Introduction
Osteoporosis is a common disease which is
characterized by the deterioration of the micro-architecture of
bone tissue, reduced bone mineral density (BMD) and an increased
risk of fragility fracture (1).
Previous studies conducted in families and twins have indicated
that BMD is strongly controlled by genes (2–6). It
is important to identify the genetic variants that result in
interindividual differences in bone traits, as this may lead to the
identification of the cellular pathways that impact bone.
Furthermore, this may lead to the formulation of novel treatments
for bone diseases and aid in the identification of populations at
risk of osteoporosis. Genetic variation serves a role in the
regulation of BMD, with a range of genetic loci observed to
contribute. In addition, certain gender-specific effects of genes
and loci upon BMD have been observed (7), as indicated by gene-by-gene
interactions (8). Collagen type I
is present at high levels in connective tissue and is required for
the normal functioning of bone and blood vessels (9). Approximately 90% of the bone matrix
protein is collagen type I, which is important for the framework
for mineralization and the tensile strength that gives bone
elasticity (9). The collagen type
I triple helix includes two α1(I) chains and one α2(I) chain,
encoded by the genes COL1A1 and COL1A2, respectively. Mutations in
these genes result in diseases such as osteogenesis imperfecta and
Ehler-Danlos syndrome, which are characterized by low BMD, moderate
to severe bone fragility and an increased tendency for bruising and
bleeding (10,11).
MicroRNAs (miRNAs) are small highly conserved
noncoding RNA species that serve important roles in numerous
cellular activities (12). The
dysregulation of miRNA expression has been associated with a
variety of human medical conditions (13). miRNAs recognize their targets
predominantly via base-pairing interactions between the 5′ end of
miRNA and complementary sequences in the 3′-untranslated regions
(3′-UTRs) of the target mRNAs (12). The binding of miRNA to mRNA is
important for the regulation of mRNA and protein expression. It has
been demonstrated that genetic polymorphisms in the 3′-UTR of mRNAs
targeted by miRNAs alters the strength of miRNA binding, impacting
upon the regulation of target genes, and as a result impacting the
individual's susceptibility to disease (14–16).
In a previous study, COL1A2 was identified as a
target gene of the miRNA, let-7g, in liver cells, and that an
insertion/deletion (INS/DEL) polymorphism in the 3′-UTR of COL1A2
interferes with the interaction between let-7g and COL1A2 (17). The rs3917 polymorphism consists of
two alleles with the wild-type being an insertion (INS) and the
minor allele being a deletion (DEL). Considering the fact that
COL1A2 and let-7 serve important roles in regulating osteogenesis,
the present study hypothesized that an INS/DEL polymorphism in the
3′-UTR of COL1A2 may compromise the physiological inhibition of
COL1A2 by let-7g, and that this may represent a potential molecular
mechanism underlying the interindividual variation in
susceptibility to osteoporosis in the population.
Materials and methods
Subjects
A total of 487 participants (42–77 years old)
including 155 women and 332 men were recruited at the Provincial
Hospital Affiliated to Shandong University (Jinan, China). Subjects
with a history of hip fracture and metabolic bone disease were
excluded from the study. In addition, subjects who were treated
with bisphosphonates, calcitonin, fluoride or hormone replacement
therapy were excluded from the study. A total of 48 underwent
surgery, during which peripheral blood was obtained with the use of
Lymphocyte Separation Medium (Human; Applygen Technologies, Inc.,
Beijing, China). The study was conducted with approval from the
Clinical Research Ethics Committee of the Provincial Hospital
Affiliated to Shandong University (Shandong, China), and informed
consent was obtained from all subjects prior to the study.
Determination of BMD
BMD was determined using a Hologic QDR 2000
dual-energy X-ray densitometer (Hologic, Inc., Waltham, MA, USA).
Measurements were taken from the proximal hip (trochanter left,
total left hip and femoral neck), and the lumbar spine (L1-L4).
DNA extraction and genotyping
A DNA extraction kit (Qiagen GmbH, Hilden, Germary)
was used to extract the DNA from peripheral blood in accordance
with the manufacturer's protocol. The COL1A2 forward primer,
5′-CTGTGGAACCATGGAAGAAG-3′ and reverse primer,
5′-GTATTGAGTTGTATCGTGTGG-3′ (Takara Biotechnology Co., Ltd.,
Dailan, China), were used for amplification of DNA fragments
containing the polymorphism. Polymerase chain reaction (PCR) was
conducted in 37.5 µl of mixed solution containing 0.5 mmol/l each
primer, 1.5 mmol/l MgCl2, 0.25 mmol/l dNTPs (Qiagen
GmbH), 3.75 ml 10X PCR buffer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), 1.5 units Taq DNA polymerase and 100 ng genomic
DNA. The cycling conditions were as follows: 94°C for 5 min, 34
cycles of 30 sec at 94°C, followed by 30 sec at 60°C, and 30 sec at
72°C, and finally 72°C for 5 min. The PCR products were then sent
for direct sequencing (Provincial Hospital Affiliated to Shandong
University, Jinan, China), and rs3917 genotypes were measured by
the peak chromograph.
Isolation and culture of primary human
osteoblasts
Of the 477 study participants, primary human
osteoblasts were isolated from 48 participants, from left-over
surgically removed bone and its use was approved by the Human
Ethics Committee at the Provincial Hospital Affiliated to Shandong
University. The bone was sectioned into 1-mm3 pieces and
washed using phosphate-buffered saline (PBS). Bone pieces were
digested using 0.02% trypsin (Sigma-Aldrich, St. Louis, MO, USA) at
37°C for 90 min. Following digestion, the cells were cultured in
complete α-minimum essential medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 100 U/ml penicillin
and streptomycin (Gibco; Thermo Fisher Scientific, Inc.), 10% (v/v)
heat-inactivated fetal calf serum (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a humid atmosphere with 5% CO2. Only
passage 2 or 3 human osteoblast cells were used in the current
study. When the cells had reached 80% confluence, cells were
transfected with 50 nM hsa-let-7g mimics (Shanghai GenePharma Co.,
Ltd., Shanghai, China) and 50 nM COL1A2 short interfering RNA
(siRNA) (anti-COL1A2 siRNA) (Shanghai GenePharma Co., Ltd.) using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in
accordance with the manufacturer's instructions. Three independent
experiments were conducted.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following culture for 48–72 h, the cells were washed
with ice-cold PBS following the removal of the medium. TRIzol
reagent (Sigma-Aldrich) was added and RNA was precipitated in
isopropanol (Sigma-Aldrich). Following centrifugation at 12,000 × g
for 20 min at 4°C, RNA pellets were washed in 70% ethanol.
Subsequently, diethylpyrocarbonate-H2O (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to dissolve the RNA and a
NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.) was
used to quantify the concentration of RNA present in the samples. A
QuantiTect ReverseTranscription kit (Qiagen GmbH) was used to
synthesize first-strand cDNA from 1 µg RNA according to the
manufacturer's instructions. The specific Taqman microRNA Assays
(Applied Biosystems, Germany) was used to perform let-7g RT-qPCR
based on the instructions by supplier. A QuantiTect SYBR Green PCR
kit (Qiagen GmbH) was used for COL1A2 qPCR in accordance with the
manufacturer's protocol. Rotor-Gene 6000 (Qiagen GmbH) was used to
analyze the expression levels of COL1A2 and let-7g. The
housekeeping gene U6 was used to normalize relative gene
expression. Data Assist software, version 2.0 (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to perform the statistical
analysis of microRNA-let-7g expression, and the 2−ΔΔCq
method was used to calculate expression levels. The primer
sequences were: COL1A2, forward 5′-TGAGGTAGTTTGTACAGTT-3′ and
reverse 5′-TCAGTCCAATGGGTACGGT-3′; U6, forward
5′-TCAGTTTGCTGTTCTGGGTG-3′ and reverse 5′ CGGTTGGCTGGAAAGGAG-3′.
The cycling conditions were as follows: 95°C for 5 min followed by
30 cycles of 30 sec at 94°C, 50 sec at 55°C or 60°C, 60 sec at 72°C
and a final elongation step for 5 min at 72°C.
Protein extraction and western blot
analysis
Following culture for 48–72 h, the cells were lysed
in radioimmunoprecipitation assay lysis buffer (Invitrogen; Thermo
Fisher Scientific, Inc.), and a bicinchoninic acid assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA) was used to measure
protein concentration. A total of 10 µg of each protein sample
containing 4X sample buffer (Invitrogen; Thermo Fisher Scientific,
Inc.) was heated at 70°C for 10 min. Subsequently, the samples were
separated by 12% SDS-PAGE (Invitrogen; Thermo Fisher Scientific,
Inc.) and transferred to a polyvinylidene fluoride membrane (EMD
Millipore, Billerica, MA, USA). The membrane was then washed using
Tris-buffered saline with Tween-20 (TBS-T; 20 mM Tris-HCl, pH 7.6
and 137 mM NaCl). The membrane was blocked in TBS-T containing 1%
bovine serum albumin (Gibco, Thermo Fisher Scientific, Inc) at room
temperature for 1 h, prior to incubation with the primary
antibodies [anti-COL1A2 antibody (ab208638; 1:4,000) and
anti-β-actin (ab8227; 1:10,000) antibody; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA] at 4°C overnight. Following three
washes, the membrane was incubated with the secondary antibody
(Santa Cruz Biotechnology Inc.) at room temperature for 1 h.
Chemiluminescence (GE Healthcare Life Sciences, Chalfont, UK) was
used to detect protein bands, which were visualized using a Bio-Rad
ChemiDoc MP Imaging system (Bio-Rad Laboratories, Inc.).
COL1A2 3′-UTR luciferase assay
A DNA extraction kit (Qiagen GmbH) was used to
extract DNA from peripheral blood in accordance with the
manufacturer's protocol. The forward PCR primer,
5′-CAGTCGTATGCGCGTATAGC-3′ and reverse primer,
5′-CGTAGTCGTAGCTAGCTAGAGA-3′ were used for amplification of the
full-length of the human COL1A2 3′-UTR. The cycling conditions were
as follows: 95°C for 30 sec, then 40 cycles of 30 sec at 95°C, 2
min at 58°C and 30 sec at 68°C, followed by 72°C for 5 min, using a
PTC-100 thermocycler (Bio-Rad Laboratories, Inc.). A TA Cloning kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to clone the
PCR product. Sanger sequencing was used to determine the accuracy
of the insert. Subsequently, an additional allele of the
polymorphism was introduced using a QuikChange XL Site-Directed
Mutagenesis kit (Agilent Technologies, Inc., La Jolla, CA, USA).
The generated wild-type and mutant 3′-UTR of COL1A2 were used to
substitute the 3′-UTR of Renilla luciferase in the pRL-SV40
vector (Promega Corporation, Madison, WI, USA). Human primary
osteoblasts were cultured at a density of 1×105
cells/well in 24-well plates. Following incubation for 12 h,
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
used to transfect the cells according to the manufacturer's
instructions. A total of 100 pmol let-7g mimics or negative control
(Ambion; Thermo Fisher Scientific, Inc.) was used to co-transfect
500 ng of the wild-type or mutant construct and 50 ng pGL3 control
vector. Following transfection for 24 h, passive lysis buffer
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to harvest
cells. The Dual-Luciferase Report Assay system (Promega
Corporation) was used to determine the luciferase activity in the
cell lysates in a Turner BioSystems TD-20/20 luminometer (Promega
Corporation).
Statistical analysis
The χ2 test was used to analyze the
genotype distribution for the Hardy-Weinberg equilibrium. The
correlation between the polymorphism and BMD, adjusted for gender,
age and body mass index (BMI), was analyzed using covariance
analysis. Student's t-test was used to compare between two groups
and one-way analysis of variance was used to compare three groups.
SPSS software, version 19.0 (IBM SPSS, Armonk, NY, USA) was used
for the statistical analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
BMD is significantly associated with
the rs3917 genotype
A total of 487 subjects were recruited in the
current study, and the age, gender and BMI stratified by the COL1A2
3′-UTR rs3917 genotypes are presented in Table I. There was no statistically
significant difference in age, gender or BMI between the two
genotype groups. When the BMD was compared between the three rs3917
genotype groups, the BMD was comparable between the INS/DEL and
DEL/DEL groups (data not shown), therefore the two genotype groups
were combined, and compared with the INS/INS genotype group. This
indicated that the BMD was increased at the four tested sites, the
femoral neck (P=0.018), total left hip (P=0.028), L1-L4 (0.025) and
intertrochanteric (P=0.018) areas, in the INS/DEL or DEL/DEL group
compared with the INS/INS group, as presented in Table I. The statistically significant
differences remained following adjustment for age, gender and
BMI.
 | Table I.The demographic and clinical
characteristics of the study participants. |
Table I.
The demographic and clinical
characteristics of the study participants.
| Category | INS/INS (n=378) | INS/DEL+DEL/DEL
(n=109) | P-value |
|---|
| Age (years) | 62.3±12.7 | 60.6±13.3 | NS |
| Gender (M/F) | 265/113 | 67/42 | NS |
| BMI
(kg/cm2) | 26.6±6.8 | 26.3±7.8 | NS |
| BMD
(g/cm2) |
|
|
|
| Femoral
neck left | 0.85±0.11 | 0.92±0.13 | 0.018 |
| Total
left hip | 0.95±0.18 | 1.02±0.21 | 0.028 |
|
Trochanter left | 0.82±0.14 | 0.93±0.16 | 0.018 |
| Lumbar
spine (L1-L4) | 1.12±0.19 | 1.23±0.22 | 0.025 |
rs3917 genotypes are associated with
the expression of COL1A2 by compromising its biding to let-7g in
human osteoblast cells
To investigate how the rs3917 genotypes affect the
expression of COL1A2, the 3′-UTR of a Renilla luciferase
reporter gene was replaced with the full-length COL1A2 3′-UTR
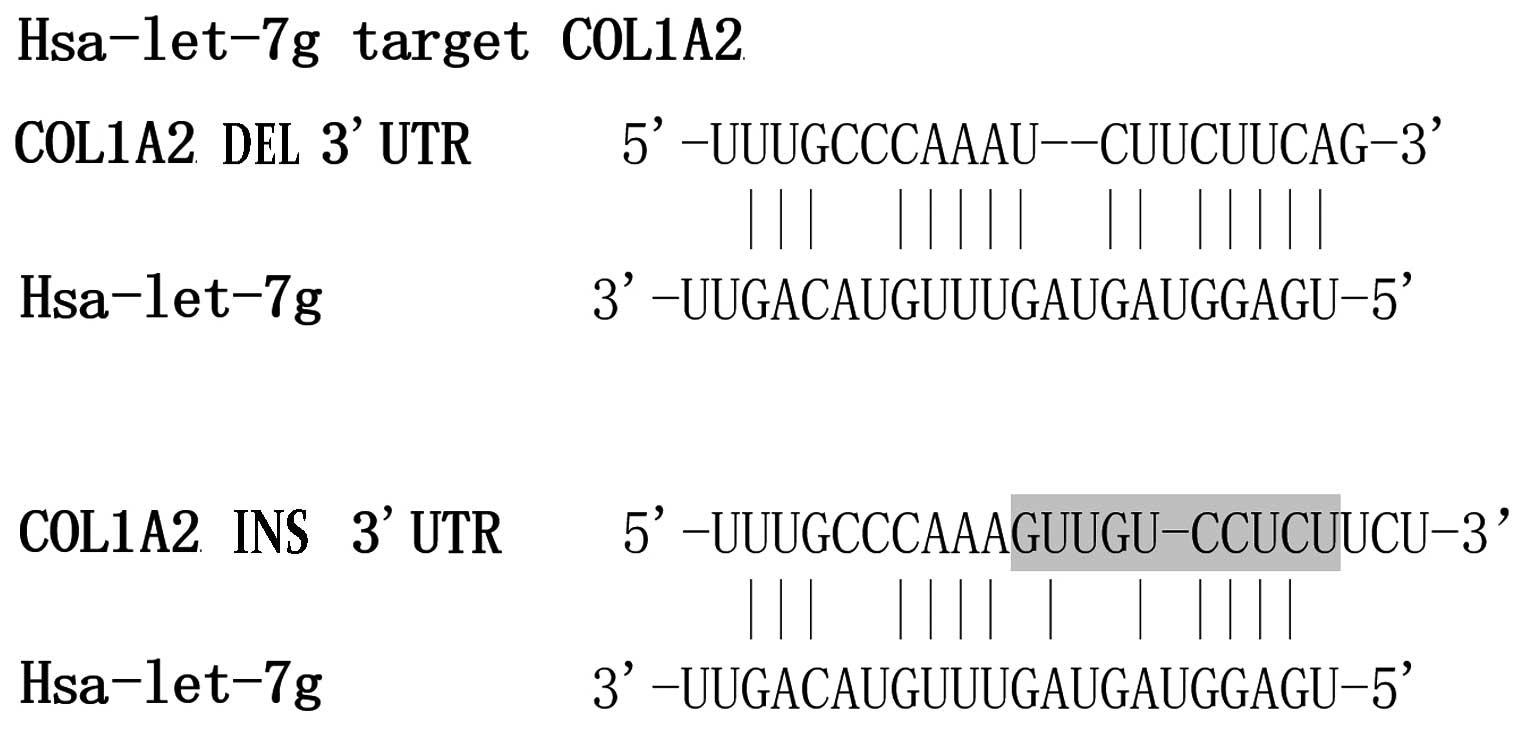
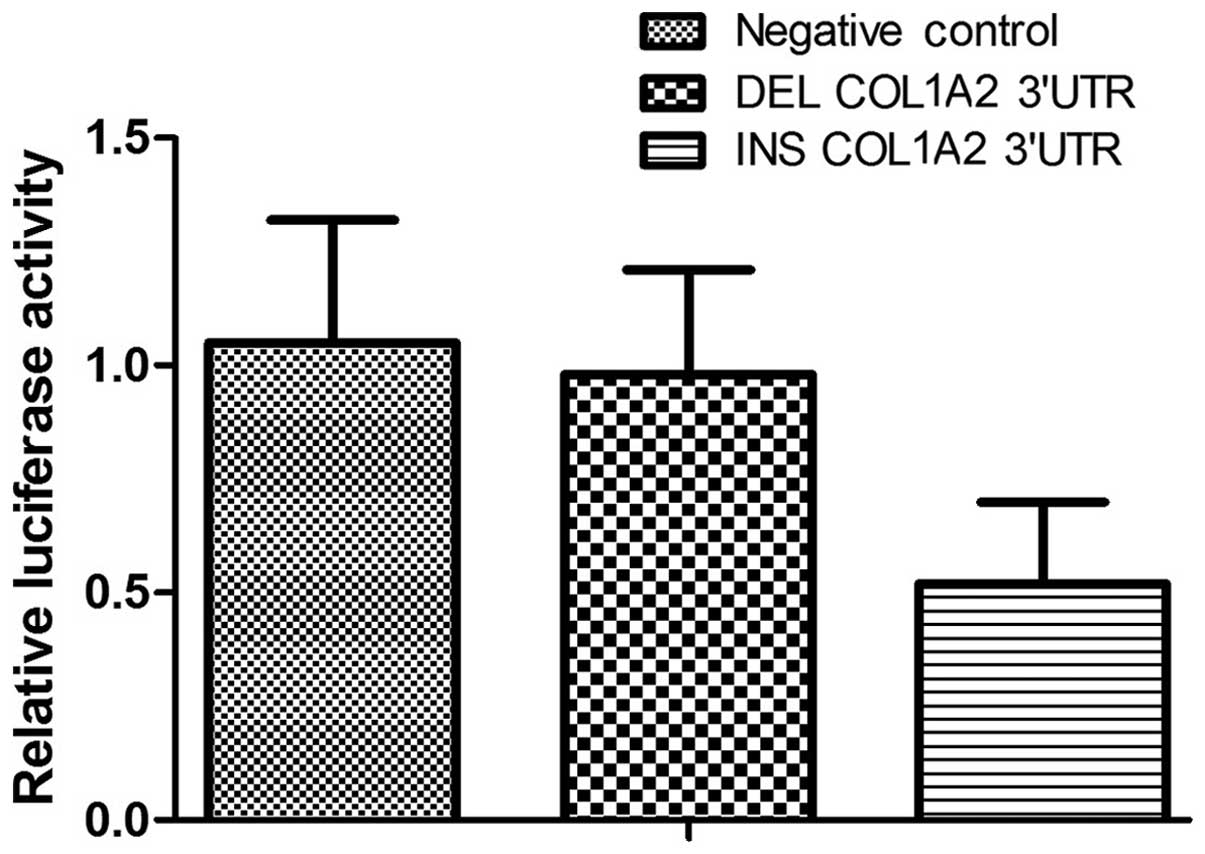
containing either allele of rs3917 (Fig. 1). In transiently transfected human
osteoblast cells, compared with the constructs containing the
wild-type allele, the luciferase activity in the cells containing
the deletion allele was significantly higher in the presence of
let-7g (Fig. 2). This result
indicates that the transcription of COL1A2 is negatively influenced
by the presence of the rs3917 deletion allele, which is suggested
to affect the binding of let-7g the COL1A2 transcript.
Association of rs3917 genotypes with
the expression levels of COL1A2 in human primary osteoblasts
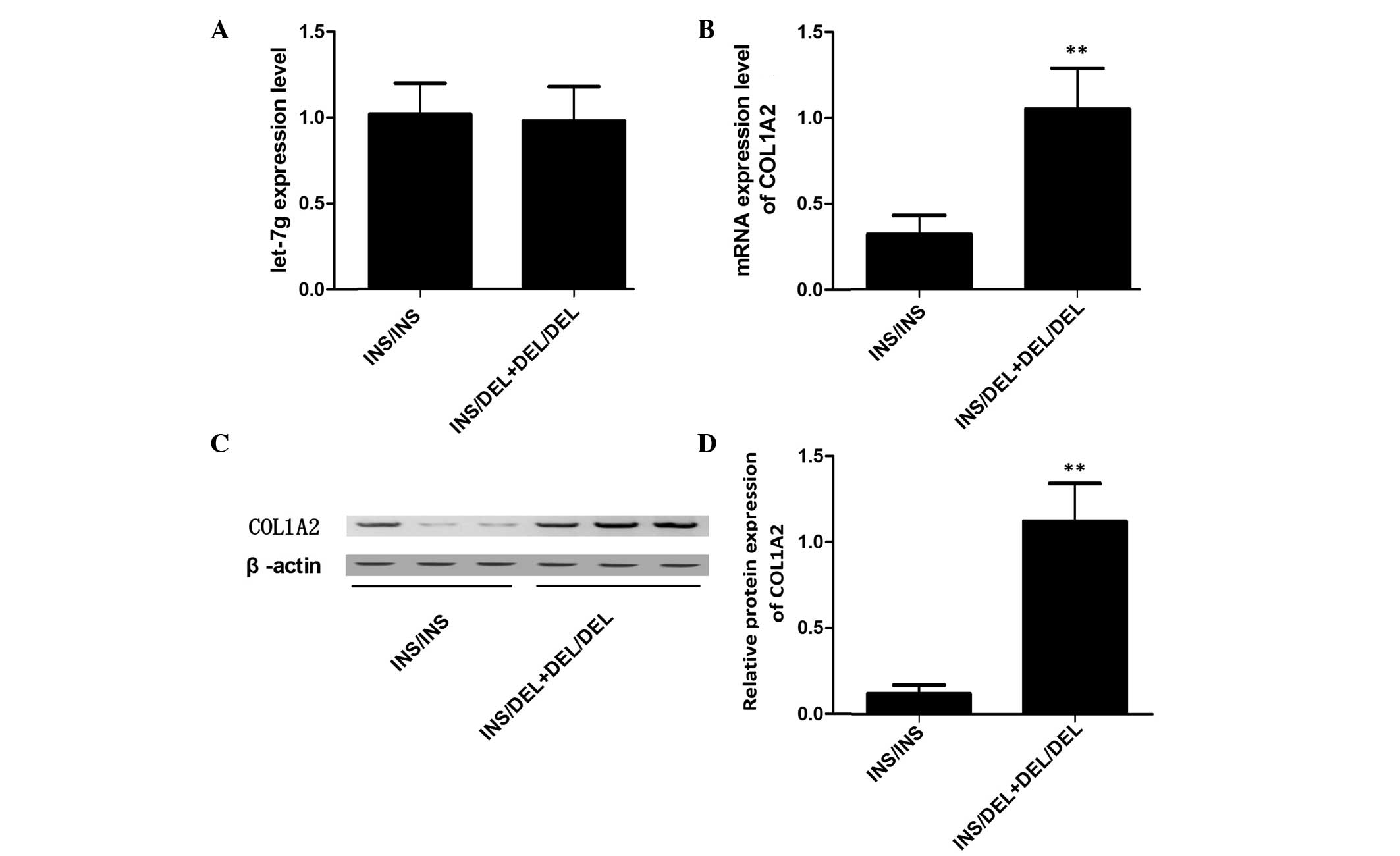
Three different genotypes of primary osteoblasts,
INS/INS (32 samples), INS/DEL (12 samples) and DEL/DEL (4 samples),
were used to further investigate the effect of rs3917 on the
transcription of COL1A2. Using RT-qPCR, the expression levels of
let-7g were observed to be similar between the INS/INS and the
INS/DEL + DEL/DEL groups (Fig.
3A). In addition, the mRNA expression levels of COL1A2 were
measured, and the expression levels of COL1A2 mRNA were observed to
be reduced in the INS/INS group compared with the INS/DEL + DEL/DEL
group (Fig. 3B). Consistent with
this, western blot analysis indicated that the protein expression
levels of COL1A2 were reduced in the INS/INS group compared with
the INS/DEL + DEL/DEL group (Fig. 3C
and D).
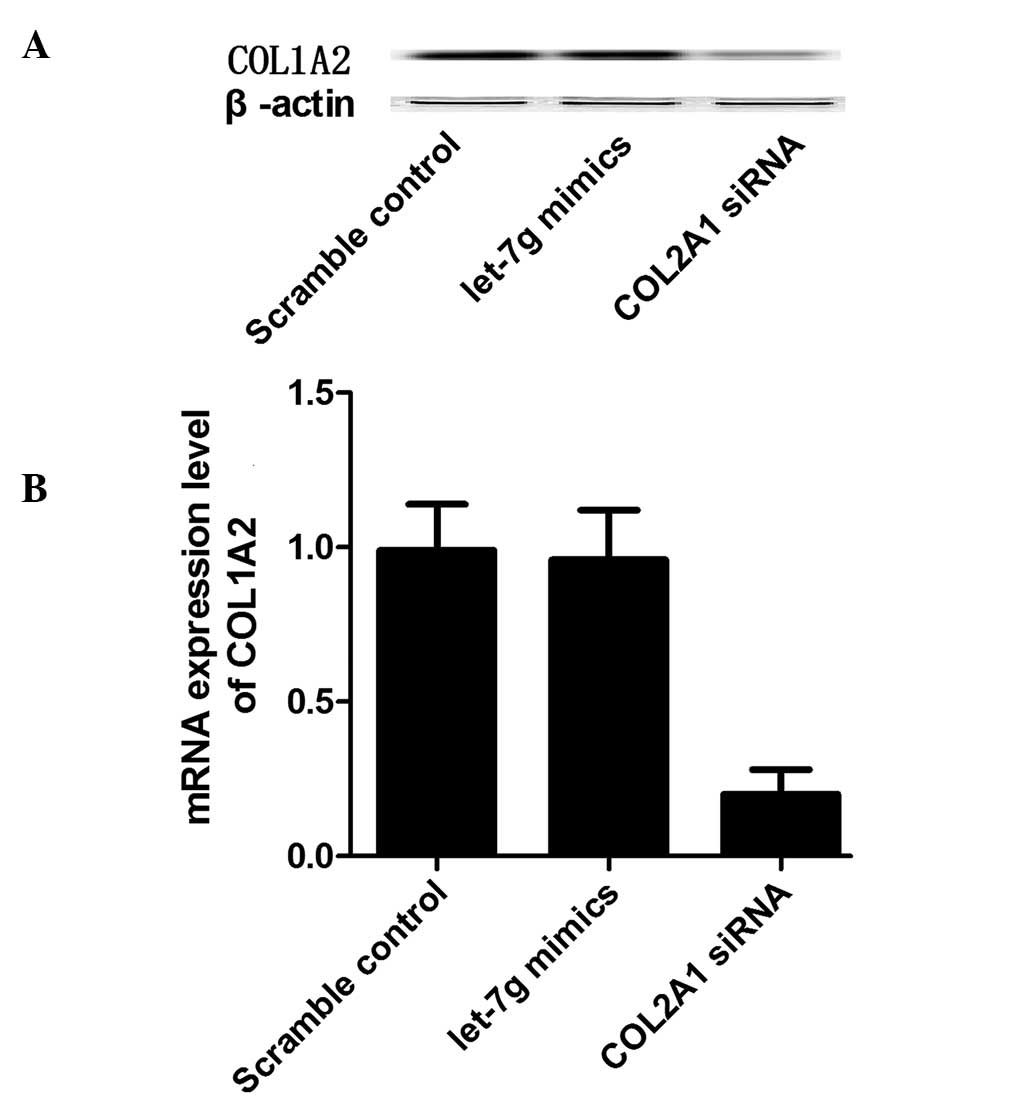
Exogenous expression of let-7g
suppresses the expression of COL1A2 in primary osteoblasts with the
INS/INS genotype, however not the DEL/DEL genotype
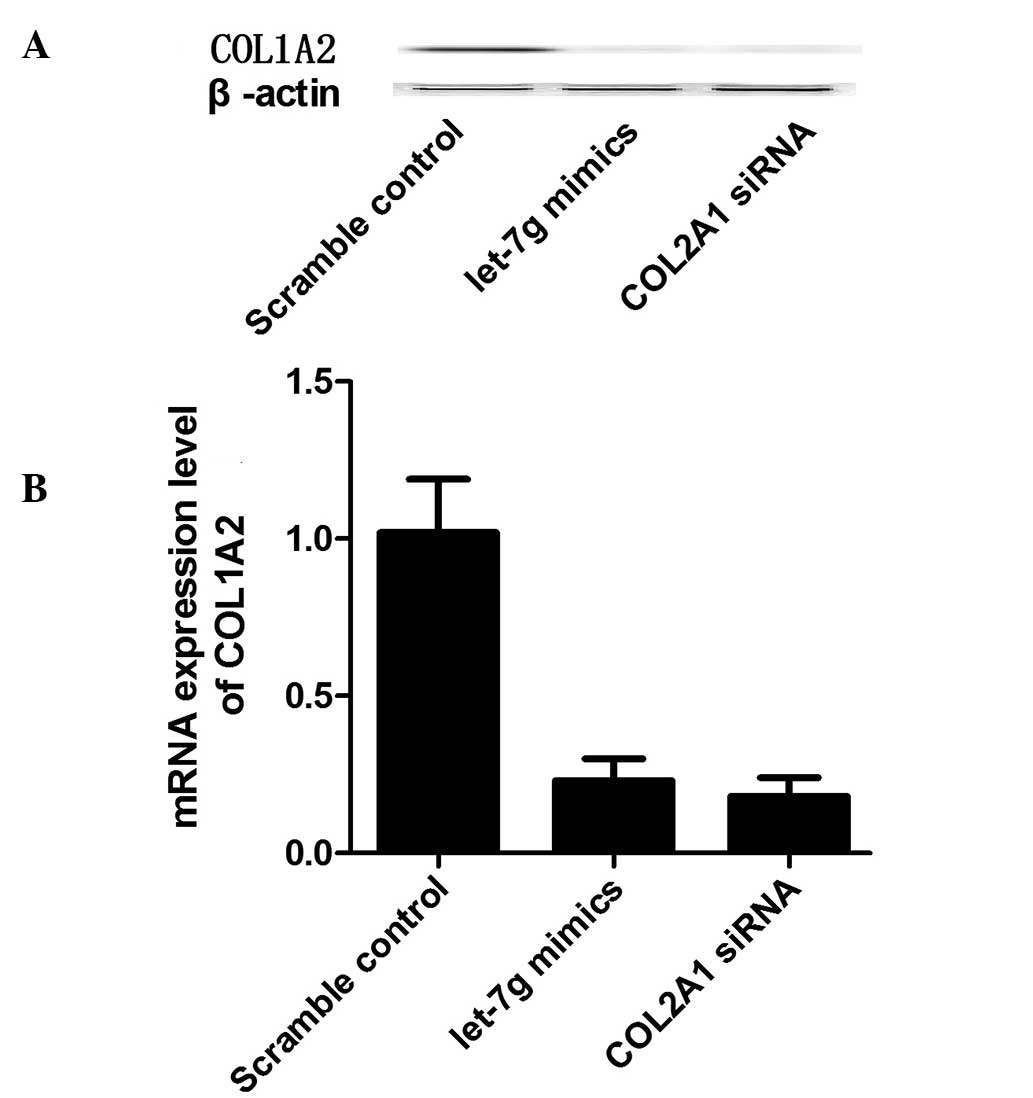
Hsa-let-7g mimics and anti-COL1A2 siRNA together
with scramble control were transfected into the osteoblast cells of
the INS/INS or DEL/DEL genotypes, and the mRNA and protein
expression levels of COL1A2 were measured in osteoblast cells. In
the primary osteoblast cells of the INS/INS genotype, the let-7g
mimics and the COL1A2 siRNA markedly reduced the mRNA and protein
expression levels of COL1A2 (Fig.
4). In the primary osteoblast cells of the DEL/DEL genotype,
whilst COL1A2 siRNA was able to reduce the mRNA and protein
expression levels of COL1A2, the let-7g mimics had no effect on the
expression levels of COL1A2 (Fig.
5).
Discussion
Osteoporosis is a common disease, characterized by
microarchitectural deterioration of bone tissue, reduced bone mass
and an increased risk of fragility fractures (1). Genetic factors have been observed to
serve an important role in the pathogenesis of osteoporosis via
multiple mechanisms associated with variation in several genes
involved in the regulation of bone geometry, quality and mineral
density (18). Following the
identification of miRNAs around 20 years ago, numerous studies
(>20,000) have been published on miRNAs (19). Certain studies (>600) have
focused on the effects of miRNAs on bone and cartilage tissues.
Numerous reviews have discussed the various roles of miRNA in
skeletal development and disease (20–25),
including regulatory roles in the growth, differentiation and
function of osteoblasts, osteoclasts, chondrocytes and other
mesenchymal cell types (for example, adipocytes and myoblasts)
(26). The miRNA, let-7g, has been
demonstrated to be associated with the regulation of ontogenesis
(27). In the current study, the
BMD at the four tested sites, the femoral neck, total left hip,
L1-L4 and intertrochanteric areas, was reduced in the COL1A2 3′-UTR
rs3917 INS/DEL or DEL/DEL group compared with the INS/INS (Table I). This statistically significant
difference remained following adjustment for age, gender and
BMI.
The COL1A2 gene encodes the pro-α2 chain of type I
collagen, whose triple helix consists of two α1 chains and one α2
chain. Type I collagen is a fibril-forming collagen, is present in
the majority of connective tissues, and is highly expressed in
dermis, bone, cornea and tendon tissue. A previous study
demonstrated that COL1A2 is a direct target of let-7g in certain
tumor cells (28). In addition,
the levels of let-7g and COL1A2 were inversely associated with
hepatocellular carcinoma clinical specimens (28). In the current study, the expression
levels of let-7g were observed to be similar between all the three
rs3917 genotype groups (Fig. 3A).
In addition, the mRNA expression levels of COL1A2 were measured and
observed to be similar between the INS/DEL and DEL/DEL groups, with
the levels greater than in the INS/INS group (Fig. 3B). The correlation between single
nucleotide polymorphisms (SNPs) in COL1A2 and BMD, and additional
surrogate phenotypes (29–32) has been investigated in numerous
studies to date. A previous study by Lindahl et al (30), conducted in older men from Sweden,
Hong Kong and the United Kingdom (n=2004), identified an
association between the COL1A2 gene and BMD. The study indicated
that rs42524 was significantly associated with BMD in older men,
with those carrying a CC or GG genotype exhibiting a higher BMD
compared with men with the CG genotype (30). However, it was demonstrated that
the same SNP was not associated with BMD or the risk for
osteoporotic fracture in postmenopausal women. As reported by Lau
et al (29), the SNPs
PvuII and EcoRI in the COL1A2 gene were associated
with BMD in older men in Hong Kong, however, no association between
these two SNPs and BMD was observed in 450 postmenopausal women.
The study by Lei et al (31) investigated the association between
MspI in the COL1A2 gene and osteoporosis according to bone
size, and reported that this SNP was associated with femoral neck
bone size in a Chinese population. The majority of studies
regarding COL1A2 have focused on two nucleotide substitutions
within the gene: G to C substitution at nucleotide 19713 in intron
24, which created a PvuII restriction site; and C to T
substitution at nucleotide 14589 at the 5′ end of intron 12, which
created an EcoR1 restriction site (GenBank sequence,
accession no. AF004877). However, there is no evidence that the
EcoR1 and PuvII loci have any function (33). Therefore, it has been suggested
that there may be additional regulatory mechanisms involved,
including miRNA, methylation and linkage equilibrium. In the
current study, the mRNA let-7g was observed to bind and negatively
regulate the transcription of COL1A2, with this regulation
negatively influenced by the presence of the rs3917 deletion
allele. To further investigate the regulation of COL1A2 by let-7g,
primary osteoblast cells of the INS/INS or DEL/DEL genotypes were
transfected with let-7g mimics and COL1A2 siRNA. In the INS/INS
genotype, let-7g mimics and anti-COL1A2 siRNA reduced the mRNA and
protein expression of COL1A2 (Fig.
4). However, in the primary osteoblast cells of the DEL/DEL
genotype, COL1A2 siRNA was able to reduce the mRNA and protein
expression levels of COL1A2, while let-7g mimics had no effect on
the expression levels of COL1A2 (Fig.
5).
The current study has limitations, as although a
large cohort demonstrated statistically significant correlations,
this is based on associations in an observational trial, with the
correlation was evidenced supported by a preliminary functional
analysis. Further in vivo experiments of this allele are
required to support the results of the current study. In addition,
the participants in the current study were selected from a single
hospital, therefore selection bias may have occurred, and so
further studies in a wider population are required to support the
observations of the current study.
In conclusion, the results of the current study
suggest that rs3917 is associated with BMD. In addition, the
present study suggests that the rs3917 polymorphism may interfere
with the interaction between let-7g and COL1A2, and that the
presence of the minor allele releases the physiological inhibition
of the target gene, which may represent a novel therapeutic or
preventive target in osteoporosis.
References
|
1
|
Kanis JA, Melton LJ III, Christiansen C,
Johnston CC and Khaltaev N: The diagnosis of osteoporosis. J Bone
Miner Res. 9:1137–1141. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guéguen R, Jouanny P, Guillemin F, Kuntz
C, Pourel J and Siest G: Segregation analysis and variance
components analysis of bone mineral density in healthy families. J
Bone Miner Res. 10:2017–2022. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arden NK, Baker J, Hogg C, Baan K and
Spector TD: The heritability of bone mineral density, ultrasound of
the calcaneus and hip axis length: A study of postmenopausal twins.
J Bone Miner Res. 11:530–534. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Christian JC, Yu PL, Slemenda CW and
Johnston CC Jr: Heritability of bone mass: A longitudinal study in
aging male twins. Am J Hum Genet. 44:429–433. 1989.PubMed/NCBI
|
|
5
|
Pocock NA, Eisman JA, Hopper JL, Yeates
MG, Sambrook PN and Eberl S: Genetic determinants of bone mass in
adults. A twin study. J Clin Invest. 80:706–710. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slemenda CW, Christian JC, Williams CJ,
Norton JA and Johnston CC Jr: Genetic determinants of bone mass in
adult women: A reevaluation of the twin model and the potential
importance of gene interaction on heritability estimates. J Bone
Miner Res. 6:561–567. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peacock M, Koller DL, Fishburn T, Krishnan
S, Lai D, Hui S, Johnston CC, Foroud T and Econs MJ: Sex-specific
and non-sex-specific quantitative trait loci contribute to normal
variation in bone mineral density in men. J Clin Endocrinol Metab.
90:3060–3066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao P, Shen H, Guo YF, Xiong DH, Liu YZ,
Liu YJ, Zhao LJ, Long JR, Guo Y, Recker RR and Deng HW: Genomic
regions identified for BMD in a large sample including epistatic
interactions and gender-specific effects. J Bone Miner Res.
21:1536–1544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prockop DJ and Kivirikko KI: Collagens:
Molecular biology, diseases, and potentials for therapy. Annu Rev
Biochem. 64:403–434. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mann V and Ralston SH: Meta-analysis of
COL1A1 Sp1 polymorphism in relation to bone mineral density and
osteoporotic fracture. Bone. 32:711–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen TV, Esteban LM, White CP, Grant SF,
Center JR, Gardiner EM and Eisman JA: Contribution of the collagen
I alpha1 and vitamin D receptor genes to the risk of hip fracture
in elderly women. J Clin Endocrinol Metab. 90:6575–6579. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pillai RS: MicroRNA function: Multiple
mechanisms for a tiny RNA? RNA. 11:1753–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wiemer EA: The role of microRNAs in
cancer: No small matter. Eur J Cancer. 43:1529–1544. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen K, Song F, Calin GA, Wei Q, Hao X and
Zhang W: Polymorphisms in microRNA targets: A gold mine for
molecular epidemiology. Carcinogenesis. 29:1306–1311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nicoloso MS, Sun H, Spizzo R, Kim H,
Wickramasinghe P, Shimizu M, Wojcik SE, Ferdin J, Kunej T, Xiao L,
et al: Single-nucleotide polymorphisms inside microRNA target sites
influence tumor susceptibility. Cancer Res. 70:2789–2798. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landi D, Gemignani F, Barale R and Landi
S: A catalog of polymorphisms falling in microRNA-binding regions
of cancer genes. DNA Cell Biol. 27:35–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Z, Jiang Y, Chen S, Jia S, Gao X, Dong
D and Gao Y: An insertion/deletion polymorphism in the 3′
untranslated region of type I collagen a2 (COL1A2) is associated
with susceptibility for hepatocellular carcinoma in a Chinese
population. Cancer Genet. 204:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Liu J, Pan Z, Du X, Li X, Ma B,
Yao W, Li Q and Liu H: The let-7g microRNA promotes follicular
granulosa cell apoptosis by targeting transforming growth factor-β
type 1 receptor. Mol Cell Endocrinol. 409:103–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stewart TL and Ralston SH: Role of genetic
factors in the pathogenesis of osteoporosis. J Endocrinol.
166:235–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ambros V: microRNAs: Tiny regulators with
great potential. Cell. 107:823–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lian JB, Stein GS, van Wijnen AJ, Stein
JL, Hassan MQ, Gaur T and Zhang Y: MicroRNA control of bone
formation and homeostasis. Nat Rev Endocrinol. 8:212–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyaki S and Asahara H: Macro view of
microRNA function in osteoarthritis. Nat Rev Rheumatol. 8:543–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia Z, Chen C, Chen P, Xie H and Luo X:
MicroRNAs and their roles in osteoclast differentiation. Front Med.
5:414–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldring MB and Marcu KB: Epigenomic and
microRNA-mediated regulation in cartilage development, homeostasis,
and osteoarthritis. Trends Mol Med. 18:109–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taipaleenmäki H, Hokland L Bjerre, Chen L,
Kauppinen S and Kassem M: Mechanisms in endocrinology: micro-RNAs:
targets for enhancing osteoblast differentiation and bone
formation. Eur J Endocrinol. 166:359–371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laine SK, Hentunen T and Laitala-Leinonen
T: Do microRNAs regulate bone marrow stem cell niche physiology?
Gene. 497:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papaioannou G, Mirzamohammadi F and
Kobayashi T: MicroRNAs involved in bone formation. Cell Mol Life
Sci. 71:4747–4761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji J, Zhao L, Budhu A, Forgues M, Jia HL,
Qin LX, Ye QH, Yu J, Shi X, Tang ZY and Wang XW: Let-7g targets
collagen type I alpha2 and inhibits cell migration in
hepatocellular carcinoma. J Hepatol. 52:690–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lau EM, Choy DT, Li M, Woo J, Chung T and
Sham A: The relationship between COLI A1 polymorphisms (Sp 1) and
COLI A2 polymorphisms (Eco R1 and Puv II) with bone mineral density
in Chinese men and women. Calcif Tissue Int. 75:133–137. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lindahl K, Rubin CJ, Brändström H,
Karlsson MK, Holmberg A, Ohlsson C, Mellström D, Orwoll E, Mallmin
H, Kindmark A and Ljunggren O: Heterozygosity for a coding SNP in
COL1A2 confers a lower BMD and an increased stroke risk. Biochem
Biophys Res Commun. 384:501–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lei SF, Deng FY, Xiao SM, Chen XD and Deng
HW: Association and haplotype analyses of the COL1A2 and ER-alpha
gene polymorphisms with bone size and height in Chinese. Bone.
36:533–541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lei SF, Deng FY, Dvornyk V, Liu MY, Xiao
SM, Jiang DK and Deng HW: The (GT)n polymorphism and haplotype of
the COL1A2 gene, but not the (AAAG)n polymorphism of the PTHR1
gene, are associated with bone mineral density in Chinese. Hum
Genet. 116:200–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Escobar-García D, Mejía-Saavedra J,
Jarquín-Yáñez L, Molina-Frechero N and Pozos-Guillén A: Collagenase
1A2 (COL1A2) gene A/C polymorphism in relation to severity of
dental fluorosis. Community Dent Oral Epidemiol. 44:162–168. 2016.
View Article : Google Scholar : PubMed/NCBI
|