Introduction
Triple-negative breast cancer (TNBC) is one of the
most common types of malignant tumors in women worldwide and is now
the third leading cause of cancer-related mortality (1). TNBC is characterized by the absence
of estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor receptor 2 (HER2) (2). Thus, TNBC patients do not benefit
from commonly used anti-estrogen and -herceptin-based therapies
(3). In addition, patients with
TNBC have been reported to have a poorer survival rate, and
recurrence and distant metastases occurs more frequently than in
patients with other types of breast cancer (4). Recent studies have revealed that TNBC
comprises a heterogeneous group of tumors encompassing several
molecular subtypes, such as luminal A, luminal B, HER2-enriched,
claudin-low and basal-like tumors (5–7).
Approximately 62% of basal-like TNBC and 43% of non-basal-like TNBC
exhibit mutations in the tumor suppressor p53 (MTp53). The majority
of p53 mutations observed in the tumor are loss-of-function
mutations; however, some patients have been shown to exhibit
oncogenic gain-of-function mutations. Therefore, targeting p53 may
be less effective in TNBC treatment (8). Thus, successful treatment of
p53-mutant TNBCs remains challenging. Doxorubicin (DOXO) and
cisplatin (CDDP) are common treatment options for TNBC. However,
acquired resistance and toxicity against these drugs eventually
occurs, preventing effective treatment (9). Thus, novel therapies that overcome
drug resistance and toxicity in p53-mutant TNBC cases are urgently
required.
p73 is a member of the p53 gene family and has been
shown to regulate p53 target genes in p53-deficient cancer cells
(10). In contrast to p53, p73 is
rarely mutated or lost in cancer (11). p53 deficient cancers are partly
resistant to chemotherapy; however, not completely chemo-resistant.
As p53 deficient cancers have other types of p53 family members
such as p73, they may be able to replace the function of p53 in
response to DNA damage (12).
Therefore, identifying anticancer agents that can activate p73 may
provide a chemotherapeutic approach for treating drug-resistant
p53-mutant cancers. Recently, nonhydrolyzable ether analog of
RRR-α-tocopherol in combination with DOXO or CDDP has been reported
to target p53-mediated genes in a p73-dependent manner, enhancing
the apoptosis of TNBC cells (9).
Traditional herbal medicines have recently been
revisited for cancer therapy as herbal extracts or mixtures based
on traditional medicines have exhibited anticancer effects with no
or fewer side effects compared with current anticancer
therapeutics, including chemical compounds and targeted antibodies
(13). Previous studies have
demonstrated the anticancer effects of herbal extracts from
Astragalus membranaceus (Am), Angelica gigas (Ag) and
Trichosanthes kirilowii Maximowicz (Tk) in different cancer
cell types, such as leukemia, hepatocellular carcinoma, colon
cancer, non-small cell lung cancer and gastric cancer cells
(14–19). Furthermore, extracts from a mixture
of Am and Ag have been shown to affect various diseases, including
hematological diseases and endocrine disorders (20–22).
The present study demonstrated that SH003 extracted
from a herbal mixture (Am, Ag and Tk) exhibited anticancer effects
on TNBC via activation of the p73 pathway. Thus, SH003 may be
useful for the treatment of TNBC.
Materials and methods
Cell culture and reagents
Established Hs578T, MDA-MB-231, ZR-75-1, MCF7 and
T47D human breast cancer cell lines were purchased from the
American Type Culture Collection (Manassas, VA, USA). All cell
lines were maintained in RPMI-1640 medium (Gibco, Thermo Fisher
Scientific Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco, Thermo Fisher Scientific Inc.), 100 units
of penicillin and 100 μl/ml streptomycin. All cells were
cultured in a 5% CO2 incubator at 37°C. SH003 was
extracted from Am, Ag or Tk, which were provided by Dr S.G. Ko
(College of Korean Medicine, University of Kyung Hee, Seoul, Korea)
as previously described (23).
Cell viability and cell death
analysis
Cells (2×105 cells per plate) were seeded
in a 60-mm plate and treated with various concentrations of SH003
(50, 100 or 200 μg/ml) for 48 h. Cell viability and cell
death were assessed using a trypan blue exclusion method. Cell
pellet was harvested and resuspend in 1 ml of phosphate-buffered
saline (PBS). A total of 10 μl 0.4% trypan blue was gently
mixed with 10 μl cell suspension. The mixture was applied to
a hemocytometer and the number of trypan blue stained and
non-stained cells were counted under a light microscope. The
percentage of viable cells was calculated.
Colony formation assay
Cells were seeded at a density of 3×102
cells per well in a 6-well plate and were treated with various
concentrations of SH003 (50, 100 or 200 μg/ml) for 24 h. The
cells were cultured for 14 days and colonies were fixed with 4%
paraformaldehyde and stained with a 0.01% crystal violet. Colony
counts were performed manually using a light microscope and images
of each plate were obtained.
RNA interference
Cells were transiently transfected with small
interfering (si)RNA using the Lipofectamine RNAi MAX reagent
(Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The siRNA sequence for
transfection was p73-siRNA, 5′-GCAAUAAUCUCUCGCAGUAUU-3′ and
scramble-siRNA, 5′-GGACUCUCGGAUUGUAAGAUU-3′
Western blot analysis
Cell lysates were prepared with
radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris-HCl,
pH 7.5; 50 mM NaCl, 1 μM EGTA and 1% Triton X-100)
containing a protease inhibitor cocktail. Protein concentrations in
extracts were determined using a Bradford assay (Bio Rad
Laboratories, Inc., Hercules, CA, USA). Total cellular proteins (20
μg) were subjected to 10–15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% non-fat dry milk in Tris-buffered saline with Tween-20
(TBST) buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20)
and probed with anti-poly ADP ribose polymerase (PARP; cat. no.
9542; 1:1,000), anti-p73 (cat. no. 14620; 1:1,000), anti-caspase 3
(cat. no. 9661; 1:1000; Cell Signaling Technology, Beverly, MA,
USA) or anti-β-actin (cat. no. sc-47778; 1:2,000; Santa Cruz
Biotechnology Inc., Dallas, TX, USA) primary antibodies at 4°C
overnight. Subsequently, the membranes were washed three times with
TBST. Primary antibodies were detected following 2 h incubation at
room temperature with a horseradish peroxidase-conjugated
anti-mouse (cat. no. 7076; 1:2,000) or anti-rabbit secondary
antibody (cat. no. 7074; 1:2,000; Cell Signaling Technology,
Danvers, MA, USA). Blots were developed with an enhanced
chemiluminescence detection kit (Amersham, Buckinghamshire,
UK).
Cell cycle analysis
In total, 3×102 cells harvested by
trypsinization were fixed in 1 ml of cold 70% ethanol for 24 h at
−20°C. After washing cell pellets with 1 ml PBS, pellets were
centrifuged at 300 × g for 5 min, discarded supernatant,
resuspended in 1 ml staining solution (50 μg/ml propidium
iodide, 50 μg/ml RNase and 0.1% Triton X 100 in citrate
buffer, pH 7.8), incubated for 30 min and washed with PBS. Cell
cycle distribution was analyzed using a FACSCalibur
fluorescence-activated cell sorter and CellQuest version 3.0
software (BD Biosciences, San Jose, CA, USA).
Statistical analysis
SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA) was
used to perform statistical analysis. Data are presented as the
mean ± standard deviation and multiple comparisons were conducted
using one-way analysis of variance followed by Newman-Keuls
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
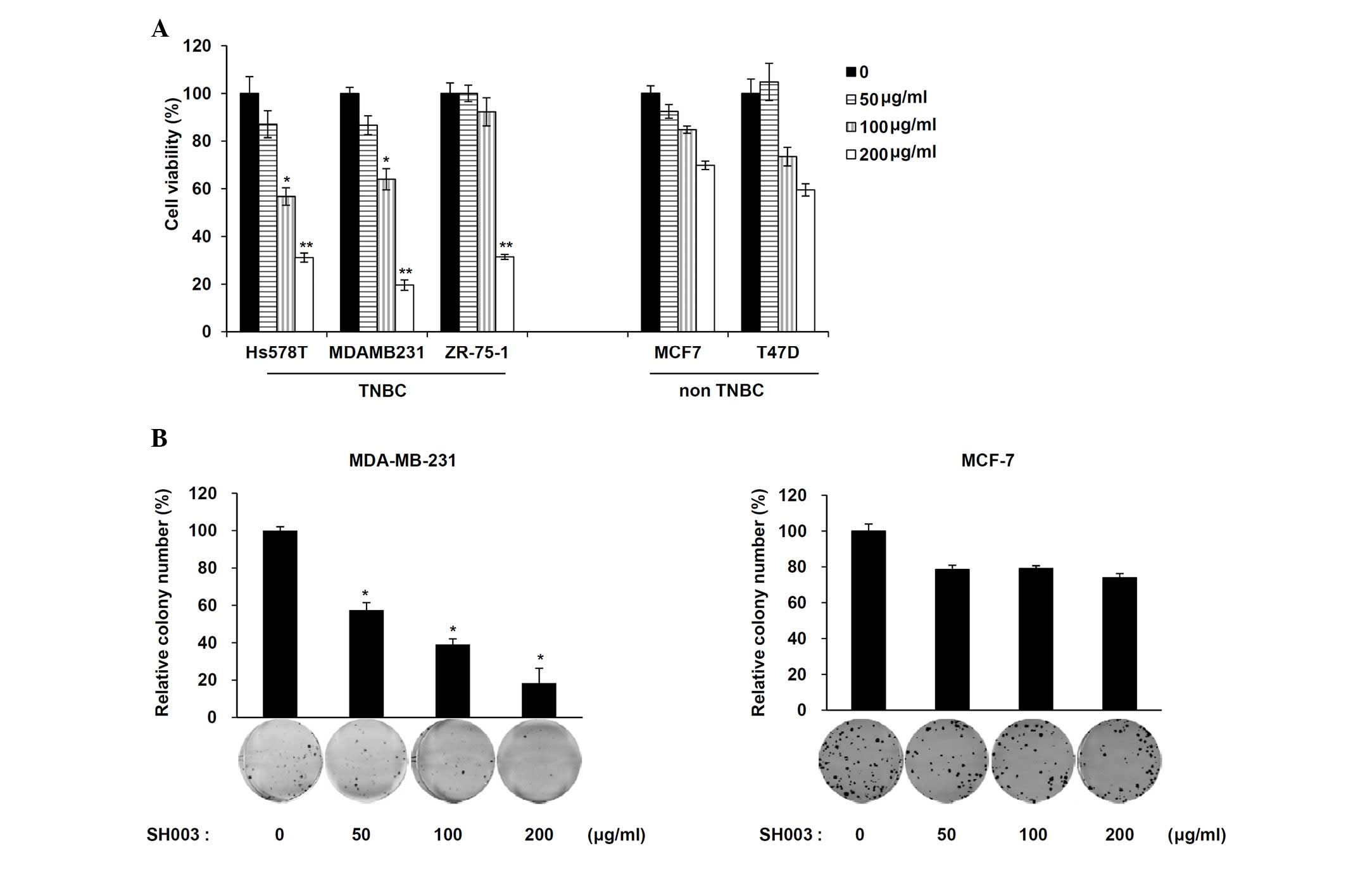
SH003 selectively inhibits the cell
viability of TNBC cells
The viability of two breast cancer cell types, TNBC
(Hs578T, MDA-MB-231 and ZR-75-1) and non-TNBC (MCF7 and T47D) was
determined following exposure to SH003. Cells were treated with
various concentrations (50, 100 and 200 μg/ml) of SH003. A
significant decrease in cell viability was observed in TNBC cells
(P<0.05 for the 100 μg/ml group and P<0.01 for the 200
μg/ml group compared with the untreated cells) but not in
non-TNBC cells (Fig. 1A).
MDA-MB-231 cells were further used as the cells showed the most
effectively reduced cell viability in a dose-dependent manner.
Additionally, colony formation analyses revealed a significant
decrease in the number of MDA-MB-231 (TNBC) cells treated with
SH003 but not MCF-7 (non-TNBC) cells (Fig. 1B; P<0.005 compared with the
untreated cells). These results indicate that SH003 selectively
decreases TNBC cell viability.
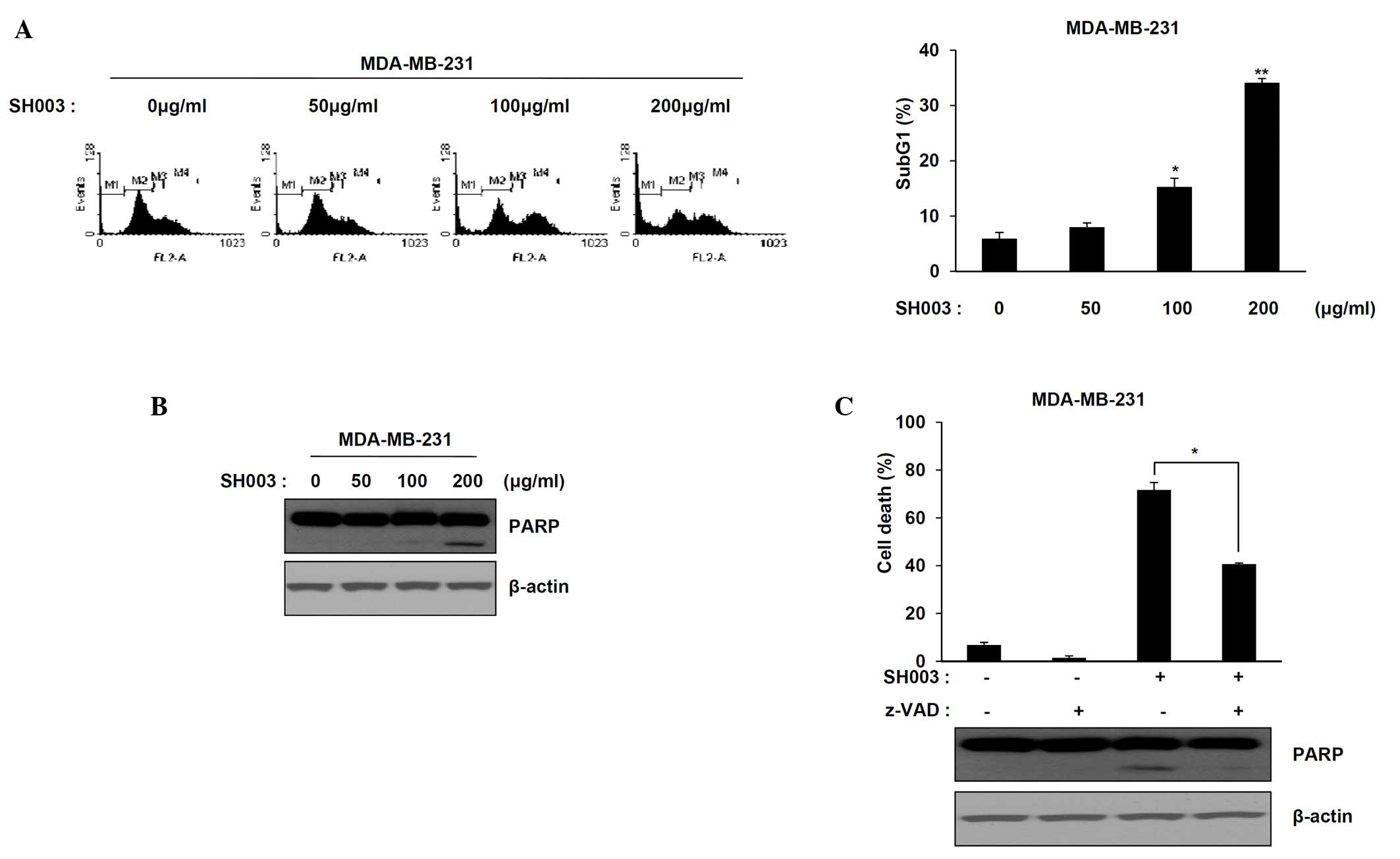
SH003 induces caspase-dependent cell
death in TNBC cells
The effect of SH003 on cell death in MDA-MB-231
cells was determined using flow cytometry. A significant increase
in the number of cells at the sub-G1 phase was observed following
SH003 treatment (50, 100 and 200 μg/ml) (Fig. 2A) (P<0.05 and P<0.01 compared
with the untreated cells). Apoptotic cell death and PARP cleavage
in response to SH003 treatment were assessed in MDA-MB-231 cells
using western blot analysis. The expression levels of cleaved PARP
increased significantly in a dose-dependent manner compared with
the untreated cells (P<0.05; Fig.
2B). SH003-induced apoptosis after pre-treatment with a
pan-caspase inhibitor, Z-VAD was then examined. Pre-treatment with
Z-VAD partially decreased the MDA-MB-231 cell death and levels of
cleaved PARP induced by SH003 (Fig.
2C; P<0.05 compared with cells treated with SH003 only).
Thus, SH003-induced cell death is partially caspase-dependent in
TNBC cells.
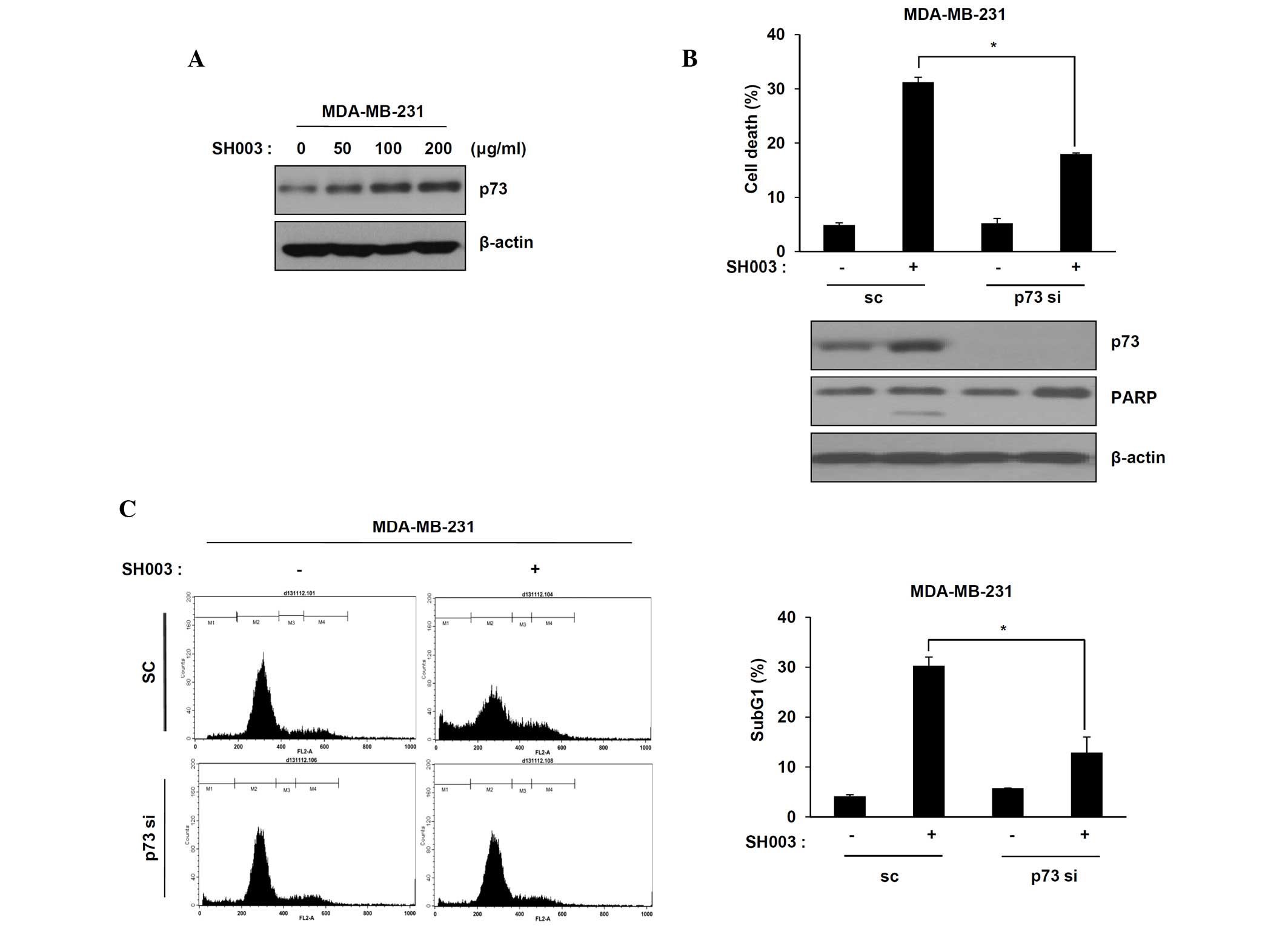
Induction of p73 expression by SH003
leads to apoptosis in TNBC
A previous study indicated that p73 expression may
prevent drug resistance and toxicity in p53-mutant TNBC (8). It was demonstrated that SH003 induced
p73-mediated apoptosis in p53 mutant MDA-MB-231 cells. p73
expression in MDA-MB-231 cells was observed following treatment
with SH003 using western blot analysis. The p73 protein levels in
MDA-MB-231 cells treated with SH003 increased in a dose-dependent
manner (Fig. 3A). To confirm that
MDA-MB-231 cell death induced by SH003 was correlated with p73, the
effect of knockdown of endogenous p73 using small interfering RNAs
in MDA-MB-231 cells was examined. Cells were transfected with
scrambled siRNA or p73 siRNA, followed by treatment with SH003.
Transfected p73 siRNA decreased cell death and PARP cleavage
compared with scrambled siRNA treatment (Fig. 3B; P<0.05 compared with SH003
single-treated scramble cells). Additionally, cell death was
confirmed using flow cytometric analysis. The number of cells in
the sub-G1 phase following SH003 treatment was decreased in the p73
siRNA-transfected cell line compared with the scrambled
siRNA-transfected cell line (Fig.
3C; P<0.05 compared with SH003 single-treated scramble
cells). These results indicated that the induction of p73
expression by SH003 leads to the apoptosis of MDA-MB-231 cells.
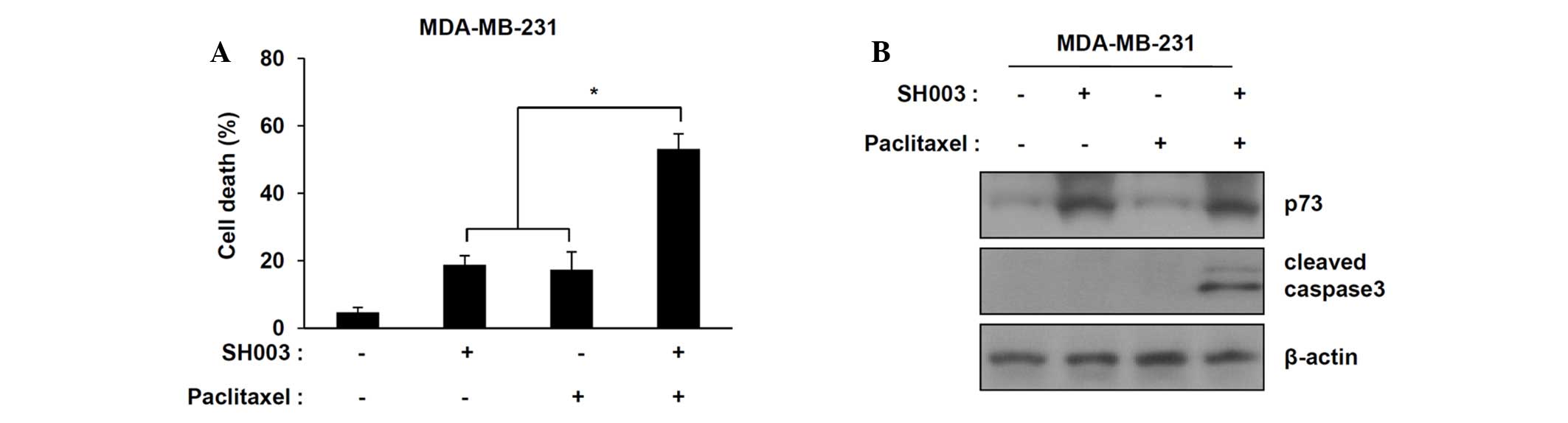
SH003 sensitizes paclitaxel-induced
MDA-MB-231 cell death
Paclitaxel (taxane) is a commonly used treatment in
conjunction with other anticancer agents for TNBC; however,
paclitaxel treatment occasionally fails due to drug resistance. It
was demonstrated that SH003 in combination with paclitaxel
synergistically increases cell death in TNBC cell compared with
individual treatments (Fig. 4A;
P<0.05 compared with cells treated with SH003 or paclitaxel
only). Western blot analyses indicated that SH003 in combination
with paclitaxel increased the levels of cleaved caspase-3 in
MDA-MB-231 cells but did not alter p73 expression (Fig. 4B). These results indicate that
SH003 in combination with paclitaxel synergistically enhances
apoptosis in TNBC cells.
Discussion
TNBC accounts for 10–20% of all types of breast
cancer (24). TNBC is an
aggressive histological subtype with limited treatment conditions
and poor prognosis following standard chemotherapy. The anticancer
effects of commonly used chemotherapeutic agents, such as
paclitaxel, doxorubicin and cisplatin are limited to cure patients
with TNBC due to acquired drug resistance and toxicity (9). The present study focused on
anticancer therapy for TNBC to overcome resistance against
conventional therapies.
The present chemotherapeutic agents for TNBC are
DNA-damaging agents (25). In the
DNA-damage pathway, tumor suppressor p53 is important in anticancer
actions of DNA-damaging agents (26). A recent study reported that
regulation of p53-mediated apoptotic signaling occurs in a
p73-dependent manner, which results in enhanced apoptosis in
p53-deficient TNBC (9). The
functional and structural similarities of p53 and p73 have been
previously reported (10). It is
also known that p73 can replace the function of p53 in response to
DNA damage in p53-deficient cancers. p73 is not frequently mutated
in cancers and regulates p53 target genes, such as Bax and Noxa in
p53-deficient cancers (27,28).
The key role of p73 in anti-cancer effects for p53-deficient TNBC
was identified.
Identification and development of traditional herbal
medicines has increased due to their potential anticancer effects
and minimal side effects. This study demonstrated that SH003
inhibited TNBC growth in a dose-dependent manner. Treatment with
SH003 resulted in apoptotic cell death as shown by increased PARP
cleavage, a caspase-dependent apoptotic marker. In addition,
SH003-induced apoptosis was validated after pretreatment with the
pan-caspase inhibitor, Z-VAD, as this partially decreased cell
death in MDA-MB-231 cells.
Notably, apoptotic cell death induced by SH003 was
associated with induction of p73 expression in TNBC. The anticancer
effect of SH003 was validated upon siRNA-mediated knockdown of p73.
The results showed that knockdown of p73 decreased apoptotic cell
death induced by SH003 treatment. In addition, single treatment
with paclitaxel did not result in any specific cell death, while
SH003 in combination with paclitaxel synergistically increased cell
death in TNBC. Therefore, SH003 in combination chemotherapies may
aid in overcoming resistance to conventional chemotherapies in
TNBC.
The apoptotic cell death induced by SH003 is
associated with p73 expression, which indicates that the anticancer
effects of SH003 are induced by p73-dependent apoptosis. This study
showed that SH003 induced the expression of p73- and
caspase-dependent apoptosis. Thus, this study revealed that a
traditional herbal medicine, SH003, has a significant anticancer
effect via p73-mediated apoptosis in TNBC cells and confirmed p73
as a promising therapeutic target for TNBC.
Abbreviations:
|
Am
|
Astragalus membranaceus
|
|
Ag
|
Angelica gigas
|
|
Tk
|
Trichosanthes kirilowii
Maximowicz
|
|
TNBC
|
triple-negative breast cancer
|
Acknowledgments
This study was supported by grants from Basic
Science Research Program through the the National Research
Foundation of Korea (NRF) grant funded by the Korean government
(MEST), Seoul, Republic of Korea (NRF-2013R1A2A2A01067394) to
Professor Dong-Hoon Jin, and the Korean Medicine R&D Project of
the Ministry of Health and Welfare to Professor Seong-Gyu Ko
(B110043).
References
|
1
|
Millis SZ, Gatalica Z, Winkler J, Vranic
S, Kimbrough J, Reddy S and O'Shaughnessy JA: Predictive Biomarker
Profiling of > 6000 Breast Cancer Patients Shows Heterogeneity
in TNBC, With Treatment Implications. Clin Breast Cancer.
15:73–481. 2015. View Article : Google Scholar
|
|
2
|
Isakoff SJ: Triple-negative breast cancer:
Role of specific chemotherapy agents. Cancer J. 16:53–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu L, Yin S, Banerjee S, Sarkar F and
Reddy KB: Enhanced anticancer effect of the combination of
cisplatin and TRAIL in triple-negative breast tumor cells. Mol
Cancer Ther. 10:550–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haffty BG, Yang Q, Reiss M, Kearney T,
Higgins SA, Weidhaas J, Harris L, Hait W and Toppmeyer D:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prat A and Perou CM: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar
|
|
8
|
Aas T, Børresen AL, Geisler S,
Smith-Sørensen B, Johnsen H, Varhaug JE, Akslen LA and Lønning PE:
Specific P53 mutations are associated with de novo resistance to
doxorubicin in breast cancer patients. Nat Med. 2:811–814. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tiwary R, Yu W, Sanders BG and Kline K:
α-TEA cooperates with chemotherapeutic agents to induce apoptosis
of p53 mutant, triple-negative human breast cancer cells via
activating p73. Breast Cancer Res. 13:R12011. View Article : Google Scholar
|
|
10
|
Levrero M, De Laurenzi V, Costanzo A, Gong
J, Wang JY and Melino G: The p53/p63/p73 family of transcription
factors: Overlapping and distinct functions. J Cell Sci.
113:1661–1670. 2000.PubMed/NCBI
|
|
11
|
Kaelin WG Jr: The p53 gene family.
Oncogene. 18:7701–7705. 1999. View Article : Google Scholar
|
|
12
|
Rödicker F and Pützer BM: p73 is effective
in p53-null pancreatic cancer cells resistant to wild-type TP53
gene replacement. Cancer Res. 63:2737–2741. 2003.PubMed/NCBI
|
|
13
|
Maurya U and Srivastava S: Traditional
Indian herbal medicine used as antipyretic, antiulcer,
anti-diabetic and anticancer: A Review. IJRPC. 1:42011.
|
|
14
|
Shin JW, Son JY, Kang JK, Han SH, Cho CK
and Son CG: Trichosanthes kirilowii tuber extract induces G2/M
phase arrest via inhibition of tubulin polymerization in HepG2
cells. J Ethnopharmacol. 115:209–216. 2008. View Article : Google Scholar
|
|
15
|
Cho WC and Leung KN: In vitro and in vivo
anti-tumor effects of Astragalus membranaceus. Cancer Lett.
252:43–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui R, He J, Wang B, Zhang F, Chen G, Yin
S and Shen H: Suppressive effect of Astragalus membranaceus Bunge
on chemical hepatocarcinogenesis in rats. Cancer Chemother
Pharmacol. 51:75–80. 2003. View Article : Google Scholar
|
|
17
|
Heo BG, Chon SU, Park YJ, Bae JH, Park SM,
Park YS, Jang HG and Gorinstein S: Antiproliferative activity of
Korean wild vegetables on different human tumor cell lines. Plant
Foods Hum Nutr. 64:257–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim T, Choi HJ, Kim NJ and Kim DH:
Anxiolytic-like effects of ginsenosides Rg3 and Rh2 from red
ginseng in the elevated plus-maze model. Planta Med. 75:836–839.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li LK, Kuang WJ, Huang YF, Xie HH, Chen G,
Zhou QC, Wang BR and Wan LH: Anti-tumor effects of Astragalus on
hepatocellular carcinoma in vivo. Indian J Pharmacol. 44:78–81.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv J, Zhao Z, Chen Y, Wang Q, Tao Y, Yang
L, Fan TP and Liu C: The chinese herbal decoction danggui buxue
tang inhibits angiogenesis in a rat model of liver fibrosis. Evid
Based Complement Alternat Med. 2012:2849632012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang M, Chan GC, Deng R, Ng MH, Cheng SW,
Lau CP, Ye JY, Wang L and Liu C: An herbal decoction of Radix
astragali and Radix angelicae sinensis promotes hematopoiesis and
thrombopoiesis. J Ethnopharmacol. 124:87–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang WL, Zheng KY, Zhu KY, Zhan JY, Bi
CW, Chen JP, Du CY, Zhao KJ, Lau DT, Dong TT and Tsim KW: Chemical
and biological assessment of Angelica herbal decoction: Comparison
of different preparations during historical applications.
Phytomedicine. 19:1042–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi YK, Cho SG, Woo SM, Yun YJ, Park S,
Shin YC and Ko SG: Herbal extract SH003 suppresses tumor growth and
metastasis of MDA-MB-231 breast cancer cells by inhibiting
STAT3-IL-6 signaling. Mediators Inflamm. 2014:4921732014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwentner L, Wolters R, Koretz K,
Wischnewsky MB, Kreienberg R, Rottscholl R and Wöckel A:
Triple-negative breast cancer: The impact of guideline-adherent
adjuvant treatment on survival - a retrospective multi-centre
cohort study. Breast Cancer Res Treat. 132:1073–1080. 2012.
View Article : Google Scholar
|
|
25
|
O'Reilly EA, Gubbins L, Sharma S, Tully R,
Guang MH, Weiner-Gorzel K, McCaffrey J, Harrison M, Furlong F, Kell
M and McCann A: The fate of chemoresistance in triple negative
breast cancer (TNBC). BBA Clin. 3:257–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Essmann F and Schulze-Osthoff K:
Translational approaches targeting the p53 pathway for anti-cancer
therapy. Br J Pharmacol. 165:328–344. 2012. View Article : Google Scholar :
|
|
27
|
Melino G, Bernassola F, Ranalli M, Yee K,
Zong WX, Corazzari M, Knight RA, Green DR, Thompson C and Vousden
KH: p73 induces apoptosis via PUMA transactivation and Bax
mitochondrial translocation. J Biol Chem. 279:8076–8083. 2004.
View Article : Google Scholar
|
|
28
|
Flinterman M, Guelen L, Ezzati-Nik S,
Killick R, Melino G, Tominaga K, Mymryk JS, Gäken J and Tavassoli
M: E1A activates transcription of p73 and Noxa to induce apoptosis.
J Biol Chem. 280:5945–5959. 2005. View Article : Google Scholar
|