Introduction
Herpes simplex virus type 2 (HSV-2) infection is a
common infectious disease in humans. HSV-2 generally causes genital
infections. The standard treatment of genital herpes is dependent
on guanosine analogues. Despite the efficacy of the treatment,
there is still no cure to prevent recurrence. Over the last few
decades, considerable efforts have been made to develop a vaccine
against genital herpes. Several candidate vaccines have been
investigated experimentally in different genital HSV model systems.
It is considered that inoculation with a HSV vaccine to promote an
immune reaction against HSV is an ideal method to prevent and treat
HSV infection.
Previous studies have demonstrated that humoral
(1,2) and cellular immune responses (3) are responsible for protective immunity
against HSV infection. During viral infection, neutralizing
antibodies can inactivate free viral particles, but are unable to
inhibit intracellular infection. Furthermore, results indicated
that antibodies at the site of mucosal infection were inadequate to
prevent invasion (4), which
indicated cellular immunity as the main factor involved in the
control of HSV infection (5,6).
Traditional candidate vaccines such as those containing live
attenuated or killed viruses have been shown to confer protective
immunity; however, due to safety concerns, the application of these
vaccines has been precluded in humans only a few have been assessed
in clinical trials (7). HSV
recombinant glycoprotein vaccines and subunit vaccines have shown
the capacity to stimulate antigen-specific immune responses.
However, they were not able to induce efficient cell-mediated
immunity, and displayed poor protective immunity in animal models
(8). DNA vaccines are the third
generation of vaccines following vaccines containing whole pathogen
bodies and recombinative protein by gene engineering. DNA vaccines
have characteristics of the safety of recombinative sub-unit
vaccines and the efficiency of live pathogen vaccines. They can
induce humoral and cellular immune responses.
CpG oligodeoxynucleotide (ODN) is a synthetic ODN
containing unmethylated cytidine-phosphate-guanosine with
appropriate flanking regions (CpG motif). Several recent studies
have demonstrated the potent adjuvant activity of CpG ODN in the
induction of systemic and mucosal immune responses (9,10).
In particular, animal challenge models showed that protective
immunity can be accelerated and enhanced by co-administering CpG
DNA with vaccines (11). Ongoing
clinical studies indicate that CpG ODNs are safe and well-tolerated
when administered as adjuvants to humans, and in certain cases they
have been shown to increase vaccine-induced immune responses
(11).
In the present study, a novel eukaryotic expression
plasmid vector was constructed containing the kanr gene
from pET-28a(+) and pcDNA3 plasmids. A gene encoding full length
HSV-2 gD was cloned into the eukaryotic expression plasmid vector
(pgD). A DNA segment containing 8 CpG motifs was also synthesized
and cloned into a eukaryotic expression plasmid vector (pCpG). Mice
were co-inoculated with pgD and pCpG by bilateral intramuscular
injection into the rear leg and the immune response was
observed.
Materials and methods
Ethics statement
The study was approved by the Ethics Committee of
Zhejiang Academy of Medical Sciences (Hangzhou, China).
Mice
Female Balb/c mice (n=48; weight, 20±2 g; age, ~7
weeks) were provided and bred by the Experimental Animal Center,
Zhejiang Academy of Medical Sciences and maintained in a
pathogen-free animal facility. Balb/c mice were maintained at
20±2°C, humidity 55±5% with a 12-h dark:light cycle. They were
given food pellets (Zhejiang Academy of Medical Sciences, Hangzhou,
China) and water ad libitum. Adequate measures were taken to
minimize animal discomfort.
Virus
HSV-2 strain Sav, obtained from the National
Institute for Viral Disease Control and Prevention (Beijing,
China), was grown in Vero cells (Institute of Biochemistry and Cell
Biology, Shanghai, China) and was stored at −80°C. Virus was
routinely prepared by infection of almost confluent Vero cells
(Institute of Biochemistry and Cell Biology) with a multiplicity of
infection of 0.1 at 37°C in a small volume of high glucose
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) without serum. After 1 hr,
virus inoculum was removed and cultures were re-fed with high
glucose DMEM. Incubation was continued until cytopathic effect was
extensive; usually for 24–48 hr. Before use, the virus particles
were released from the cells by freezing and thawing cycles and
cellular debris was removed by centrifugation (640 × g for 10 min
at 4°C). The method of titration was a plaque assay in Vero cells
and results were expressed as PFU/ml (12).
Bacterial strains and plasmids
E. coli DH5a and E. coli Tg1 (Beijing
ComWin Biotech Co., Ltd., Beijing, China) were used as hosts during
the cloning experiments and for propagation of the plasmids.
Bacterial strains were grown at 37°C in Luria Bertani (LB) media,
supplemented with ampicillin or kanamycin when required. pCDNA3
(Invitrogen; Thermo Fisher Scientific Inc.), pET-28a(+) (Novagen,
EMD Millipore, Billerica, MA, USA) and pcDNA3-gD (HSV-2
glycoprotein D gene was inserted) plasmids [pcDNA3-gD (HSV-2
glycoprotein D gene was inserted)] were constructed by the
Institute of Bioengineering, Zhejiang Academy of Medical Sciences
(13). Plasmids were amplified by
E. coli DH5a, purified by the pure plasmid mini kit (Beijing
ComWin Biotech Co., Ltd., Beijing, China), and sequenced by Sangon
Biotech (Shanghai) Co., Ltd. (Shanghai, China).
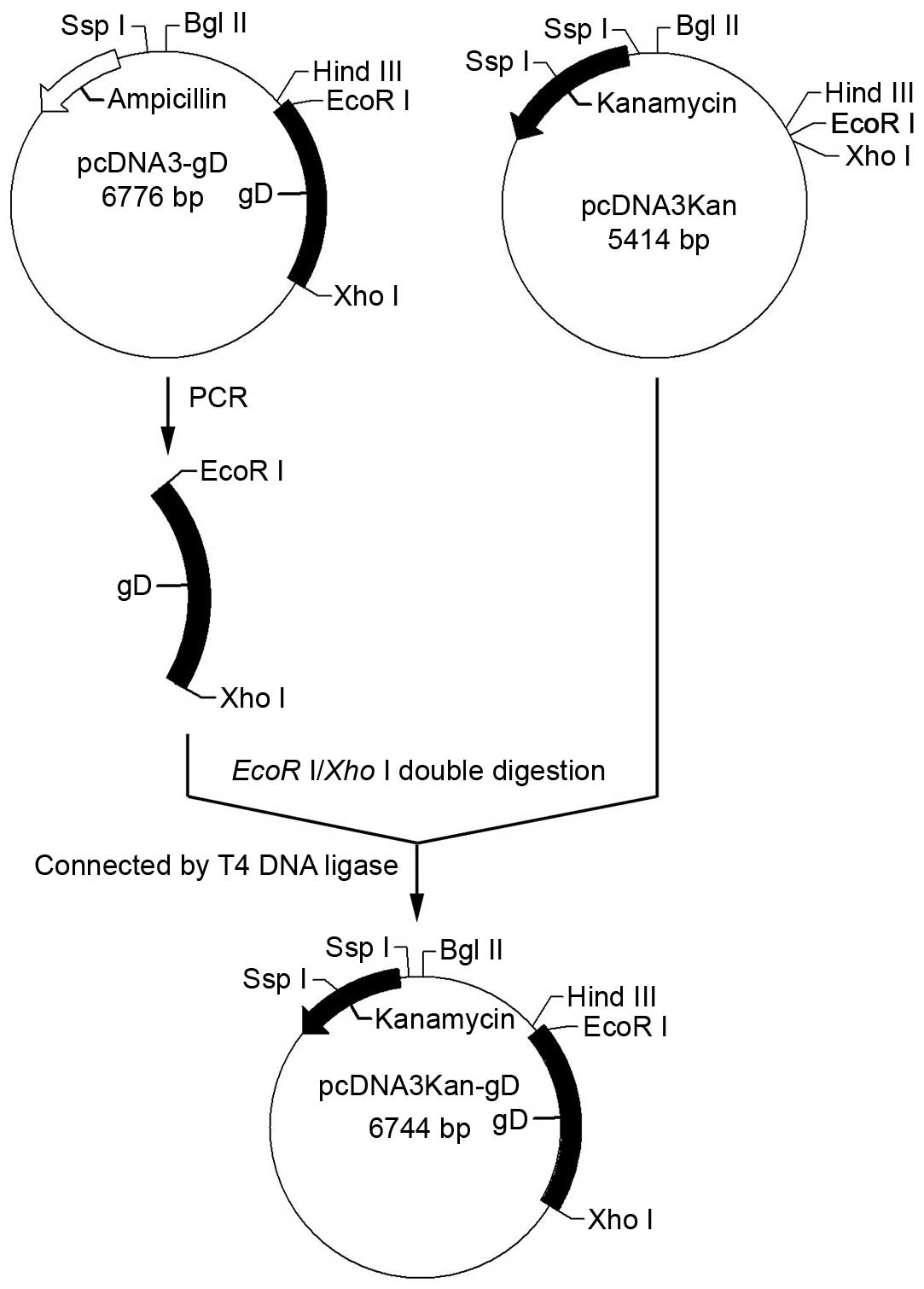
Constructing a new eukaryotic expression
plasmid vector (pcDNA3Kan) containing the kanr gene from
plasmid pET-28a(+) and pcDNA3
The kanr gene was amplified from the
pET-28a(+) plasmid by polymerase chain reaction according to
standard protocol (14) using the
following primers: Forward,
5′-GCCCTTAAGATGAGCCATATTCAACGG-3′ (bold section indicates
restriction enzyme site of AflII) and reverse,
5′-AGTCCGCGGTTAGAAAAACTCATCGAG-3′ (bold section indicates
restriction enzyme site of SacII). The whole sequence of the
pcDNA3 plasmid except the Amp+ gene was amplified from
pcDNA3 by PCR according to standard protocols using the following
primers: Forward, 5′-GCGGCTTAAGACTCTTCCTTTTTCAAT-3′ (bold
section indicates restriction enzyme site of AflII) and
reverse, 5′-ATACCGCGGCTGTCAGACCAAGTTTAC-3′ (bold section
indicates restriction enzyme site of SacII). The two PCR
products that were digested with AflII and SacII were
sealed together by T4 DNA ligase and transformed into E.
coli DH5a. After selection with kanamycin, a new eukaryotic
expression plasmid vector, pcDNA3Kan, was obtained (Fig. 1). The new eukaryotic expression
plasmid vector pcDNA3Kan was identified by restriction enzyme
BglII/XhoI or SspI digestion analysis.
Cloning of gD into the eukaryotic
expression vector pcDNA3Kan
A gene encoding full length HSV-2 gD was amplified
from the pcDNA3-gD plasmid by polymerase chain reaction using the
following primers: Forward, 5′-ATCGAATTCAACCACTAGTCGCCG-3′
(bold section indicatesrestriction enzyme site of EcoRI) and
reverse, 5′-CGCTCGAGACTCCCTTTATGC-3′ (bold section indicates
Xho restriction enzyme site of I). The PCR product and
plasmid pcDNA3Kan were digested with EcoRI and XhoI.
The two DNA strands were then joined at their sticky ends and were
sealed together by T4 DNA ligase, to form the recombinant plasmid
pcDNA3Kan-gD (pgD) (Fig. 2). The
plasmids pgD and pCpG was sequenced by Sangon Biotech (Shanghai)
Co. Ltd.
Constructing a new DNA vaccine adjuvant
pcDNA3Kan-CpG containing CpG motifs
Two ssDNA segments containing 8 CpG motifs (the
sequence of CpG motif was according to ODN 1826): Forward,
5′-AGCTT TCCAT GACGTT CCT GACGTT CCT GACGTT CCT GACGTT CCT GACGTT CCTC GTCGTT TT GTCGTT TT GTCGTT G-3′ (bold section
indicates restriction enzyme site of HindIII; underline
section indicates CpG motif) and reverse, 5′-AATTC
AACGAC AA
AACGAC AA
AACGAC GAGG
AACGTC AGG
AACGTC AGG
AACGTC AGG
AACGTC AGG
AACGTC ATGGA A-3′
(bold section indicates restriction enzyme site of EcoRI;
underline section indicates CpG motif) were synthesized. The two
ssDNA segments were integrated into one DNA double-stranded segment
and cloned into the pcDNA3Kan eukaryotic expression plasmid vector
using HindIII and EcoRI restriction enzymes, to form
a recombinant pcDNA3Kan-CpG (pCpG) plasmid (Fig. 3). The plasmid pCpG was then
sequenced.
Immunization and sample collection
Forty-eight mice were divided into the following
four groups, with 12 in each group: i) Control, inoculated
intraperitoneally with 100 µg pcDNA3Kan; ii) pgD, inoculated
intraperitoneally with 100 µg pgD; iii) pgD+pCpG, inoculated
intraperitoneally with 100 µg pgD and 30 µg pCpG; and
iv) pgD+pcDNA3Kan, inoculated intraperitoneally with 100 µg
pgD and 30 µg pcDNA3Kan. All groups were inoculated every 3
weeks for 6 weeks. DNA and adjuvant were all dissolved in normal
saline with a final volume of 100 µl. Blood samples (0.5 ml
per mouse) were collected at day 14 after each inoculation and
divided it into two; one for use in flow cytometry and the other
was left standing in room temperature. After standing for 1 h,
blood sample were divided into upper and lower layers, the upper of
which was the serum and was collected for use in enzyme linked
immunosorbent assay (ELISA).
ELISA of antibodies
Serum specimens were assessed for IgG antibodies to
HSV-2 using an indirect ELISA method. Using recombinant HSV-2 gD
protein (produced in the Institute of Bioengineering, Zhejiang
Academy of Medical Sciences) according to a previously described
protocol by Zhou et al (15) and affinity-purified polyclonal goat
anti-mouse IgG labeled with horseradish peroxidase (HRP; 1:4,000;
cat no. 80U00120; Beijing Dingguochangsheng Biotech Co., Ltd.,
Beijing, China) as antigen and secondary antibody. Absorbance was
determined at 450 nm using a Multiskan MK3 (Thermo Fisher
Scientific, Inc.).
CD4+ and CD8+ cell
subset detection in peripheral blood by flow cytometry
CD4+ and CD8+ cell subset
levels were detected in peripheral blood samples by flow cytometry
(16) (BD FACSCalibur, BD
Biosciences, Franklin Lakes, NJ, USA).
Virus challenge
Karber's method was used to test the lethal dose
(LD)50 of HSV-2 strain Sav in Balb/c mice by
intraperitoneal injection. All 4 groups of mice were
intra-peritoneally injected with 50 LD50 (50/50% lethal
dose) HSV-2 strain Sav at days 21 after the third
inoculation. Adverse reactions and the number of fatalities were
observed daily in the 4 groups of mice after HSV-2 challenge and
the results were recorded. All surviving mice were sacrificed at
day 14 after HSV-2 challenge.
Statistical analysis
Statistical differences for antibody titers, T
lymphocyte assays and the duration of survival were determined
using one-way analysis of variance followed by a Bonferroni
correction test using GraphPad Prism (version 4; San Diego, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference. All IgG levels and the percentage of
CD4+ or CD8+ T cells are presented as the
mean ± standard deviation.
Results
Identification of plasmid vector
pcDNA3Kan by restriction enzymes
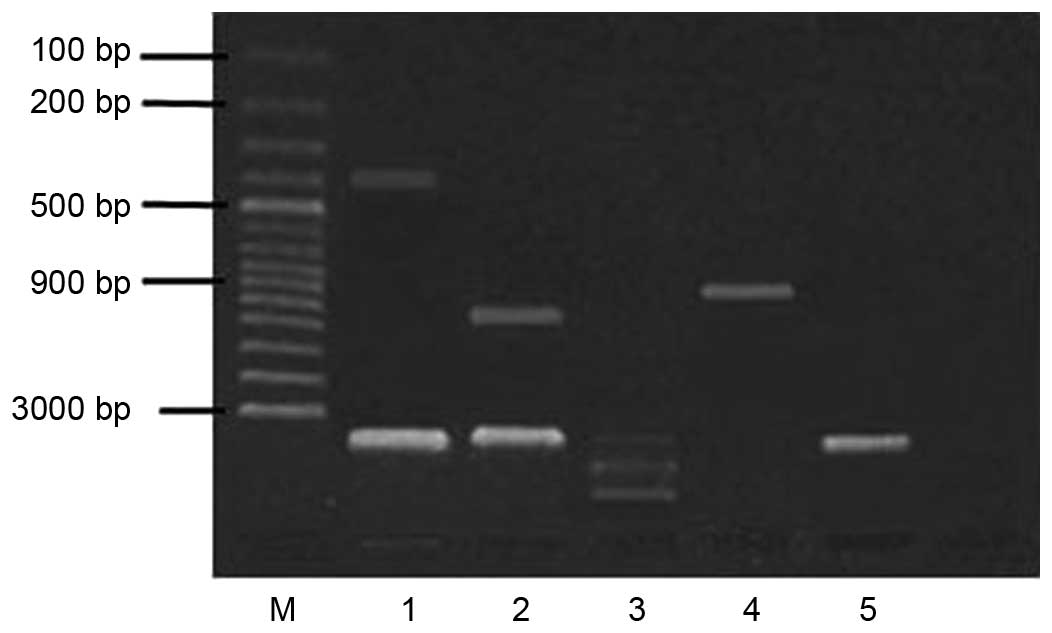
Based on the gene content of pcDNA3Kan, there are
two consensus restriction site sequences (SspI) at ~20 bp
upstream of the kanr insertion site and ~360 bp in the
kanr gene. pcDNA3Kan treated with SspI showed a
380 bp fragment in agarose gel electrophoresis (Fig. 4). The pcDNA3Kan digested with
BglII (existed upstream of the kanr insertion
site) and XhoI (existed at multiple cloning sites) showed a
960 bp fragment in agarose gel electrophoresis. Thus, the sequences
of pcDNA3Kan were interconnected successfully. The E. coli
Tg1 cells transfected with pcDNA3Kan grew well on LB medium
supplemented with 50 µg/ml kanamycin. Therefore, the
eukaryotic expression vector containing the kanr and gD
genes was successfully constructed.
Identification of the recombinant
plasmids pgD and pCpG by DNA sequencing
The gD gene in the pgD recombinant plasmids was
successfully cloned with the correct open reading fragment. The
fragment containing 8 CpG motifs in the recombinant plasmid pCpG
had the same DNA sequence as that designed.
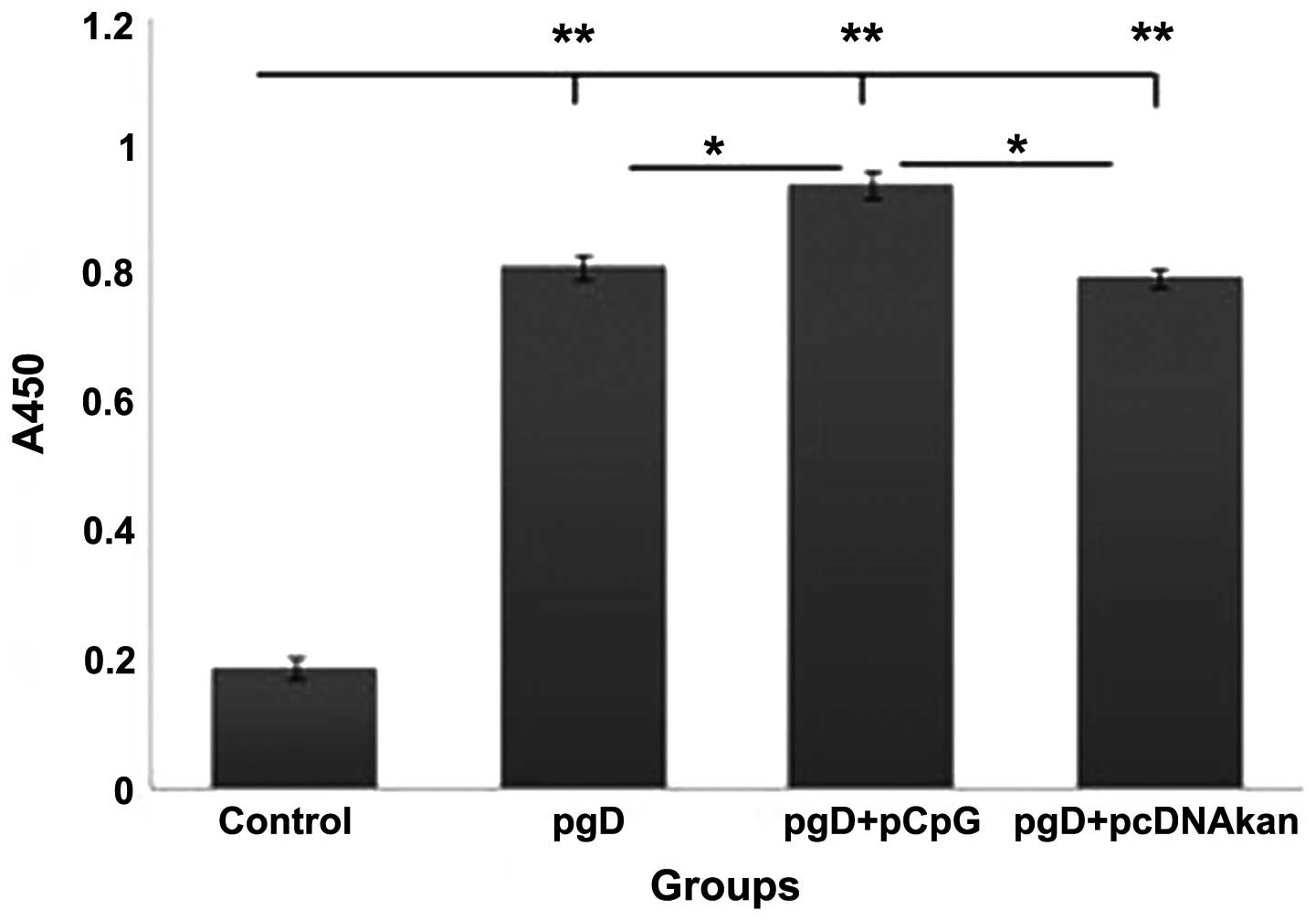
Enhancement of the anti-HSV-2-gD titer by
pgG+pCpG
Serum anti-HSV-2-gD specific total IgG was assessed
by ELISA. As shown in Fig. 5, IgG
levels were significantly increased in the pgD+pCpG group, compared
with the pgD and pgD+pcDNA3Kan groups (P<0.05), at week 8
post-inoculation. The levels in the control group were
significantly reduced compared with the remaining groups
(P<0.001).
Difference in CD4+ and
CD8+ T cells in each group
To determine the importance of these T cell subsets
in the protection of pgD-immune mice against infection with HSV-2,
CD4+ and CD8+ T cells were analyzed from all
groups prior to virus challenge. As shown in Fig. 6, the percentage of CD8+
T cells from the pgD+pCpG group was only marginally higher than in
other groups. However, the percentage of CD4+ T cells in
the pgD+pCpG group was significantly higher than in the other
groups (P<0.05), particularly compared with the control-treated
pcDNA3Kan group (P<0.001).
Protection against HSV-2 challenge
To evaluate the level of protection conferred by
immunization, mice were challenged with a lethal dose of
1×105 PFU of HSV-2, 3 weeks following the last
immunization. Mice in the pgD, pgD+pCpG and pgD+pcDNA3Kan groups
showed significantly higher survival rates compared with the
control group (Fig. 7).
Discussion
Mice were intramuscularly immunized with eukaryotic
expression plasmids encoding gD to induce protective immune
responses (17–19). Certain immune adjuvants such as
interleukin-12, chemokines, cytokines and the E. coli heat
labile enterotoxin can enhance the immunogenicity of the HSV DNA
vaccine (20–23). At present, there is no way to
provide complete immunity against HSV in mice, and there is no
appropriate HSV vaccine for humans.
The present study designed and constructed a
recombinant plasmid pCpG containing 8 CpG motifs. These were
investigated as immune adjuvants for a DNA vaccine. According to
the results, the recombinant plasmid pCpG in combination with the
DNA vaccine could protect mice infected with lethal doses of HSV-2
virus. The HSV-2 antigen specific antibodies were detected by
ELISA, and the IgG levels were moderately increased in the pgD+pCpG
group, compared with other groups, at week 8 post-inoculation.
Peripheral T-lymphocyte subsets were examined by flow cytometric
analysis, and the percentage of CD4+ T cells from the
pgD+pCpG group was significantly increased compared with the other
groups (P<0.05). Test results proved that these mice could
induce more notable cellular immunity compared with pgD+pcDNA3Kan
and pgD alone in mice.
The experimental results demonstrated that cloning
CpG motifs into plasmid DNA is an effective way to apply CpG motifs
as adjuvants for DNA vaccines. Sato et al (24) demonstrated that the characteristics
of CpG existed in plasmid DNA. Human monocytes transfected with
plasmid DNA containing CpG motifs, transcribed large amounts of
interferon-α, interferon-β, and interleukin-12. This type of immune
response is highly important.
In the present study, a new recombinant plasmid pCpG
based on CpG motifs was constructed. pCpG could significantly
improve cell-mediated immunity induced by the HSV-2 DNA vaccine.
Thus, pCpG has shown great potential as an adjuvant for the HSV-2
DNA vaccine, and may also be used for other DNA vaccines.
Acknowledgments
This study was supported by the Science and
Technology Foundation of Zhejiang Province (grant nos. 2011F20015
and 2011C23002), and the Natural Science Foundation of Zhejiang
Province (grant nos. LQ12C01002 and LY12H19009).
References
|
1
|
Eis-Hübinger AM, Schmidt DS and Schneweis
KE: Anti-glycoprotein B monoclonal antibody protects T
cell-depleted mice against herpes simplex virus infection by
inhibition of virus replication at the inoculated mucous membranes.
J Gen Virol. 74:379–385. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherwood JK, Zeitlin L, Whaley KJ, Cone RA
and Saltzman M: Controlled release of antibodies for long-term
topical passive immunoprotection of female mice against genital
herpes. Nat Biotechnol. 14:468–471. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Milligan GN, Dudley-McClain KL, Chu CF and
Young CG: Efficacy of genital T cell responses to herpes simplex
virus type 2 resulting from immunization of the nasal mucosa.
Virology. 318:507–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuklin N, Daheshia M, Karem K, Manickan E
and Rouse BT: Induction of mucosal immunity against herpes simplex
virus by plasmid DNA immunization. J Virol. 71:3138–3145.
1997.PubMed/NCBI
|
|
5
|
Manickan E, Rouse RJ, Yu Z, Wire WS and
Rouse BT: Genetic immunization against herpes simplex virus.
Protection is mediated by CD4+ T lymphocytes. J Immunol.
155:259–265. 1995.PubMed/NCBI
|
|
6
|
McDermott MR, Goldsmith CH, Rosenthal KL
and Brais LJ: T lymphocytes in genital lymph nodes protect mice
from intravaginal infection with herpes simplex virus type 2. J
Infect Dis. 159:460–466. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoshino Y, Dalai SK, Wang K, Pesnicak L,
Lau TY, Knipe DM, Cohen JI and Straus SE: Comparative efficacy and
immunogenicity of replication-defective, recombinant glycoprotein,
and DNA vaccines for herpes simplex virus 2 infections in mice and
guinea pigs. J Virol. 79:410–418. 2005. View Article : Google Scholar :
|
|
8
|
Ramachandran S and Kinchington PR:
Potential prophylactic and therapeutic vaccines for HSV infections.
Curr Pharm Des. 13:1965–1973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harandi AM: The potential of
immunostimulatory CpG DNA for inducing immunity against genital
herpes: Opportunities and challenges. J ClinVirol. 30:207–210.
2004.
|
|
10
|
Kwant A and Rosenthal KL: Intravaginal
immunization with viral subunit protein plus CpG
oligodeoxynucleotides induces protective immunity against HSV-2.
Vaccine. 22:3098–3104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harandi AM: The potential of
immunostimulatory CpG DNA for inducing immunity against genital
herpes: Opportunities and challenges. J Clin Virol. 30:207–210.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spear PG and Roizman B: Proteins specified
by herpes simplex virus. V. Purification and structural proteins of
the herpesvirion. J Virol. 9:143–159. 1972.PubMed/NCBI
|
|
13
|
Hong Y, Yang LH, Chen Y, Jing L, Jiang JH
and Wang YT: Immune response induced by herpes simplex virus-2 DNA
vaccine in mice. Chin J Publ Health. 19:1079–1080. 2003.
|
|
14
|
Sambrook J and Russell D: Molecular
Cloning: A Laboratory Manual. 3rd Edition. Cold Spring Harbor
Laboratory Press; New York: pp. 1632001
|
|
15
|
Zhou C, Cao CL, Fan JY and Yang HL:
Prokaryotic expression of full length HSV-2 gD antigen and its
antienicity. J Pract Med. 24:1668–1670. 2008.
|
|
16
|
Prince HE, Arens L and Kleinman SH: CD4
and CD8 subsets defined by dual-color cytofluorometry which
distinguish symptomatic from asymptomatic blood donors seropositive
for human immunodeficiency virus. Diagn Clin Immunol. 5:188–193.
1987.PubMed/NCBI
|
|
17
|
Görander S, Ekblad M, Bergström T and
Liljeqvist JÅ: Anti-glycoprotein g antibodies of herpes simplex
virus 2 contribute to complete protection after vaccination in mice
and induce antibody-dependent cellular cytotoxicity and
complement-mediated cytolysis. Viruses. 6:4358–4372. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Awasthi S, Balliet JW, Flynn JA, Lubinski
JM, Shaw CE, DiStefano DJ, Cai M, Brown M, Smith JF, Kowalski R, et
al: Protection provided by a herpes simplex virus 2 (HSV-2)
glycoprotein C and D subunit antigen vaccine against genital HSV-2
infection in HSV-1-seropositive guinea pigs. J Virol. 88:2000–2010.
2014. View Article : Google Scholar :
|
|
19
|
Delagrave S, Hernandez H, Zhou C,
Hamberger JF, Mundle ST, Catalan J, Baloglu S, Anderson SF,
DiNapoli JM, Londoño-Hayes P, et al: Immunogenicity and efficacy of
intra-muscular replication-defective and subunit vaccines against
herpes simplex virus type 2 in the mouse genital model. PLoS One.
7:e467142012. View Article : Google Scholar
|
|
20
|
Sin JI, Kim JJ, Arnold RL, Shroff KE,
McCallus D, Pachuk C, McElhiney SP, Wolf MW, Pompa-de Bruin SJ,
Higgins TJ, et al: IL-12 gene as a DNA vaccine adjuvant in a herpes
mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated
protective immunity against herpes simplex virus-2 challenge. J
Immunol. 162:2912–2921. 1999.PubMed/NCBI
|
|
21
|
Eo SK, Lee S, Chun S and Rouse BT:
Modulation of immunity against herpes simplex virus infection via
mucosal genetic transfer of plasmid DNA encoding chemokines. J
Virol. 75:569–578. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee S, Gierynska M, Eo SK, Kuklin N and
Rouse BT: Influence of DNA encoding cytokines on systemic and
mucosal immunity following genetic vaccination against herpes
simplex virus. Microbes Infect. 5:571–578. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haynes JR, Arrington J, Dong L, Braun RP
and Payne LG: Potent protective cellular immune responses generated
by a DNA vaccine encoding HSV-2 ICP27 and the E. coli heat labile
enterotoxin. Vaccine. 24:5016–5026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato Y, Roman M, Tighe H, Lee D, Corr M,
Nguyen MD, Silverman GJ, Lotz M, Carson DA and Raz E:
Immunostimulatory DNA sequences necessary for effective intradermal
gene immunization. Science. 273:352–354. 1996. View Article : Google Scholar : PubMed/NCBI
|