Introduction
Lumbar disc herniation (LDH) that causes back and
leg pain, numbness, muscle loss and sphincter disturbances, amongst
other symptoms, is a common orthopedic disease (1). Waist LDH is divided into three types:
i) Nuclear protrusion does not damage the fiber ring; ii)
subligamentous extrusion of nucleus pulposus does not damage
posterior longitudinal ligaments; iii) sequestration is a posterior
longitudinal ligament rupture, in which nucleus pulposus is
dislocated to the spinal canal (2). It is currently accepted that
intervertebral disc degeneration or damage and inflammation of the
surrounding soft tissue are the source of lower back pain (3). Oppression of nerves in locations away
from the intervertebral disc causes nerve damage and degeneration
of outstanding intervertebral discs. After the intervertebral disc
ruptures, dislocation of nucleus pulposus stimulates nerve to
produce chemical nerve root inflammation, which leads to sciatica
(4). Therefore, the aim of
conservative treatment should be regeneration of degenerated
intervertebral discs and neural protection, with a focus on disc
nucleus pulposus tissue reuptake (5).
Intervertebral disc degeneration, caused by a series
of spinal degenerative diseases and their secondary pathological
effects, including vascular injury and oxidative stress is common;
however, the cause and specific mechanism remains unclear. The
excessive apoptosis of intervertebral disc cells directly decreases
the number of intervertebral disc cells, which results in
intervertebral disc degeneration. Nucleus pulposus cells (NPCs) are
important, as they maintain the normal intervertebral disc
environment and repair degenerated intervertebral discs. Therefore,
excessive apoptosis of NPCs is the direct cause of intervertebral
disc degeneration (6). The
apoptosis process is triggered through mitochondrial signaling
pathways, which are dependent on hydrogen peroxide
(H2O2)-mediated oxidative stress (7).
Cannabidiol was isolated from marijuana during the
1940s, and in vivo experiments demonstrated that cannabidiol
stimulated cannabinoid receptor type I, which affected mental
activity, whilst also exerting anticonvulsant (8), sedative hypnotic (9), anti-anxiety (10), antipsychotic (11), anti-inflammatory (12) and nerve protective (13) effects. Preclinical and clinical
studies have indicated that the pharmacokinetic properties of
cannabidiol are good; following injection it quickly passes through
the blood-brain barrier, exerting a marked protective effect on the
cranial nerve (14). Furthermore,
cannabidiol exerts its neuroprotective effects through multiple
channels (15). The aim of the
current study was to evaluate the protective effect of cannabidiol
by investigating whether its administration prevented
H2O2-induced apoptosis, inflammation and
oxidative stress in NPCs.
Materials and methods
Reagents
Gibco Dulbecco's modified Eagle's medium/Ham's F-12
(DMEM/F-12) was obtained from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA) and HyClone fetal bovine serum (FBS) was
obtained from GE Healthcare Life Sciences (Logan, UT, USA).
Cannabidiol (>98% purity) was supplied by Sigma-Aldrich (St.
Louis, MO, USA) and its chemical structure is demonstrated in
Fig. 1. The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was obtained from Beyotime Institute of Biotechnology
(Haimen, China) and the Invitrogen TRIzol reagent was obtained from
Thermo Fisher Scientific, Inc. A reverse transcription-polymerase
chain reaction (RT-PCR) kit was obtained from Takara Biotechnology
Co., Ltd. (Dalian, China) and the Applied Biosystems ABI Prism
7900HT Real-Time PCR system was obtained from Thermo Fisher
Scientific, Inc. Invitrogen interleukin (IL)-1β and IL-6 assay kits
were obtained from Thermo Fisher Scientific, Inc.
Animals
A total of 40 healthy male Sprague-Dawley rats
(weight, 362±35 g, 10 rats per treatment group) were obtained from
the Center of Experimental Animals of Xi'an Jiaotong University
(Xi'an, China) and selected as NPC donors. All rats were housed
with their respective groups at a temperature of 22±1°C under a
12-h light/dark cycle with food and water available ad
libitum. The study protocol was approved by the Animal Use and
Care Committee for Research and Education of Xi'an Jiaotong
University.
Cell isolation and culture
Rat NPCs were isolated according to a previously
described explant culture method (16). Briefly, rats were anesthetized with
10% chloral hydrate (i.p.; 4 ml/kg body weight). The lumbar
intervertebral discs were resected from the spinal column. The
gel-like nucleus pulposus tissue was separated from the annulus
fibrosus under aseptic conditions. The gelatinous nucleus pulposus
tissue samples were obtained from the rats and sliced into small
sections. Under aseptic conditions, skin and tissue were separated
at the thigh, and then the annulus fibrosus was incised to separate
the gel-like nucleus pulposus tissue. The tissues were digested
with 0.1% type-2 collagenase (Sigma-Aldrich) in DMEM/F-12 at 2,000
×g. The cells were cultivated with 0.1% type-2 collagenase in
DMEM/F-12 in an incubator at 37°C under an atmosphere of 5% carbon
dioxide for 4 h. Following cultivation, the suspension was filtered
through mesh (pore size, a 70-μm; Thermo Fisher Scientific,
Inc.). The filtered cells were washed with DMEM/F-12 3 times and
then placed in 25-cm2 culture flasks (Thermo Fisher
Scientific, Inc.). Finally, the NPCs were incubated with DMEM/F-12,
10% FBS, 100 U/ml streptomycin, 100 U/ml penicillin at 37°C under
an atmosphere of 5% carbon dioxide. The NPCs were chondrocyte-like
cells, identified by immunohistostaining of type II collagen and
aggrecan.
Establishment of NPC apoptosis
models
Briefly, NPCs were plated onto a 6-well plate at a
density of 1×106 cells/well. NPCs were exposed to 200
μM H2O2 for 24 h to induce damage.
Cell viability assay
NPCs were plated in a 96-well plate at a density of
2×104 cells/well. After 24 h the medium was replaced
with phosphate-buffered saline (PBS; Sangon Biotech Co., Ltd.,
Shanghai, China; control group), DMEM/F-12 containing
H2O2 (model group) or cannabidiol (2.5 or 5
μM group) (17). For
quantitative analysis of cell viability, 0.5% MTT solution (20
μl; Beyotime Institute of Biotechnology) with PBS was added
to each well and incubated for 4 h at 37°C under an atmosphere of
5% carbon dioxide. The culture medium was replaced, and 200
μl dimethyl sulfoxide (Sangon Biotech Co., Ltd.) was added
to each well and agitated for 20 min at room temperature. The
optical density as measured at 450 nm using a microplate reader
(ELx800; BioTek Instruments, Winooski, VT, USA).
Annexin V-fluorescein isothiocyanate
(FITC)/propidium-iodide (PI) staining
NPCs (1×106 cells/well) were plated onto
a 6-well plate. The medium was replaced after 24 h with PBS
(control group), DMEM/F-12 containing H2O2
(model group) or cannabidiol (2.5 or 5 μM group) (17). Annexin V-FITC (5 μl) and PI
(5 μl) were added and incubated for 10 min in the dark at
room temperature, both were obtained from BD Biosciences (Franklin
Lakes, NJ, USA). Cell apoptosis was examined using a BD FACSCanto
II flow cytometer (BD Biosciences). Cell Quest Pro software was
used for this data (version 4.01; BD Biosciences)
RT-quantitative PCR of caspase-3 and
COX-2 mRNA expression levels
NPCs were plated onto 6-well plates at a density of
1×106 cells/well. After 24 h, the medium was replaced
with PBS (control group), DMEM/F-12 containing
H2O2 (model group) or cannabidiol (2.5 or 5
μM group) (17). Total RNA
was extracted from NPCs using TRIzol reagent according to the
manufacturer's protocol and cDNA was transcribed from RNA using the
RT-PCR kit according to the manufacturer's instructions. The qPCR
system was performed using an ABI Prism 7900HT Real-Time PCR system
according to the manufacturer's instructions. The SYBR Green I
florescent dye (Thermo Fisher Scientific, Inc.) method was
conducted to quantify the cDNA. Table
I demonstrates the primer sequences(Sangon Biotech Co.,
Ltd.).
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis of
gene expression. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis of
gene expression.
| Gene | Primer
sequence |
|---|
| Caspase-3 | Forward
5′-GGCCTGCTTTTTACCTCAGA3′ |
| Reverse
5′-CGTTTCCGCACAGGCTGCTT-3′ |
| COX-2 | Forward
5′-GTGTATCCCCCCACAGTCAAA-3′ |
| Reverse
5′-ACACTCTGTTGTGCTCCCGAA-3′ |
| GAPDH | Forward
5′-TGTCTCCTGCGACTTCAACAG3′ |
| Reverse
5′-GAGGCCATGTAGGCCATGAG-3′ |
Measurement of IL-1β and IL-6
NPCs were plated onto 96-well plates at a density of
1×104 cells/well. The medium was replaced 24 h later
with PBS (control group), DMEM/F-12 containing
H2O2 (model group) or cannabidiol (2.5 or 5
μM group) (17). An
Invitrogen ELISA kit (Thermo Fisher Scientific, Inc.) was used to
measure the quantities of IL-1β and IL-6 according to the
manufacturer's instructions.
Western blot analysis of Bcl-2 and
inducible nitric oxide synthase (iNOS)
NPCs were plated onto 6-well plates at a density of
1×106 cells/well. After 24 h, the medium was replaced
PBS (control group), DMEM/F-12 containing
H2O2 (model group) or cannabidiol (2.5 or 5
μM group) (17). The NPCs
were prepared in RIPA lysis buffer (Beyotime Institute of
Biotechnology) for 30 min on ice. The cytochylema was subsequently
centrifuged at 12,000 x g for 10 min at 4°C and the supernate was
collected. The cytochylema protein concentration was determined
using a commercial bicinchoninic acid protein assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Equal protein (50 ng) was
resolved on 12% SDS-PAGE gel (Thermo Fisher Scientific, Inc.) and
transferred to nitrocellulose membranes (EMD Millipore, Billerica,
MA, USA). After the membranes were washed 3 times for 5 min, the
membranes were incubated with polyclonal rabbit anti-Bcl-2 (cat.
no. sc-492; 1:1,500; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) polyclonal rabbit anti-iNOS (cat. no. sc-650; 1:1,500; Santa
Cruz Biotechnology, Inc.) and polyclonal rabbit anti-β-actin (cat.
no. D110007; 1:500; Sangon Biotech Co., Ltd.) overnight at 4°C. The
membranes were subsequently incubated with corresponding monoclonal
mouse anti-rabbit horseradish peroxidase-conjugated secondary
antibody (cat. no. D110059-0100; 1:5,000; Sangon Biotech Co., Ltd.)
for 2 h at room temperature.
Statistical analysis
All statistical analysis was conducted using SPSS
18.0 software (SPSS, Inc., Chicago, IL, USA) and data are presented
as means ± standard deviation. Values were evaluated by one way
analysis of variance, followed by Duncan's multiple range tests and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of cannabidiol on cell viability
in H2O2-treated NPCs
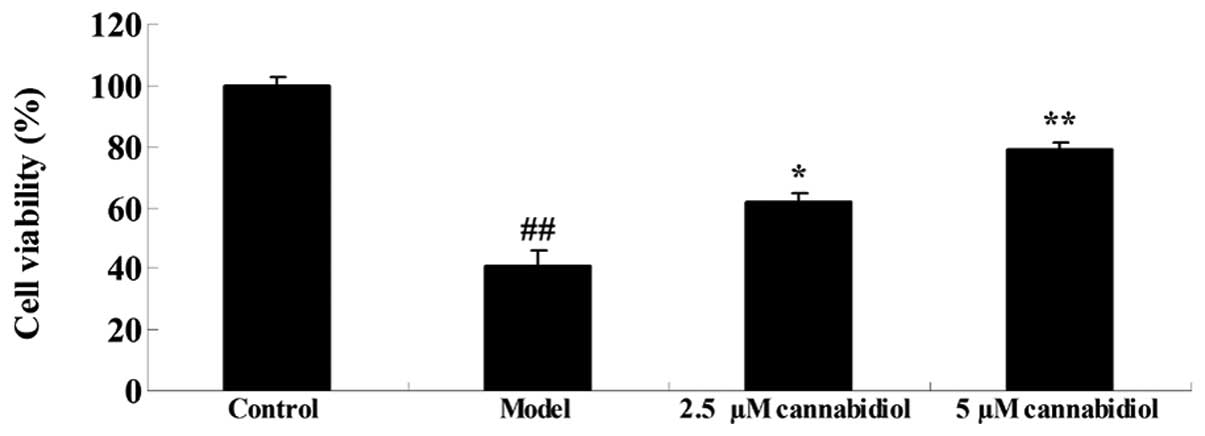
NPC viability was analyzed by MTT assay. The results
indicated reduced cell viability in NPCs treated with 200 μM
H2O2 when compared with the control group
(P<0.01; Fig. 2). A significant
increase was observed in the 2.5 and 5 μM
cannabidiol-treated groups, compared with cell viability of NPC
following 200 μM H2O2 treatment
(Fig. 2). These results indicate
that cannabidiol may exert protective effects on NPCs.
Effect of cannabidiol on apoptosis in
H2O2-treated NPCs
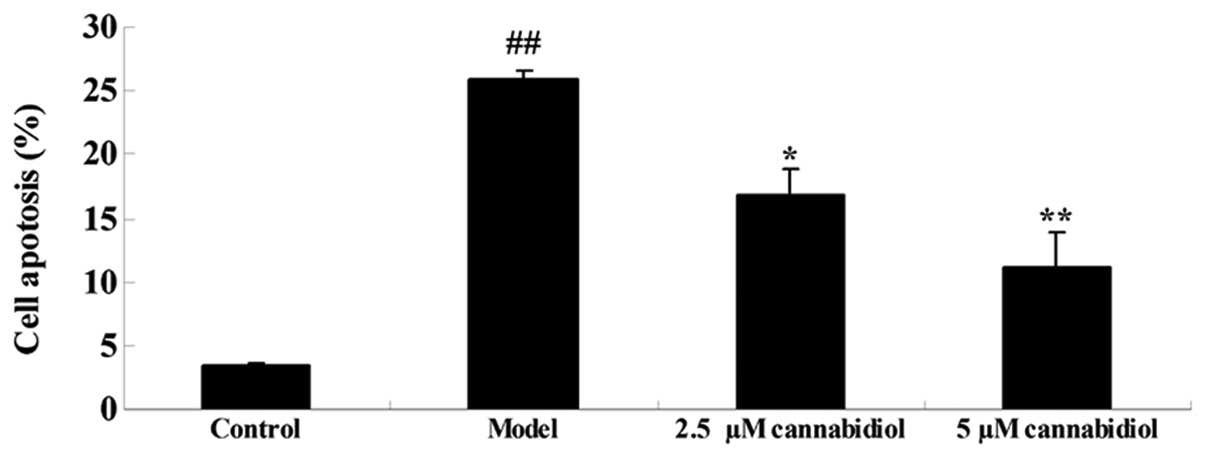
To analyze the effect of cannabidiol on apoptosis in
H2O2-treated NPCs, the rate of apoptosis was
quantified by flow cytometry. As presented in Fig. 3, a significant increase in the rate
of apoptosis was observed after 24 h of exposure to 200 μM
H2O2. The increased apoptotic rate was
suppressed by 2.5 or 5 μM cannabidiol treatment in the NPCs
exposed to 200 μM H2O2.
Effect of cannabidiol on caspase-3 gene
expression in H2O2-treated NPCs
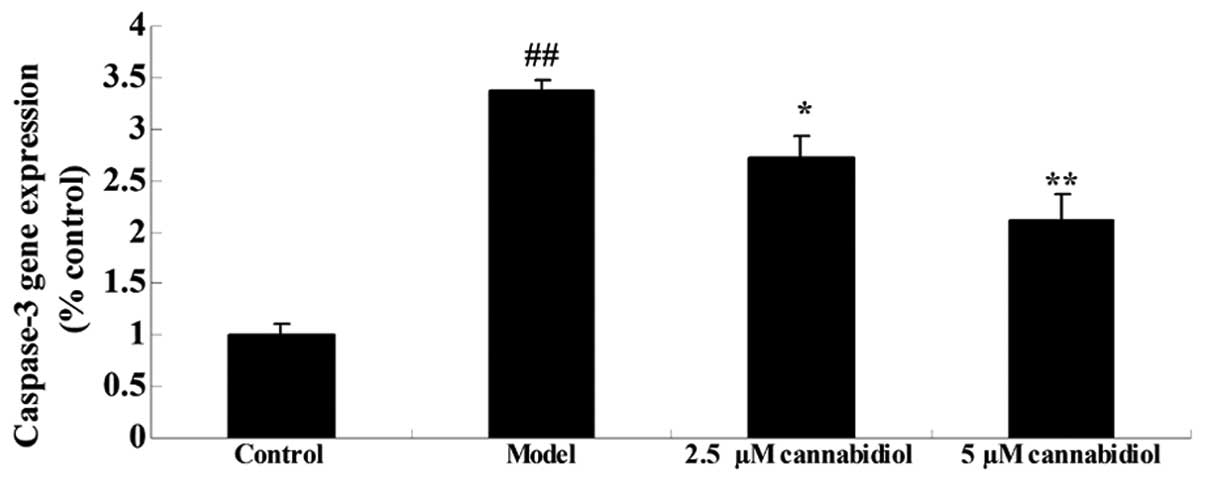
To further investigate the effect of cannabidiol on
apoptosis in H2O2-treated NPCs, caspase-3
gene expression was quantified using qPCR. As presented in Fig. 4, H2O2
exposure (200 μM) increased caspase-3 gene expression of the
NPCs when compared with that of the control group. Treatment with
2.5 or 5 μM cannabidiol significantly inhibited
H2O2-induced caspase-3 gene expression
(Fig. 4).
Effect of cannabidiol on the expression
levels of Bcl-2 in H2O2-treated NPCs
To elucidate the effect of cannabidiol on the
expression levels of Bcl-2 in H2O2-treated
NPCs, the protein expression levels of Bcl-2 were measured by
western blot analysis. The results indicated that
H2O2 exposure (200 μM) induced a
significant reduction in the Bcl-2 protein expression level in NPCs
following treatment for 24 h compared with the control group
(Fig. 5A and B). Treatment with
2.5 or 5 μM cannabidiol was demonstrated to prevent this
reduction (Fig. 5A and B).
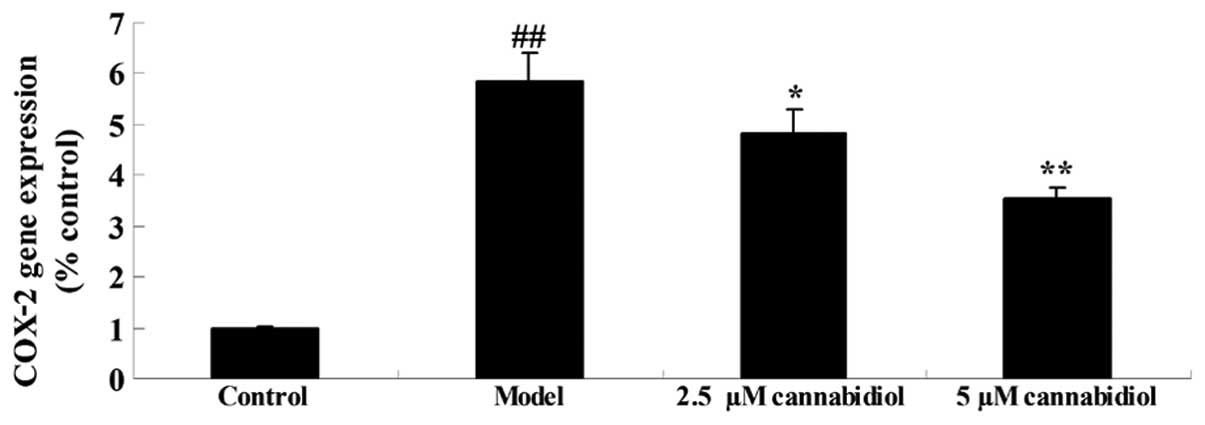
Effect of cannabidiol on COX-2 gene
expression in H2O2-treated NPCs
To investigate the effect of cannabidiol on COX-2 in
H2O2-treated NPCs, COX-2 gene expression was
quantified by qPCR. As presented in Fig. 6, 200 μM
H2O2 significantly increased the COX-2 gene
expression compared with the control group. Treatment with 2.5 or 5
μM cannabidiol inhibited this increase (Fig. 6).
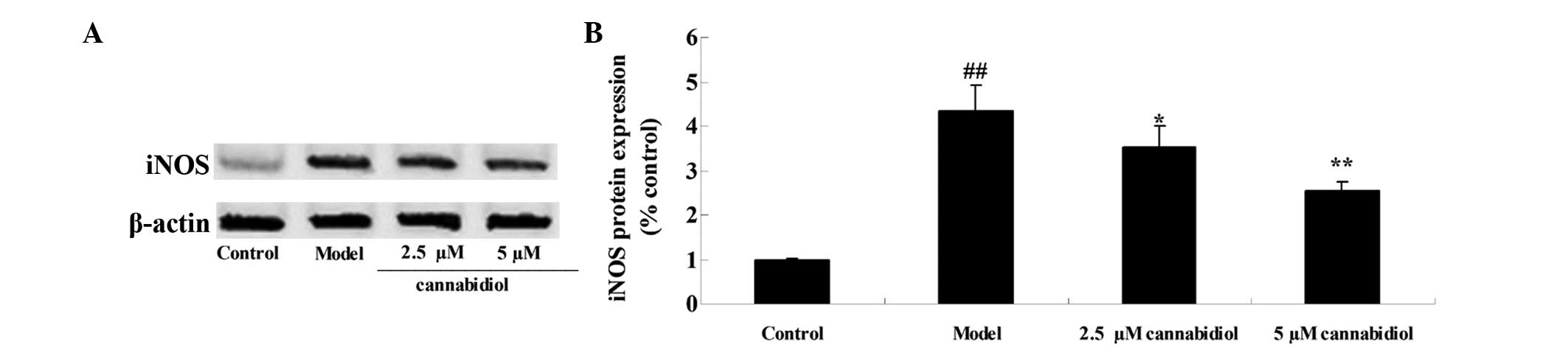
Effect of cannabidiol on the expression
level of iNOS in H2O2-treated NPCs
To clarify the effect of cannabidiol on the
expression level of iNOS in H2O2-treated
NPCs, the protein expression levels of iNOS were measured by
western blot analysis. As presented in Fig. 7A and B, 200 μM
H2O2 significantly increased the level of
iNOS protein compared with the control group. Notably, treatment
with 2.5 or 5 μM cannabidiol inhibited the increased iNOS
protein expression in H2O2-treated NPCs
(Fig. 7A and B).
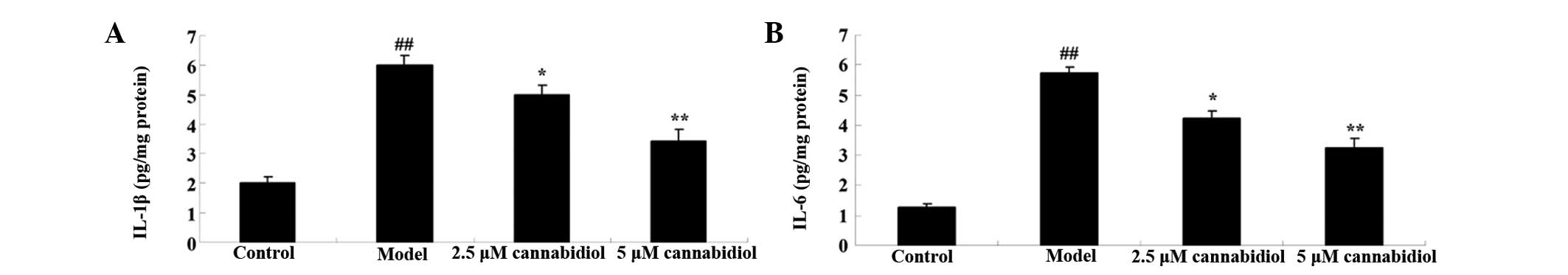
Effect of cannabidiol on IL-1β and IL-6
in H2O2-treated NPCs
The effect of cannabidiol on inflammation in
H2O2-treated NPCs was also investigated in
the present study by measurement of IL-1β and IL-6 levels. As
presented in Fig. 8A and B, 200
μM H2O2 significantly increased the
levels of IL-1β and IL-6 in the NPCs. Notably, administration of
2.5 or 5 μM cannabidiol to the
H2O2-treated NPCs inhibited the increase in
IL-1β and IL-6 levels (Fig. 8A and
B).
Discussion
LDH is a common type of clinical disease. Due to
changes in the way individuals work and live, the incidence of LDH
is increasing and is affecting individuals at a younger age
(2). According to statistics,
~85–90% of the patients exhibiting LDH demonstrate a satisfactory
recovery following appropriate non-surgical treatment (2). The present study demonstrated that
cannabidiol treatment exerted a protective effect on NPCs in
vitro by increasing cell viability and decreasing apoptosis
following H2O2 exposure. Kwiatkoski et
al (18) confirmed that
cannabidiol administered to treat cryogenic spinal cord injury
resulted in higher motor scores in rats.
Previous studies found that apoptosis may be
involved in intervertebral disc tissue degeneration of
pathophysiological changes, and indicates that apoptosis was
important in the process of intervertebral disc degeneration
(19). Excessive cell apoptosis
results in a reduction in the activity of intervertebral disc cells
and a subsequent decrease in extracellular matrix change in
synthesis and composition, contributing to the pathology of
intervertebral disc degeneration (20). Numerous studies regarding signal
transduction pathways have indicated that intervertebral disc cells
may be associated with caspase-3 and Bcl-2 signal transduction and
apoptosis, regulation of apoptosis may be a method of preventing
invertebral disc degeneration (21). In the present study, it was
observed that treatment with cannabidiol markedly reduced the level
of caspase-3 gene expression and increased the Bcl-2 protein
expression level in the NPCs that had undergone
H2O2 exposure. Mecha et al (17) demonstrated that cannabidiol
treatment protects oligodendrocyte progenitor cells by decreasing
caspase-3 gene expression and increasing the expression of
anti-apoptotic Bcl-2.
In normal circumstances within the body, the
generation of reactive oxygen species (ROS) and the active oxygen
removal systems are in a state of dynamic balance. Various
circumstances can result in increased ROS production and/or a
reduced ability of the body to remove ROS, causing a state of
oxidative stress. When the body is in a state of oxidative stress,
tissue cells contain elevated levels of molecular oxygen, which the
body is unable to remove. This leads to increased tissue lipid
peroxidation levels, causes abnormal oxidative DNA damage and
protein expression, and ultimately damages the body (22). In the present study,
H2O2-treated NPCs exhibited an increased
COX-2 gene expression level. Administration of cannabidiol
significantly inhibited the H2O2-stimulated
increase in COX-2 gene expression. Castillo et al (23) reported that cannabidiol inhibited
hypoxic-ischemic brain damage by reducing COX-2 and iNOS expression
levels. Wheal et al (24)
demonstrated that cannabidiol improves vasorelaxation through
COX-1/2 activation in Zucker diabetic fatty rats.
During LDH, persistent compression of the spinal
cord or nerve root by an inflamed outstanding intervertebral disc,
activates iNOS, producing an increased quantity of nitric oxide
(NO), thus increasing the NOS expression levels in the damaged area
(25). As compression of the
spinal cord or nerve root by the outstanding intervertebral disc
cannot be removed, spinal cord ischemia, hypoxia and blocked energy
generation are observed. This results in increased enzyme activity
and insufficient substrate for NO synthesis. Thus, the NOS
(resulting from insufficient substrate) promotes superoxide anion
and H2O2 production, tissue damage, kills
neurons surrounding the spinal cord and leads to nerve cell
degeneration and necrosis. Finally, iNOS induces motor neuron
death, causing the loss of cells that synthesize NO, resulting in
reduced NOS activity and NO content in the serum (26,27).
The present study provides evidence that treatment with cannabidiol
significantly suppressed the enhanced level of iNOS protein
resulting from H2O2 exposure. Furthermore, De
Filippis et al (28)
indicated that cannabidiol reduced intestinal inflammation via
suppression of iNOS expression. Esposito et al (29) confirmed that cannabidiol suppressed
IL-1β and iNOS expression in vivo, which inhibits β-amyloid
induced neuroinflammation.
The major clinical manifestation of intervertebral
disc degeneration is lower back pain, and clinical treatments
include conservative treatment, intervertebral disc resection and
spinal fusion; however, these treatments cannot fundamentally
prevent degeneration (30). At
present, the study of lumbar disc degeneration is concentrated
within the field of molecular biology (31). During investigation of the
mechanisms underlying lumbar disc degeneration, the focus is
predominantly on genetic factors, high loading of intervertebral
discs and nutritional disorders; analyzing cytokines and
inflammatory transmitters is also considered to be important
(32,33). IL-1, IL-4, and tumor necrosis
factor-α expression levels, within the intervertebral discs of the
lumbar region, contribute to synthesis and decomposition of the
matrix, inflammation and neurotransmission, and are important in
disc cell apoptosis (34). The
findings of the present study indicate that cannabidiol decreased
the increased levels of IL-1β and IL-6 in
H2O2-treated rats. Barichello et al
(35) demonstrated that
cannabidiol reduced the host immune response and exerted an
anti-inflammatory effect in Wistar rats that were submitted to
pneumococcal meningitis. Hao et al (36) suggested that cannabidiol protects
against doxorubicin-induced cardiomyopathy by modulating
antioxidant and anti-inflammatory activity.
In conclusion, the current study demonstrated that
cannabidiol treatment exerts a protective effect on
H2O2-treated NPCs. However, the underlying
mechanism of the protective effect of cannabidiol remains unknown,
but appears to be partly mediated by anti-apoptosis,
anti-inflammation and anti-oxidative activities, as well as by
protecting mitochondrial function. These data provide a potential
mechanism of cannabidiol reversing the effects upon
H2O2-exposed NPCs, and support the
therapeutic rationale for the use of cannabidiol to treat human
LDH.
Acknowledgments
The present study was supported by Shannxi Province
Natural Science Foundation Grants (grant no. 2011k14-08-06).
References
|
1
|
Emel E, Karagöz F, Aydin IH, Hacisalihoğlu
S and Seyithanoğlu MH: Alkaptonuria with lumbar disc herniation: A
report of two cases. Spine (Phila Pa 1976). 25:2141–2144. 2000.
View Article : Google Scholar
|
|
2
|
Zhu Z, Huang P, Chong Y, George SK, Wen B,
Han N, Liu Z, Kang L and Lin N: Nucleus pulposus cells derived
IGF-1 and MCP-1 enhance osteoclastogenesis and vertebrae disruption
in lumbar disc herniation. Int J Clin Exp Pathol. 7:8520–8531.
2014.
|
|
3
|
Lee SH, Kim SY, Kim JH, Jung HY, Moon JH,
Bae KH and Choi BT: Phosphoproteomic analysis of electroacupuncture
analgesia in an inflammatory pain rat model. Mol Med Rep.
6:157–162. 2012.PubMed/NCBI
|
|
4
|
Greg Anderson D, Li X, Tannoury T, Beck G
and Balian G: A fibronectin fragment stimulates intervertebral disc
degeneration in vivo. Spine (Phila Pa 1976). 28:2338–2345. 2003.
View Article : Google Scholar
|
|
5
|
Zhang CC, Cui GP, Hu JG, Xiao YZ, Zhou XS,
Shao C, Lin Q and Zhou JS: Effects of adenoviral vector expressing
hIGF-1 on apoptosis in nucleus pulposus cells in vitro. Int J Mol
Med. 33:401–405. 2014.
|
|
6
|
Erwin WM, Islam D, Inman RD, Fehlings MG
and Tsui FW: Notochordal cells protect nucleus pulposus cells from
degradation and apoptosis: Implications for the mechanisms of
intervertebral disc degeneration. Arthritis Res Ther. 13:R2152011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen JW, Ni BB, Li B, Yang YH, Jiang SD
and Jiang LS: The responses of autophagy and apoptosis to oxidative
stress in nucleus pulposus cells: implications for disc
degeneration. Cell Physiol Biochem. 34:1175–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Consroe PF and Wolkin AL: Anticonvulsant
interaction of cannabidiol and ethosuximide in rats. J Pharm
Pharmacol. 29:500–501. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Casarotto PC, Gomes FV, Resstel LB and
Guimarães FS: Cannabidiol inhibitory effect on marble-burying
behaviour: Involvement of CB1 receptors. Behav Pharmacol.
21:353–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moreira FA, Aguiar DC and Guimarães FS:
Anxiolytic-like effect of cannabidiol in the rat Vogel conflict
test. Prog Neuropsychopharmacol Biol Psychiatry. 30:1466–1471.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuardi AW, Crippa JA, Hallak JE,
Bhattacharyya S, Atakan Z, Martin-Santos R, McGuire PK and
Guimarães FS: A critical review of the antipsychotic effects of
cannabidiol: 30 years of a translational investigation. Curr Pharm
Des. 18:5131–5140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oláh A, Tóth BI, Borbíró I, Sugawara K,
Szöllõsi AG, Czifra G, Pál B, Ambrus L, Kloepper J, Camera E, et
al: Cannabidiol exerts sebostatic and antiinflammatory effects on
human sebocytes. J Clin Invest. 124:3713–3724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iuvone T, Esposito G, De Filippis D,
Scuderi C and Steardo L: Cannabidiol: A promising drug for
neurodegenerative disorders? CNS Neurosci Ther. 15:65–75. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hind WH, England TJ and O'Sullivan SE:
Cannabidiol protects an in vitro model of the blood brain barrier
from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br
J Pharmacol. 173:815–825. 2015. View Article : Google Scholar
|
|
15
|
Deiana S, Watanabe A, Yamasaki Y, Amada N,
Arthur M, Fleming S, Woodcock H, Dorward P, Pigliacampo B, Close S,
et al: Plasma and brain pharmacokinetic profile of cannabidiol
(CBD), cannabidivarine (CBDV), Δ(9)-tetrahydrocannabivarin (THCV)
and cannabigerol (CBG) in rats and mice following oral and
intraperitoneal administration and CBD action on
obsessive-compulsive behaviour. Psychopharmacology (Berl).
219:859–873. 2012. View Article : Google Scholar
|
|
16
|
Risbud MV, Guttapalli A, Stokes DG,
Hawkins D, Danielson KG, Schaer TP, Albert TJ and Shapiro IM:
Nucleus pulposus cells express HIF-1 alpha under normoxic culture
conditions: A metabolic adaptation to the intervertebral disc
microenvironment. J Cell Biochem. 98:152–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mecha M, Torrao AS, Mestre L,
Carrillo-Salinas FJ, Mechoulam R and Guaza C: Cannabidiol protects
oligodendrocyte progenitor cells from inflammation-induced
apoptosis by attenuating endoplasmic reticulum stress. Cell Death
Dis. 3:e3312012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwiatkoski M, Guimarães FS and Del-Bel E:
Cannabidiol-treated rats exhibited higher motor score after
cryogenic spinal cord injury. Neurotox Res. 21:271–280. 2012.
View Article : Google Scholar
|
|
19
|
Alini M, Eisenstein SM, Ito K, Little C,
Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I and Wilke HJ:
Are animal models useful for studying human disc
disorders/degeneration? Eur Spine J. 17:2–19. 2008. View Article : Google Scholar
|
|
20
|
Kermani HR, Hoboubati H, Esmaeili-Mahani S
and Asadi-Shekaari M: Induction of intervertebral disc cell
apoptosis and degeneration by chronic unpredictable stress. J
Neurosurg Spine. 20:578–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang SD, Bai ZL, Zhang F, Ma L, Yang DL
and Ding WY: Levofloxacin increases the effect of serum deprivation
on anoikis of rat nucleus pulposus cells via Bax/Bcl-2/caspase-3
pathway. Toxicol Mech Methods. 24:688–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou G, Lu H, Chen M, Yao H and Zhao H:
Oxidative stress participates in age-related changes in rat lumbar
intervertebral discs. Arch Gerontol Geriatr. 59:665–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castillo A, Tolón MR, Fernández-Ruiz J,
Romero J and Martinez-Orgado J: The neuroprotective effect of
cannabidiol in an in vitro model of newborn hypoxic-ischemic brain
damage in mice is mediated by CB(2) and adenosine receptors.
Neurobiol Dis. 37:434–440. 2010. View Article : Google Scholar
|
|
24
|
Wheal AJ, Cipriano M, Fowler CJ, Randall
MD and O'Sullivan SE: Cannabidiol improves vasorelaxation in Zucker
diabetic fatty rats through cyclooxygenase activation. J Pharmacol
Exp Ther. 351:457–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rannou F, Richette P, Benallaoua M,
François M, Genries V, Korwin-Zmijowska C, Revel M, Corvol M and
Poiraudeau S: Cyclic tensile stretch modulates proteoglycan
production by intervertebral disc annulus fibrosus cells through
production of nitrite oxide. J Cell Biochem. 90:148–157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furusawa N, Baba H, Miyoshi N, Maezawa Y,
Uchida K, Kokubo Y and Fukuda M: Herniation of cervical
intervertebral disc: Immunohistochemical examination and
measurement of nitric oxide production. Spine (Phila Pa 1976).
26:1110–1116. 2001. View Article : Google Scholar
|
|
27
|
Hassanin A, Malek HA and Saleh D: Heparin
modulation on hepatic nitric oxide synthase in experimental
steatohepatitis. Exp Ther Med. 8:1551–1558. 2014.PubMed/NCBI
|
|
28
|
De Filippis D, Esposito G, Cirillo C,
Cipriano M, De Winter BY, Scuderi C, Sarnelli G, Cuomo R, Steardo
L, De Man JG and Iuvone T: Cannabidiol reduces intestinal
inflammation through the control of neuroimmune axis. PLoS One.
6:e281592011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esposito G, Scuderi C, Savani C, Steardo L
Jr, De Filippis D, Cottone P, Iuvone T, Cuomo V and Steardo L:
Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation
by suppressing IL-1beta and iNOS expression. Br J Pharmacol.
151:1272–1279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davis RJ, Nunley PD, Kim KD, Hisey MS,
Jackson RJ, Bae HW, Hoffman GA, Gaede SE, Danielson GO 3rd, Gordon
C and Stone MB: Two-level total disc replacement with Mobi-C
cervical artificial disc versus anterior discectomy and fusion: A
prospective, randomized, controlled multicenter clinical trial with
4-year follow-up results. J Neurosurg Spine. 22:15–25. 2015.
View Article : Google Scholar
|
|
31
|
Hwang MH, Shin JH, Kim KS, Yoo CM, Jo GE,
Kim JH and Choi H: Low level light therapy modulates inflammatory
mediators secreted by human annulus fibrosus cells during
intervertebral disc degeneration in vitro. Photochem Photobiol.
91:403–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu K, Chen W, Wang X, Peng Y, Liang A,
Huang D, Li C and Ye W: Autophagy attenuates the catabolic effect
during inflammatory conditions in nucleus pulposus cells, as
sustained by NF-κB and JNK inhibition. Int J Mol Med. 36:661–668.
2015.PubMed/NCBI
|
|
33
|
Cai A, Qi S, Su Z, Shen H, Ma W and Dai Y:
Tripterygium glycosides inhibit inflammatory mediators in the rat
synovial RSC-364 cell line stimulated with interleukin-1β. Biomed
Rep. 3:763–766. 2015.PubMed/NCBI
|
|
34
|
Wang SL, Yu YL, Tang CL and Lv FZ: Effects
of TGF-β1 and IL-1β on expression of ADAMTS enzymes and TIMP-3 in
human intervertebral disc degeneration. Exp Ther Med. 6:1522–1526.
2013.PubMed/NCBI
|
|
35
|
Barichello T, Ceretta RA, Generoso JS,
Moreira AP, Simões LR, Comim CM, Quevedo J, Vilela MC, Zuardi AW,
Crippa JA and Teixeira AL: Cannabidiol reduces host immune response
and prevents cognitive impairments in Wistar rats submitted to
pneumococcal meningitis. Eur J Pharmacol. 697:158–164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hao E, Mukhopadhyay P, Cao Z, Erdélyi K,
Holovac E, Liaudet L, Lee WS, Haskó G, Mechoulam R and Pacher P:
Cannabidiol protects against doxorubicin-induced cardiomyopathy by
modulating mitochondrial function and biogenesis. Mol Med.
21:38–45. 2015. View Article : Google Scholar : PubMed/NCBI
|