Introduction
Over the last 30 years, the number of patients with
diabetes has increased >2-fold, which renders it a major threat
to human health (1). Among the
complications associated with diabetes, diabetic cardiomyopathy
(DCM) is characterized by structural and functional alterations in
the myocardium, and is one of the leading causes of morbidity and
mortality in patients with diabetes worldwide (2,3).
Hyperglycemia-induced cardiac inflammation and cytotoxicity are the
major pathological causes of DCM. In the hearts of leptin
receptor-deficient db/db and streptozotocin (STZ)-induced diabetic
mice, hyperglycemia was demonstrated to induce cytotoxicity of
cardiac myocytes (4).
Recently, an increasing number of studies have
focused on elucidating the signal transduction pathways associated
with high glucose (HG)-induced inflammation and cytotoxicity in
cardiac tissues (2,5,6).
Hyperglycemia may activate the p38 mitogen-activated protein kinase
(MAPK) pathway (7), which serves a
critical role in the activation of nuclear factor-κB (NF-κB). NF-κB
is an essential transcription factor that regulates the expression
of proinflammatory genes, including inducible nitric oxide synthase
(iNOS), cyclooxygenase-2 (COX-2), interleukin (IL)-1β and IL-6
(8). In addition, activation of
NF-κB may upregulate the transcription of specific genes involved
in the inflammatory response (9).
iNOS mediates the nitrosylation of caspase-3, which may lead to
cardiomyocyte cell death (10).
COX-2 has been demonstrated to induce cardiomyocyte apoptosis in
several mouse models of cardiomyopathy (11,12).
Soetikno et al (13)
demonstrated that curcumin prevents DCM in STZ-induced diabetic
rats by inhibiting the activation of the p38MAPK/NF-κB signaling
pathway. Therefore, molecules that function to inhibit
p38MAPK/NF-κB, COX-2 and iNOS activation may protect against
HG-induced cardiomyocyte injury.
Hydrogen sulfide (H2S) is synthesized
from cysteine by cystathionine gamma lyase, as well as additional
naturally occurring enzymes. Along with nitric oxide and carbon
monoxide, H2S forms part of a group of biologically
active gases termed gasotransmitters or gasomediators (14–16).
An increasing number of previous studies have demonstrated that
H2S is an important cardioprotective agent (17,18).
One such study indicated that exogenous H2S exhibits
cardioprotective effects by inhibiting oxidative stress and
enhancing heat shock protein 90 expression levels (17). Guo et al (19) reported that H2S may
protect against doxorubicin-induced inflammation and cytotoxicity
in cardiomyocytes by inhibiting the p38MAPK/NF-κB signaling
pathway. In diabetic rats and in vitro models,
H2S was reported to protect cardiomyocytes from
inflammation and cell death (20–22).
However, the mechanisms underlying these protective effects of
H2S in diabetic cardiomyocytes remain unclear.
Therefore, the aim of the present study was to investigate the
cardioprotective effects of H2S against HG-induced
injury in cardiomyocytes, and to determine whether this may involve
the p38MAPK/NF-κB, COX-2 and iNOS signaling pathways.
Materials and methods
Reagents
The sodium H2S donor (NaHS), the selective inhibitor
of p38MAPK (SB203580), the selective inhibitor of NF-κB
(pyrrolidine dithiocarbamate, PDTC), and glucose were purchased
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). The Cell
Counting Kit-8 (CCK-8) assay was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). Primary antibodies specific
to the phosphorylated (p)-p65 as a measure of NF-κB protein
expression levels, COX-2, caspase-3, and p38 were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA; cat. nos. 3033,
12282, 9665 and 4511, respectively). The horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody (cat.
no. KC-RB-035), and the enzyme linked immunosorbent assay (ELISA)
kits for assessing IL-1β and IL-6 expression levels were purchased
from Zhejiang Kangchen Biotech Co., Inc. (Hangzhou, China). The
primary antibody against iNOS (cat no. sc650) was purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The primary
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (cat. no.
HJTW0125) was purchased from Guangzhou Jetway Biotech Co., Ltd.
(Guangzhou, China). The interleukin-1 receptor antagonist (IL-1Ra)
was purchased from Prospec-Tany TechnoGene, Ltd. (East Brunswick,
NJ, USA). The bicinchoninic acid (BCA) protein assay kit was
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
The radioimmunoprecipitation assay (RIPA) buffer was purchased from
Beyotime Institute of Biotechnology (Shanghai, China). Low-glucose
Dulbecco's modified Eagle's medium-Ham's F12 (DMEM-F12),
phosphate-buffered saline (PBS) and fetal bovine serum (FBS) were
purchased from Gibco; Thermo Fisher Scientific, Inc. H9c2 cells
were obtained from the Sun Yat-sen University Experimental Animal
Center (Guangzhou, China).
Cell culture and treatments
H9c2 cardiac cells were cultured in DMEM-F12 medium
supplemented with 10% FBS at 37°C and 5% CO2. In order to
investigate the cytotoxic effects of HG, H9c2 cells were treated
with 11, 22, 33 or 44 mM glucose for 24 h. To investigate the
cardioprotective effects of H2S on HG-induced injury, the cells
were pretreated with 400 µM NaHS for 30 min prior to HG treatment.
To further determine whether the anti-inflammatory effects of H2S
were associated with inhibition of p38MAPK/NF-κB pathway, H9c2
cells were pretreated with 3 µM SB203580 (a selective inhibitor of
p38MAPK) for 60 min or 100 µM PDTC (a selective inhibitor of NF-κB)
for 30 min prior to HG treatment, or were co-treated with HG plus
20 ng/ml IL-1Ra (a selective antagonist of the IL-1β receptor) for
24 h.
Cell viability assay
H9c2 cells were cultured in 96-well plates (1×104
cells/well) and were divided into the following groups: HG,
HG+NaHS, HG+SB203580, HG+PDTC, HG+IL-Ra, NaHS, SB203580, PDTC or
IL-1Ra) before 10 µl CCK-8 solution was added to each well at a
dilution of 1:10, and the cells were incubated for 2 h. The
absorbance was measured at 450 nm using a Multiskan MK3 microplate
reader (Thermo Fisher Scientific, Inc.). The mean optical density
(OD) of five wells for each treatment group was used to calculate
the percentage cell viability relative to untreated control cells,
according to the following formula: Cell viability (%) =
(ODtreatment/ODcontrol) ×100. The experiments were performed in
triplicate.
Detection of IL-1β and IL-6 production
in the cell culture media using ELISA
H9c2 cells were plated in 60 mm dishes at a density
of 1×106 cells/well. Cells were divided into the following
treatment groups: HG, HG+NaHS, HG+SB203580, HG+PDTC, HG+IL-Ra,
NaHS, SB203580, PDTC or IL-1Ra. The level of IL-1β and IL-6 in the
culture media was determined using ELISA, according to the
manufacturer's instructions. The absorbance was read at 450 nm, and
experiments were performed >5 times.
Extraction of cytoplasmic and nuclear
proteins
H9c2 cells were plated in 60 mm dishes at a density
of 1×106 cells/well and divided into the following treatment
groups: HG, HG+NaHS, HG+SB203580, HG+PDTC, HG+IL-Ra, NaHS,
SB203580, PDTC or IL-1Ra. Cytoplasmic and nuclear proteins from
H9c2 cells were isolated using the NE-PER Nuclear and Cytoplasmic
Extraction Reagent kit, according to the manufacturer's
instructions (Thermo Fisher Scientific, Inc.). Briefly, after
washing three times with cold PBS, the cells were treated with
cytoplasmic protein extraction buffer to isolate cytoplasmic
protein fraction proteins. The nuclear proteins were subsequently
extracted by adding the nuclear protein extraction buffer.
Cytoplasmic and nuclear protein extracts were subject to western
blot analysis.
Western blot analysis
H9c2 cells were seeded in 60-mm dishes at a density
of 1×106 cells/well and divided into the following treatment
groups: HG, HG+NaHS, HG+SB203580, HG+PDTC, HG+IL-Ra, NaHS,
SB203580, PDTC or IL-1Ra, Cells were washed in PBS and lysed in
RIPA buffer for 30 min, and the homogenate was centrifuged at
21,380 × g for 10 min at 4°C. The total protein
concentration of the sample supernatant was determined using a BCA
protein assay kit. Total protein (30 µg) was separated by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The
proteins were then transferred onto a polyvinylidene difluoride
membrane and the membrane was blocked with 5% non-fat milk diluted
in Tris-buffered saline-0.1% Tween 20 (TBS-T) for 1 h at room
temperature. The membranes were subsequently incubated with primary
antibodies specific to phosphorylated (p)-p65 (dilution, 1:500),
iNOS (dilution, 1:1,000), COX-2 (dilution, 1:1,000), caspase-3
(dilution, 1:1,000), p-p38 (dilution, 1:1,000) or GAPDH (dilution,
1:10,000) with gentle agitation at 4°C overnight. The following
day, the membranes were incubated with secondary antibodies
(dilution, 1:5,000) for 1.5 h at room temperature. Following three
washes with TBS-T, the membranes were developed using enhanced
chemiluminescence and exposed to X-ray films. To quantify protein
expression levels, the X-ray films were visualized and analyzed
using ImageJ software (version, 1.41; National Institutes of
Health, Bethesda, MA, USA).
Statistical analysis
The data are presented as the mean ± standard error.
Differences among treatment groups were analyzed using one-way
analysis of variance with the least significant difference test.
Statistical analyses were performed using the SPSS software program
(version, 13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
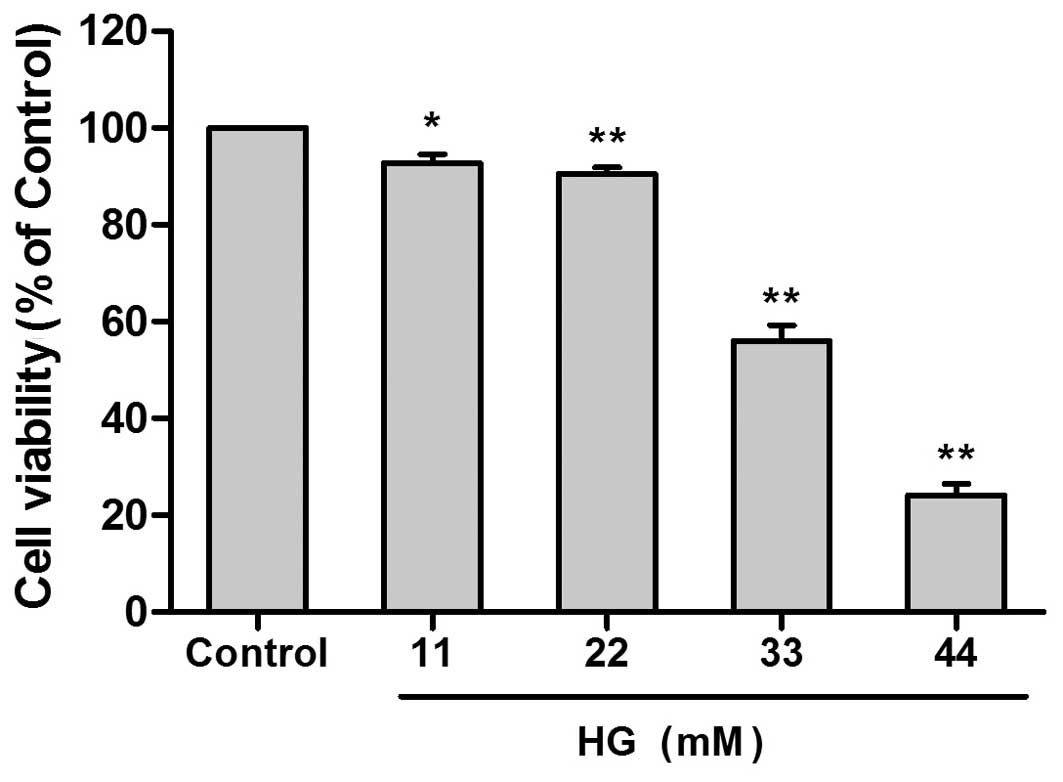
HG decreased cell viability in a
concentration-dependent and time-dependent manner
In order to investigate the cytotoxic effects of HG,
H9c2 cells were treated with 11, 22, 33 or 44 mM glucose for 24 h
As shown in Fig. 1, a significant
reduction in cell viability was observed following treatment with
33 mM HG when compared with untreated controls (58.10±2.24%
reduction; P<0.01). Based on these results, together with those
of a previous study (21),
treatment of cells with 33 mM HG for 24 h was selected to model the
effects of hyperglycemia on cardiac cells in vitro.
Exogenous H2S attenuates
HG-induced cytotoxicity in H9c2 cells
As shown in Fig.
2A, exposure of H9c2 cells to HG for 24 h induced cytotoxicity,
as evidenced by the significant decrease in cell viability
(P<0.01). However, this effect was significantly diminished when
the cells were pretreated with 400 µM NaHS for 30 min prior to HG
treatment (P<0.01), which suggests that H2S may attenuate
HG-induced cytotoxicity. Similarly, pretreatment of cells with
SB203580 or PDTC prior to HG treatment, significantly attenuated
the cytotoxic effects of HG treatment (SB203580 and PDTC,
P<0.01; Fig. 2A). These results
demonstrate that the p38MAPK/NF-κB signaling pathway may be
involved in HG-induced cytotoxicity. Notably, treatment of H9c2
cells with NaHS alone did not have a significant effect on cell
viability (Fig. 2A).
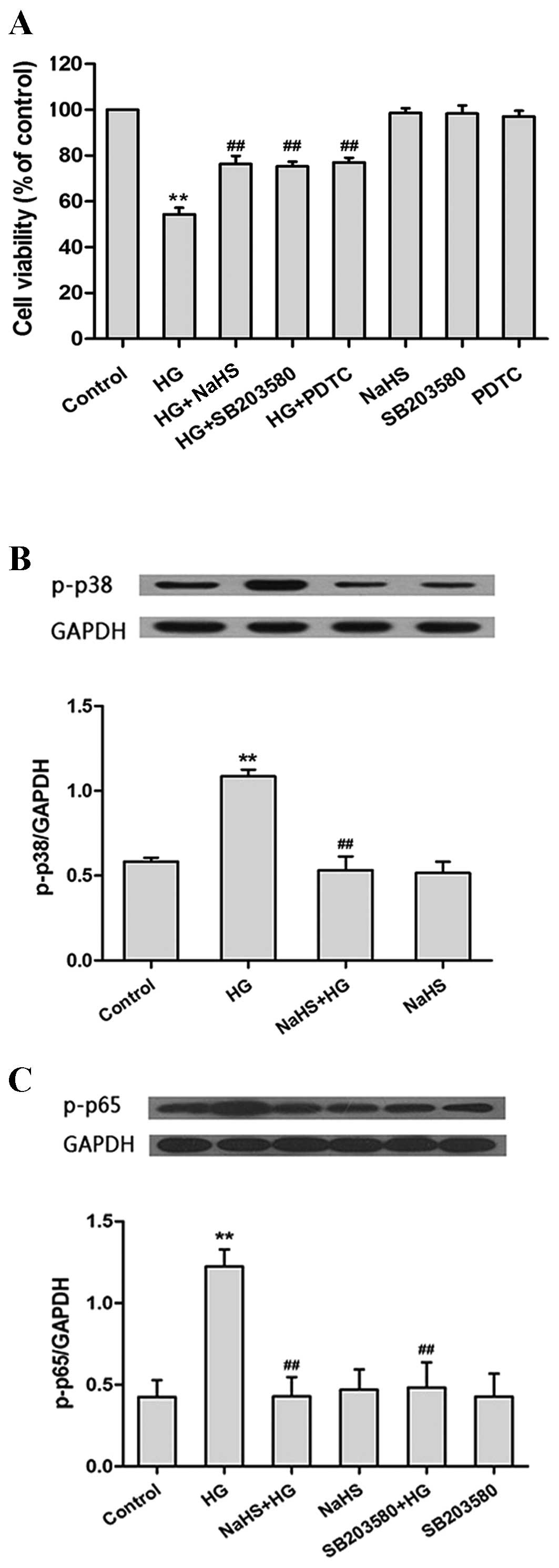 | Figure 2.H2S attenuates HG-induced
cytotoxicity by inhibiting the p38MAPK/NF-κB signaling pathway in
H9c2 cells. (A) The viability of rat H9c2 myocardium cells
following pretreatment with NaHS, SB203580 (a selective inhibitor
of p38MAPK) or PDTC (a selective inhibitor of NF-κB) prior to HG
treatment was significantly increased compared with the cells
treated with HG alone, as determined using the Cell Counting Kit-8
assay (n=6). (B) Pretreatment with NaHS significantly diminished
the HG-induced increase in p-p38 protein expression levels in H9c2
cells, as determined by western blotting (n=3). (C) Pretreatment
with NaHS or SB203580 significantly reduced the HG-induced increase
in p-p65 protein expression in H9c2 cells, as determined by western
blotting (n=3). Protein expression levels are presented relative to
GAPDH. The data are presented as the mean ± standard error.
(**P<0.01 vs. the control group; ##P<0.01 vs. the
HG treatment group). MAPK, mitogen-activated protein kinase; NF-κB,
nuclear factor-κB; PDTC, pyrrolidine dithiocarbamate; HG, high
glucose; p-, phosphorylated-; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Exogenous H2S inhibits the
p38MAPK/NF-κB signaling pathway in HG-treated H9c2 cells
The next aim of the present study was to investigate
the effect of H2S on the p38MAPK/NF-κB signaling pathway. As shown
in Fig. 2B, exposure of H9c2 cells
to HG for 24 h significantly enhanced the protein expression levels
of p-p38MAPK when compared with untreated controls (P<0.01). In
contrast, pretreatment of cells with 400 µM NaHS for 30 min prior
to HG treatment was associated with a significant reduction in
p-p38 expression when compared with HG-treated cells (P<0.01;
Fig. 2B).
Exposure of H9c2 cells to HG for 24 h significantly
increased the expression of NF-κB (P<0.01; Fig. 2C). However, pretreatment of cells
with 400 µM NaHS for 30 min or 3 µM SB203580 for 60 min prior to HG
treatment was associated with a significant reduction in p-p65
expression levels (NaHS, P<0.01; SB203580, P<0.01; Fig. 2C). These findings suggest that NaHS
may inhibit the expression of NF-κB and the p38MAPK/NF-κB signaling
pathway following exposure to HG.
Exogenous H2S suppresses
the HG-induced production of proinflammatory cytokines by
inhibiting the p38MAPK/NF-κB pathway in H9c2 cells
Following exposure to HG for 24 h, the production of
COX-2 (P<0.01; Fig. 3A), iNOS
(P<0.01; Fig. 3B), IL-1β
(P<0.01; Fig. 3C) and IL-6
(P<0.01; Fig. 3D) were
significantly increased when compared with the untreated control
group. However, pretreatment of cells with 400 µM NaHS for 30 min
prior to HG exposure, significantly ameliorated the HG-induced
increase in COX-2, iNOS, IL-1β and IL-6 expression levels (COX-2,
iNOS, IL-1β and IL-6, P<0.01; Fig.
3A-D). These results suggest that H2S may suppress the
production of proinflammatory cytokines in HG-treated H9c2
cells.
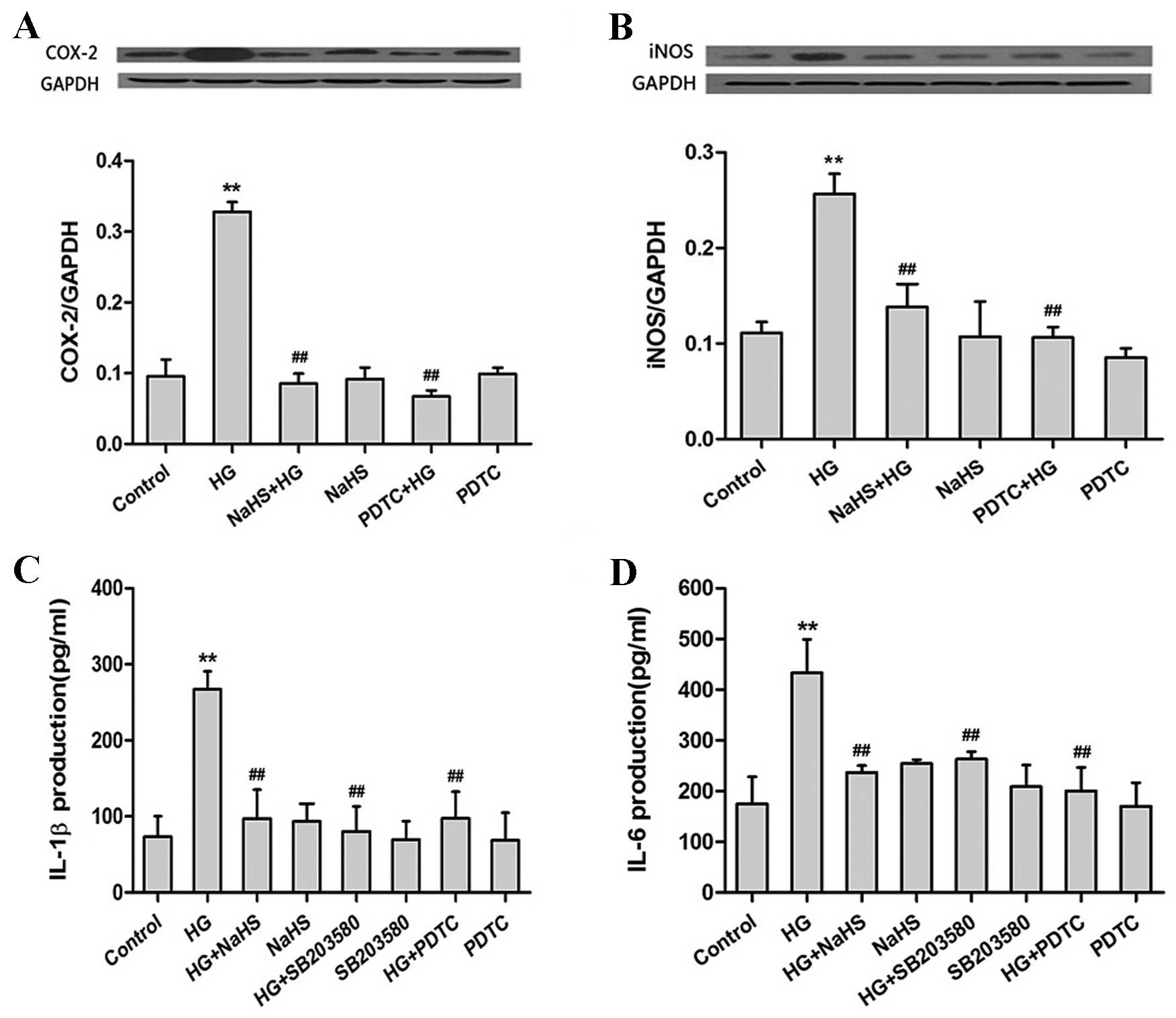 | Figure 3.H2S downregulates the
HG-induced increase in proinflammatory cytokine expression via the
p38MAPK/NF-κB signaling pathway. Pretreatment of rat H9c2 cells
with NaHS or PDTC (a selective inhibitor of NF-κB) prior to HG
exposure significantly reduced the HG-induced increase in (A)
COX-2, (B) iNOS, (C) IL-1β and (D) IL-6 protein expression levels,
as determined by (A and B) western blotting and (C and D)
enzyme-linked immunosorbent assay analyses. Protein expression
levels are presented relative to GAPDH. Pretreatment of cells with
SB203580 (a selective inhibitor of p38MAPK) prior to HG exposure
significantly attenuated the HG-induced increase in (C) IL-1β and
(D) IL-6 expression levels. The data are presented as the mean ±
standard error (n=3; **P<0.01 vs. the untreated control group;
##P<0.01 vs. the HG treatment group). HG, high
glucose; MAPK, mitogen-activated protein kinase; NF-κB, nuclear
factor-κB; PDTC, pyrrolidine dithiocarbamate; COX-2,
cyclooxygenase-2; iNOS, inducible nitric oxide synthase; IL,
interleukin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Pretreatment of H9c2 cells with 100 µM PDTC for 30
min prior to HG exposure attenuated the HG-induced production of
COX-2, iNOS, IL-1β and IL-6 (COX-2, iNOS, IL-1β and IL-6,
P<0.01; Fig. 3A-D). In
addition, H9c2 cells pretreated with 3 µM SB203580 for 60 min prior
to HG exposure demonstrated a significant reduction in IL-1β and
IL-6 expression (IL-1β and IL-6, P<0.01; Fig. 3C and D) when compared with
HG-treated controls. This suggests that H2S may suppress
the HG-induced production of COX-2, iNOS, IL-1β and IL-6 by
inhibiting the p38MAPK/NF-κB signaling pathway in H9c2 cells.
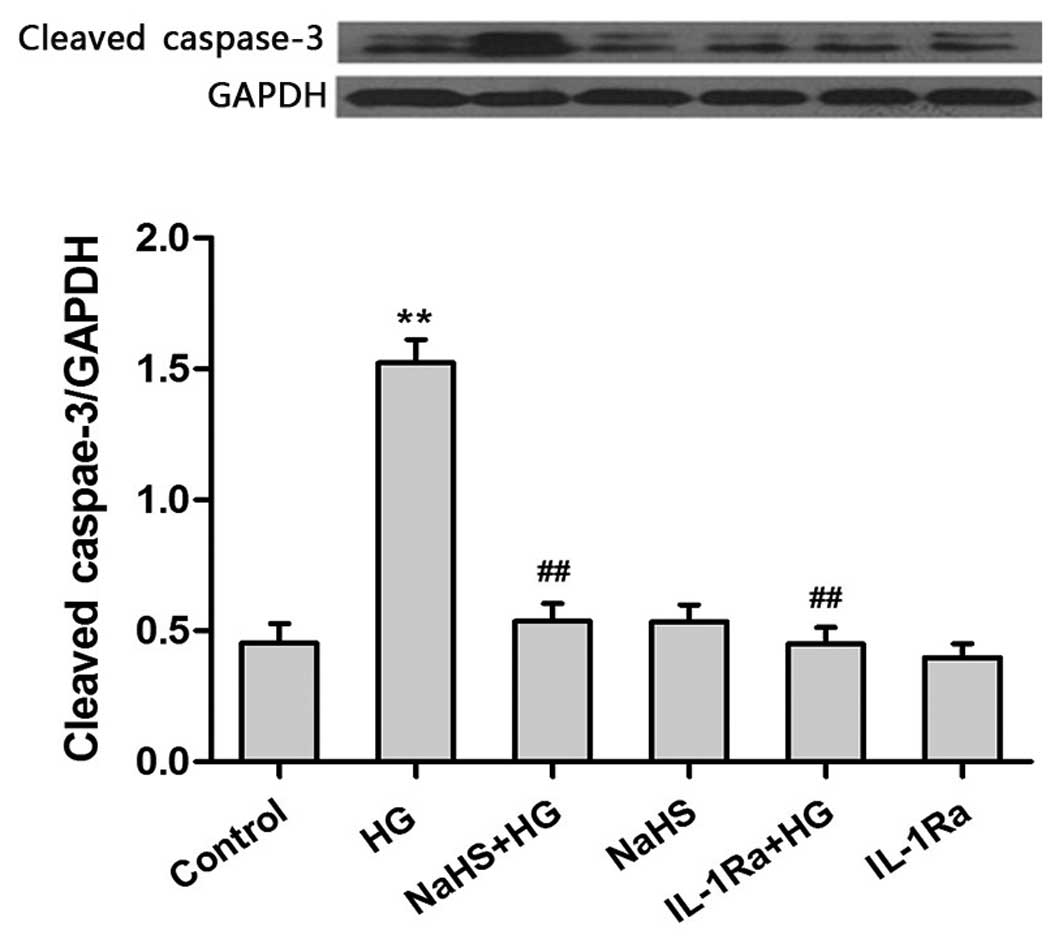
Exogenous H2S exhibits
anti-inflammatory effects by ameliorating the HG-induced increase
in caspase-3 expression
The results presented so far suggest that exogenous
H2S may protect against HG-induced cytotoxicity (Fig. 2A) and reduce the production of
proinflammatory cytokines (Fig. 3)
in H9c2 cells. Therefore, the final aim of the present study was to
investigate whether the anti-inflammatory effects of H2S may
attenuate HG-induced apoptosis. Following exposure of H9c2 cells to
HG for 24 h, the expression levels of caspase-3, which is an
important inducer of apoptosis (23), were significantly increased
(Fig. 4). However, pretreatment of
cells with 400 µM NaHS prior to HG exposure was associated with a
significant reduction in caspase-3 expression levels when compared
with cells treated with HG alone (P<0.01). Similarly, treatment
of cells with HG plus 20 ng/ml IL-1Ra for 24 h significantly
attenuated the production of caspase-3 when compared with cells
exposed to HG alone (P<0.01; Fig.
4). This suggests that the IL-1 receptor may be involved in
mediating the HG-induced increase in caspase-3 expression. These
results indicate that exogenous H2S may exhibit anti-inflammatory
effects by ameliorating the HG-induced increase in caspase-3
expression, which protects against HG-induced apoptosis.
Discussion
The results of the present study suggest that
exogenous H2S protects cardiac cells against HG-induced
inflammation and cytotoxicity, and inhibition of the p38MAPK/NF-κB,
COX-2 and iNOS signaling pathways may be involved in mediating
these cardioprotective effects of H2S in cardiac cells
in vitro. In addition, H2S exhibited
anti-inflammatory effects by significantly attenuating the
HG-induced increase in caspase-3 expression.
Several factors have been implicated in the
development of DCM, including metabolic disturbances, myocardial
fibrosis, small vessel disease, autonomic dysfunction and insulin
resistance (2). Metabolic
disturbances, particularly hyperglycemia, initiate the development
of DCM (24). Consistent with
previous studies (20,25), exposure to 33 mM glucose for 24 h
in the present study, was associated with cytotoxicity and the
induction of inflammatory responses in H9c2 cells, as evidenced by
a decrease in cell viability, increased expression of the apoptosis
inducing factor-caspase 3, and an increase in the expression of
multiple pro-inflammatory cytokines, including COX-2, iNOS, IL-1β
and IL-6. In addition, HG-treated cardiac cells demonstrated an
increase in p38MAPK and p-p65 expression, which suggests that the
p38MAPK/NF-κB signaling pathway may have been involved in mediating
the pro-apoptotic and cytotoxic effects of HG in H9c2 cells.
The physiological importance of H2S was
recognized in the last 15 years. Previous studies have demonstrated
that H2S influences a wide range of physiological and
pathological processes, including blood vessel dilation (26–28),
arterial contraction (27,29,30),
neurotransmission (31), the
regulation of inflammation (32,33)
and cardioprotection (34,35). In patients with diabetes (36) and in rats with STZ-induced diabetes
(36,37), the levels of H2S in the
circulation were significantly reduced. Therefore, a significant
amount of attention has focused on investigating whether exogenous
H2S supplementation may protect cardiac cells from
diabetes-induced cardiac injury. Several studies have revealed that
exogenous H2S protects against HG-induced cytotoxicity
and inflammation in cardiac cells (20,21).
A previous in vivo study demonstrated that intraperitoneal
or oral administration of H2S reduced myocardial
hypertrophy, as well as the degree of fibrosis (18). Consistent with previous studies,
the results of the present study demonstrate that exogenous
H2S may protect against cytotoxicity and inflammation in
HG-treated H9c2 cells.
The present study investigated the mechanisms
underlying the cardioprotective effects of H2S against
HG-induced cytotoxicity in H9c2 cells. Exogenous H2S
inhibited the HG-induced activation of the p38MAPK/NF-κB signaling
pathway, and significantly attenuated the HG-induced cytotoxicity
of HG-treated H9c2 cells. Consistent with these observations,
H2S has been demonstrated to inhibit p38MAPK activity in
various cell types, including rat aortic vascular smooth muscle
cells (38), microglia (32) and neuroblastoma cells (39). In addition, H2S has been
demonstrated to inhibit NF-κB activation and nuclear translocation
in macrophages (40), kidney cells
(41), pancreatic acinar cells
(42) and heart cells (43). It is possible that activation of
the p38MAPK signaling pathway may be a common mechanism involved in
the pathogenesis of chronic complications associated with diabetes,
as the elevation of p-p38MAPK in mouse diabetic cardiomyocytes
activates the production of several cytokines (44). NF-κB is a key factor involved in
inflammation, which regulates the transcription of >100 genes
associated with immune and inflammatory responses (16). The functional consequence of
reduced NF-κB activation in cells is a downregulation in the number
of activated proinflammatory genes and cytokines, including COX-2,
iNOS, IL-1β and IL-6. Zheng et al (45) observed that downregulation of the
NF-κB pathway reduced the downstream expression of proinflammatory
genes, including IL-1β, IL-6 and tumor necrosis factor-α, which led
to attenuation of inflammation-mediated injury of the vascular
wall. These data support the role of H2S as a
cardioprotective agent against HG-induced inflammation and
cytotoxicity through modulating the activity of the p38MAPK/NF-κB
signaling pathway in H9c2 cells.
iNOS is an enzyme and can be induced in all cells
and tissues through the action of cytokines (46). Activation of iNOS has been
associated with the development of cardiovascular complications in
rat models of diabetes. Bardell et al (47) demonstrated that the expression of
iNOS in the mesenteric arteries is increased in rat models of
diabetes. In the present study, the expression levels of iNOS were
significantly elevated in cardiac cells following exposure to HG,
which were significantly attenuated by H2S treatment or
following inhibition of NF-κB. A previous study, involving
STZ-induced diabetic rats, demonstrated that iNOS mediated the
nitrosylation of GAPDH and caspase-3, which led to cardiomyocyte
death (5). Therefore, the
p38MAPK/NF-κB and iNOS signaling pathways are likely to be involved
in mediating the cardioprotective effects of exogenous
H2S against HG-induced inflammation and cytotoxicity in
H9c2 cells.
COX is an enzyme involved in the metabolism of
arachidonic acid (AA), and the COX-2 isoform is a key enzyme in the
conversion of AA to prostaglandins (48). A previous study demonstrated that
overexpression of COX-2 is correlated with induction of
inflammatory processes (49). In
the present study, the level of COX-2 expression was significantly
elevated in cardiac cells following HG exposure. In addition,
exposure of cardiac H9c2 cells to H2S or a specific
inhibitor to NF-κB, abolished the HG-induced increase in COX-2
expression. In a rat model of heart ischemia/reperfusion injury
(50), inhibition of COX-2 was
associated with a reduction in cellular apoptosis. Consistent with
these observations, the results of the present study suggest that
H2S may attenuate apoptosis of H9c2 cells following HG
exposure by reducing caspase-3 expression levels. Therefore,
H2S may protect cardiac cells by inhibiting COX-2
expression potentially through modulating the p38MAPK/NF-κB
signaling pathway.
In the present study, pretreatment of H9c2 cells
with an IL-1Ra prior to HG exposure significantly reduced caspase-3
expression. This suggests that inhibition of inflammation may
protect against HG-induced apoptosis in H9c2 cells. Consistent with
this observation, inhibition of the inflammatory response
significantly reduced doxorubicin-induced cytotoxicity of H9c2
cells (19). In addition, p38MAPK
was demonstrated to promote apoptosis in a number of in
vitro models, such as pulmonary microvascular endothelial cells
(51) and breast cancer cells
(52), while H2S
prevented apoptosis by inhibition of p38MAPK (53). Therefore, the p38MAPK/NF-κB
pathway-mediated activation of the anti-inflammatory response may
be associated with the cardioprotective effects of exogenous
H2S treatment against HG-induced cytotoxicity in H9c2
cells.
In conclusion, the results of the present study
demonstrated that exogenous H2S exhibits
cardioprotective effects against HG-induced cytotoxicity and
inflammation in rat cardiac cells in vitro. H2S
exerted cytoprotective effects potentially via the
p38MAPK/NF-κB-mediated anti-inflammatory and antiapoptotic
signaling pathways. The results provide evidence to suggest that
H2S may have a potential therapeutic value in
hyperglycemia-induced cardiac lesions, which are predominant in
DCM.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81270296) and the
Social Development Project of Guangdong Province in China (grant
no. 2014SC107).
References
|
1
|
Chen L, Magliano DJ and Zimmet PZ: The
worldwide epidemiology of type 2 diabetes mellitus-present and
future perspectives. Nat Rev Endocrinol. 8:228–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goyal BR and Mehta AA: Diabetic
cardiomyopathy: Pathophysiological mechanisms and cardiac
dysfuntion. Hum Exp Toxicol. 32:571–590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poornima IG, Parikh P and Shannon RP:
Diabetic cardiomyopathy: The search for a unifying hypothesis. Circ
Res. 98:596–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen E, Li Y, Li Y, Shan L, Zhu H, Feng Q,
Arnold JM and Peng T: Rac1 is required for cardiomyocyte apoptosis
during hyperglycemia. Diabetes. 58:2386–2395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Puthanveetil P, Zhang D, Wang Y, Wang F,
Wan A, Abrahani A and Rodrigues B: Diabetes triggers a PARP1
mediated death pathway in the heart through participation of FoxO1.
J Mol Cell Cardiol. 53:677–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans JL, Goldfine ID, Maddux BA and
Grodsky GM: Oxidative stress and stress-activated signaling
pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev.
23:599–622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Igarashi M, Wakasaki H, Takahara N, Ishii
H, Jiang ZY, Yamauchi T, Kuboki K, Meier M, Rhodes CJ and King GL:
Glucose or diabetes activates p38 mitogen-activated protein kinase
via different pathways. J Clin Invest. 103:185–195. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crowell JA, Steele VE, Sigman CC and Fay
JR: Is inducible nitric oxide synthase a target for
chemoprevention? Mol Cancer Ther. 2:815–823. 2003.PubMed/NCBI
|
|
9
|
Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok
SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, et al: Quercetin
suppresses proinflammatory cytokines production through MAP kinases
and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage.
Mol Cell Biochem. 243:153–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puthanveetil P, Zhang D, Wang Y, Wang F,
Wan A, Abrahani A and Rodrigues B: Diabetes triggers a PARP1
mediated death pathway in the heart through participation of FoxO1.
J Mol Cell Cardiol. 53:677–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jenke A, Wilk S, Poller W, Eriksson U,
Valaperti A, Rauch BH, Stroux A, Liu P, Schultheiss HP,
Scheibenbogen C and Skurk C: Adiponectin protects against Toll-like
receptor 4-mediated cardiac inflammation and injury. Cardiovasc
Res. 99:422–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Streicher JM, Kamei K, Ishikawa TO,
Herschman H and Wang Y: Compensatory hypertrophy induced by
ventricular cardiomyocyte-specific COX-2 expression in mice. J Mol
Cell Cardiol. 49:88–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soetikno V, Sari FR, Sukumaran V,
Lakshmanan AP, Mito S, Harima M, Thandavarayan RA, Suzuki K, Nagata
M, Takagi R and Watanabe K: Curcumin prevents diabetic
cardiomyopathy in streptozotocin-induced diabetic rats: Possible
involvement of PKC-MAPK signaling pathway. Eur J Pharm Sci.
47:604–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YH, Lu M, Hu LF, Wong PT, Webb GD and
Bian JS: Hydrogen sulfide in the mammalian cardiovascular system.
Antioxid Redox Signal. 17:141–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holwerda KM, Karumanchi SA and Lely AT:
Hydrogen sulfide: Role in vascular physiology and pathology. Curr
Opin Nephrol Hypertens. 24:170–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Bhatia M and Moore PK: Hydrogen
sulphide-A novel mediator of inflammation? Curr Opin Pharmacol.
6:125–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen SL, Yang CT, Yang ZL, Guo RX, Meng
JL, Cui Y, Lan AP, Chen PX and Feng JQ: Hydrogen sulphide protects
H9c2 cells against chemical hypoxia-induced injury. Clin Exp
Pharmacol Physiol. 37:316–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Seweidy MM, Sadik NA and Shaker OG:
Role of sulfurous mineral water and sodium hydrosulfide as potent
inhibitors of fibrosis in the heart of diabetic rats. Arch Biochem
Biophys. 506:48–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo R, Wu K, Chen J, Mo L, Hua X, Zheng D,
Chen P, Chen G, Xu W and Feng J: Exogenous hydrogen sulfide
protects against doxorubicin-induced inflammation and cytotoxicity
by inhibiting p38MAPK/NFκB pathway in H9c2 cardiac cells. Cell
Physiol Biochem. 32:1668–1680. 2013.PubMed/NCBI
|
|
20
|
Xu W, Wu W, Chen J, Guo R, Lin J, Liao X
and Feng J: Exogenous hydrogen sulfide protects H9c2 cardiac cells
against high glucose-induced injury by inhibiting the activities of
the p38 MAPK and ERK1/2 pathways. Int J Mol Med. 32:917–925.
2013.PubMed/NCBI
|
|
21
|
Wei WB, Hu X, Zhuang XD, Liao LZ and Li
WD: GYY4137, a novel hydrogen sulfide-releasing molecule, likely
protects against high glucose-induced cytotoxicity by activation of
the AMPK/mTOR signal pathway in H9c2 cells. Mol Cell Biochem.
389:249–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou X and Lu X: Hydrogen sulfide inhibits
high-glucose-induced apoptosis in neonatal rat cardiomyocytes. Exp
Biol Med (Maywood). 238:370–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fulda S: Targeting apoptosis for
anticancer therapy. Semin Cancer Biol. 31:84–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mortuza R and Chakrabarti S:
Glucose-induced cell signaling in the pathogenesis of diabetic
cardiomyopathy. Heart Fail Rev. 19:75–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodrigues B, Cam MC and McNeill JH:
Metabolic disturbances in diabetic cardiomyopathy. Mol Cell
Biochem. 180:53–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng Y, Ndisang JF, Tang G, Cao K and
Wang R: Hydrogen sulfide-induced relaxation of resistance
mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol.
287:H2316–H2323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ali MY, Ping CY, Mok YY, Ling L, Whiteman
M, Bhatia M and Moore PK: Regulation of vascular nitric oxide in
vitro and in vivo; a new role for endogenous hydrogen sulphide? Br
J Pharmacol. 149:625–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hosoki R, Matsuki N and Kimura H: The
possible role of hydrogen sulfide as an endogenous smooth muscle
relaxant in synergy with nitric oxide. Biochem Biophys Res Commun.
237:527–531. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kiss L, Deitch EA and Szabo C: Hydrogen
sulfide decreases adenosine triphosphate levels in aortic rings and
leads to vasorelaxation via metabolic inhibition. Life Sci.
83:589–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lim JJ, Liu YH, Khin ES and Bian JS:
Vasoconstrictive effect of hydrogen sulfide involves downregulation
of cAMP in vascular smooth muscle cells. Am J Physiol Cell Physiol.
295:C1261–C1270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abe K and Kimura H: The possible role of
hydrogen sulfide as an endogenous neuromodulator. J Neurosci.
16:1066–1071. 1996.PubMed/NCBI
|
|
32
|
Hu LF, Wong PT, Moore PK and Bian JS:
Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation
by inhibition of p38 mitogen-activated protein kinase in microglia.
J Neurochem. 100:1121–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L, Bhatia M and Moore PK: Hydrogen
sulphide-a novel mediator of inflammation? Curr Opin Pharmacol.
6:125–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johansen D, Ytrehus K and Baxter GF:
Exogenous hydrogen sulfide (H2S) protects against regional
myocardial ischemia-reperfusion injury-Evidence for a role of K ATP
channels. Basic Res Cardiol. 101:53–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elrod JW, Calvert JW, Morrison J, Doeller
JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, et al:
Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury
by preservation of mitochondrial function. Proc Natl Acad Sci USA.
104:15560–15565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jain SK, Bull R, Rains JL, Bass PF, Levine
SN, Reddy S, McVie R and Bocchini JA: Low levels of hydrogen
sulfide in the blood of diabetes patients and
streptozotocin-treated rats causes vascular inflammation? Antioxid
Redox Signal. 12:1333–1337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yusuf M, Huat BT Kwong, Hsu A, Whiteman M,
Bhatia M and Moore PK: Streptozotocin-induced diabetes in the rat
is associated with enhanced tissue hydrogen sulfide biosynthesis.
Biochem Biophys Res Commun. 333:1146–1152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Du J, Hui Y, Cheung Y, Bin G, Jiang H,
Chen X and Tang C: The possible role of hydrogen sulfide as a
smooth muscle cell proliferation inhibitor in rat cultured cells.
Heart Vessels. 19:75–80. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu LF, Lu M, Wu ZY, Wong PT and Bian JS:
Hydrogen sulfide inhibits rotenone-induced apoptosis via
preservation of mitochondrial function. Mol Pharmacol. 75:27–34.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim
HR, Jeon SB, Jeon WK, Chae HJ and Chung HT: Hydrogen sulfide
inhibits nitric oxide production and nuclear factor-kappaB via heme
oxygenase-1 expression in RAW264.7 macrophages stimulated with
lipopolysaccharide. Free Radic Biol Med. 41:106–119. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tripatara P, Patel NS, Collino M,
Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown
PA, Yaqoob MM, et al: Generation of endogenous hydrogen sulfide by
cystathionine gamma-lyase limits renal ischemia/reperfusion injury
and dysfunction. Lab Invest. 88:1038–1048. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tamizhselvi R, Moore PK and Bhatia M:
Inhibition of hydrogen sulfide synthesis attenuates chemokine
production and protects mice against acute pancreatitis and
associated lung injury. Pancreas. 36:e24–e31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sivarajah A, McDonald MC and Thiemermann
C: The production of hydrogen sulfide limits myocardial ischemia
and reperfusion injury and contributes to the cardioprotective
effects of preconditioning with endotoxin, but not ischemia in the
rat. Shock. 26:154–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boudina S and Abel ED: Diabetic
cardiomyopathy, causes and effects. Rev Endocr Metab Disord.
11:31–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng X, Zhu S, Chang S, Cao Y, Dong J, Li
J, Long R and Zhou Y: Protective effects of chronic resveratrol
treatment on vascular inflammatory injury in steptozotocin-induced
type 2 diabetic rats: Role of NF-kappaB signaling. Eur J Pharmacol.
720:147–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bardell AL and MacLeod KM: Evidence for
inducible nitric-oxide synthase expression and activity in vascular
smooth muscle of streptozotocin-diabetic rats. J Pharmacol Exp
Ther. 296:252–259. 2001.PubMed/NCBI
|
|
48
|
Alhouayek M and Muccioli GG: COX-2-derived
endocannabinoid metabolites as novel inflammatory mediators. Trends
Pharmacol Sci. 35:284–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zamorano B and Carmona MT:
Prostaglandin-E2 and cyclic adenosine 3′-5′ monophosphate levels in
the hypertrophied rat heart. Biol Res. 25:85–89. 1992.PubMed/NCBI
|
|
50
|
Song ZF, Chen DY, DU B and Ji XP: Poly
(ADP-ribose) polymerase inhibitor reduces heart
ischaemia/reperfusion injury via inflammation and Akt signalling in
rats. Chin Med J (Engl). 126:1913–1917. 2013.PubMed/NCBI
|
|
51
|
Liu ZF, Zheng D, Fan GC, Peng T and Su L:
Heat stress prevents lipopolysaccharide-induced apoptosis in
pulmonary microvascular endothelial cells by blocking calpain/p38
MAPK signalling. Apoptosis. 21:896–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dong Y, Yin S, Song X, Huo Y, Fan L, Ye M
and Hu H: Involvement of ROS-p38-H2AX axis in novel curcumin
analogues-induced apoptosis in breast cancer cells. Mol Carcinog.
55:323–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han OJ, Joe KH, Kim SW, Lee HS, Kwon NS,
Baek KJ and Yun HY: Involvement of p38 mitogen-activated protein
kinase and apoptosis signal-regulating kinase-1 in nitric
oxide-induced cell death in PC12 cells. Neurochem Res. 26:525–532.
2001. View Article : Google Scholar : PubMed/NCBI
|