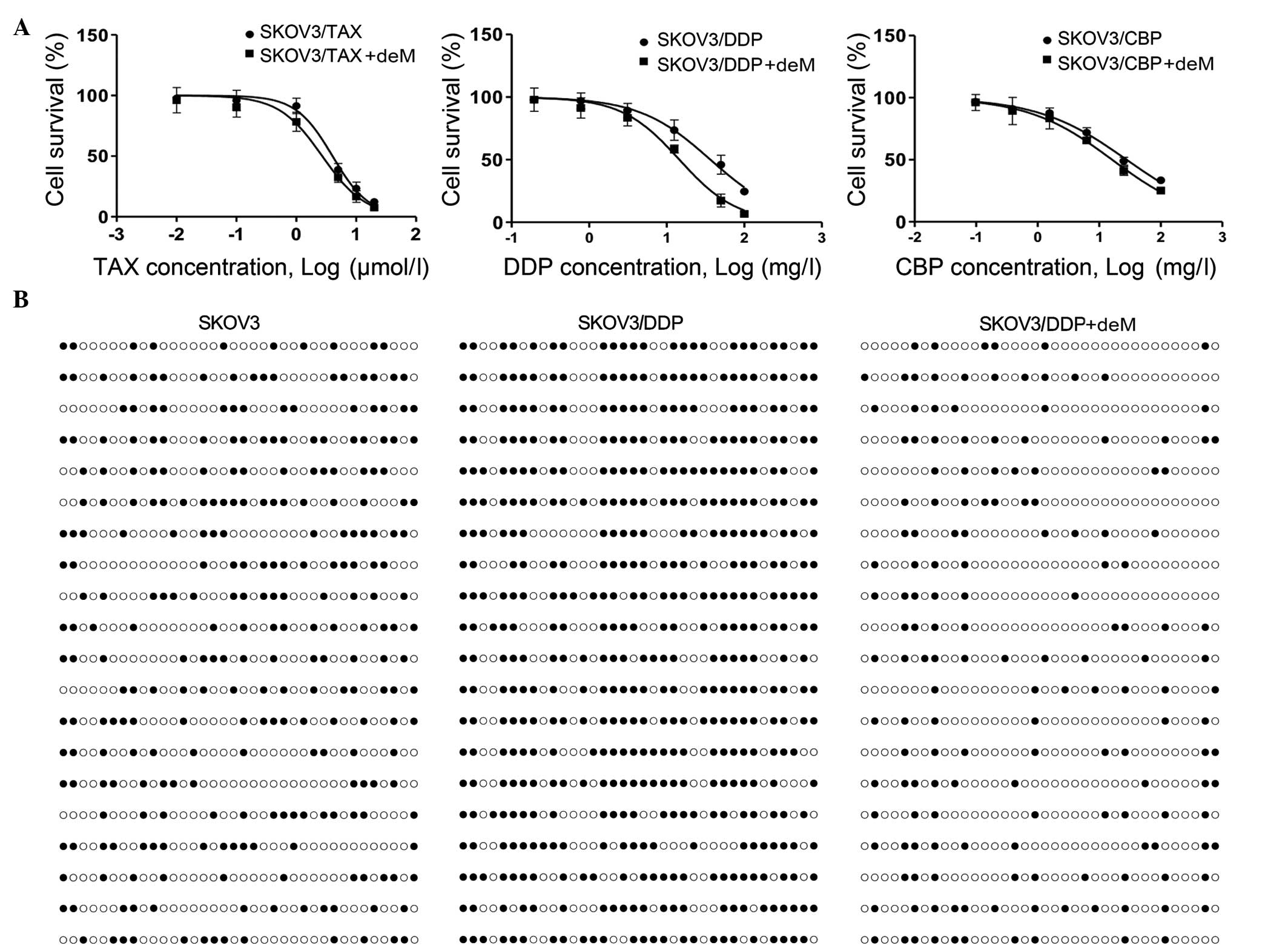
5-aza-2′-deoxyeytidine (5-aza) reverses
the low level expression of p27 in SKOV3/DDP cells
To examine whether the expression levels of p27 were
associated with drug resistance, we measured the expression of p27
in chemoresistant and 5-aza-treated cell lines using RT-qPCR and
western blotting (Fig. 2).
Notably, SKOV3/TAX (0.5134±0.0389, P<0.001; 0.63±0.05,
P<0.001), SKOV3/DDP (0.2552±0.0596, P<0.001; 0.35±0.03,
P<0.001), and SKOV3/CBP (0.3790±0.0150, P=0.004; 0.77±0.07,
P=0.003) showed significant reductions in mRNA and protein
expression levels of p27 compared with the control (1.0020±0.0745;
0.98±0.09). However, this was reversed when 5-aza was added to the
chemoresistant cell lines, as presented in Fig. 2A and B. The 5-aza treatment
significantly increased p27 mRNA expression levels in SKOV3/TAX
(0.5134±0.0389, P=0.002), SKOV3/DDP (0.8156±0.0239, P<0.001) and
SKOV3/CBP (0.7360±0.0908, P=0.001) compared with their
chemoresistant counterparts, respectively. The protein expression
of P27 was increased only in SKOV3/DDP groups (0.8156±0.0239,
P<0.001) compared with their chemoresistant counterparts.
However, no significant differences were observed between
5-aza-treated and untreated SKOV3/TAX cells (0.6994±0.0604,
P=0.052). Together, these data demonstrated that chemoresistance
resulted in the reduction in the expression levels of p27 in OC
cell lines, and that 5-aza treatment was able to reverse the
reduced expression of p27 in DDP-resistant SKOV3 cells.
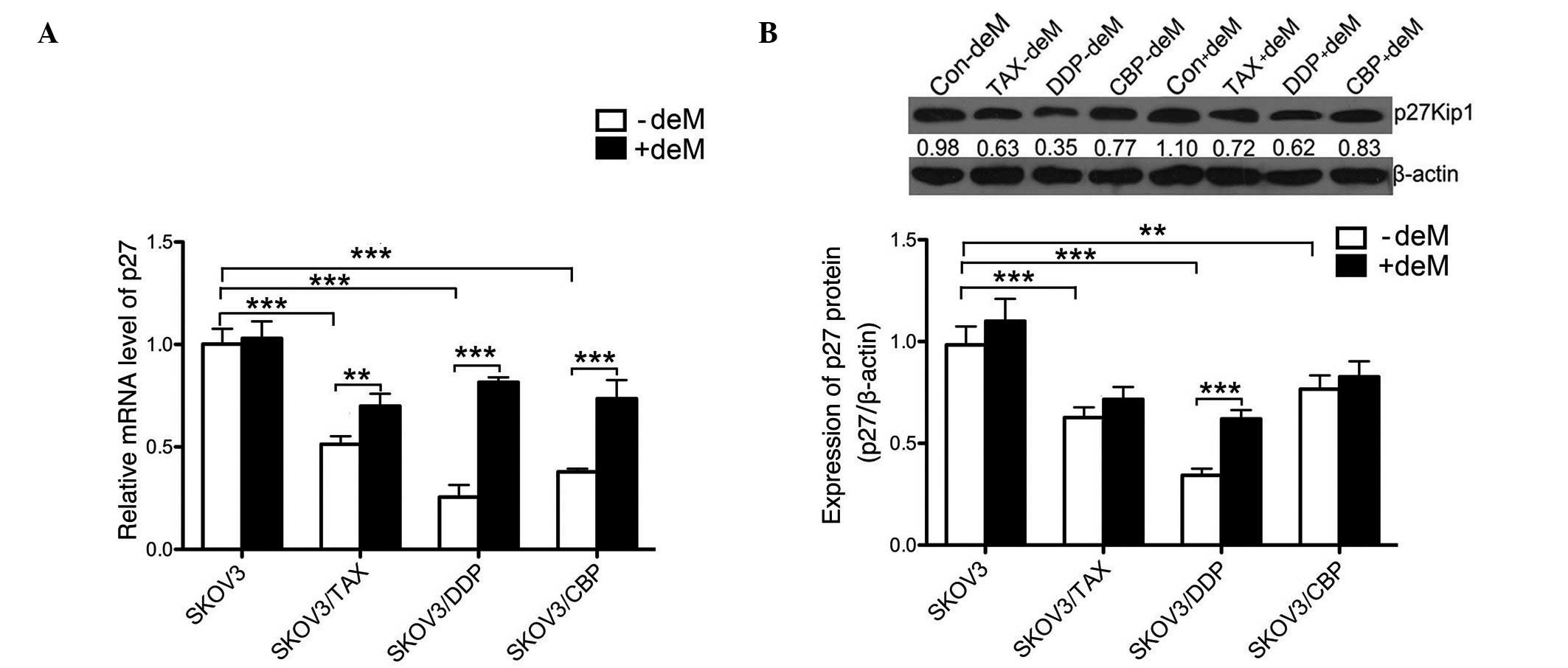 | Figure 2mRNA and protein expression levels of
p27 in SKOV3 cell lines. (A) Relative mRNA level of p27 in SKOV3
cells. All cell lines with or without 5-aza treatment were analyzed
by reverse transcription-quantitative polymerase chain reaction for
the measurement of p27 mRNA. (B) Protein expression of p27 in SKOV3
cells. The expression levels of p27 protein was measured in all
SKOV3 resistant cell lines with or without 5-aza treatment by
western blotting. Data are presented as the mean ± standard
deviation, n=3. **P<0.01, ***P<0.001,
comparison indicated by brackets. 5-aza, 5-aza-2′-deoxyazacytidine;
-deM, without 5-aza treatment; +deM, with 5-aza treatment; Con,
control; TAX, paclitaxal; DDP, cisplatin; CBP, carboplatin. |
5-aza treatment sensitizes OC SKOV3/DDP
cells to DDP by the demethylation of p27
The effect of 5-Aza-treatment on the sensitivity to
chemotherapy was next investigated in the chemoresistant cell lines
using an MTT assay. The results indicated that the differences
between SKOV3/TAX, SKOV3/CBP and their counterpart 5-aza-treated
cells were not significant (Fig.
3A). By contrast, the IC50 value in the
5-aza-treated SKOV3/DDP cells was markedly reduced compared with
the untreated SKOV3/DDP cells. This indicates that the 5-aza
treatment resulted in the DDP-resistant OC cells being more
sensitive to DDP.
To confirm the mechanism of the sensitizing effect
of 5-aza, the level of p27 methylation was investigated in SKOV3,
SKOV3/DDP, and 5-aza-treated SKOV3/DDP cells using BSP (Fig. 3B). The data indicated that the
frequency of methylation in SKOV3/DDP cells was higher than in
SKOV3 cells, with this reduced that following 5-aza treatment. This
indicated that drug resistance to DDP resulted in increased
methylation of p27 in SKOV3 cells, which may be reduced by 5-aza
treatment. Taken together, these data indicate that 5-aza resulted
in an increased sensitivity to DDP by downregulating the
methylation of P27.
Overexpression of p27 leads to cell cycle
alterations and increased apoptotic response to DDP in
SKOV3/DDP
p27-overexpression plasmids (pCDNA3.1-P27) were
transfected into the SKOV3 and SKOV3/DDP cell lines, and the
expression levels of p27 protein were measured by western blotting
(Fig. 4A).
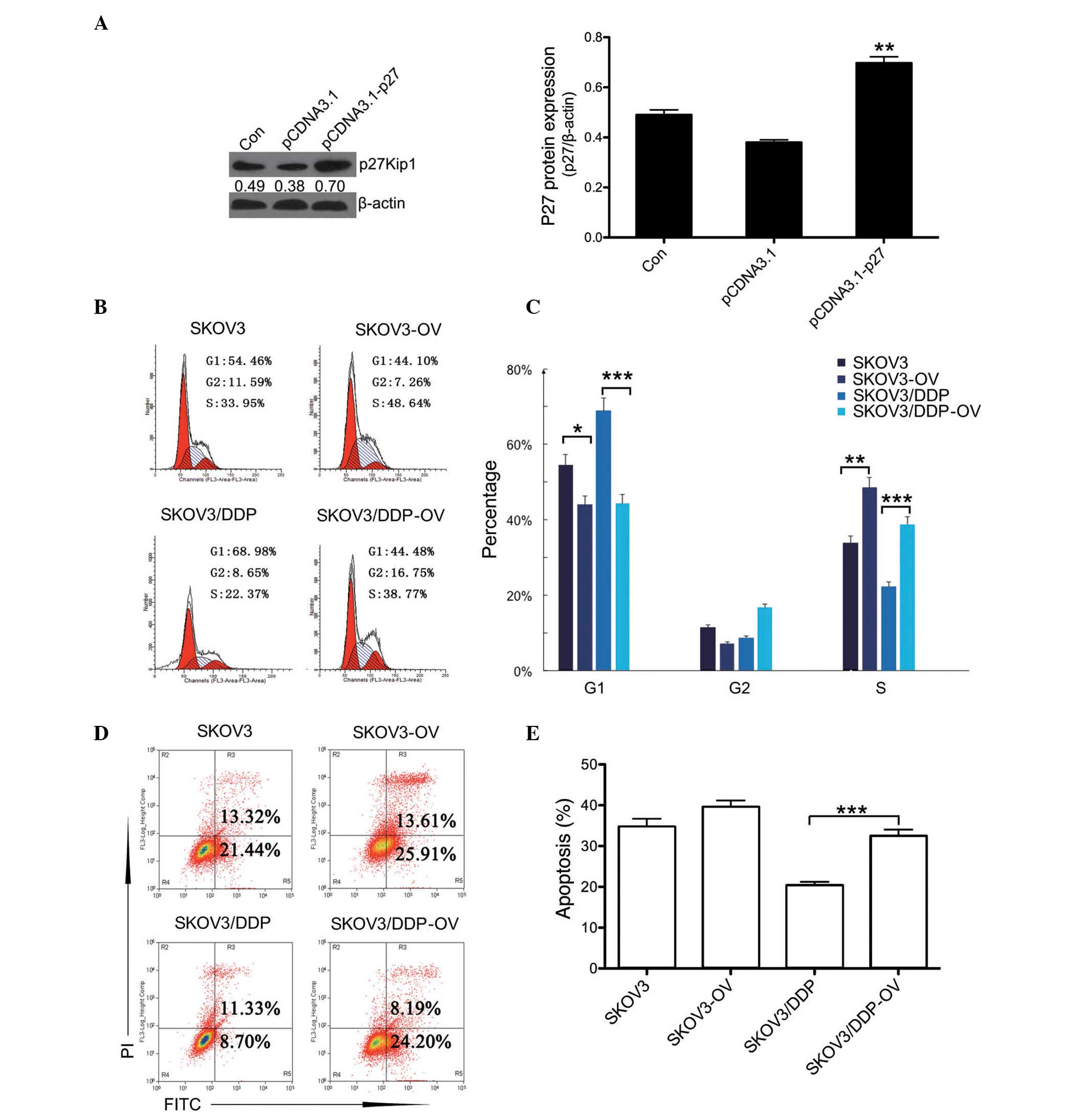 | Figure 4Overexpression of p27 leads to cell
cycle alterations and an increased apoptotic response to DDP in
SKOV3/DDP cells. (A) Plasmid pCDNA3.1-p27 was transfected into
SKOV3 cell lines to establish the p27-OV line. Following
transfection, western blotting was used to measure the expression
levels of p27 protein. **P<0.01 vs. pCDNA3.1 group.
(B) Cell-cycle analysis for SKOV3, SKOV3/DDP and their p27-OV
counterparts was conducted using flow cytometry. The red peak on
the left of each histogram represents G1 phase and the
red peak on the right indicates G2 phase. The blue
slashed area indicates S phase. (C) Quantification of cell-cycle
distribution. (D) The level of apoptosis in SKOV3, SKOV3-OV,
SKOV3/DDP and SKOV3/DDP-OV cells was measured by flow cytometry.
The right portion of the quadrant indicates the apoptotic cells.
(E) Quantification of the levels of apoptosis. Data are presented
as the mean ± standard deviation, n=3. *P<0.05,
**P<0.01, ***P<0.01, comparison
indicated by brackets. DDP, cisplatin; p27-OV, p27-overexpression;
Con, control; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
Additionally, the cell cycle was analyzed in
p27-transfected and untransfected SKOV3 and SKOV3/DDP cells
(Fig. 4B). The results indicated
that the proportion of cells in G1 phase were markedly
reduced, with an increase proportion of cells in S phase in
p27-transfected SKOV3 and SKOV3/DDP cells compared with their
untransfected counterparts (Fig.
4C). Furthermore, the apoptotic response to DDP was examined by
flow cytometry (Fig. 4D). The
apoptotic rate in p27-transfected SKOV3/DDP cells was significantly
increased compared with the untransfected SKOV3/DDP cells. The data
indicated that the overexpression of p27 was able to enhance the
sensitivity of SKOV3/DDP cells to DDP (Fig. 4E). Together, these results suggest
that the overexpression of p27 led to the increased sensitivity of
SKOV3/DDP cells to DDP via cell cycle arrest.
Discussion
In the present study, OC cell lines demonstrated
increased survival, enhanced proliferation and an increase of cells
in G1 phase and a reduction in S phase following
step-wise incubation-induced chemoresistance. In addition, the
redution in p27 expression was observed in chemoresistant cells.
Following demethylation using 5-aza, expression levels of p27 were
increased in DDP-resistant cells. These cells also became more
sensitive to DDP. p27 overexpression confirmed the enhancing effect
of p27 on DDP cytotoxicity.
p27 has been previously observed to be an accurate
prognostic marker in OC (2). Using
multivariate analysis in 99 primary tumors cases, Belletti et
al (8) proposed that p27 is a
novel potential prognostic marker for patients with advanced
epithelial OC, as its loss was significantly associated with a
shorter time to progression and a reduced overall survival. These
data were confirmed in a study by Hershko (18), which demonstrated that p27
expression was positively associated with overall survival of
patients with OC. Furthermore, there is growing evidence suggesting
that loss of p27 can mediate a drug-resistance phenotype (7). These previous studies support the
results of the current study, which demonstrated that drug
resistance resulted in a significant reduction in p27 protein
expression, with p27 overexpression in DDP-resistant cell lines
leading to an enhanced sensitivity to DDP.
It has been suggested that the silence of tumor
suppressor genes, such as p27, is associated with promoter
methylation. Li et al (19)
investigated cisplatin-sensitive and -resistant OC cells, and
observed that DNA hyper-methylation may contribute to
drug-resistance in OC. In addition, p27 expression in tumors was
significantly reduced, however, a number of previous studies have
demonstrated that rare methylation of p27 in tumor patients
(20,21). Li et al (22) observed no hypermethylation in the
p27 gene promoter in 5 patients with pancreatic carcinoma.
Stanganelli et el (23)
reported that all the patients with multiple myeloma they tested
lacked methylation at the p27 gene. By contrast, the present study
observed that the drug-resistant SKOV3 cell lines exhibited high
levels of methylation of p27. This indicates that the
cheomresistance-induced reduction in p27 in SKOV3 cells may not
only occur as the result of p27 methylation. Notably, 5-aza
treatment induced demethylation and resulted in upregulation of the
expression of p27, similar to the effect of p27 overexpression.
This suggests additional mechanisms may lead to the downregulation
of p27 in these patients (22).
The drugs investigated in the current study posses
different target points in the cell cycle. Paclitaxel binds to
microtubules and prevents their depolymerization, blocking cell
division at the G2/M phase of the cell cycle (24,25).
Platinum containing drugs, including cisplatin and carboplatin,
react with DNA to form intra- and interstrand crosslinks that also
block cell division. Therefore, factors that can reduce the
reaction of platinum-containing drugs with DNA will reduce the
toxicity of the drug (26). In the
present study, it was observed that chemoresistant cells altered
their cell cycle distribution, with the proportion of cells in the
G1 phase increased and reduced in S phase. This means
that the majority of chemoresistant cell lines reduce the ability
of platinum-containing drugs to bind to DNA. With the reduced
binding of platinum-containing drugs, tumor cells may be activated,
which may ultimately result in tumor recurrence and poor prognosis
(5,26,27).
Furthermore, overexpression of p27 restored the cell-cycle
distribution in DDP-resistant cells, enabling the cells to divide.
This suggests that the target points of the chemotherapeutic drugs
were exposed following the return of the cells into the cell cycle,
therefore increasing the sensitivity of chemoresistant cells to
DDP.
Additionally, it was observed that the protein
levels of p27 in SKOV3 cells chemoresistant to TAX and CBP were not
significantly restored by 5-aza treatment. This may indicate that
the chemoresistance in SKOV3 cells to TAX and CBP is not due to p27
methylation. As mentioned, TAX is a drug that blocks cell division
by binding to microtubules and preventing their depolymerization.
However, the differential effects on CBP-resistant and
DDP-resistant cells implies that different mechanisms are
associated with chemoresistance to these drugs. Further research on
the different mechanisms may aid in the understanding of the
difference between CBP and DDP in clinical applications.
In conclusion, demethylation of p27 was able to
enhance the DDP-induced cytotoxicity in human OC cells through the
regulation of the cell cycle. In addition, p27 may represent a
potential biological marker for OC prognosis, and a potential
target for overcoming chemoresistance in OC cells.
Abbreviations:
|
TAX
|
paclitaxel
|
|
DDP
|
cisplatin
|
|
CBP
|
carboplatin
|
|
5-Aza
|
5-aza-2′-deoxycytidine
|
|
p27
|
p27Kip1
|
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Andrilli G, Kumar C, Scambia G and
Giordano A: Cell cycle genes in ovarian cancer: Steps toward
earlier diagnosis and novel therapies. Clin Cancer Res.
10:8132–8141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matei D: Novel agents in ovarian cancer.
Expert Opin Investig Drugs. 16:1227–1239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrandina G, Zannoni GF, Martinelli E,
Paglia A, Gallotta V, Mozzetti S, Scambia G and Ferlini C: Class
III beta-tubulin overexpression is a marker of poor clinical
outcome in advanced ovarian cancer patients. Clin Cancer Res.
12:2774–2779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonelli P, Tuccillo FM, Borrelli A,
Schiattarella A and Buonaguro FM: CDK/CCN and CDKI alterations for
cancer prognosis and therapeutic predictivity. Biomed Res Int.
2014:3610202014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abukhdeir AM and Park BH: P21 and p27:
Roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Belletti B, Nicoloso MS, Schiappacassi M,
Chimienti E, Berton S, Lovat F, Colombatti A and Baldassarre G:
p27(kip1) functional regulation in human cancer: A potential target
for therapeutic designs. Curr Med Chem. 12:1589–1605. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vidal A and Koff A: Cell-cycle inhibitors:
Three families united by a common cause. Gene. 247:1–15. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang LW, Chao SL, Hwang JL and Chou YY:
Down-regulation of p27 is associated with malignant transformation
and aggressive phenotype of cervical neoplasms. Gynecol Oncol.
85:524–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Paola F, Vecci AM, Granato AM, Liverani
M, Monti F, Innoceta AM, Gianni L, Saragoni L, Ricci M, Falcini F,
et al: p27/kip1 expression in normal epithelium, benign and
neoplastic breast lesions. J Pathol. 196:26–31. 2002. View Article : Google Scholar
|
|
12
|
Chu I, Sun J, Arnaout A, Kahn H, Hanna W,
Narod S, Sun P, Tan CK, Hengst L and Slingerland J: p27
phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell.
128:281–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt M and Fan Z: Protection against
chemotherapy-induced cytotoxicity by cyclin-dependent kinase
inhibitors (CKI) in CKI-responsive cells compared with
CKI-unresponsive cells. Oncogene. 20:6164–6171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xing H, Wang S, Hu K, Tao W, Li J, Gao Q,
Yang X, Weng D, Lu Y and Ma D: Effect of the cyclin-dependent
kinases inhibitor p27 on resistance of ovarian cancer multicellular
spheroids to anticancer chemotherapy. J Cancer Res Clin Oncol.
131:511–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le XF, Mao W, He G, Claret FX, Xia W,
Ahmed AA, Hung MC, Siddik ZH and Bast RC Jr: The role of p27(Kip1)
in dasatinib-enhanced paclitaxel cytotoxicity in human ovarian
cancer cells. J Natl Cancer Inst. 103:1403–1422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pohl PC, Carvalho DD, Daffre S, Vaz Ida S
Jr and Masuda A: In vitro establishment of ivermectin-resistant
rhipicephalus microplus cell line and the contribution of ABC
transporters on the resistance mechanism. Vet Parasitol.
204:316–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Hershko DD: Cyclin-dependent kinase
inhibitor p27 as a prognostic biomarker and potential cancer
therapeutic target. Future Oncol. 6:1837–1847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Balch C, Montgomery JS, Jeong M,
Chung JH, Yan P, Huang TH, Kim S and Nephew KP: Integrated analysis
of DNA methylation and gene expression reveals specific signaling
pathways associated with platinum resistance in ovarian cancer. BMC
Med Genomics. 2:342009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ling Y, Zhang C, Xu Y, Zhu J, Zhu C, Lu M,
Liu Y and Zhou T: Promoter methylation-associated silencing of
p27kip1 gene with metastasis in esophageal squamous cell carcinoma.
Mol Med Rep. 9:1075–1079. 2014.PubMed/NCBI
|
|
21
|
Qian X, Jin L, Kulig E and Lloyd RV: DNA
methylation regulates p27kip1 expression in rodent pituitary cell
lines. Am J Pathol. 153:1475–1482. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li G, Ji Y, Liu C, Li J and Zhou Y:
Reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 in
pancreatic carcinoma. Mol Med Rep. 5:1106–1110. 2012.PubMed/NCBI
|
|
23
|
Stanganelli C, Arbelbide J, Fantl DB,
Corrado C and Slavutsky I: DNA methylation analysis of tumor
suppressor genes in monoclonal gammopathy of undetermined
significance. Ann Hematol. 89:191–199. 2010. View Article : Google Scholar
|
|
24
|
Horwitz SB: Taxol (paclitaxel): Mechanisms
of action. Ann Oncol. 5(Suppl 6): S3–S6. 1994.PubMed/NCBI
|
|
25
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Branch P, Masson M, Aquilina G, Bignami M
and Karran P: Spontaneous development of drug resistance: Mismatch
repair and p53 defects in resistance to cisplatin in human tumor
cells. Oncogene. 19:3138–3145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mekhail TM and Markman M: Paclitaxel in
cancer therapy. Expert Opin Pharmacother. 3:755–766. 2002.
View Article : Google Scholar : PubMed/NCBI
|