Introduction
Raynaud's phenomenon (RP) is characterized by
prolonged vasoconstriction and reduced blood flow in cutaneous
capillaries and arterioles upon cold stress (1,2).
This intensified contraction leads to a chilly sensation at the
extremities of the hands or feet, although contributing to minimize
heat loss from the body (3,4),
suggesting that the development of drugs for relieving an abnormal
vasospasm would be useful for RP treatment. This vessel contraction
is regulated by RhoA-mediated signaling pathways in vascular cells,
including endothelial cells (ECs), vascular smooth muscle cells
(VSMCs) and pericytes (5–7). In RP, cold-induced RhoA activation in
ECs regulates the production of endothelin-1 (ET-1), which is
considered to be a key vasoconstrictor in RP-like disease (8–10).
In capillary vessels, ET-1 released from ECs promotes actin
reorganization in pericytes that are undergoing constriction
(11). EC-derived ET-1 also
activates the RhoA pathway in VSMCs within arterioles, and this
activation is responsible for translocation of the α2c adrenergic
receptor (α2c-AR) from the Golgi apparatus to the plasma membrane
(12–14). α2c-AR is predominantly involved in
cold-induced vasoconstriction in RP (14–18).
Therefore, inhibiting the cold-induced RhoA pathway may be a useful
strategy for RP treatment.
Traditional Chinese medicine (TCM) theory-based
formulas have been used for RP patient therapy, and one of the
TCM-based herbal medicines, Danggui-Sayuk-Ga-Osuyu-Senggang-Tang
(DSGOST), has been clinically applied to patients in order to
relieve coldness and other RP-like symptoms (19–22).
DSGOST led to an improvement in the peripheral circulation with an
elevated temperature in the rat (23). Furthermore, a previous in
vitro study performed in our laboratory revealed that DSGOST
inhibits cold-derived contraction of ECs and VSMCs through the
inhibition of both RhoA activation and production of ET-1 and
translocation of α2c-AR (24).
Therefore, DSGOST may be an effective agent for RP treatment.
However, the effects of DSGOST on RP treatment have yet to be fully
elucidated via either in vitro or in vivo
studies.
Based on the results of our previous in vitro
study (24), the present study
aimed to further evaluate the mitigative effects of DSGOST on
cold-induced vasoconstriction. DSGOST inhibited cold- and
ET-1-provoked contraction of both pericytes and ECs, with decreased
activity of the RhoA-associated contractile pathway. In addition,
orally administered DSGOST prevented the reduction in the vessel
area of the mouse tail from occurring. Furthermore, DSGOST
mitigated cold-induced vasoconstriction with a reduced staining
intensity for CD31 and α2c-AR in mice. Therefore, our present data
suggest that DSGOST exerts beneficial effects in terms of relieving
the cold response in microvessels in RP-like disease.
Materials and methods
Preparation of DSGOST
DSGOST powder was prepared as previously described
(24). In brief, herbal components
of DSGOST were mixed and subsequently extracted by hot water
(10-fold volume), comprising 1 g Angelica radix, 1 g Cinnamomi
cortex, 1 g Paeoniae root, 1 g Akebia root, 0.67 g Asarum,
0.67 g Glycyrrhiza, 1.67 g Zizyphus jujuba, 0.67 g
Evodia fruit and 1.33 g Ginger root. The dried mixture was stored
at −80°C until use. The composition of the DSGOST herbal
prescription is shown in Table
I.
 | Table I.Composition of the
Danggui-Sayuk-Ga-Osuyu- Senggang-Tang herbal prescription (total,
9.01 g). |
Table I.
Composition of the
Danggui-Sayuk-Ga-Osuyu- Senggang-Tang herbal prescription (total,
9.01 g).
| Korean name | Chinese name | Scientific/common
name | Amount (g) |
|---|
| Danggui | Danggui | Angelica radix | 1 |
| Geji | Guizhi | Cinnamomi
cortex | 1 |
| Jakyak | Bai shao | Paeoniae
root | 1 |
| Moktong | Mutong | Akebia root | 1 |
| Sesin | Xixin | Asarum | 0.67 |
| Gamcho | Gancao |
Glycyrrhiza | 0.67 |
| Daechu | Dazao | Zizyphus
jujuba | 1.67 |
| Osuyu | Wuzhuyu | Evodia fruit | 0.67 |
| Saenggang | Shengjian | Ginger root | 1.33 |
Cell culture
Human umbilical vein endothelial cells (HUVECs),
human dermal microvascular endothelial cells (HDMECs) and pericytes
were purchased from ScienCell Research Laboratories, Inc.
(Carlsbad, CA, USA). HUVECs and HDMECs were cultured in endothelial
medium supplemented with 5% fetal bovine serum and 1% endothelial
cell growth supplement (ScienCell Research Laboratories, Inc.).
Pericytes from human brain microvessels were cultured in pericyte
medium (ScienCell Research Laboratories, Inc.) supplemented with 5%
fetal bovine serum (ScienCell Research Laboratories, Inc.), 1%
pericyte growth supplement and 1% penicillin/streptomycin
solution.
In vitro studies
For cold-mediated contraction, endothelial cells and
pericytes were pretreated with DSGOST (100 µg/ml) for 30 min, and
subsequently cultured at physiological temperature (37°C) or a
cooler temperature (25°C) for 30 min. In case of ET-1-mediated
pericyte contraction, instead of the cooler condition (i.e. at
25°C), pericytes were incubated with ET-1 (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany). Following the incubation at the
cooler temperature with ET-1, cells were harvested for protein
extraction using iced radioimmunoprecipitation assay (RIPA) buffer.
The extracted proteins were mixed with sample buffer and boiled at
100°C for 10 min. The boiled samples were separated using 8–12%
SDS-PAGE according to their molecular weights, and then transferred
to a nitrocellulose membrane for western blotting. The proteins
were blotted using the following antibodies: Rabbit
anti-phospho-cofilin (Ser3) monoclonal (cat. no. 3313; 1:1,000),
rabbit anti-phospho-LIM domain kinase (LIMK)1 (Thr508)/LIMK2
(Thr505) polyclonal (cat. no. 3841; 1:1,000), rabbit
anti-testis-specific kinase 1 (TESK1) monoclonal (cat. no. 4655;
1:1,000), rabbit anti-PDXP monoclonal (cat. no. 4686; 1:1,000) and
rabbit anti-phospho-myosin light chain 2 (Ser19; cat. no. 3671;
1:1,000) antibodies (Cell Signaling Technology, Inc., Danvers, MA,
USA) and mouse-anti-RhoA-GTP monoclonal antibody (cat. no. 26904;
1:500; NewEast Biosciences, Malvern, PA, USA).
In vivo studies
Six-week-old BALB/c mice were purchased from Jungang
Lab Animal, Inc. (Seoul, Korea). Mice were housed under conditions
of a constant temperature (24±2°C) with 12 h-light/12 h-dark cycle.
All experimental procedures were approved by the Kyung Hee
University Institutional Animal Care and Use Committee (KHU-IACUC).
Mice were randomly grouped and administered orally with drinking
water or DSGOST (10 mg/kg). After 30 min, mice were anesthetized
using Avertin solution which was prepared by mixing
2,2,2-tribromoethanol (Sigma-Aldrich; Merck Millipore) and
2-methyl-2-butanol, tertiary amyl alcohol 99% (Sigma-Aldrich; Merck
Millipore), according to the manufacturer's protocol. Subsequently,
their tails were soaked under cool (25±2°C) or warm (37±2°C) water
for 30 min, using a heat block to maintain constant temperature.
The 10 mm length of the segment from extremities of the tail were
fixed in formaldehyde and embedded in paraffin. The paraffin blocks
were sectioned at 15 µm and mounted on slide glasses. For
hematoxylin and eosin (H&E) staining, the prepared slides were
immersed in H&E solution for staining the nuclei and the
cytoplasm. The stained sections were photographed at a
magnification of 40x, and subsequently the area of the vessels from
each group was measured using ImageJ software (National Institutes
of Health, Bethesda, MD, USA). Immunohistochemistry was performed
using a Vextastain® ABC-AP kit, according to the
manufacturer's protocol (Vector Laboratories, Burlingame, CA, USA).
Rabbit anti-α2c-AR polyclonal antibody (1:100) and rabbit anti-CD31
polyclonal antibody (1:100; Abcam, Cambridge, UK) were used, and
the histological expression patterns of α2c-AR and CD31 were
analyzed. Following staining with each of the antibodies, the
sections were photographed at a magnification of 40x.
Statistical analysis
Statistical analysis was performed using SPSS V22.0
(IBM SPSS, Armonk, NY, USA). The differences of the means between
the groups were analyzed using one-way analysis of variance and a
Student's t-test. P<0.05 was used to indicate a statistically
significant difference.
Results
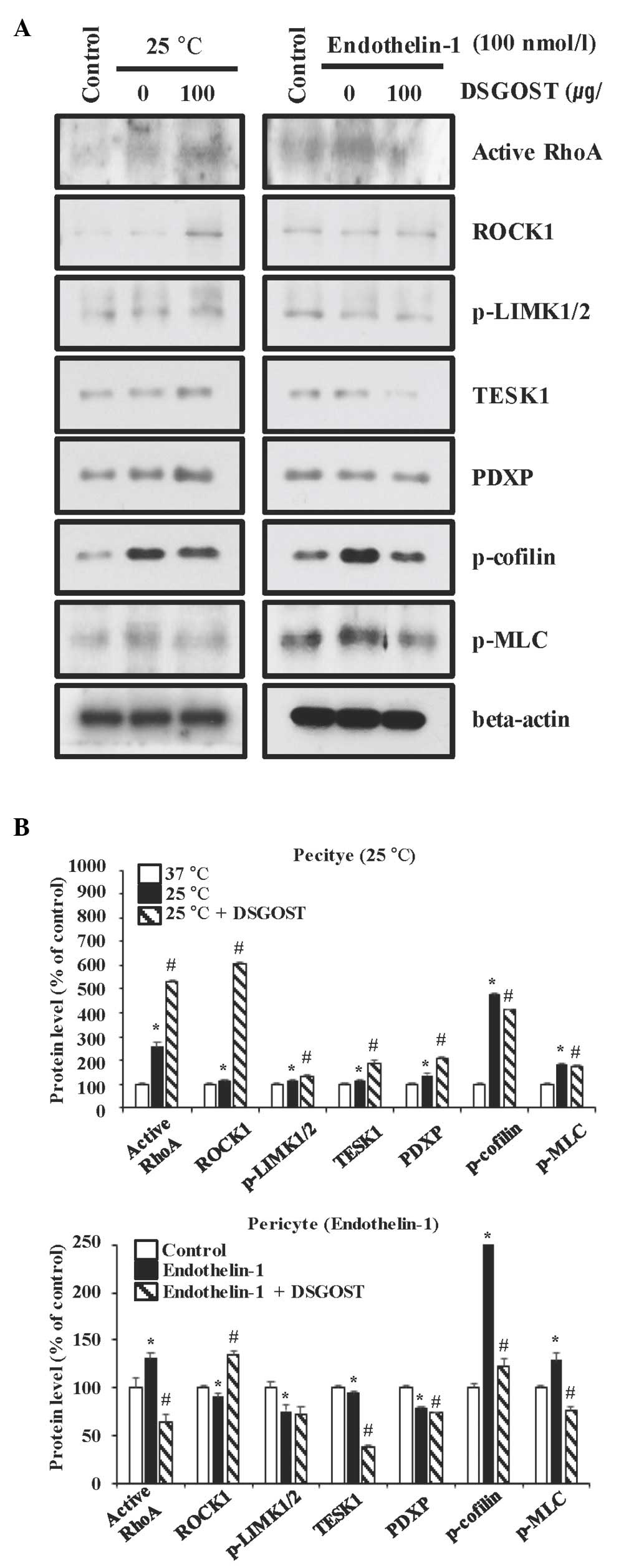
Inhibitory effect of DSGOST on the
cold- and ET-1-induced contractile pathway in pericytes
Since pericytes cause vessel contraction that is
important for the processes of RP (1,25),
whether treatment with DSGOST inhibited contraction of either cold
(25°C)- or ET-1-exposed pericytes was first examined. DSGOST
enhanced RhoA activation and increased the levels of ROCK1, TESK1
and PDXP in pericytes exposed to the cold, but not to ET-1, while
slightly reducing the levels of LIMK1/2, cofilin and MLC for the
two different conditions (Fig. 1).
Therefore, DSGOST activation of the RhoA/ROCK/TESK1/PDXP pathway
appeared to be unique to the cold-exposed pericytes.
Inhibitory effect of DSGOST on the
cold-induced contractile pathway in vascular endothelial cells
Based on our previous work on the mitigative effects
of DSGOST on the cold response in ECs (24), whether DSGOST affects the
cold-induced RhoA pathway in ECs was subsequently investigated.
DSGOST inhibited RhoA activation, the phosphorylation of LIMK1/2
and MLC, and reduced the level of ROCK1, with no effect on TESK1
and PDXP in cold-exposed ECs (Fig.
2).
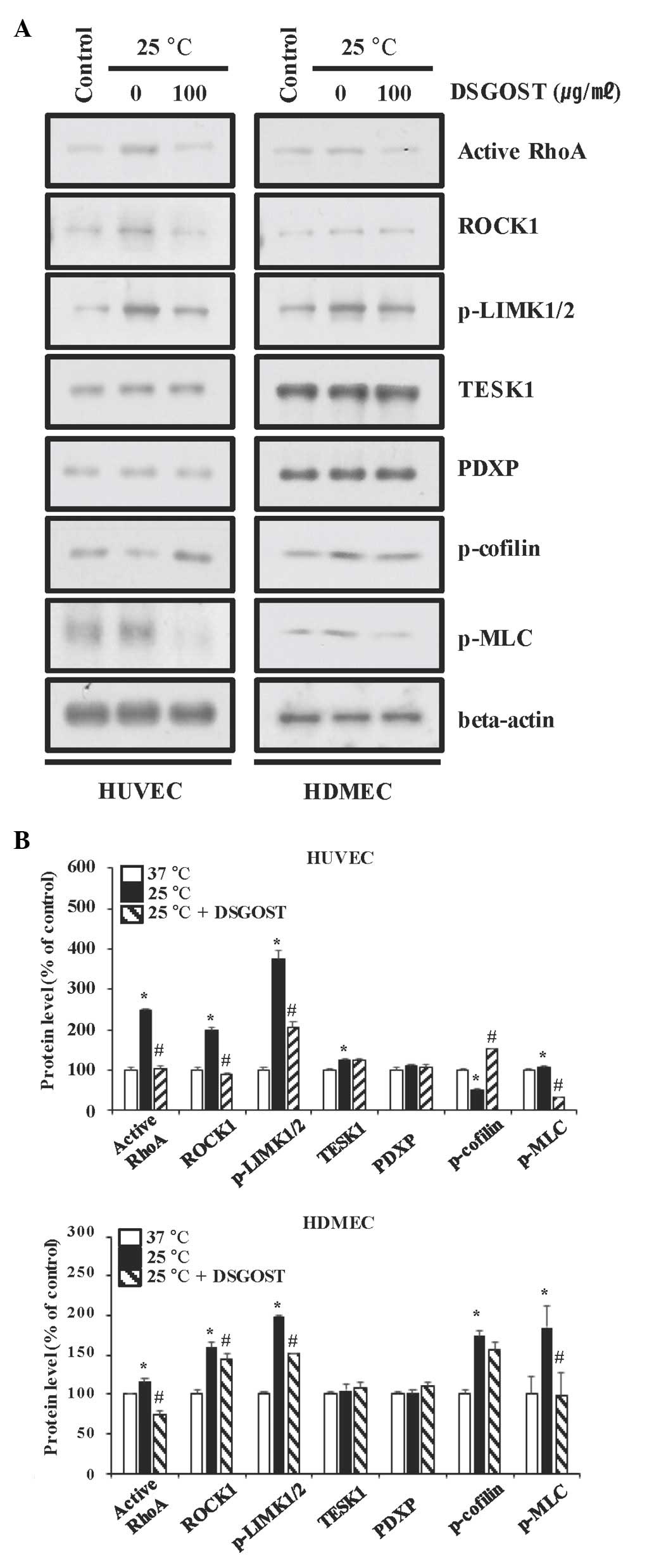 | Figure 2.Inhibitory effects of DSGOST on
cold-induced contraction-associated signaling molecules in vascular
endothelial cells. (A) Protein expression in cold-exposed HUVECs
(left panels) and HDMECs (right panels) were determined by western
blot analysis. Three independent experiments were performed.
β-actin was used as a loading control. (B) The protein expression
in (A) was quantified by densitometric analysis of western blots.
Values are expressed as the mean ± standard deviation. *P<0.05
vs. 37°C; #P<0.05 vs. 25°C. DSGOST,
Danggui-Sayuk-Ga-Osuyu-Senggang-Tang; ET-1, endothelin-1; GTP,
guanosine triphosphate; ROCK1, Rho-associated,
coiled-coil-containing protein kinase 1; p-LIMK1/2, phosphorylated
LIM domain kinase 1/2; TESK1, testis-specific kinase 1; PDXP,
pyridoxal phosphatase; MLC, myosin light-chain kinase; HDMEC, human
dermal microvascular cell; HUVEC, human umbilical vein endothelial
cell. |
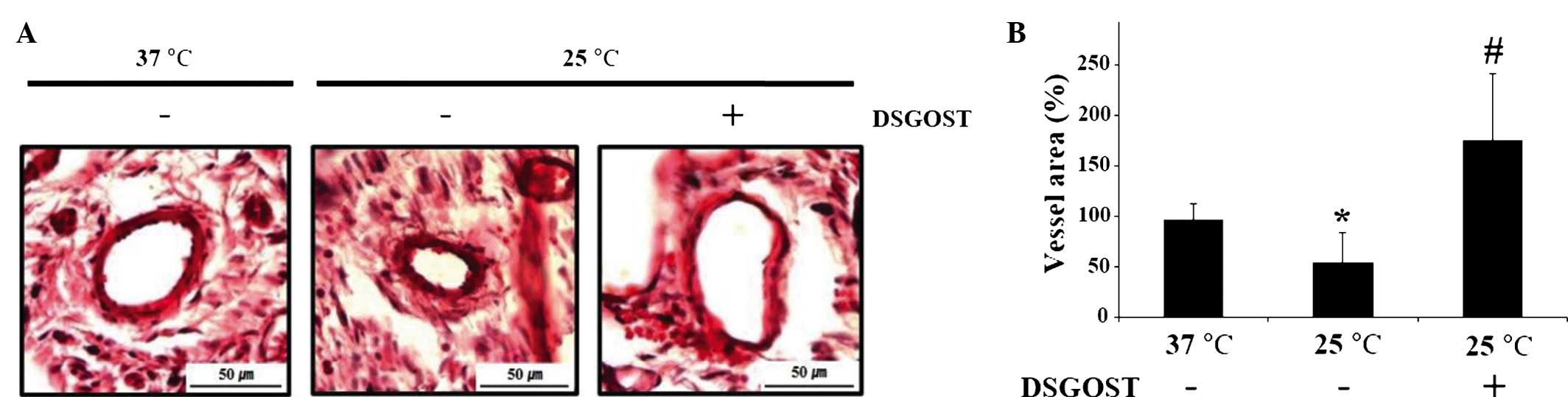
Mitigative effects of DSGOST on
cold-induced vasoconstriction
To investigate DSGOST efficacy on cold-mediated
vessel contraction, the vessel area was measured following
incubation under each condition. DSGOST treatment rescued
cold-induced vessel contraction and appeared to markedly promote
vasodilation (Fig. 3). Thus, our
data suggested that DSGOST may be useful for relieving cold-induced
vessel contraction.
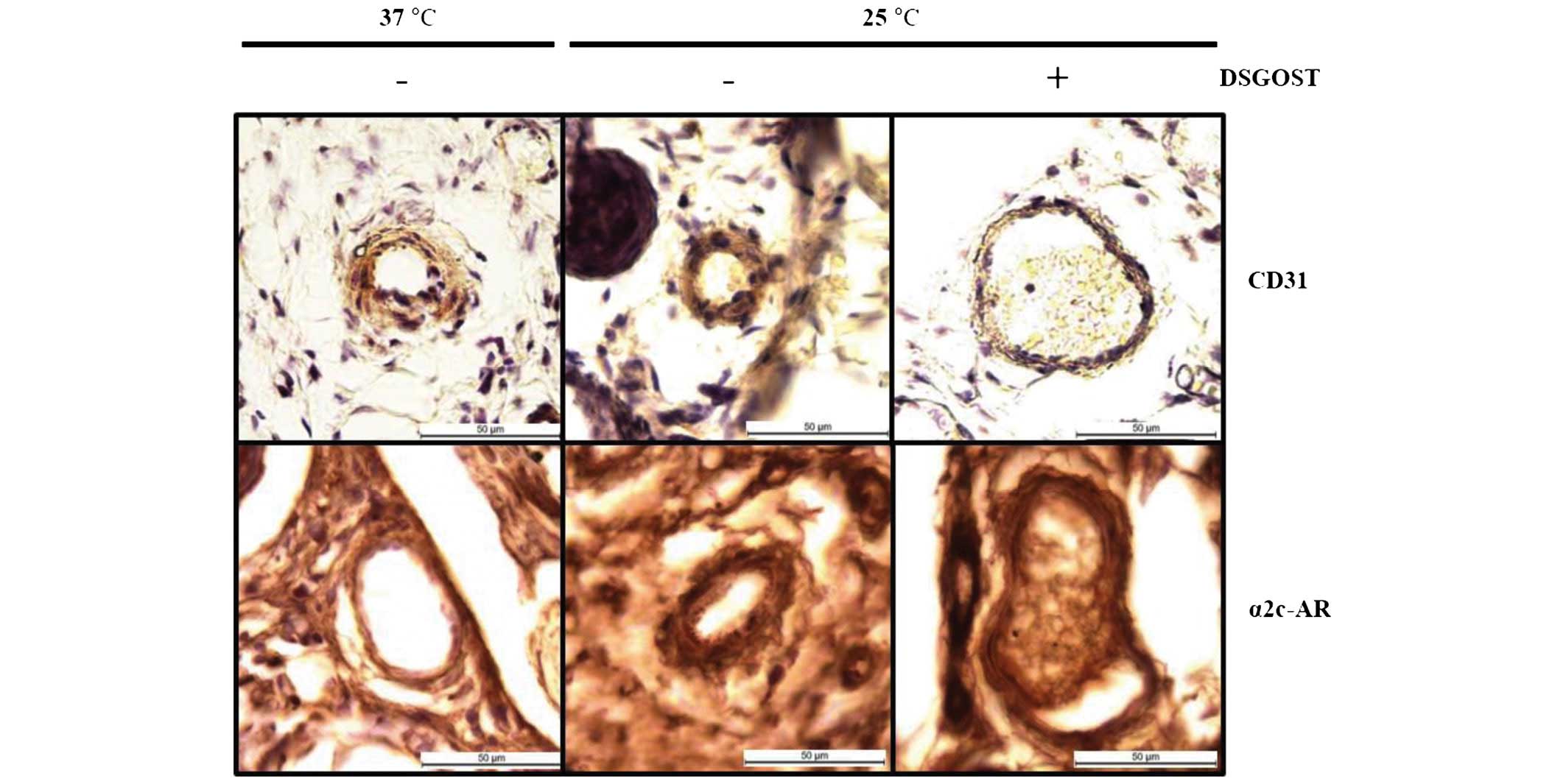
Effect of DSGOST on α2c-AR in the tail
vein
As increases in α2c-AR protein expression and the
anterograde translocation of this protein to the plasma membrane
occurs when cells are exposed to cooler temperatures (12–14),
this protein was selected for the current investigation. In the
immunohistochemical analysis, although α2c-AR was evidently
detected in mouse tail vessels at 25°C, DSGOST treatment reduced
its induction in the vessel (Fig.
4). Therefore, the oral administration of DSGOST was
demonstrated to be effective at reducing cold-induced vessel
contraction.
Discussion
DSGOST has long been used to treat cold syndrome,
including RP (19–23). Nevertheless, the molecular
mechanisms underpinning its effects in RP have yet to be fully
elucidated. In the present study, it has been revealed that DSGOST
activates the RhoA/ROCK/TESK1/PDXP pathway in cold-exposed
pericytes.
The findings in the present study are slightly
different from those obtained previously by our group with respect
to RhoA-mediated intracellular signaling (24). Although a role for pericytes in the
regulation of EC function and blood flow is in common with a role
of VSMCs, certain phenotypic differences between pericytes and
VSMCs may afford a possible reason to account for the contrasting
results of DSGOST in pericytes and VSMCs (5–7). For
example, pericytes do not possess the calcium-binding protein,
calponin, which is an important regulator of VSMC contraction
(26). In addition, capillaries
and arterioles derived from endothelial cells are covered and
regulated by pericytes and VSMCs, respectively (5,27).
Furthermore, the source of VSMCs used in our previous study was
human umbilical veins (24),
whereas pericytes in the present study were derived from human
brain microvessels. Understanding those above-mentioned differences
may make it possible to explain the DSGOST effect on the
RhoA-mediated pathway in cold-exposed pericytes. Furthermore, the
results in the present study concerning DSGOST inhibition of the
cold response in ECs are consistent with our previous findings that
DSGOST suppresses cold-induced RhoA activation in ECs. From the
in vivo experiments, DSGOST effectively inhibited
cold-induced constriction of mouse tail vessels with decreased
expression of α2c-AR, which was used to evaluate coldness in RP
(12–14,28,29).
Taken together, the in vitro and in vivo data in the
present study suggested that DSGOST may mitigate cold-stimulated
vasoconstriction via inhibition of the RhoA-mediated contractile
pathway in both pericytes and ECs for RP treatment, whereas it is
has yet to be fully determined which active compounds of DSGOST
have an inhibitory effect on pathological vasospasms in RP.
It was also revealed that DSGOST inhibits
RhoA-mediated pathways for the phosphorylation of cofilin and MLC
in cold- and ET-1-exposed vascular cells, indicating that DSGOST
may suppress contraction of vascular cells via regulation of actin
reorganization (30–33). Based on DSGOST inhibition of either
RhoA activation or cytoskeletal reorganization, it is hypothesized
that DSGOST may be useful in the management of other diseases with
dysregulation of actin cytoskeleton in vascular cells. For example,
dysregulation of actin dynamics in vascular cells is likely to be
important for tumor migration, invasion, angiogenesis and
metastasis (34–36). Additionally, endothelial
dysfunction with cytoskeletal dynamics is closely associated with
vascular failure (37). Therefore,
DSGOST is possibly useful for the treatment of diseases that are
associated with dysregulation of actin reorganization.
In conclusion, it has been shown, to the best of our
knowledge for the first time, that DSGOST inhibits the
RhoA-mediated contractile pathway in cold- or ET-1-exposed vascular
cells, and mitigates cold-induced vasoconstriction in mouse tail
vein. Although the precise biological mechanism of DSGOST on RP
treatment has yet to be fully elucidated, and there remains a
requirement to know which metabolites of DSGOST effectively relieve
cold-induced vasoconstriction, based on our findings it is
hypothesized that DSGOST is beneficial for the treatment of RP-like
disease.
Acknowledgements
This study was supported by the Korean Medicine
R&D Project of the Ministry of Health and Welfare (no.
HI13C0530).
References
|
1
|
Cooke JP and Marshall JM: Mechanisms of
Raynaud's disease. Vasc Med. 10:293–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
LeRoy EC and Medsger TA Jr: Raynaud's
phenomenon: A proposal for classification. Clin Exp Rheumatol.
10:485–488. 1992.PubMed/NCBI
|
|
3
|
Kellogg DL Jr: In vivo mechanisms of
cutaneous vasodilation and vasoconstriction in humans during
thermoregulatory challenges. J Appl Physiol (1985). 100:1709–1718.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Marken Lichtenbelt WD and Schrauwen P:
Implications of nonshivering thermogenesis for energy balance
regulation in humans. Am J Physiol Regul Integr Comp Physiol.
301:R285–R296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamilton NB, Attwell D and Hall CN:
Pericyte-mediated regulation of capillary diameter: A component of
neurovascular coupling in health and disease. Front
Neuroenergetics. 2:52010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rajendran P, Rengarajan T, Thangavel J,
Nishigaki Y, Sakthisekaran D, Sethi G and Nishigaki I: The vascular
endothelium and human diseases. Int J Biol Sci. 9:1057–1069. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Webb RC: Smooth muscle contraction and
relaxation. Adv Physiol Educ. 27:201–206. 2003.PubMed/NCBI
|
|
8
|
Zamora MR, O'Brien RF, Rutherford RB and
Weil JV: Serum endothelin-1 concentrations and cold provocation in
primary Raynaud's phenomenon. Lancet. 336:1144–1147. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barman SA: Vasoconstrictor effect of
endothelin-1 on hypertensive pulmonary arterial smooth muscle
involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell
Mol Physiol. 293:L472–L479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakurada S, Okamoto H, Takuwa N, Sugimoto
N and Takuwa Y: Rho activation in excitatory agonist-stimulated
vascular smooth muscle. Am J Physiol Cell Physiol. 281:C571–C578.
2001.PubMed/NCBI
|
|
11
|
Dehouck MP, Vigne P, Torpier G,
Breittmayer JP, Cecchelli R and Frelin C: Endothelin-1 as a
mediator of endothelial cell-pericyte interactions in bovine brain
capillaries. J Cereb Blood Flow Metab. 17:464–469. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chotani MA, Flavahan S, Mitra S, Daunt D
and Flavahan NA: Silent alpha(2C)-adrenergic receptors enable
cold-induced vasoconstriction in cutaneous arteries. Am J Physiol
Heart Circ Physiol. 278:H1075–1083. 2000.PubMed/NCBI
|
|
13
|
Jeyaraj SC, Chotani MA, Mitra S, Gregg HE,
Flavahan NA and Morrison KJ: Cooling evokes redistribution of
alpha2C-adrenoceptors from golgi to plasma membrane in transfected
human embryonic kidney 293 cells. Mol Pharmacol. 60:1195–1200.
2001.PubMed/NCBI
|
|
14
|
Bailey SR, Eid AH, Mitra S, Flavahan S and
Flavahan NA: Rho kinase mediates cold-induced constriction of
cutaneous arteries: Role of alpha2C-adrenoceptor translocation.
Circ Res. 94:1367–1374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aburto TK, Lajoie C and Morgan KG:
Mechanisms of signal transduction during alpha-2-adrenergic
receptor-mediated contraction of vascular smooth-muscle. Circ Res.
72:778–785. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aubdool AA, Graepel R, Kodji X, Alawi KM,
Bodkin JV, Srivastava S, Gentry C, Heads R, Grant AD, Fernandes ES,
et al: TRPA1 is essential for the vascular response to
environmental cold exposure. Nat Commun. 5:57322014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ekenvall L, Lindblad LE, Norbeck O and
Etzell BM: Alpha-adrenoceptors and cold-induced vasoconstriction in
human finger skin. Am J Physiol. 255:H1000–H1003. 1988.PubMed/NCBI
|
|
18
|
Faber JE: Effect of local tissue cooling
on microvascular smooth muscle and postjunctional alpha
2-adrenoceptors. Am J Physiol. 255:H121–H130. 1988.PubMed/NCBI
|
|
19
|
Ninomiya F: Clinical evaluation of
perspiration reducing effects of a kampo formula, shigyaku-san, on
palmoplantar hidrosis. Evid Based Complement Alternat Med.
5:199–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumura Y, Tanaka A and Sato H: Efficacy of
kampo formula tokishigyakukagoshuyushokyoto for cold syndrome
evaluated with a novel clinical method using a patient-based
questionnaire database. Kampo Med. 63:299–304. 2012. View Article : Google Scholar
|
|
21
|
Tsukada R, Yamaguchi T, Hang L, Iseki M,
Kobayashi H and Inada E: Effect of a traditional Japanese medicine
goshajinkigan, tokishigyakukagoshuyushokyoto on the warm and cold
sense threshold and peripheral blood flow. Health (NY). 6:757–763.
2014. View Article : Google Scholar
|
|
22
|
Hayashi A: A case of atypical facial pain
treated with toki-shigyaku-ka-goshuyu-shokyo-to. Kampo Medicine.
50:257–260. 1999. View Article : Google Scholar
|
|
23
|
Kanai S, Okano H and Abe H: Efficacy of
toki-shigyakuka-gosyuyu-syokyo-to
(danggui-sini-jia-wuzhuyu-shengjiang-tang) on peripheral
circulation in autonomic disorders. Am J Chin Med. 25:69–78. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho SG, Go HY, Park JS, Jung KY, Sun SH,
Choi YK, Song YK, Park JH, Jun CY and Ko SG: Herbal Prescription,
DSGOST, prevents cold-induced RhoA activation and endothelin-1
production in endothelial cells. Evid Based Complement Alternat
Med. 2014:5493072014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toi M, Takayanagi T, Souma R and Tominaga
T: Inhibition of vascular endothelial growth-factor induced
cell-growth by an angiogenesis inhibitor agm-1470 in capillary
endothelial-cells. Oncol Rep. 1:423–426. 1994.PubMed/NCBI
|
|
26
|
Bandopadhyay R, Orte C, Lawrenson JG, Reid
AR, Silva S and Allt G: Contractile proteins in pericytes at the
blood-brain and blood-retinal barriers. J Neurocytol. 30:35–44.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cleaver O and Melton DA: Endothelial
signaling during development. Nat Med. 9:661–668. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chotani MA, Mitra S, Su BY, Flavahan S,
Eid AH, Clark KR, Montague CR, Paris H, Handy DE and Flavahan NA:
Regulation of alpha (2)-adrenoceptors in human vascular smooth
muscle cells. Am J Physiol Heart Circ Physiol. 287:H59–H67.
2004.
|
|
29
|
Thompson-Torgerson CS, Holowatz LA,
Flavahan NA and Kenney WL: Cold-induced cutaneous vasoconstriction
is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart
Circ Physiol. 292:H1700–H1705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsai CH and Lee YJ: Focus on ADF/Cofilin:
Beyond actin cytoskeletal regulation. ISRN Cell Biol. 2012:1–7.
2012. View Article : Google Scholar
|
|
31
|
Wysolmerski RB and Lagunoff D: Involvement
of myosin light-chain kinase in endothelial-cell retraction. Proc
Natl Acad Sci USA. 87:16–20. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen XS, Pavlish K and Benoit JN: Myosin
phosphorylation triggers actin polymerization in vascular smooth
muscle. Am J Physiol Heart Circ Physiol. 295:H2172–H2177. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dubus I, L'Azou B, Gordien M, Delmas Y,
Labouyrie JP, Bonnet J and Combe C: Cytoskeletal reorganization by
mycophenolic acid alters mesangial cell migration and
contractility. Hypertension. 42:956–961. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lamalice L, Le Boeuf F and Huot J:
Endothelial cell migration during angiogenesis. Circ Res.
100:782–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bergers G, Song S, Meyer-Morse N,
Bergsland E and Hanahan D: Benefits of targeting both pericytes and
endothelial cells in the tumor vasculature with kinase inhibitors.
J Clin Invest. 111:1287–1295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raza A, Franklin MJ and Dudek AZ:
Pericytes and vessel maturation during tumor angiogenesis and
metastasis. Am J Hematol. 85:593–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hirase T and Node K: Endothelial
dysfunction as a cellular mechanism for vascular failure. Am J
Physiol Heart Circ Physiol. 302:H499–H505. 2012. View Article : Google Scholar : PubMed/NCBI
|