Introduction
Somatic mutations have been demonstrated to be major
factors in cancer development and progress, resulting in cell
proliferation, tissue invasion, and an increased risk of metastasis
(1). Increasing evidence indicates
that the behavior of tumor cells is also influenced by their
surrounding microenvironment, thus, a theory first presented in
1889, the 'seed and soil' hypothesis, has refreshed the view of
tumorigenesis (2). Tumors have
been demonstrated to be organ-like structures composed of various
cell types whose interactions are crucial for driving and promoting
tumor development and progression (3). As evidenced in the early literature,
an aberrant microenvironment contributes to tumor initiation
(4). Solid tumors are in synergy
with their surrounding microenvironment to promote tumor growth and
metastasis. Thus, targeting tumor cells and components in the
microenvironment may provide a more effective method of early
diagnosis and prevention of cancer. Improved understanding of the
cancer microenvironment and its components allows further
elucidation of the mechanisms underlying carcinogenesis in a
novel.
Regulation of normal physiological processes during
development of tumors may be destroyed and normal structures of
cells, including apical-basal polarity, are lost (5). It is well known that cell polarity is
vital to tissue homeostasis, thus, lost normal cell polarity
promotes the initiation and progression of multiple cancer types
(5). Common markers of polarity
include E-cadherin (6), Crumbs3
(CRB3) (7,8) and proteinase activated receptor 3
(PAR-3) (8), they function in
tumorigenesis and cell migration and these markers were detected in
the current study.
Cancer cells develop and progress in an aberrant
microenvironment that favors growth and progression, and changes in
the extracellular matrix (ECM) are one of numerous components that
contribute to carcinogenesis (9).
Abnormal tumor microenvironment (TME) consists of aberrant ECM
components, including collagens, hyaluronic acid, and various
cells, including fibroblasts, myofibroblasts, macrophages,
lymphocytes and endothelial cells, these components are key in the
initiation and progression of cancer (10–13).
The ECM comprises a variety of proteins that contribute to the
stability of tissue structure and physiological functions. Collagen
type I (Col-I) is a major ECM protein (14), thus, it was selected to be
investigated in order to elucidate the role of the ECM in cancer
development. The current study also examined the expression levels
of hyaluronidase-1 (Hyal-1), a major tumor-derived hyaluronidase
(15). Dysregulation of different
ECM components may favor the neoplastic process (16). Various cells, belonging to the ECM,
are also important in tumor initiation and progression. α-Smooth
muscle actin (α-SMA) is a common marker for myofibroblasts
(17) and cyclin D1 (CD1) is
considered to be an oncogenic marker in a number of types of human
cancer (18). Cluster of
differentiation (CD)133 is a marker for adult stem cells in various
types of tissue and tumors (19).
Vimentin is an intermediate filament of mesenchymal tissue
(20), and, similarly to
cytokeratins (CKs), are markers of epithelial cells or epithelial
cancer (21). These markers were
investigated in the present study in order to analyze the
importance of different cell types and proteins in the ECM on
carcinogenesis.
The current study analyzed the expression levels of
different components in the tissue microenvironment adjacent to
colorectal cancer (CRC) lesions using immunohistochemistry (IHC) to
investigation their association with early tumorigenesis. IHC,
which is widely used in clinical cancer diagnostics, can preserve
the morphology of tissue structures and demonstrate protein
expression in various cell populations of complex tissues. For
analysis of the process of cancer development, samples of different
distances from the CRC lesion were collected.
Materials and methods
Sample collection
A total of 48 patients (age range, 49–73 years old)
undergoing colonoscopic polypectomy in The First Affiliated
Hospital of Guangzhou University of TCM (Guangzhou, China) between
September 2013 and October 2014 were enrolled in the present study.
Patients who had undergone neoadjuvant chemotherapy or radiotherapy
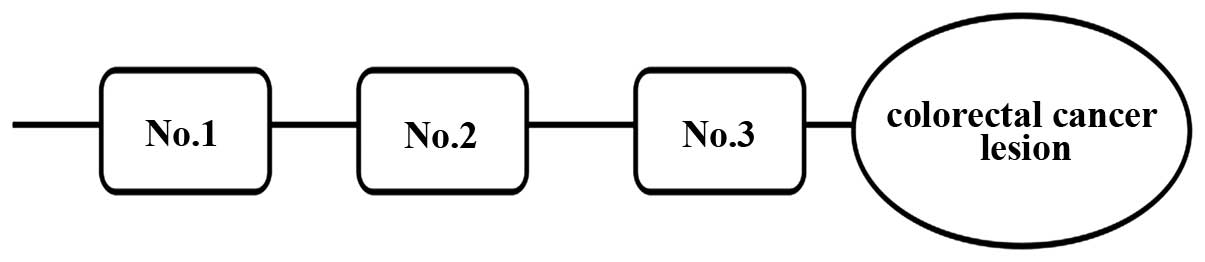
were excluded from the study. Samples were collected from three
different sites from each patient, and termed No. 1, No. 2 and No.
3, which were ≥10 cm, 5 cm and ≤2 cm distance from the proximal
lesion of CRC tissue, respectively (Fig. 1). A total of 144 samples were
collected. Informed consent was obtained from all participants and
the present study was approved by the Institutional Ethics
Committee of Guangzhou University of Chinese Medicine. The current
study was performed in accordance with the ethical guidelines of
the Declaration of Helsinki.
Hematoxylin and eosin staining of colonic
crypts
The collected tissues were fixed with 4%
paraformaldehyde, and then paraffin-embedded samples were cut into
4-µm sections. After 10 min and 3 min treatments with
dimethylbenzene and ethanol, respectively, the sections were
stained with hematoxylin for 10 min, and subsequently with 0.5%
eosin for 3 min, and then again treated with ethanol. The sections
were sealed with neutral gum and observed for obtaining images with
an inverted phase contrast microscope (Olympus).
IHC staining
The primary antibodies used in the IHC staining in
the present study were as follows: Rabbit polyclonal against
E-cadherin (Abcam, Cambridge, MA, USA; cat. no. ab15148), rat
monoclonal against CRB3 (Abcam; cat. no. ab180835), rabbit
monoclonal against α-SMA (Abcam; cat. no. 124964), rabbit
polyclonal against PAR-3 (Bioss, Inc., Woburn, MA, USA; cat. no.
bs-9510R), rabbit polyclonal against Hyal-1 (Abcam; cat. no.
ab103977), mouse monoclonal against Col-I (Abcam; cat. no. ab6308),
rabbit polyclonal against cytokeratin 18 (CK18; Bioss, Inc.; cat.
no. bs-1339R), rabbit polyclonal against vimentin (Bioss, Inc.;
cat. no. bs-8533R), rabbit polyclonal against CD1 (Bioss, Inc.;
cat. no. bs-0623R) and rabbit polyclonal against CD133 (Bioss,
Inc.; cat. no. bs-0395R). Biotin-conjugated goat anti-rabbit (Wuhan
Boster Biological Technology, Ltd., Wuhan, China; cat. no. SA1022),
horseradish peroxidase-conjugated goat anti-mouse (Wuhan Boster
Biological Technology, Ltd.; cat. no. BA1051) and anti-rat (Wuhan
Boster Biological Technology, Ltd.; cat. no. SA1025) secondary
antibodies were used.
All the biomarkers in the tissue samples were
detected using IHC. Specimens were confirmed by hematoxylin and
eosin staining of the sections. Formalin-fixed, paraffin-embedded
sections (4-µm) were deparaffinized in xylene, rehydrated in
graded alcohol and rinsed in phosphate-buffered saline (PBS).
Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide in methanol for 20 min. Epitope retrieval was performed in
citrate buffer for 5 min at 100°C. Slides were blocked in 5% bovine
serum albumin (Sigma-Aldrich, St. Louis, MO, USA) for 2 h at 37°C.
Slides were incubated with the primary antibodies (1:100) at 4°C
overnight, washed with PBS 0.05% Tween 20, and then incubated with
secondary antibodies (1:1,000) at 37°C for 30 min, prior to
staining with diaminobenzidine, counterstaining with hematoxylin,
dehydrated, coverslipped and then imaged using an inverted phase
contrast microscope.
For immunostaining intensity, the staining was
scored by two pathologists from 0 to 3 (0, negative; 1, weak
staining; 2, moderate staining; and 3, strong staining). The
percentage of reactivity area was scored as follows: 1, <25%; 2,
25–50%; 3, 51–75% and 4, >75%. Immunoreactive score was
calculated by multiplying the scores of intensity and the scores of
reactivity area. Immunoreactive score was classified into four
degrees: Negative, 0, weak, 1–4, moderate, 6–8, and strong, 9–12.
The reactivity of all the markers was evaluated using an
immunoreactive score.
PBS was used in place of the primary antibodies in
each sample to serve as a negative control. Pathology image
analysis software, IPP 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA), was applied to detect absorbance value (A) of the
immune-positive product in each group. A total of six fields from
each slide were randomly measured using value A of the same slide
as a standard in order to obtain the corrected A (CCA) value of the
sample. This was obtained subtracting the A value of the standard
from the A value of the immunoreaction product producing the real
optical density of the positive reaction, and the mean of six
fields served as the mean CCA value of each sample. To avoid
unnecessary error due to non-specific staining, data analysis and
comparison was conducted using the mean CCA value. These data were
used to perform related functional analysis using hierarchical
index cluster, as described previously by with Knösel et al
(22), using GenePix Pro software
(version 6.0; Molecular Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
All data were analyzed using Stata 12.0 (StataCorp
LP, College Station, TX, USA) software. The CCA values are
presented as the mean ± standard deviation. One-way analysis of
variance was conducted to accomplish the comparison among the three
groups, assuming that the data were normally distributed, with
post-hoc least significance difference test. Non-parametric rank
sum tests were performed when data were not normally distributed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Observation of crypt structures
demonstrated the degradation of normal colonic crypt
architecture
Colonic crypts are stereotypical structures
containing distinct stem cell proliferating, and stem cell
differentiating compartments (23). Generally, although CRC derives from
colonic crypt epithelia, it exhibits morphologically disarrayed
glands (24). Thus, aberrant crypt
foci (ACF) may be an early indicator of colon cancer development
(25). The size and number of ACF
is considered to be associated with CRC development.
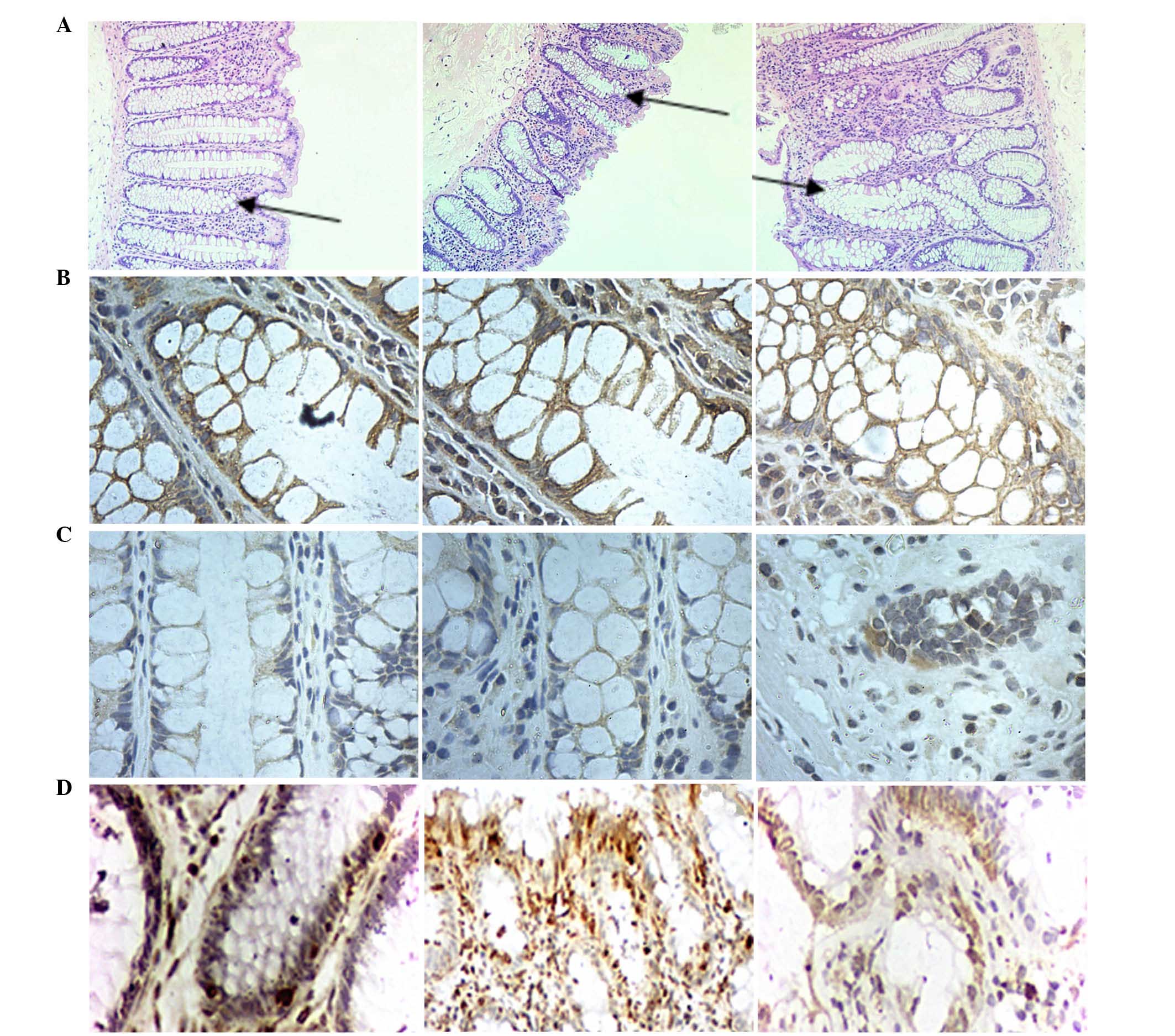
In the current study, the crypts were vertically cut
to observe histopathological characteristics of normal colon crypts
(Fig. 2). The results indicated
that the colon crypts without branching or protuberances presented
a test-tube shape and smooth in surface (stained with hematoxylin
and eosin; Fig. 2-A1). The crypt
structures in sample No. 2 were larger than in sample No. 1.
Compared with sample No. 1, the shape of crypts in sample No. 2
were lost (Fig. 2-A2), which is
consistent with previous data demonstrating marked discrepancies
from normal colonic crypt architecture (23).
Expression of biomarkers in tissue
microenvironment differed with sample location
Increasing evidence demonstrates that genetic
aberrations in cancer cells are key in the pathophysiology of
cancer, however, the crosstalk among cancer cells, non-malignant
cells, cytokines, growth factors and other participants in the TME
(26). In all sections that were
examined, the presence of E-cadherin, CRB3, PAR-3, Col-I, Hyal-1,
CD1, CD133, vimentin, CK18 and α-SMA were observed by IHC (Figs. 3 and 4). The expression of E-cadherin, CRB3 and
PAR-3 in No. 1 and No. 2 was significantly higher than in No. 3
(P<0.05), and the majority of the expression was observed in the
membrane of crypt cells (Fig.
2).
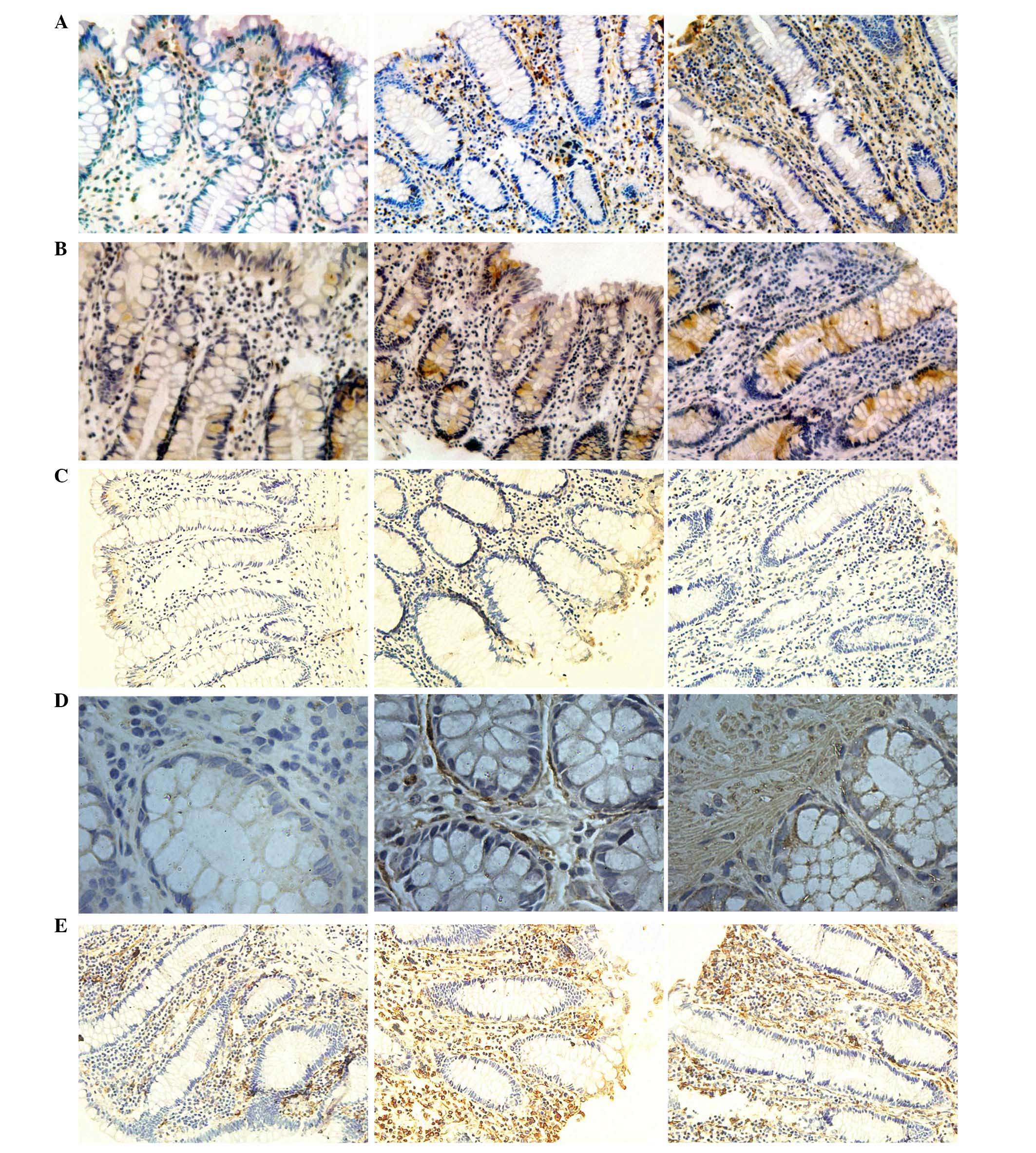 | Figure 3Expression levels of CD1, CD133,
CK18, α-SMA and vimentin indicated by immunohistochemical staining.
Expression levels of (A) CD1, (B) CD133, (C) CK18, (D) α-SMA and
(E) vimentin, in samples no. 1, 2 and 3 (left to right). CD1,
cyclin D1; CD133, cluster of differentiation 133; CK18, cytokeratin
18; α-SMA, α-smooth muscle actin. |
E-cadherin is a marker of epithelial-mesenchymal
transition (EMT) (27), and it
contributes to the development and metastasis of cancer, as
decreased expression levels are observed in cancer tissues, it is
considered an inhibitor of EMT (28). E-cadherin expression is regulated
by a variety of protein molecules, including osteopontin (OPN)
(29), and N-Myc
downstream-regulated gene 2 (NDRG2) (30). An in vitro model
demonstrates that ectopic expression of the onco-protein OPN
induced colorectal cancer cell migration via E-cadherin repression
(29), which may elucidate an
underlying mechanism for the decreased expression of E-cadherin in
sample No. 3. However, NDRG2, a differentiation-associated tumor
suppressor, positively regulates the expression of E-cadherin
(30). This suggests E-cadherin
regulation is mediated by positive feedback and negative feedback
through different signaling pathways.
Three evolutionary conserved polarity complexes, the
Scribble, PAR and CRB complexes act as regulators of apical-basal
polarity, however, tight junction integrity is predominantly
maintained by the CRB and PAR polarity complexes (31). When normal tissues undergo
canceration, cell polarity is also altered. For example, immortal
neonatal mouse kidney epithelial cells with tumorigenicity
exhibited repressed CRB3, associated with disruption of tight
junctions and apicobasal polarity, in addition to promoting
migration and metastasis (32).
PAR-3, a PDZ protein, involved in the formation of junctional
complexes in epithelial cells (33), has been observed in the distal
resection margin of rectal cancer in a similar study that
demonstrated that samples taken 3 cm from the tumor tissues had
marked expression of PAR-3, while expression in the samples taken 2
cm away and in the tumor samples was relatively weak (34), which is consistent with the results
of the present study. Interaction of PAR-3 and other proteins is
frequently observed. T-lymphoma invasion and metastasis-inducing
protein 1 (Tiam1) and its interactor β2-syntrophin are required for
optimal cell-cell adhesion, notably, the repression of Tiam1-Rac
activity by PAR-3 facilitates tight-junction assembly (35), in addition to the interaction of
PAR-3 and CRB3 (8) suggesting that
the two proteins may have similar functions in a synergetic manner,
as demonstrated in the present study, which demonstrated that the
expression levels of the two proteins were associated (Fig. 5).
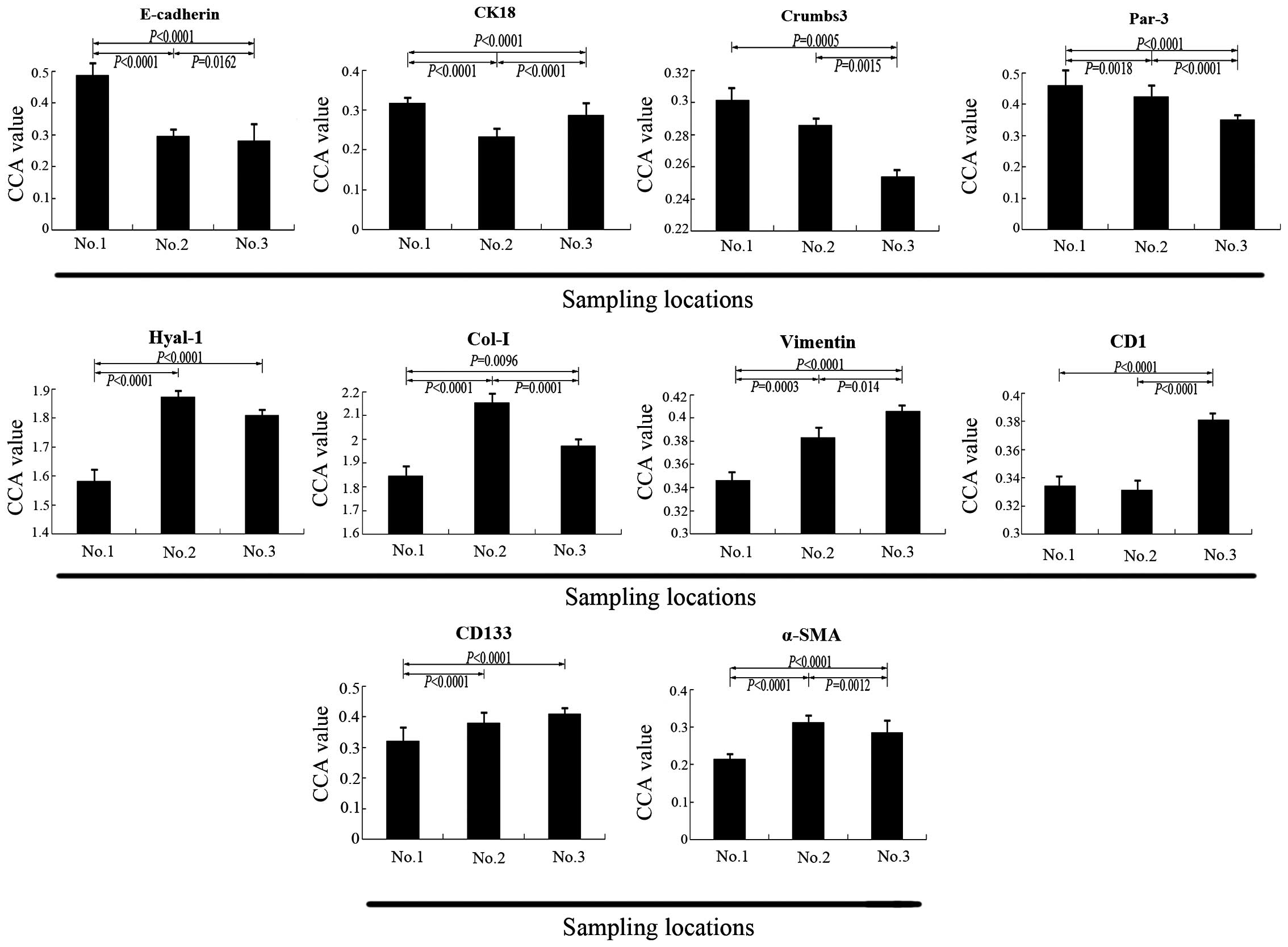 | Figure 5CCA value of ten biomarkers in
samples No. 1, No. 2 and No. 3. Demonstrated by the CCA value, the
expression levels of E-cadherin, CK18, Crumbs-3 and Par-3 in sample
No. 3 were lower compared with sample No. 1 (P<0.05). The
expression of CK18 in sample No. 2 was lower than in sample No. 3
(P<0.0001). The expression levels of Hyal-1, Col-I, vimentin,
CD1, CD133 and α-SMA in sample No. 3 were higher than in sample No.
1 (P<0.05), however, the expression levels of Hyal-1, Col-I,
α-SMA in sample No. 3 were lower than that in sample No. 2, the
result for Hyal-1 was not significant. CCA, corrected absorbance
value; CK18, cytokeratin 18; Par-3, proteinase activated receptor
3; Hyal-1, hyaluronidase 1; Col-I, collagen type I; CD1, cyclin D1;
CD133, cluster of differentiation 133; α-SMA, α-smooth muscle
actin. |
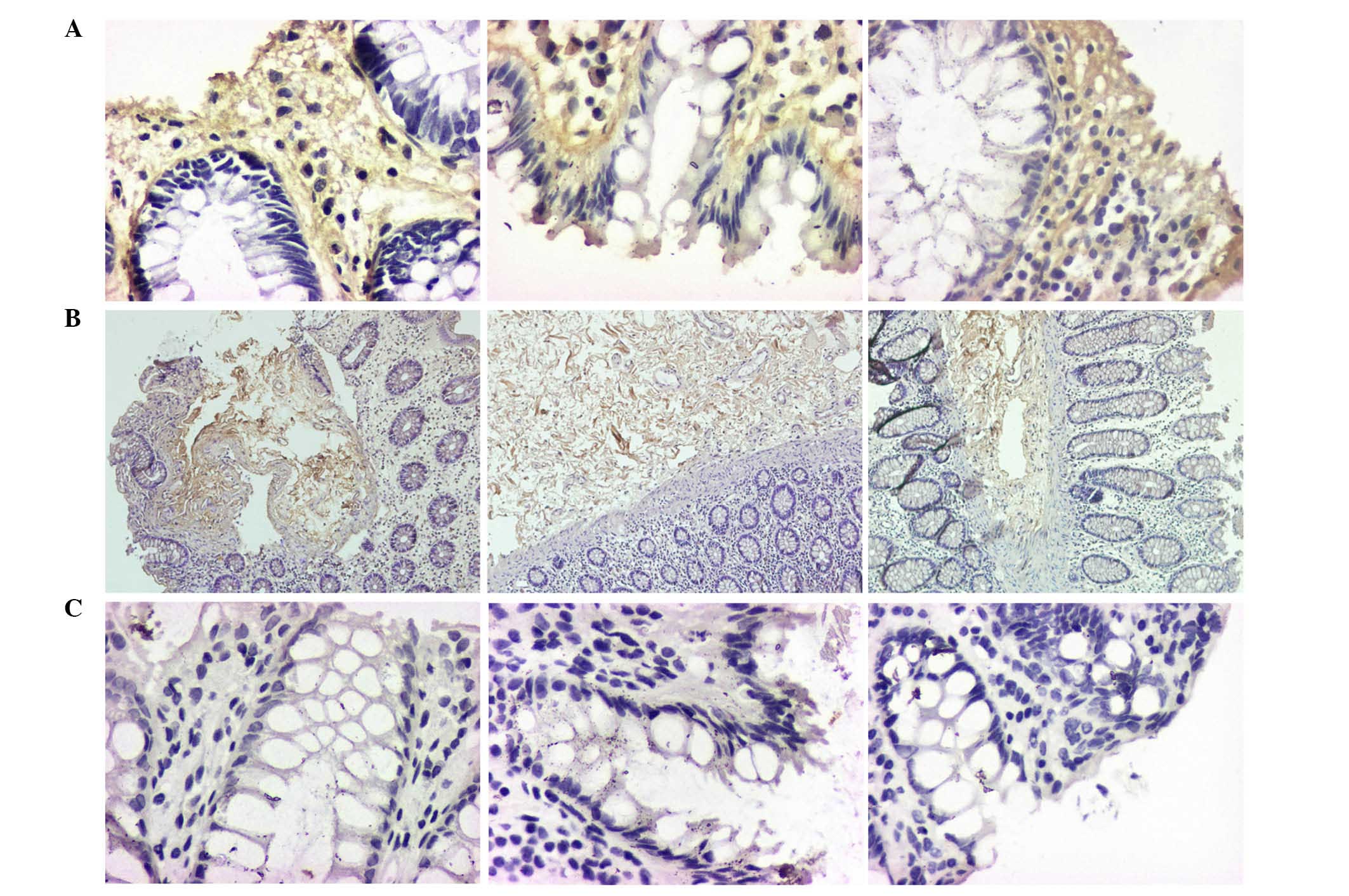
By contrast, the expression levels of Col-I, Hyal-1,
CD1, CD133, vimentin and α-SMA in samples No. 2 and No. 3 were
significantly higher than in sample No. 1 (P<0.05). However, the
expression profile of CK18 was different, in samples No. 2 and No.
3 its expression was lower than in sample No. 1 (P<0.05).
Downregulation of CK18 was also demonstrated in the CRC SW480 cell
line (36) and 468 CRC samples
(22). The above results and
functional cluster analysis, as presented in Figs. 5 and 6 suggests CK18 and CRB3 may have certain
similar but not identical regulatory mechanisms. Furthermore, the
expression levels of CK18, CD1, vimentin and α-SMA were observed in
mesenchymal tissue and CD133 was predominantly distributed in the
crypt (Fig. 3).
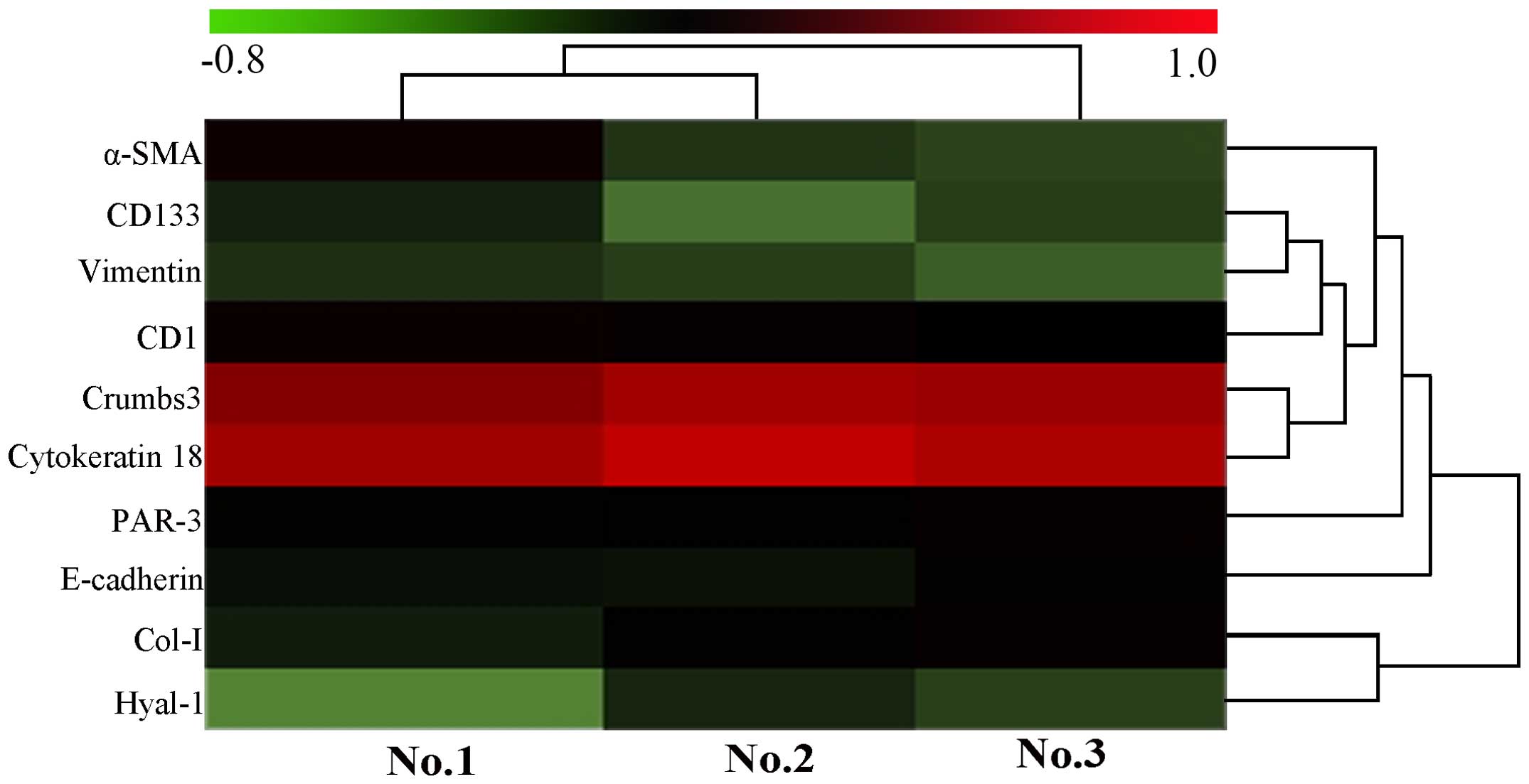 | Figure 6Heatmap of biomarker expression in
the present study according to the CCA value of samples No. 1, No.
2 and No. 3. The heatmap diagram indicates the result of the
two-way hierarchical clustering of markers and samples. Each row
represents a marker and each column represents a sample. The marker
clustering tree is presented on the right, and the sample
clustering tree at the top. Cluster analysis arranges samples and
markers into groups based on their CCA values, which allows
formation of hypotheses regarding the association between markers
and samples. The color of each pattern indicates the CCA value,
green to red is −0.8 to 1.0. CCA, corrected absorbance value;
α-SMA, α-smooth muscle actin; CD1, cyclin D1; CD133, cluster of
differentiation 133; PAR-3, proteinase activated receptor 3; Col-I,
collagen type I; Hyal-1, hyaluronidase 1. |
Vimentin, a mesenchymal marker, is notably increased
in CRC, accompanied by reduced epithelial markers, E-cadherin and
β-catenin, and is associated with regulation of dachshund homolog 1
(DACH1), a tumor suppressor (37)
whose inhibition markedly increases cell growth, migration and
invasion of SW480 cells. Its expression is also associated with
proline rich 11, a newly identified oncogene that is overexpressed
in colorectal cancer (38). In
addition to the regulation of DACH1, the regulator transforming
growth factor-β (TGF-β), a factor in microenvironment, also exerts
an effect on vimentin. For example, in an in vitro study,
TGF-β1 reduces E-cadherin expression and increases the vimentin
expression (39). A previous study
has demonstrated that vimentin is induced by Bcl-2-like protein 2 a
pro-survival protein, via activating the transcription factors,
β-catenin, Twist-related protein 1 and zinc finger protein Snai1
(40). Together with the above
data, this suggests that vimentin is an important mediator of
multiple signal proteins, thus exerting a vital effect in
tumorigenesis.
CD133 also has a close association with vimentin,
and is emerging as a marker of cancer stem cells (CSCs) (19). As presented in Figs. 3, 5 and 6,
they have the similar profiles of expression, suggesting that they
may have similar roles in cancer. However, CD133 is markedly
affected by micromilieu, including hypoxia in TME (19). In CRC cell lines, downregulated
CD133 expression is observed at 0.1% oxygen (41), consistent with an emerging
hypothesis that CD133 is predominantly located in the inner core of
the cancer mass (42) in which the
hypoxic environment is located. Regulation of stem cell state is
dynamic with the environment, as demonstrated in the present study
where CD133 expression in sample No. 3 is increased. In addition,
CD133 expression is also associated with EMT as previously
demonstrated in a highly migratory subclone of the Capan-1
pancreatic cancer cell line (43).
Stromal cells in the TME, including fibroblasts
contribute to human cancer cell engraftment and cancer progression
(44). In the TME, interaction of
activated cancer-associated fibroblasts (CAFs), with α-SMA as a
characteristic marker, and tumor cells promotes the production of
CSCs exhibiting multipotency, indefinite self-renewal and
asymmetric cell division (19),
resulting in growth of cancer masses and cancer progression. This
underlies CD133 being predominantly observed in ACF.
Snail1, a transcriptional factor that is important
in EMT, is upregulated in CAFs and is associated with α-SMA
(45). Researches have found that
Snail1 is required for the protumorigenic effects of activated
fibroblasts on CRC cells (45). In
addition, the presence of α-SMA expression at E-cadherin
downregulated sites is demonstrated by IHC staining (46), consistent with the present study as
α-SMA expression in samples No. 3 and No. 2 were increased compared
with sample No. 1, which implies activation of fibroblasts and
induction of EMT.
CD1 is a positive cell cycle regulator involved in
G1/S and G2/M checkpoint transitions,
resulting in CRC cell proliferation (47), and is positively regulated by
transcription factor paired box 2 via activator protein 1 (48) and by β-catenin (49). Increased expression of CD1 is
observed in tumor tissues. However, its upregulation was also
observed in precancerous lesions in the present study, suggesting
that CD1 may serve as a pro-oncogene via interaction with oncogene
c-Myc and associated signals involving phosphoinositide
3-kinase/Akt/glycogen synthase kinase-3β signaling (49,18).
The current study demonstrates that Col-I and Hyal-1
expression was predominantly distributed in the connective tissues
(Fig. 4). Col-I is secreted from
activated hepatic stellate cells, and it is considered to be
associated with EMT (46). A
previous study has demonstrated that Col-I may interact with
integrin αvβ8 leading to the upregulation of the latter, and
resulting in proliferation and invasion of carcinoma cells via
activation of the mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase signaling pathway
(50). There are fewer similar
studies investigating the role of Hyal-1 in colorectal cancer is
relatively few, however, notably, a previous study suggests that
reduced Hyal-1 is observed in human endometrial cancer and is
associated with cancer progression involving a possible mechanism
involving reduced E-cadherin expression (51). In addition, Hyal-1 expression is
likely to be dependent on cancer types (51), consistently with this previous
study, the expression profile of Hyal-1 was the same as E-cadherin
in samples No. 1 and No. 2, however, it was different in sample No.
3, which suggests Hyal-1 expression in pre-malignant tissues is not
regulated via the above mechanism.
Related function analysis using
hierarchical index cluster indicated the importance of Col-I and
Hyal-1 in tumorigenesis
For functional analysis of the proteins, index
clustering was used. As presented in Fig. 6, all these protein molecules
mentioned were divided into two large groups, one containing Hyal-1
and Col-I, and the other containing was the other investigated
proteins. From this clustering, it can be determined that certain
proteins may have the same, similar or synergetic functions during
canceration, in addition to the same or similar regulatory
mechanisms. For example, E-cadherin upregulation, and
downregulation of vimentin, cyclin-D1, and α-SMA are mediated by
microRNA-494 in breast cancer cells (52).
Cell polarity, an essential feature in eukaryotic
cells, is the determinant of cellular morphology. It is important
in asymmetric division and tight junctions. Disrupted polarity is
also considered to be a characteristic of cancer (53).
Hyal-1 and Col-I are key in tumorigenesis. Col-I is
produced by fibroblasts (54),
remodeling of the ECM may result in a pre-cancerous niche, which
could lead to a chronic stress escape strategy (CSES) during
carcinogenesis. The terminal consequence is a normal-cell to
cancer-cell transition (1).
Notably, the TME and ECM must generate conditions in favor of
metastatic cancers proliferation at certain locations but not at
others (55). The expression of
the Hya1-1 and Col-I presents different profiles in different
sites, which suggests, together with the functional analysis, that
Hyal-1 and Col-I may be important in tumorigenesis.
Discussion
There is an emerging hypothesis that tumors function
as a complex organ similar to normal tissues. The various cell
types and components of surrounding tumor microenvironment are
involved in tumorigenesis (56).
Phenotypic and epigenetic changes have been observed in the cells
and the stroma during tumor initiation and progression. The
microenvironment, or niche, in tumor tissue is suggested to provide
essential support for the aberrant growth of tumor stem cells
(57). Cells are associated with
the surrounding environment and stroma are associated with the
basal membrane from which information can be transmitted and
processed (55).
The asymmetrical distribution of cell polarity
divides the cellular domains into different structural and
functional areas in which cells can interact with surrounding
extracellular environments. Cell polarity is key for controlling
cell behavior and loss of cell polarity promotes tumor initiation.
E-cadherin, which localizes at the lateral membrane, is vital to
the formation of the adherens junctions. However, CRB3 is located
in the apical pole and its loss contributes to phenotypic changes,
such as EMT. Lateral growth factor receptors are exposed to apical
ligands following downregulation of CRB3 and disruption of tight
junctions. This process may deregulate cell growth and promote
proliferation. Furthermore, decreased CRB3 may alter cellular
behaviors contributing to motility and migration (58). Tight junctions separate the
basolateral and apical cellular domains in epithelial cells vital
for establishing and maintaining cell morphology. PAR-atypical
protein kinase C complex is recruited to the cell cortex from the
cytoplasm during the formation of tight junctions (59). PAR3 combines the junctional
adhesion molecules, including junctional adhesion molecule-1 and
nectin (60,61). Cell polarity is important in the
maintenance of normal crypt organization and structure.
Downregulation of E-cadherin, CRB3 and PAR3 in sample No. 3
suggests that polarity proteins may promote tumorigenesis in human
carcinomas via controlling cell behavior.
In the progression of cancer, the expression of ECM
components is upregulated, downregulated or lost, promoting tumor
initiation and progression. ECM is important in cell growth and
interaction. Cell-cell and cell-ECM interaction is involved in
tumorigenesis. Cell-cell communication in microenvironment is
necessary for morphogenesis, cell differentiation, homeostasis,
cell growth, and cell-cell interaction. Notably, cell-cell
communication is described as 'the music that the nucleus hears'
and, the health of the organism is likely to be damaged by aberrant
cell-cell communication (62).
Hyaluronan is key in cancer development and
tumorigenesis, including tumor growth, infiltration and
angiogenesis (63,64). Hyal-1 is a primary tumor-derived
enzyme expressed by various different types of tumor cell.
Hyaluronan interacts with other proteoglycans to maintain the
structural integrity required in tissue homeostasis. Hyaluronan
contributes to basic cellular behavior, such as cell adhesion, cell
recognition and cell migration (65,66).
Thus, hyaluronan is important in tissue morphogenesis, tissue
remodeling and tumor growth. In CRC, hyaluronan promotes tumor cell
migration and proliferation. It may also stimulate
anchorage-independent growth and protect against immune
surveillance (67). Furthermore,
tumor cells may be more aggressive to invade into surrounding
tissues with hyaluronan-rich ECM. Hyaluronidases have been reported
to function like tumor inhibitors in vivo (68). Collagens are part of connective
tissues and the predominant component of the ECM. They have a
marked effect on structural stability and integrity. Degradation of
collagen and aberrant metabolism are important in tumorigenesis.
Collagens contribute to tissue repair and regeneration by their
binding capacity with other growth factors, including hepatocyte
growth factor (69–73). In numerous types of malignant
tumor, including colorectal adenocarcinoma, Col-I and III are the
primary components of ECM. Increased Col-I has been demonstrated to
be associated with tumor malignancy (74). Increasing expression of Hyal-1 and
Col-I from sample No. 1 to sample No. 3 in the present study also
indicates these may promote tumor initiation and progression.
Fibroblasts and myofibroblasts produce the increased
quantity of collagen in cancer (75). Myofibroblasts and abnormal
fibroblasts are recruited during tumorigenesis, promoting tumor
cell proliferation, angiogenesis, and metastasis. Cancer-associated
fibroblasts (CAFs) are also important in the tumor
microenvironment, functioning in cancer initiation and progression,
which has been previously investigated (76). α-SMA is a common marker for
detecting the expression of myofibroblasts and vimentin.
Furthermore, vimentin enhances motility. The tumor size and growth
rate of MCF7 breast cancer cells suspended in fibroblast
conditioned medium were increased, demonstrating that the soluble
factors secreted by fibroblasts may contribute to tumor progression
(77). A large number of CAFs,
distinct from normal fibroblasts, exist in the cancer
microenvironment. TGF-β and hepatocyte growth factor secreted by
CAFs promote EMT and tumor metastasis. In addition, the transition
from one cell function to another, or the transition of one cell
type to another appears to be a routine event rather than a rare
one. EMT can induce non-cancer stem cells to become cancer stem
cells (78). Colorectal cancer
stem cells are a small subpopulation of tumor cells that have been
proposed to be tumor-initiating cells in CRC (79). Furthermore, CAFs stimulate tumor
cell proliferation directly by secreting a variety of growth
factors, hormones and cytokines (80). A number of these secreted factors
promote transformation of epithelial cells. The expression levels
of α-SMA and vimentin were upregulated in samples No. 2 and No. 3
suggesting that myofibroblasts and fibroblasts served as positive
factors in tumor initiation. CD1, an important regulator of the
G1- to S-phase transition and cell proliferation
(81), has been reported to be
important in cancer progression (82). CD1 is also considered to be an
oncogenic marker in a number of types of human cancer. CD133 has
been identified as a biomarker for cancer stem cells, which can
promote tumorigenesis, tumor cell proliferation, differentiation,
metastasis, self-renewal and treatment resistance. The results of
the present study demonstrated that expression of CD1 and CD133 was
increased in sample No. 3 compared with No. 1 and No. 2, indicating
that they may be important indicators for colorectal cancer.
It has been increasingly recognized that ECM
regulates gene expression by cell surface integrin receptors
(83,84). Intermediate filaments are
transducers between cell surface integrins and nuclei (85,86).
Integrins with bidirectional communicating features mediating the
information exchanged between cells and their surrounding
components allow a flow of information in two directions, enabling
the signal transduction of bidirectional information exchange
between the ECM and the cell (87,88).
CK18 is the predominant intermediate filament protein of epithelial
cells and epithelial-derived tumors, accounting for ~5% of the
total cell protein. CK18 promotes cellular collapse and apoptosis.
The potential of increased CK18 as a marker for CRC has been tested
by qPCR in CRC tissue samples (89). The loss of epithelial CK18 and the
overexpression of vimentin in sample No. 3 indicates that EMT may
be occurring during tumorigenesis.
In conclusion, to the best of our knowledge, the
present study indicates that co-evolution of a tumor and its
microenvironment occurs spontaneously in human colorectal cancer.
Targeting the components of the cancer microenvironment may be a
potential approach for cancer diagnosis and prevention. Future
investigations in this field should aim to integrate tissue culture
studies and analyses in animal models representing early stages of
cancer development. The present study increases the understanding
of communication within the cancer microenvironment, which may aid
the development of early diagnosis or preventive strategies based
on this.
Acknowledgments
The authors would like to thank Liyun Wu and Meng Su
for their technical assistance. The present study is funded by the
National Natural Science Foundation of China (grant no. 81173257)
and The Central Government Funds Supporting the Development of
Local Colleges and Universities For the Prevention Collaborative
Innovation Platform of Major Refractory Spleen and Stomach Diseases
[grant no. (2013) 338].
References
|
1
|
Brücher BL and Jamall IS: Epistemology of
the origin of cancer: A new paradigm. BMC Cancer. 14:3312014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paget S: The distribution of secondary
growths in cancer of the breast 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
3
|
Korkaya H, Liu S and Wicha MS: Breast
cancer stem cells, cytokine networks, and the tumor
microenvironment. J Clin Invest. 121:3804–3809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Felsher DW: Cancer revoked: Oncogenes as
therapeutic targets. Nat Rev Cancer. 3:375–380. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li R and Pendergast AM: Arg kinase
regulates epithelial cell polarity by targeting β1-integrin and
small GTPase pathways. Curr Biol. 21:1534–1542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammoudi A, Song F, Reed KR, Jenkins RE,
Meniel VS, Watson AJ, Pritchard DM, Clarke AR and Jenkins JR:
Proteomic profiling of a mouse model of acute intestinal Apc
deletion leads to identification of potential novel biomarkers of
human colorectalcancer (CRC). Biochem Biophys Res Commun.
440:364–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elsum IA, Martin C and Humbert PO:
Scribble regulates an EMT polarity pathway through modulation of
MAPK-ERK signaling to mediate junction formation. J Cell Sci.
126:3990–3999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sfakianos J, Togawa A, Maday S, Hull M,
Pypaert M, Cantley L, Toomre D and Mellman I: Par3 functions in the
biogenesis of the primary cilium in polarized epithelial cells. J
Cell Biol. 179:1133–1140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reed MJ, Damodarasamy M, Chan CK, Johnson
MN, Wight TN and Vernon RB: Cleavage of hyaluronan is impaired in
aged dermal wounds. Matrix Biol. 32:45–51. 2013. View Article : Google Scholar :
|
|
10
|
Tlsty TD and Hein PW: Know thy neighbor:
Stromal cells can contribute oncogenic signals. Curr Opin Genet
Dev. 11:54–59. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weinberg R and Mihich E: Eighteenth annual
pezcoller symposium: Tumor microenvironment and heterotypic
interactions. Cancer Res. 66:11550–11553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roncucci L, Pedroni M, Vaccina F, Benatti
P, Marzona L and De Pol A: Aberrant crypt foci in colorectal
carcinogenesis. Cell and crypt dynamics. Cell Prolif. 33:1–18.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo F, Wang Q, Zhou Y, Wu L, Ma X, Liu F,
Huang F and Qin G: Lentiviral vector-mediated FoxO1 overexpression
inhibits extracellular matrix protein secretion under high glucose
conditions in mesangial cells. J Cell Biochem. 117:74–83. 2016.
View Article : Google Scholar
|
|
15
|
Kolliopoulos C, Bounias D, Bouga H,
Kyriakopoulou D, Stavropoulos M and Vynios DH: Hyaluronidases and
their inhibitors in the serum of colorectal carcinoma patients. J
Pharm Biomed Anal. 83:299–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barreto SC, Hopkins CA, Bhowmick M and Ray
A: Extracellular matrix in obesity-cancer interactions. Horm Mol
Biol Clin Investig. 22:63–77. 2015.PubMed/NCBI
|
|
17
|
Abu El-Asrar AM, De Hertogh G, van den
Eynde K, Alam K, Van Raemdonck K, Opdenakker G, Van Damme J, Geboes
K and Struyf S: Myofibroblasts in proliferative diabetic
retinopathy can originate from infiltrating fibrocytes and through
endothelial-to-mesenchymal transition (EndoMT). Exp Eye Res.
132:179–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noah TK, Lo YH, Price A, Chen G, King E,
Washington MK, Aronow BJ and Shroyer NF: SPDEF functions as a
colorectal tumor suppressor by inhibiting β-catenin activity.
Gastroenterology. 144:1012–1023.e6. 2013. View Article : Google Scholar
|
|
19
|
Grosse-Gehling P, Fargeas CA, Dittfeld C,
Garbe Y, Alison MR, Corbeil D and Kunz-Schughart LA: CD133 as a
biomarker for putative cancer stem cells in solid tumours:
Limitations, problems and challenges. J Pathol. 229:355–378. 2013.
View Article : Google Scholar
|
|
20
|
Wang P: Suppression of DACH1 promotes
migration and invasion of colorectal cancer via activating
TGF-β-mediated epithelial-mesenchymal transition. Biochem Biophys
Res Commun. 460:314–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai DW, Liu SH, Karlsson AI, Lee WJ, Wang
KB, Chen YC, Shen CC, Wu SM, Liu CY, Tien HR, et al: The novel Aryl
hydrocarbon receptor inhibitor biseugenol inhibits gastric tumor
growth and peritoneal dissemination. Oncotarget. 5:7788–7804. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knösel T, Emde V, Schlüns K, Schlag PM,
Dietel M and Petersen I: Cytokeratin profiles identify diagnostic
signatures in colorectal cancer using multiplex analysis of tissue
microarrays. Cell Oncol. 28:167–175. 2006.PubMed/NCBI
|
|
23
|
Cernat L, Blaj C, Jackstadt R, Brandl L,
Engel J, Hermeking H, Jung A, Kirchner T and Horst D: Colorectal
cancers mimic structural organization of normal colonic crypts.
PLoS One. 9:e1042842014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Milicic A, Harrison LA, Goodlad RA, Hardy
RG, Nicholson AM, Presz M, Sieber O, Santander S, Pringle JH,
Mandir N, et al: Ectopic expression of P-cadherin correlates with
promoter hypomethylation early in colorectal carcinogenesis and
enhanced intestinal crypt fission in vivo. Cancer Res.
68:7760–7768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Whiteman EL, Liu CJ, Fearon ER and
Margolis B: The transcription factor snail represses Crumbs3
expression and disrupts apicobasal polarity complexes. Oncogene.
27:3875–3879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ribeiro AL and Okamoto OK: Combined
effects of pericytes in the tumor microenvironment. Stem Cells Int.
2015:8684752015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MJ, Lee YS, Han GY, Lee HN, Ahn C and
Kim CW: Profilin 2 promotes migration, invasion, and stemness of
HT29 human colorectal cancer stem cells. Biosci Biotechnol Biochem.
79:1438–1446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen CC, Sureshbabul M, Chen HW, Lin YS,
Lee JY, Hong QS, Yang YC and Yu SL: Curcumin suppresses metastasis
via Sp-1, FAK inhibition and E-Cadherin upregulation in colorectal
cancer. Evid Based Complement Alternat Med. 2013:5416952013.
View Article : Google Scholar
|
|
29
|
Ng L, Wan TM, Lam CS, Chow AK, Wong SK,
Man JH, Li HS, Cheng NS, Pak RC, Cheung AH, et al: Post-operative
plasma osteopontin predicts distant metastasis in human colorectal
cancer. PLoS One. 10:e01262192015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim YJ, Kang HB, Yim HS, Kim JH and Kim
JW: NDRG2 positively regulates E-cadherin expression and prolongs
overall survival in colon cancer patients. Oncol Rep. 30:1890–1898.
2013.PubMed/NCBI
|
|
31
|
Elsum IA, Martin C and Humbert PO:
Scribble regulates an EMT polarity pathway through modulation of
MAPK-ERK signaling to mediate junction formation. J Cell Sci.
126:3990–3999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karp CM, Tan TT, Mathew R, Nelson D,
Mukherjee C, Degenhardt K, Karantza-Wadsworth V and White E: Role
of the polarity determinant crumbs in suppressing mammalian
epithelial tumor progression. Cancer Res. 68:4105–4115. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sfakianos J, Togawa A, Maday S, Hull M,
Pypaert M, Cantley L, Toomre D and Mellman I: Par3 functions in the
biogenesis of the primary cilium in polarized epithelial cells. J
Cell Biol. 179:1133–1140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Zhang W, Liu J, Lu X, Long Y, Zhou
Y and Liu S: Expressions of connexin and par-3 in the distal margin
of rectal cancer after ultra-low anterior resection. J Huazhong
Univ Sci Technolog Med Sci. 29:330–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mack NA, Porter AP, Whalley HJ, Schwarz
JP, Jones RC, Khaja AS, Bjartell A, Anderson KI and Malliri A:
β2-syntrophin and Par-3 promote an apicobasal Rac activity gradient
at cell-cell junctions by differentially regulating Tiam1 activity.
Nat Cell Biol. 14:1169–1180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wong CS, Wong VW, Chan CM, Ma BB, Hui EP,
Wong MC, Lam MY, Au TC, Chan WH, Cheuk W and Chan AT:
Identification of 5-fluorouracil response proteins in colorectal
carcinoma cell line SW480 by two-dimensional electrophoresis and
MALDI-TOF mass spectrometry. Oncol Rep. 20:89–98. 2008.PubMed/NCBI
|
|
37
|
Wang P: Suppression of DACH1 promotes
migration and invasion of colorectal cancer via activating
TGF-β-mediated epithelial-mesenchymal transition. Biochem Biophys
Res Commun. 460:314–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Cha Z, Fang W, Qian B, Yu W, Li W,
Yu G and Gao Y: The prognostic potential and oncogenic effects of
PRR11 expression in hilar cholangiocarcinoma. Oncotarget.
6:20419–20433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L,
Jiang H, Ren J, Cai J and Li Q: Resveratrol suppresses
epithelial-to-mesenchymal transition in colorectal cancer through
TGF-β1/Smads signaling pathway mediated Snail/E-cadherin
expression. BMC Cancer. 15:972015. View Article : Google Scholar
|
|
40
|
Lee WS, Woo EY, Kwon J, Park MJ, Lee JS,
Han YH and Bae IH: Bcl-w enhances mesenchymal changes and
invasiveness of glioblastoma cells by inducing nuclear accumulation
of β-catenin. PLoS One. 8:e680302013. View Article : Google Scholar
|
|
41
|
Matsumoto K, Arao T, Tanaka K, Kaneda H,
Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y, et al:
mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133
expression in cancer cells. Cancer Res. 69:7160–7164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pistollato F, Abbadi S, Rampazzo E,
Persano L, Della Puppa A, Frasson C, Sarto E, Scienza R, D'avella D
and Basso G: Intratumoral hypoxic gradient drives stem cells
distribution and MGMT expression in glioblastoma. Stem Cells.
28:851–862. 2010.PubMed/NCBI
|
|
43
|
Ding Q, Yoshimitsu M, Kuwahata T, Maeda K,
Hayashi T, Obara T, Miyazaki Y, Matsubara S, Natsugoe S and Takao
S: Establishment of a highly migratory subclone reveals that CD133
contributes to migration and invasion through
epithelial-mesenchymal transition in pancreatic cancer. Hum Cell.
25:1–8. 2012. View Article : Google Scholar
|
|
44
|
Tlsty TD and Coussens LM: Tumor stroma and
regulation of cancer development. Annu Rev Pathol. 1:119–150. 2006.
View Article : Google Scholar
|
|
45
|
Herrera A, Herrera M, Alba-Castellón L,
Silva J, García V, Loubat-Casanovas J, Alvarez-Cienfuegos A, Miguel
García J, Rodriguez R, Gil B, et al: Protumorigenic effects of
Snail-expression fibroblasts on colon cancer cells. Int J Cancer.
134:2984–2990. 2014. View Article : Google Scholar
|
|
46
|
Yang MC, Wang CJ, Liao PC, Yen CJ and Shan
YS: Hepatic stellate cells secretes type I collagen to trigger
epithelial mesenchymal transition of hepatoma cells. Am J Cancer
Res. 4:751–763. 2014.PubMed/NCBI
|
|
47
|
Feng Y, Xu X, Zhang Y, Ding J, Wang Y,
Zhang X, Wu Z, Kang L, Liang Y, Zhou L, et al: HPIP is upregulated
in colorectal cancer and regulates colorectal cancer cell
proliferation, apoptosis and invasion. Sci Rep. 5:94292015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang HS, Yan B, Li XB, Fan L, Zhang YF,
Wu GH, Li M and Fang J: PAX2 protein induces expression of cyclin
D1 through activating AP-1 protein and promotes proliferation of
colon cancer cells. J Biol Chem. 287:44164–44172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park EJ, Chung HJ, Park HJ, Kim GD, Ahn YH
and Lee SK: Suppression of Src/ERK and GSK-3/β-catenin signaling by
pinosylvin inhibits the growth of human colorectal cancer cells.
Food Chem Toxicol. 55:424–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hayashido Y, Kitano H, Sakaue T, Fujii T,
Suematsu M, Sakurai S and Okamoto T: Overexpression of integrin αv
facilitates proliferation and invasion of oral squamous cell
carcinoma cells via MEK/ERK signaling pathway that is activated by
interaction of integrin αvβ8 with type I collagen. Int J Oncol.
45:1875–1882. 2014.PubMed/NCBI
|
|
51
|
Nykopp TK, Pasonen-Seppänen S, Tammi MI,
Tammi RH, Kosma VM, Anttila M and Sironen R: Decreased
hyaluronidase 1 expression is associated with early disease
recurrence in human endometrial cancer. Gynecol Oncol. 137:152–159.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Song L, Liu D, Wang B, He J, Zhang S, Dai
Z, Ma X and Wang X: miR-494 suppresses the progression of breast
cancer in vitro by targeting CXCR4 through the Wnt/β-catenin
signaling pathway. Oncol Rep. 34:525–531. 2015.PubMed/NCBI
|
|
53
|
Cao F, Miao Y, Xu K and Liu P: Lethal (2)
giant larvae: An indispensable regulator of cell polarity and
cancer development. Int J Biol Sci. 11:380–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mayne R, Vail MS and Miller EJ:
Characterization of the collagen chains synthesized by cultured
smooth muscle cells derived from rhesus monkey thoracic aorta.
Biochemistry. 17:446–452. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brücher BL and Jamall IS: Cell-cell
communication in the tumor microenvironment, carcinogenesis and
anticancer treatment. Cell Physiol Biochem. 34:213–243. 2014.
View Article : Google Scholar
|
|
56
|
Yamanaka T, Horikoshi Y, Suzuki A,
Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T,
Ishikawa H and Ohno S: Par-6 regulates aPKC activity in a novel way
and mediates cell-cell contact-induced formation of the epithelial
junctional complex. Genes Cells. 6:721–731. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo J, Niu R, Huang W, Zhou M, Shi J,
Zhang L and Liao H: Growth factors from tumor microenvironment
possibly promote the proliferation of glioblastoma-derived
stem-like cells in vitro. Pathol Oncol Res. 18:1047–1057. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Itoh M, Sasaki H, Furuse M, Ozaki H, Kita
T and Tsukita S: Junctional adhesion molecule (JAM) binds to PAR-3:
A possible mechanism for the recruitment of PAR-3 to tight
junctions. J Cell Biol. 154:491–497. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ebnet K, Suzuki A, Horikoshi Y, Hirose T,
Meyer Zu, Brickwedde MK, Ohno S and Vestweber D: The cell polarity
protein ASIP/PAR-3 directly associates with junctional adhesion
molecule (JAM). EMBO J. 20:3738–3748. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lokeshwar VB, Rubinowicz D, Schroeder GL,
Forgacs E, Minna JD, Block NL, Nadji M and Lokeshwar BL: Stromal
and epithelial expression of tumor markers hyaluronic acid and
HYAL1 hyaluronidase in prostate cancer. J Biol Chem.
276:11922–11932. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lokeshwar VB, Young MJ, Goudarzi G, Iida
N, Yudin AI, Cherr GN and Selzer MG: Identification of bladder
tumor-derived hyaluronidase: Its similarity to HYAL1. Cancer Res.
59:4464–44670. 1999.PubMed/NCBI
|
|
62
|
McCrea PD, Gu D and Balda MS: Junctional
music that the nucleus hears: Cell-cell contact signaling and the
modulation of gene activity. Cold Spring Harb Perspect Biol.
1:a0029232009. View Article : Google Scholar
|
|
63
|
Menzel EJ and Farr C: Hyaluronidase and
its substrate hyaluronan: Biochemistry, biological activities and
therapeutic uses. Cancer Lett. 131:3–11. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Turley EA, Noble PW and Bourguignon LY:
Signaling properties of hyaluronan receptors. J Biol Chem.
277:4589–4592. 2002. View Article : Google Scholar
|
|
65
|
Paiva P, Van Damme MP, Tellbach M, Jones
RL, Jobling T and Salamonsen LA: Expression patterns of hyaluronan,
hyaluronan synthases and hyaluronidases indicate a role for
hyaluronan in the progression of endometrial cancer. Gynecol Oncol.
98:193–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang F, Grigorieva EV, Li J, Senchenko VN,
Pavlova TV, Anedchenko EA, Kudryavtseva AV, Tsimanis A, Angeloni D,
Lerman MI, et al: HYAL1 and HYAL2 inhibit tumour growth in vivo but
not in vitro. PLoS One. 3:e30312008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lokeshwar VB, Cerwinka WH and Lokeshwar
BL: HYAL1 hyaluronidase: A molecular determinant of bladder tumor
growth and invasion. Cancer Res. 65:2243–2250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kramer MW, Golshani R, Merseburger AS,
Knapp J, Garcia A, Hennenlotter J, Duncan RC, Soloway MS, Jorda M,
Kuczyk MA, et al: HYAL-1 hyaluronidase: A potential prognostic
indicator for progression to muscle invasion and recurrence in
bladder cancer. Eur Urol. 57:86–93. 2010. View Article : Google Scholar :
|
|
69
|
Wakitani S, Kimura T, Hirooka A, Ochi T,
Yoneda M, Yasui N, Owaki H and Ono K: Repair of rabbit articular
surfaces with allograft chodnrocytes embedded in collagen gel. J
Bone Joint Surg Br. 71:74–80. 1989.PubMed/NCBI
|
|
70
|
Frenkel SR, Toolan B, Menche D, Pitman MI
and Pachence JM: Chondrocyte transplantation using a collagen
bilayer matrix for cartilage repair. J Bone Joint Surg Br.
79:831–836. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schuppan D, Schmid M, Somasundaram R,
Ackermann R, Ruehl M, Nakamura T and Riecken EO: Collagens in the
liver extracellular matrix bind hepatocyte growth factor.
Gastroenterology. 114:139–152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kauppila S, Stenbäck F, Risteli J, Jukkola
A and Risteli L: Aberrant type I and III collagen gene expression
in human breast cancer in vivo. J Pathol. 186:262–268. 1998.
View Article : Google Scholar
|
|
73
|
Minamoto T, Ooi A, Okada Y, Mai M, Nagai Y
and Nakanishi I: Desmoplastic reaction of gastric carcinoma: A
light- and electron microscopic immunohistochemical analysis using
collagen type-specific antibodies. Hum Pathol. 19:815–821. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bosman FT, de Bruïne A, Flohil C, van der
Wurff A, ten Kate J and Dinjens WW: Epithelial-stromal interactions
in colon cancer. Int J Dev Biol. 37:203–211. 1993.PubMed/NCBI
|
|
75
|
Dahlman T, Lammerts E, Wik M, Bergström D,
Grimelius L, Westermark K, Rubin K and Heldin NE: Fibrosis in
undifferentiated (anaplastic) thyroid carcinomas: Evidence for a
dual action of tumour cells in collagen type I synthesis. J Pathol.
191:376–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pietras K and Ostman A: Hallmarks of
cancer: Interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Niehans GA, Kratzke RA, Froberg MK, Aeppli
DM, Nguyen PL and Geradts J: G1 checkpoint protein and p53
abnormalities occur in most invasive transitional cell carcinomas
of the urinary bladder. Br J Cancer. 80:1175–1184. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Huang R, Wang G, Song Y, Tang Q, You Q,
Liu Z, Chen Y, Zhang Q, Li J, Muhammand S, et al: Colorectal cancer
stem cell and chemo-resistant colorectal cancer cell phenotypes and
increased sensitivity to Notch pathway inhibitor. Mol Med Rep.
12:2417–2424. 2015.PubMed/NCBI
|
|
80
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
82
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar
|
|
83
|
Gilcrease MZ: Integrin signaling in
epithelial cells. Cancer Lett. 247:1–25. 2007. View Article : Google Scholar
|
|
84
|
Takada Y, Ye X and Simon S: The integrins.
Genome Biol. 8:2152007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Iwatsuki H, Sasaki K, Suda M and Itano C:
Vimentin intermediate filament protein as differentiation marker of
optic vesicle epithelium in the chick embryo. Acta Histochem.
101:369–382. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gilles C, Polette M, Zahm JM, Tournier JM,
Volders L, Foidart JM and Birembaut P: Vimentin contributes to
human mammary epithelial cell migration. J Cell Sci. 112:4615–4625.
1999.PubMed/NCBI
|
|
87
|
Shattil SJ, Kim C and Ginsberg MH: The
final steps of integrin activation: The end game. Nat Rev Mol Cell
Biol. 11:288–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Box C, Rogers SJ, Mendiola M and Eccles
SA: Tumour-microenvironmental interactions: Paths to progression
and targets for treatment. Semin Cancer Biol. 20:128–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hammoudi A, Song F, Reed KR, Jenkins RE,
Meniel VS, Watson AJ, Pritchard DM, Clarke AR and Jenkins JR:
Proteomic profiling of a mouse model of acute intestinal Apc
deletion leads to identification of potential novel biomarkers of
human colorectal cancer (CRC). Biochem Biophys Res Commun.
440:364–370. 2013. View Article : Google Scholar : PubMed/NCBI
|