Introduction
Nonalcoholic fatty liver disease (NAFLD), which
includes nonalcoholic steatohepatitis (NASH), is one of the hepatic
manifestations of metabolic syndrome, and is becoming a public
health concern as NAFLD and NASH progress to hepatic failure and
hepatocellular carcinoma (1–3).
Epidemiological studies have demonstrated that 10% of patients with
NASH develop cirrhosis over a 15-year period (4), and NAFLD is a common reason for liver
transplantation in the United States (5). By contrast, nonalcoholic fatty liver,
or simple steatosis, rarely progresses to advanced disease. The
mechanism underlying the occurrence and progression of NAFLD is
currently considered to be a ‘multiple hit process’ involving
insulin resistance (IR), oxidative stress, apoptosis and
perturbations of adipokine levels (6,7).
Predictive factors of advanced fibrosis in patients with NAFLD also
include increasing age, type 2 diabetes mellitus, and hypertension.
However, the precise mechanism underlying the progression of NAFLD
remains to be fully elucidated.
Metabolic syndrome is associated with IR and other
metabolic risk factors, including central obesity, dyslipidemia and
hypertension. The coexistence of these disorders is correlated with
cardiovascular disease (CVD) (8–11).
IR is also important in the pathogenesis of NAFLD, and patients
with NAFLD have reduced insulin sensitivity, not only in muscle,
but also in the liver (12). In
addition, NAFLD is an independent risk factor for CVD and predicts
future cardiac events (13). It
was previously reported that there is an apparent association
between non-obese hypertensive patients with NAFLD and increases in
IR (14). The increased risk of
all-cause mortality rates in patients with NAFLD appears to be
associated with hypertension in addition to IR, however, the
association between hypertension and IR in patients with NAFLD
remains to be fully elucidated.
Our previous study (15) investigated whether high-salt (HS)
diet-induced hypertension exacerbated the pathophysiology of
steatohepatitis induced by a choline-deficient, L-amino
acid-defined (CDAA) diet in an animal model. It was found that
hypertension was a potential risk factor for liver injury and
hepatic fibrosis. Using spontaneously hypertensive rats (SHRs) fed
a CDAA diet, it was demonstrated that the levels of alanine
aminotransferase (ALT) and alkaline phosphatase (ALP) were
significantly higher, and hepatic mRNA levels of interleukin
(IL)-10 were significantly lower in the HS group, compared with the
normal-salt group. Immune function analysis showed that healthy
human subjects on a HS diet (12 g/day) had higher levels of
proinflammatory cytokines, IL-6 and IL-23, and lower levels of the
anti-inflammatory cytokine, IL-10, compared with those receiving
the recommended salt intake (6 g/day), suggesting that a HS diet
may contribute to an excessive immune response (16). Pro-inflammatory serum cytokines,
including IL-6, may be increased and anti-inflammatory cytokines,
including IL-10, may be decreased in NASH due to imbalanced
cytokine production in the liver and/or extrahepatic production
(17–21). However, the association between IR
and the production of cytokines, including IL-6 and IL-10 in NAFLD
remain to be fully elucidated.
In the present study, it was hypothesized that
HS-induced hypertension affects IR through imbalanced cytokine
production in SHRs with CDAA-diet-induced steatohepatitis, and that
IR or imbalanced cytokine production can be reversed by
antihypertensive therapy. It was shown that HS-induced hypertension
was positively correlated with blood glucose and levels of serum
insulin, and with homeostasis model assessment (HOMA)-IR,
suggesting that hypertension induced IR in this NASH model. It was
also demonstrated that the expression of cytokine IL-6 was
correlated with hypertension and was attenuated by antihypertensive
therapy, which was accompanied by the expression of IL-10. The
cytokine expression and IR induced by hypertension may be
associated with the progression of NASH (22), and the experimental animal model
used in the present study may be useful for investigating NASH with
metabolic syndrome.
Materials and methods
Animals
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Kagoshima University
(Kagoshima, Japan). Male, 6-week-old SHRs were obtained from
Charles River Laboratories (Yokohama, Japan). The rats were allowed
to acclimatize to the laboratory conditions for 1 week at a
constant temperature of 24°C with a 12-h light-dark cycle and
40–70% humidity, and were fed standard chow (control diet)
containing 0.27% NaCl (CE-2; Kyudo, Kumamoto, Japan) and water
ad libitum. Following the acclimatization period, the rats
were divided into groups (n=10 per group) and fed five different
diets ad libitum, as follows: 6 weeks standard chow with
normal salt concentration (0.27% NaCl), followed by a standard chow
or CDAA diet containing normal salt concentration for an additional
8 weeks (control and CDAA groups, respectively), and 6 weeks
standard chow with a HS concentration (8.0% NaCl), followed by a
CDAA diet containing HS for an additional 8 weeks, with or without
the antihypertensive agents, amlodipine (Aml; Wako, Osaka, Japan;
10 mg/day in food) or hydralazine (Hyd; Sigma-Aldrich; Thermo
Fisher Scientific, Inc., Waltham, MA, USA; 20 mg/day in food).
These were termed the CDAA+HS, CDAA+HS+Aml and CDAA+HS+Hyd groups,
respectively. Thus, all experiments were performed for 14 weeks and
then all rats were sacrificed by exsanguination under pentobarbital
anesthesia (12–18 mg/body; intraperitoneal administration; Kyoritsu
Seiyaku Corporation, Tokyo, Japan). The diets were obtained from
Dyets, Inc. (Bethlehem, PA, USA) and 30 g per day was administered
to ensure equal total food intake.
Measurement of systolic blood pressure
(SBP) and serum markers
SBP was determined using the tail-cuff method once
every 2 weeks between 7 and 21 weeks (MK-1030; Muromachi, Tokyo,
Japan).
Blood was collected by vena cava puncture following
a 12-h fast and then centrifuged at 1,800 × g for 5 min at
4°C. The resulting serum was stored at −80°C. Serum levels of ALT,
ALP, total cholesterol and triglyceride were determined
commercially at SRL, Inc. (Tokyo, Japan). Fasting blood glucose
(FBG) and serum immunoreactive insulin (IRI) levels were determined
using Spotchem II-Glucose (Arkray, Kyoto, Japan) and ELISA
(Morinaga Institute of Biological Science, Kanagawa, Japan),
respectively. HOMA-IR was calculated using the following formula:
HOMA-IR = FBG (mg/dl) × fasting IRI (µU/ml) / 405. Serum levels of
IL-6 and IL-10 were determined using a rat IL-6 or IL-10 Quantikine
ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA).
Histological and immunohistochemical
analyses
The resected livers and spleens were weighed, and
thin slices were immersed in 10% neutralized formalin and embedded
in paraffin to produce 4-µm-thick sections for staining with
hematoxylin and eosin. Immunohistochemical analyses using anti-CD68
antibody (ED-1; cat. no. ab31630; Abcam, Cambridge, MA, USA) were
performed using the paraffin-embedded sections. The samples were
blocked with protein block (Dako Japan Co., Ltd., Tokyo, Japan) for
10 min, followed by incubation with the primary antibody at a 1:400
dilution overnight at 4°C. The samples were incubated with goat
anti-mouse/rabbit IgG (Histofine Simple Stain Rat MAX PO MULTI;
cat. no. 414191; Nichirei Biosciences, Tokyo, Japan) to detect
bound antibodies. After the signal was visualized by the 3,
3-diaminobenzidine, the CD68+ cells were counted
(magnification, ×400) in five randomly selected fields of the
periportal and perivenular areas of the liver lobules using a
fluorescence microscope (BZ-9000; Keyence Corporation, Osaka,
Japan).
Measurement of the levels of hepatic
monocyte chemoattractant protein-1 (MCP-1)
The protein concentration was standardized at 8
mg/ml following extraction of total protein from the liver tissues
according to a previous report (23). Rat MCP-1 in the homogenate was
measured using an ELISA kit (R&D Systems, Inc.) according to
the manufacturer's protocol.
Cell isolation and flow cytometry
Single-cell suspensions of splenic cells were
prepared using cell strainers and incubated for 5 min in 1X RBC
lysis buffer (eBioscience, Inc., San Diego, CA, USA). The cells
were washed twice using flow cytometry staining buffer
(eBioscience, Inc.) and resuspended in flow cytometry staining
buffer (eBioscience, Inc.). The splenic cells were analyzed by
three-color intracellular flow cytometry using fluorescein
isothiocyanate-CD4 (1:100; cat. no. 11-0040; 4°C, 20 min
incubation), phycoerythrin-CD25 (1:100; cat. no. 12-0390; 4°C, 20
min incubation) and allophycocyanin-forkhead box P3 (Foxp3; 1:100;
cat. no. 77-5775; room temperature, 30 min incubation) antibodies
(eBioscience, Inc.). The expression of antigens were analyzed on a
CyAnTM ADP flow cytometer (Beckman Coulter, Inc., Brea, CA,
USA).
Statistical analysis
Data are presented as mean ± standard deviation, or
the 10th, 25th, 50th (median), 75th and 90th percentiles.
Statistical comparisons among groups were performed using one-way
analysis of variance followed by Tukey's post-hoc test.
Correlations between continuous variables were calculated using
Pearson's correlation. The χ2 test was used to compare
the categorical variables among groups. All analyses were performed
using SPSS v.18 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Antihypertensive therapy in SHRs fed a
HS CDAA diet
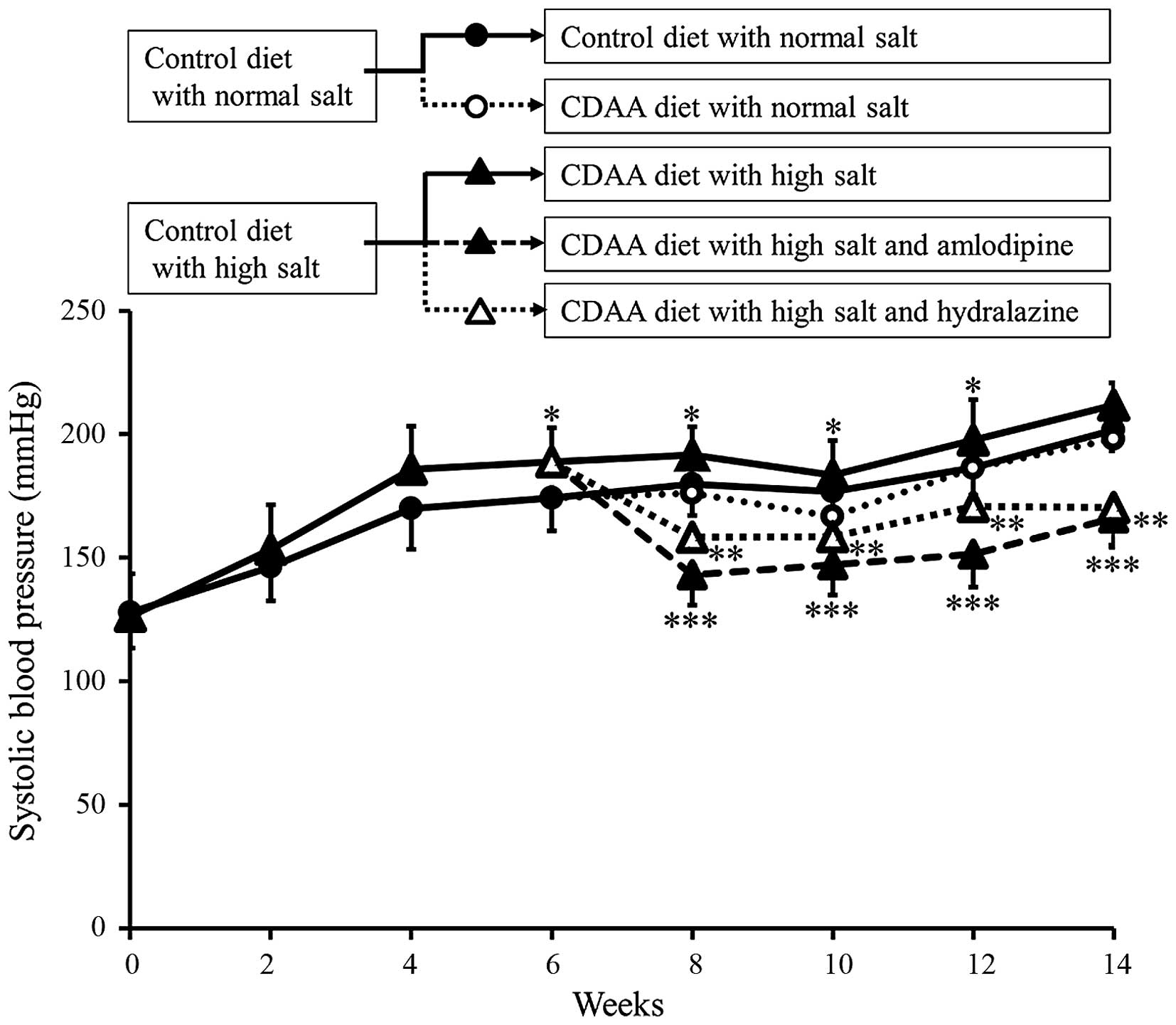
The SBP of the animals in each group were assessed
between 0 and 14 weeks. The SBPs in the control and CDAA groups
gradually increased in a similar manner (Fig. 1). In addition, as our previous
study showed (15), administration
of a HS diet induced severe hypertension. By contrast, the
antihypertensive agents, Aml and Hyd, significantly decreased SBP
(Fig. 1).
At week 14, the liver weight to body weight ratio in
the CDAA+HS group was significantly higher, compared with the
ratios in the control and CDAA groups, and was decreased by
antihypertensive therapy (Table
I).
 | Table I.Body, liver and spleen weights, and
SBP at week 14 in each group. |
Table I.
Body, liver and spleen weights, and
SBP at week 14 in each group.
| Group | Body weight
(g) | Liver weight
(g) | Liver/body weight
(%) | Spleen weight
(g) | SBP (mmHg) |
|---|
| Control | 357.00±19.93 | 10.23±0.60 | 2.87±0.09 | 0.66±0.07 | 201.80±8.95 |
| CDAA | 362.40±17.89 | 9.87±0.59 | 2.72±0.11 | 0.64±0.03 | 198.12±13.03 |
| CDAA+HS |
325.80±17.83b,e | 10.40±1.01 |
3.18±0.21c,f | 0.64±0.08 | 211.80±9.56 |
| CDAA+HS+Aml |
334.30±20.33d | 9.63±0.58 |
2.89±0.17g | 0.62±0.05 |
166.16±12.48c,f,h |
| CDAA+HS+Hyd |
329.90±15.42a,e | 10.00±0.64 |
3.03±0.16e | 0.59±0.07 |
170.42±16.14cfh |
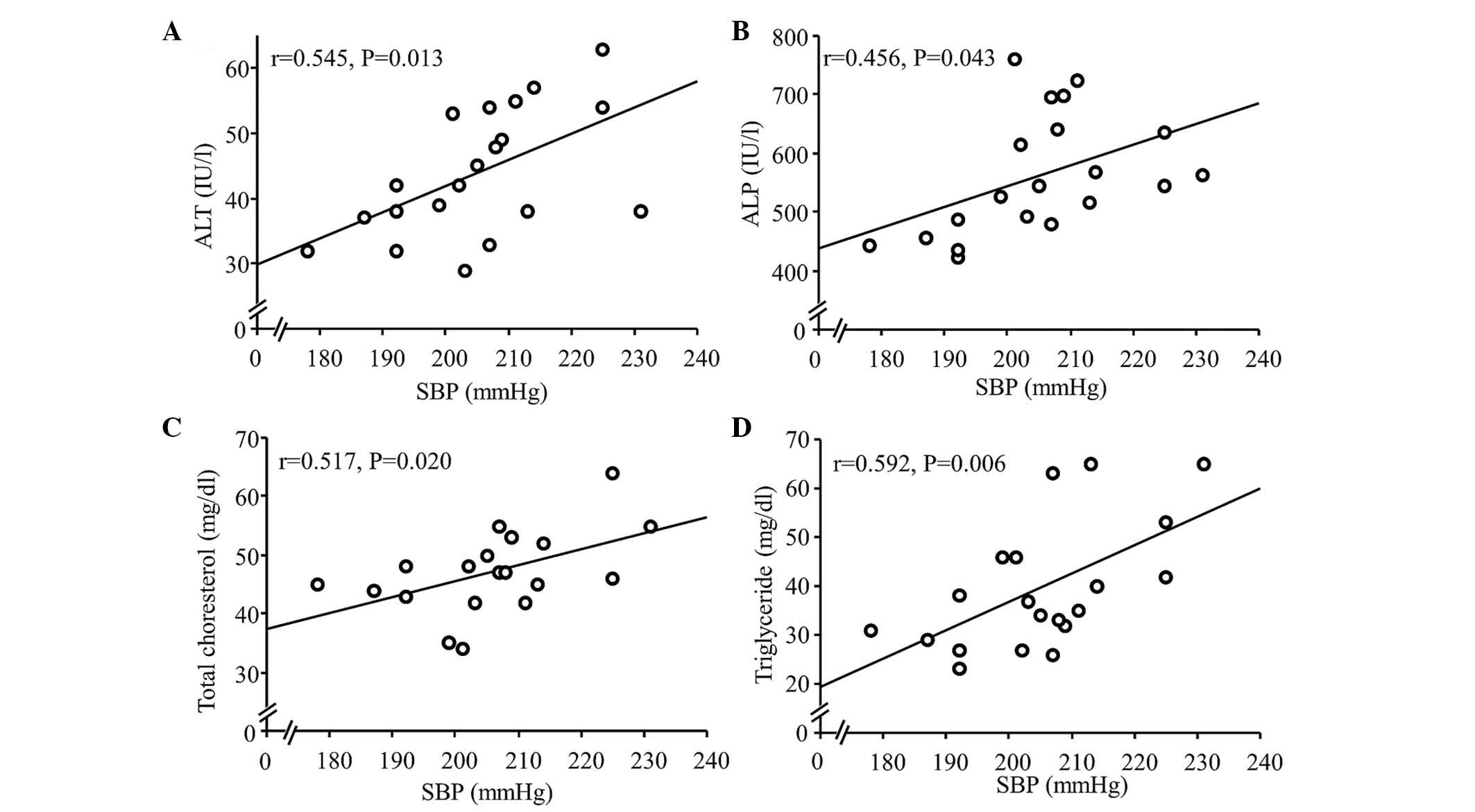
Association between SBP and blood
chemistry analysis
Using a similar model as that used in the present
study, our previous study (15)
reported that serum levels of ALT and ALP were significantly higher
in the HS group, compared with the normal-salt group. In the
present study, the correlation between SBP and serum levels of ALT
and ALP were investigated further. In the CDAA and CDAA+HS groups,
SBP was significantly correlated with ALT and ALP (Fig. 2A and B), as expected, and with
total cholesterol (TC) and triglyceride (TG; Fig. 2C and D). Antihypertensive therapy
lowered serum levels of ALP (Fig.
3A), but did not lower serum levels of ALT (Fig.3B). The serum levels of TC and TG
were also reduced, although not significantly (Fig. 3C and D).
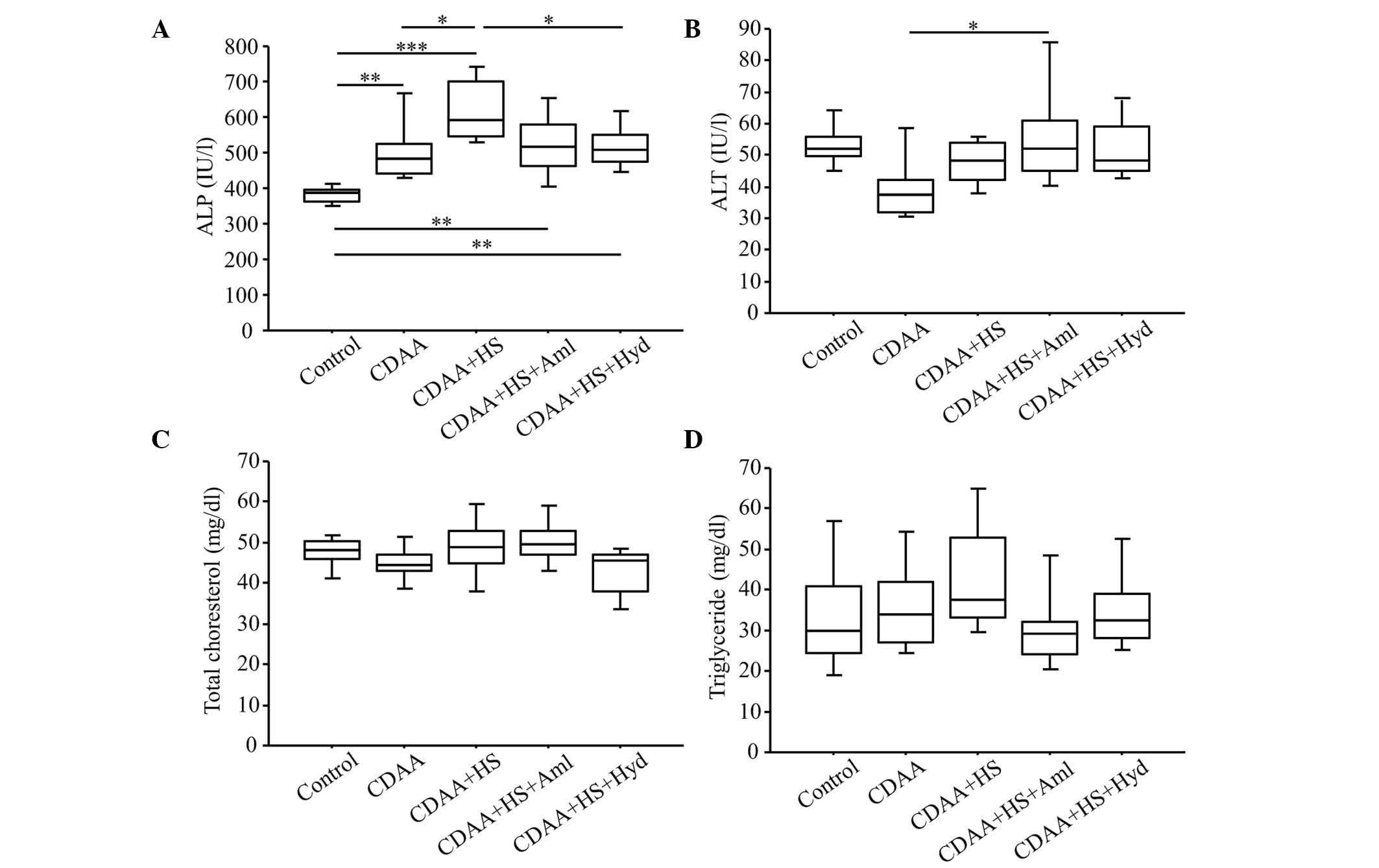 | Figure 3.Serum levels of ALT, ALP, total
cholesterol and triglyceride at week 14 (with or without CDAA, HS
or anti-hypertensive agents). (A) Serum levels of ALP were
significantly higher in the CDAA+HS group, compared with the
Control and CDAA groups. Elevation of serum levels of ALP were
significantly attenuated by antihypertensive therapy. These changes
in ALP were not observed with serum levels of (B) ALT, (C) total
cholesterol or (D) triglyceride. Box plots show the 25th, 50th
(median), and 75th percentiles, with whiskers representing the 10th
and 90th percentiles (n=10 in each group). *P<0.05, **P<0.01
and ***P<0.001. ALT, alanine aminotransferase; ALP, alkaline
phosphatase; CDAA, choline-deficient, L-amino acid-defined diet;
HS, high-salt diet. Aml, amlodipine; Hyd, hydralazine. |
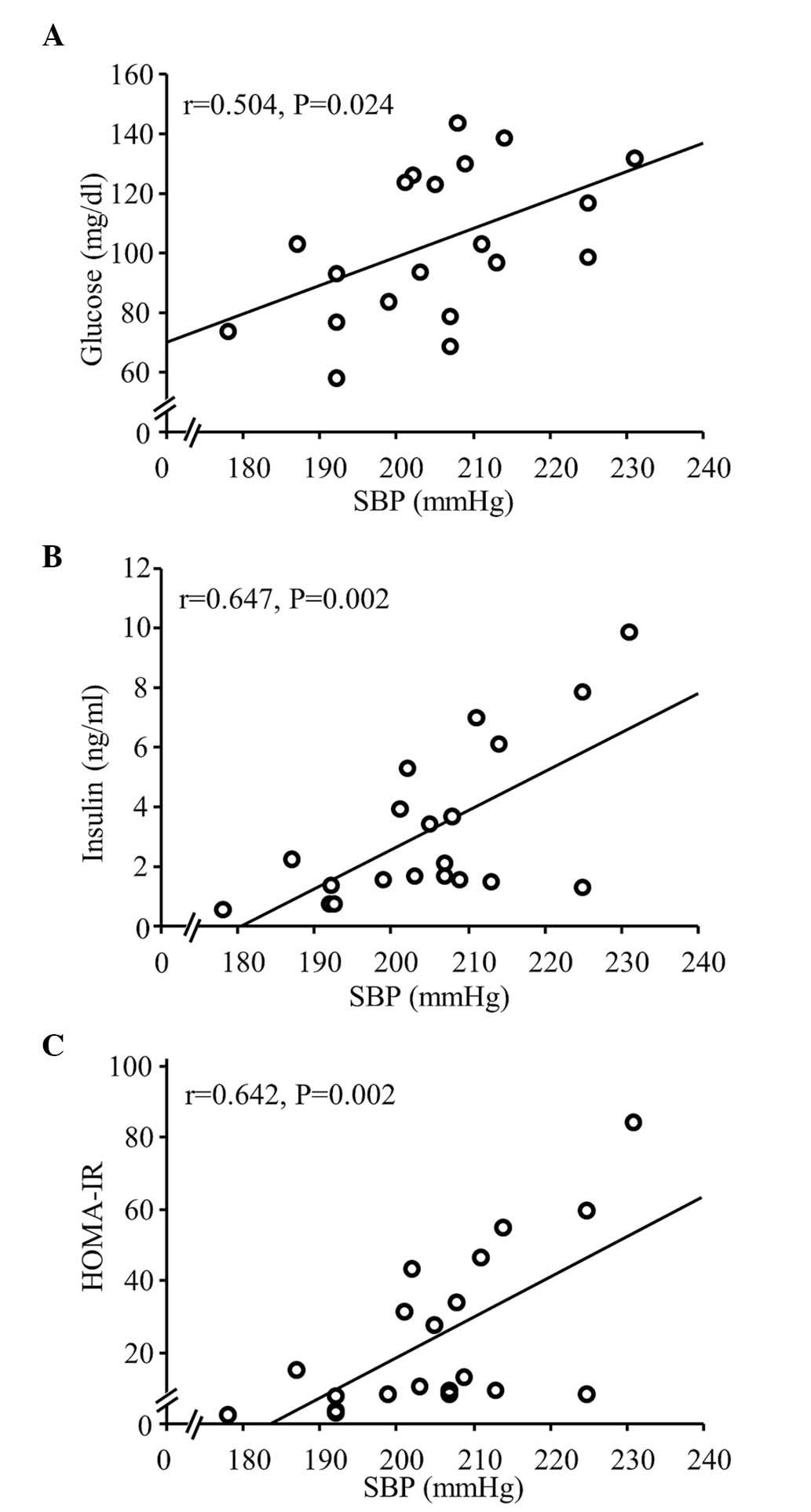
Effects of antihypertensive therapy on
blood chemistry analysis
Our previous study (15) reported that FBG levels were
significantly higher in the HS group, compared with the normal-salt
group. The present study examined the effect of antihypertensive
therapy on IR in SHRs fed a CDAA diet. Serum levels of glucose and
insulin, and HOMA-IR were significantly correlated with SBP among
the rats fed either a normal-salt or HS CDAA diet (Fig. 4), however, the CDAA diet did not
affect these levels in the SHRs (control, vs. CDAA; Fig. 5). In addition, HS increased HOMA-IR
in the SHRs fed a CDAA diet, indicating IR (Fig. 5C). Of note, treatment with the
antihypertensive agents, Aml and Hyd, caused a significant decrease
in serum levels of glucose and insulin (Fig. 5A and B), and significantly
ameliorated HOMA-IR (Fig. 5C).
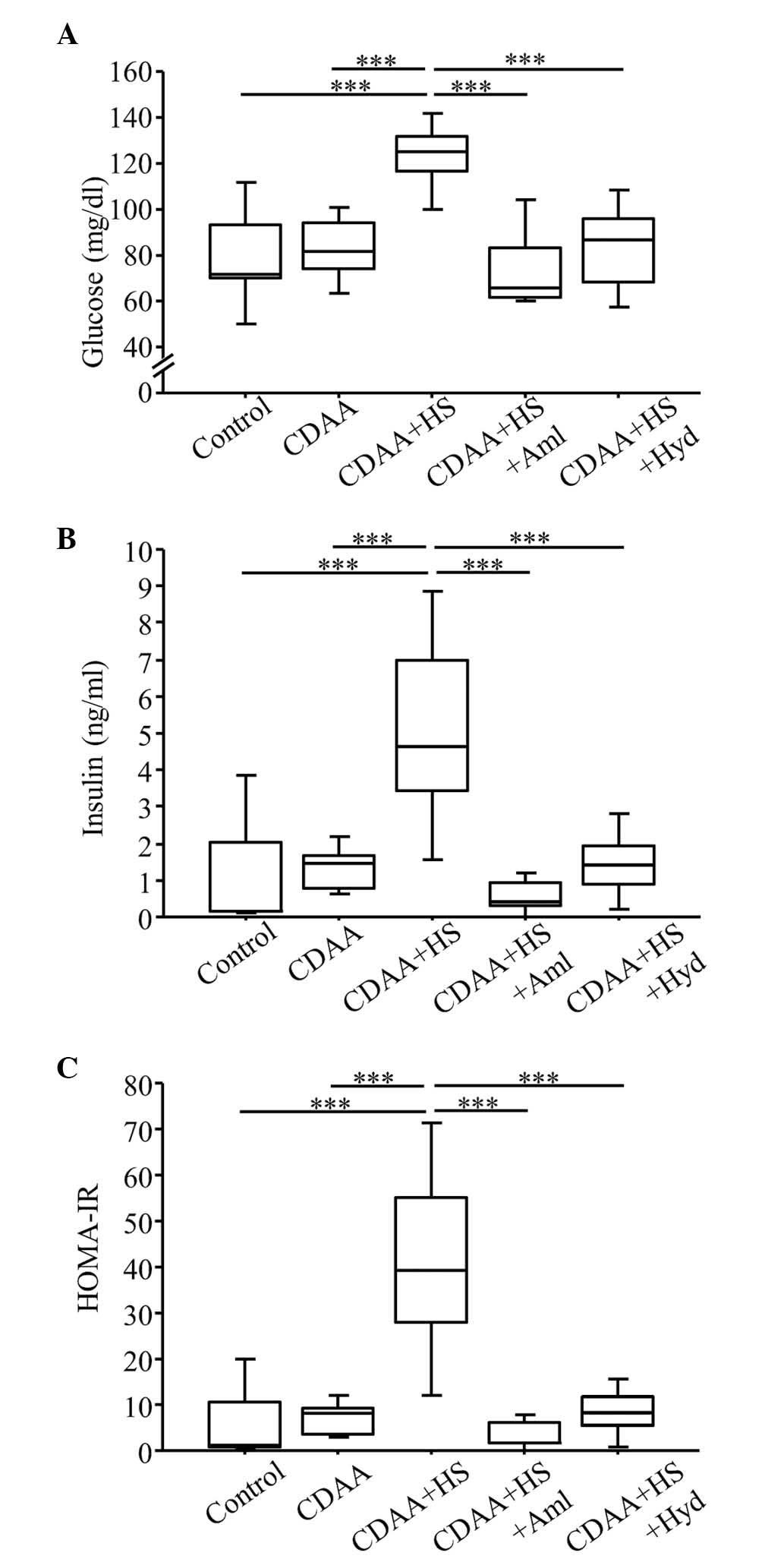 | Figure 5.Fasting blood glucose, serum insulin
and HOMA-IR at week 14 (with or without CDAA, HS or
anti-hypertensive agents). (A) Fasting glucose and (B) serum
insulin in the CDAA+HS group were higher, compared with those in
the Control and CDAA groups. Increased glucose and insulin levels
in the CDAA+HS group were attenuated by antihypertensive therapy
with Aml or Hyd. (C) Similarly, the CDAA+HS diet induced IR, as
assessed by HOMA-IR, and this IR was ameliorated by
antihypertensive therapy. Box plots show the 25th, 50th (median),
and 75th percentiles, with whiskers representing the 10th and 90th
percentiles (n=10 in each group). ***P<0.001. HOMA-IR,
homeostasis model assessment-insulin resistance; CDAA,
choline-deficient, L-amino acid-defined diet; HS, high-salt diet;
Aml, amlodipine; Hyd, hydralazine. |
Antihypertensive therapy reduces
levels of IL-6 and induces levels of IL-10 in SHRs fed a HS CDAA
diet
The serum levels of IL-6 were increased in the SHRs
fed a CDAA diet, and HS further increased these levels (Fig. 6A). By contrast, antihypertensive
therapy with Aml significantly decreased the elevated levels of
serum IL-6 induced by the CDAA and HS diets, and Hyd had a similar,
although less pronounced, effect (Fig.
6A). Neither the normal nor the HS CDAA diet affected serum
levels of IL-10 in the SHRs (Fig.
6B), however, antihypertensive therapy increased the levels of
IL-10 in the HS groups (Fig. 6B).
The number of SHRs with a level of IL-10 >10 pg/ml was
significantly higher when either Aml (n=8; 80%) or Hyd (n=8; 80%)
was administered with a HS CDAA diet, compared with the rats fed
the HS CDAA diet only (n=2; 20%; P<0.01).
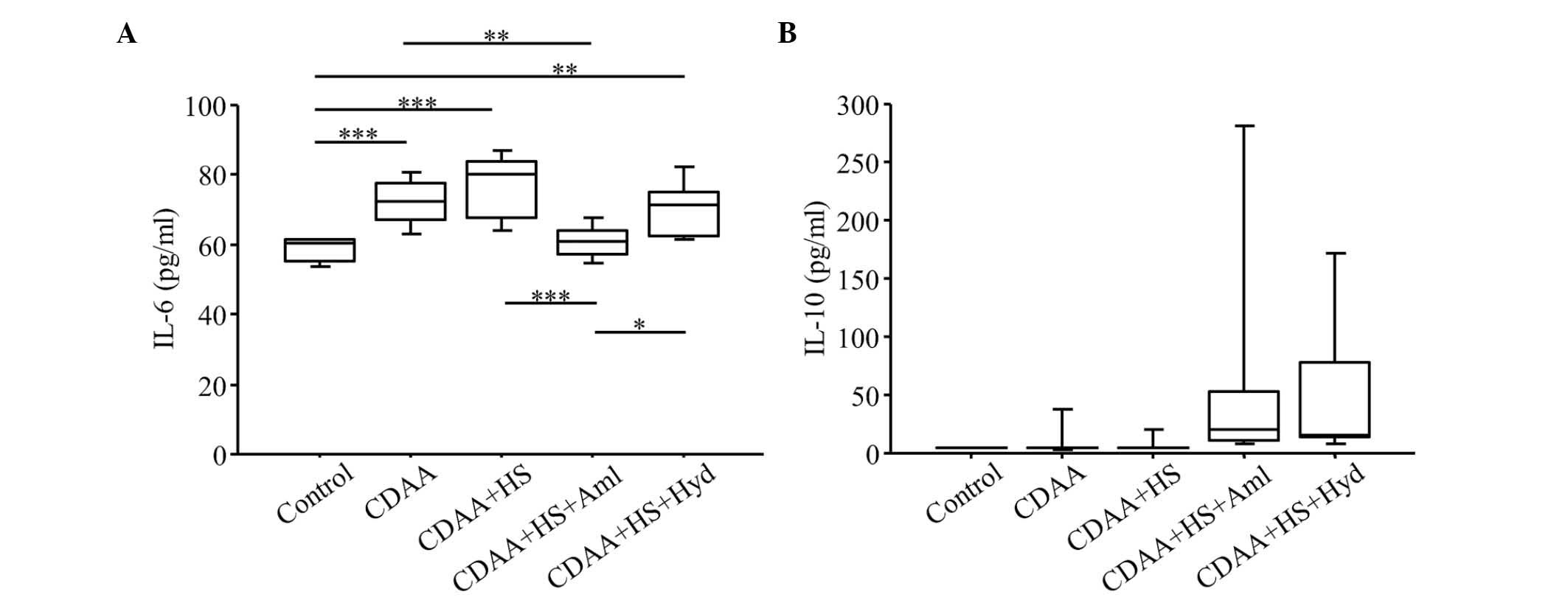 | Figure 6.Serum levels of IL-6 and IL-10. Serum
levels of (A) IL-6 in the CDAA and CDAA+HS groups were higher,
compared with those in the Control group. Increased serum levels of
IL-6 in the CDAA or CDAA+HS groups were attenuated by
antihypertensive therapy with Aml or Hyd. (B) CDAA or CDAA+HS diets
did not affect the serum levels of IL-10, but antihypertensive
therapy increased serum levels of IL-10. Box plots show the 25th,
50th (median), and 75th percentiles, with whiskers representing the
10th and 90th percentiles (n=10 in each group). *P<0.05,
**P<0.01 and ***P<0.001. IL, interleukin; CDAA,
choline-deficient, L-amino acid-defined diet; HS, high-salt diet;
Aml, amlodipine; Hyd, hydralazine. |
Increased CD68+ cells and protein
levels of MCP-1 in the liver are decreased by Aml in SHRs fed a HS
CDAA diet
There was a significant increase in the number of
CD68+ cells in the liver of the SHRs fed a HS CDAA diet,
compared with the control groups (Fig.
7A). By contrast, antihypertensive therapy with Aml tended to
decrease the elevated number of CD68+ cells induced by
the CDAA and HS diets. In addition, hepatic protein levels of MCP-1
in the liver tissue were increased in the SHRs fed a CDAA diet, and
HS further increased these levels (Fig. 7B). Antihypertensive therapy with
Aml significantly decreased the elevated levels of MCP-1 caused by
the CDAA and HS diets.
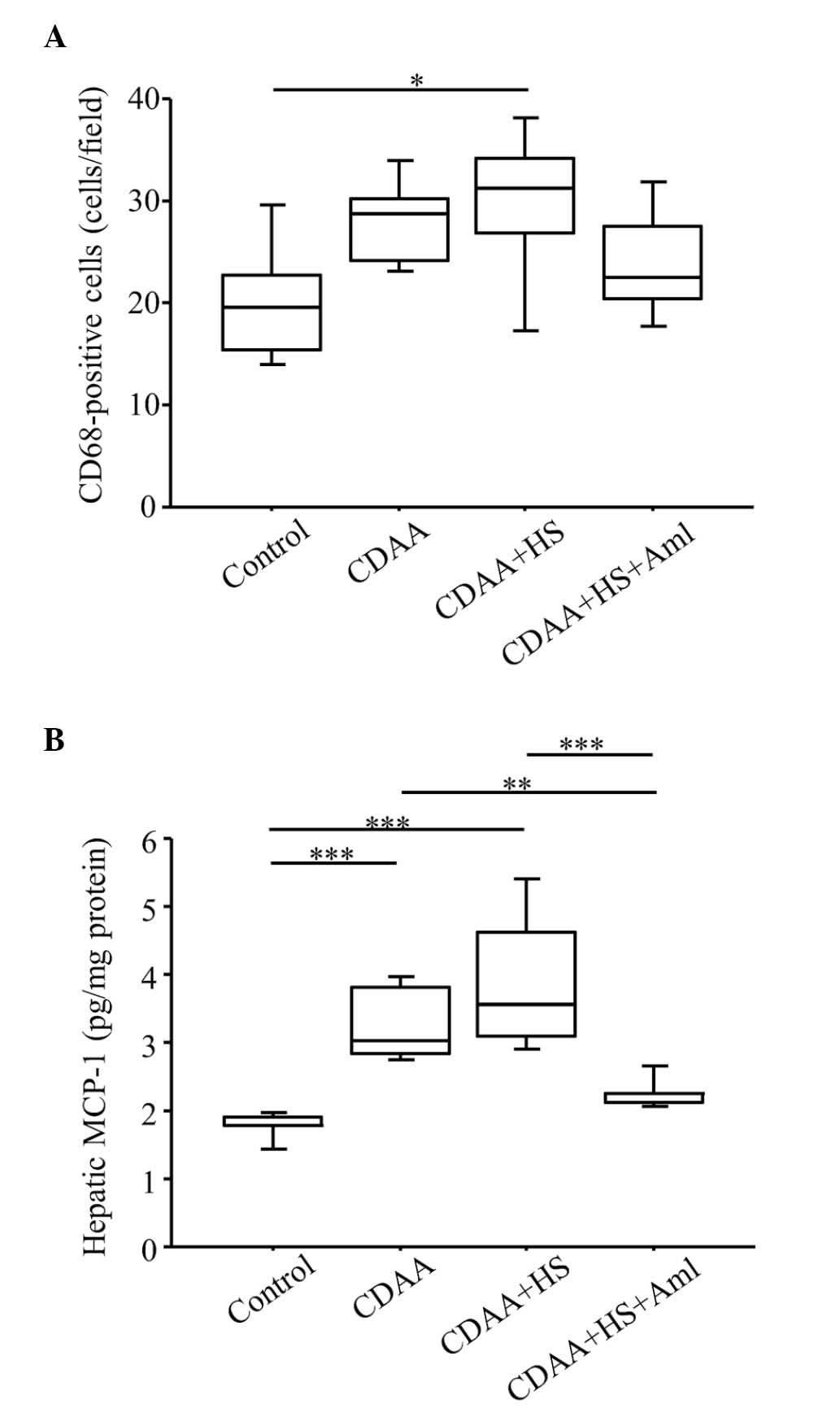 | Figure 7.Quantitation of CD68+
cells and protein expression levels of MCP-1 in the liver. (A)
Numbers of CD68+ cells increased significantly in SHRs
fed a HS CDAA diet, compared with control groups, and increased
numbers of CD68+ cells were decreased by Aml. (B) HS
CDAA diet increased hepatic expression levels of MCP-1 in SHRs, and
Aml significantly attenuated this effect. Box plots show the 25th,
50th (median), and 75th percentiles, with whiskers representing the
10th and 90th percentiles (n=10 in each group). *P<0.05,
**P<0.01 and ***P<0.001. MCP-1, monocyte chemotactic
protein-1; HS, high-salt diet; CDAA, choline-deficient, L-amino
acid-defined diet; Aml, amlodipine. |
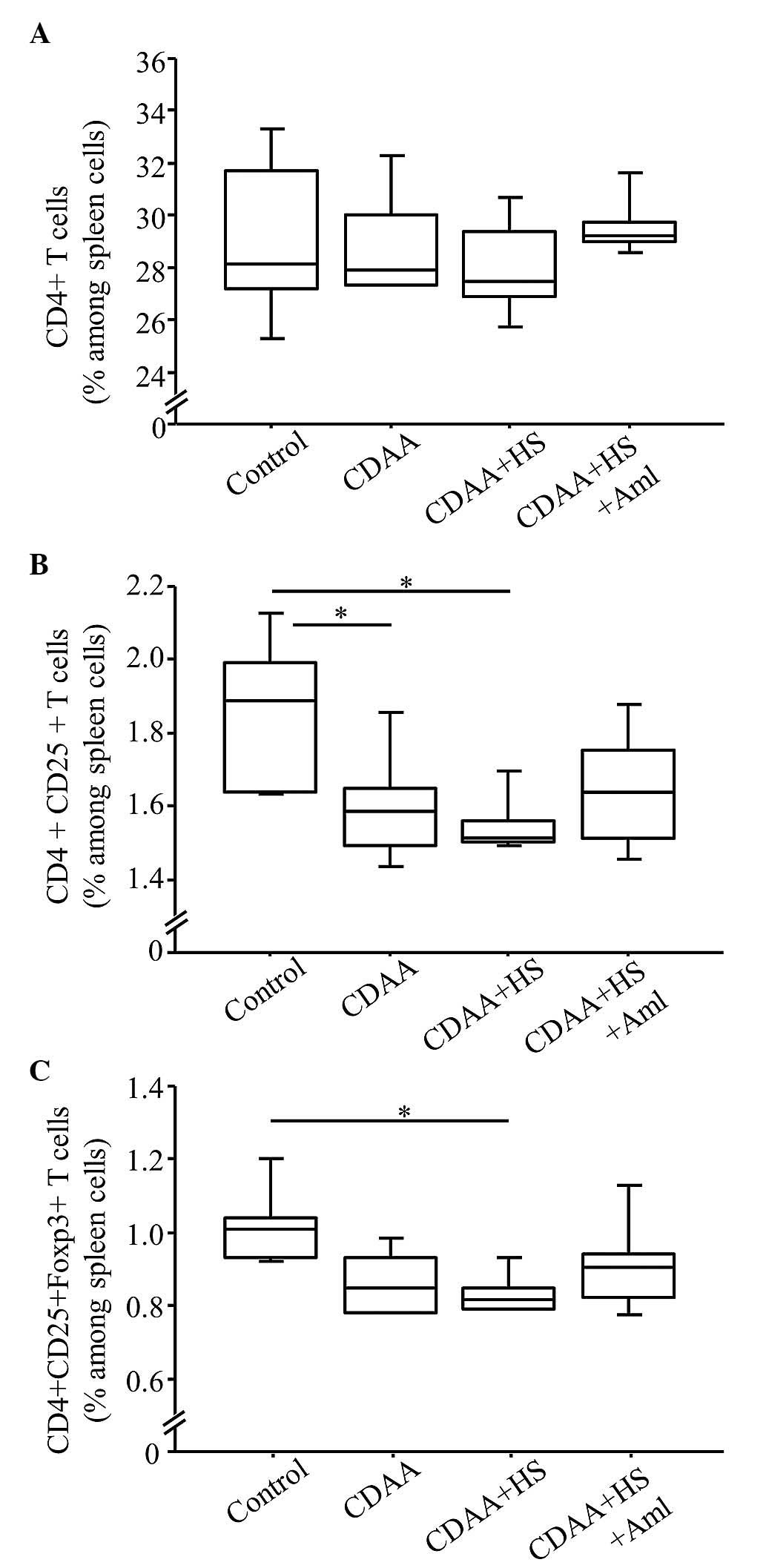
T cell profiles in the spleen of SHRs
fed a CDAA diet
The frequency of CD4+ T cells in the
spleen was similar regardless of diet (Fig. 8A). By contrast, the frequencies of
CD4+CD25+ T cells and
CD4+CD25+Foxp3+ T cells in the
SHRs fed a normal or HS CDAA diet were significantly lower,
compared with those in the control group (Fig. 8B and C). These decreases were
ameliorated by Aml (Fig. 8B and
C), however the differences were not significant.
Discussion
In the present study, a HS diet induced severe
hypertension in SHRs, although SHRs are known to exhibit
hypertension as they age (24).
Furthermore, the combination of the HS-induced hypertension and
CDAA-diet-induced steatohepatitis was associated with increased
serum levels of glucose and insulin, and IR. These responses were
accompanied by high levels of serum IL-6 and hepatic MCP-1 protein,
and low frequencies of CD4+CD25+ and
CD4+CD25+Foxp3+ T cells in the
spleen. Furthermore, antihypertensive therapy reduced the levels of
proinflammatory IL-6 and hepatic MCP-1 protein, and increased the
level of anti-inflammatory IL-10. It also restored the frequencies
of CD4+CD25+ and
CD4+CD25+Foxp3+ T cells, although
the changes in IL-10 and the indicated T cell frequencies were not
significant. These results indicated that hypertension induced by a
HS diet may cause immune-response-mediated IR in patients with
NASH, suggesting one of the molecular mechanisms underlying the
progression of NASH in patients with metabolic syndrome.
Pro-inflammatory cytokines, including IL-6, may be
crucial in the pathogenesis and development of hypertension
(25). However, it was previously
reported that the deletion of IL-6 did not affect blood pressure in
hypertension induced by a combination of angiotensin II and a HS
diet (26). In the present study
involving SHRs, a CDAA diet affected the serum levels of IL-6, but
did not affect blood pressure. The effect of IL-6 on hypertension
may vary among animal models and physiological conditions, and this
effect may not be present in the experimental model. By contrast,
the present study found that the overexpression of IL-6 induced by
a HS CDAA diet was reduced by antihypertensive therapy. Serum
levels of IL-10 were increased by antihypertensive therapy, but
were not affected by a HS diet. Therefore, pathways other than
those involving the IL-6 signaling may have been active in
HS-induced hypertension in the present study. The reduction of IL-6
may be associated with the overexpression of IL-10 caused by
antihypertensive therapy, and these cytokine expression patterns
may affect systemic pathogenesis, including IR, in NASH.
Systemic inflammation is a characteristic feature of
metabolic syndrome and CVD. A common serum or plasma marker of
systemic inflammation is C-reactive protein (CRP) (27). It has been reported that
high-sensitivity CRP is an independent predictor of the risk of
cardiovascular events (28).
Furthermore, IL-6 has been reported to be an independent predictor
of future cardiovascular events in high-risk Japanese patients
(29). The most marked
correlations between serum levels of CRP and IL-6 are observed in
men with angiographically-documented coronary heart disease
(30). Serum levels of IL-6 and
high-sensitivity CRP are also shown to be higher in patients with
NAFLD, compared with healthy controls (17,31).
Therefore, the present study hypothesized that NAFLD-associated
IL-6 contributes to hypertensive heart disease, which can result in
extrahepatic disease-associated mortality. Longitudinal
investigations are required to investigate whether the inflammatory
cytokines associated with hypertension affect mortality rates in
patients with NAFLD.
It was previously reported that the short-term
infusion of IL-6 does not induce IR or impair insulin signaling in
rats (32). By contrast, IL-6 has
been reported to induce IR in 3T3-L1 adipocytes and be
overexpressed in human fat cells from subjects with IR (33). Chronic IL-6 treatment increases the
secretion of IL-6 and induces IR in adipocytes (34). In addition, the depletion of IL-6
improves insulin responsiveness in insulin tolerance tests in mice
with diet-induced obesity (35).
Long-term exposure to high levels of IL-6 may be required prior to
IR being affected. Furthermore, Klover et al (36) suggested that the major targets for
cross-talk between IL-6 and insulin may be adipose tissue and the
liver, rather than skeletal muscle. In the present study, insulin
signaling, assessed by the phosphorylation of insulin receptor
substrate-1 in liver tissue, was inhibited in the HS CDAA group,
compared with the controls. This inhibition was attenuated by
antihypertensive therapy, which was accompanied by lower serum
levels of IL-6 (data not shown). Thus, although the entire
molecular mechanism remains to be fully elucidated, IL-6 and IR in
the liver were linked in the present study, and may affect
pathological hepatic conditions, including hepatic fibrosis
(22).
IL-10 is known to have anti-inflammatory effects in
various organs and tissues under pathological conditions, however,
studies investigating the effect of endogenous IL-10 in the
pathogenesis of NAFLD are limited (37). Kim et al (38) found that treatment with IL-10
prevents IL-6-induced defects in hepatic insulin action and
signaling activity. Hong et al (39) showed that transgenic mice with
muscle-specific overexpression of IL-10 are protected from
diet-induced IR in skeletal muscle, and this is associated with
reduced levels of cytokines, including IL-6, in the skeletal
muscle. In another study, endogenous IL-10 inhibition was found to
impair insulin signaling and promote the increased expression of
inflammatory cytokines, including IL-6 in an animal model of liver
disease induced by a high-fat diet (19). The above findings are consistent
with the observations in the present study, that the induction of
IL-10 and suppression of IL-6 by antihypertensive therapy were at
least partly associated with improvement of IR.
It was previously reported that mice lacking T and B
cells (Rag1−/−mice) do not develop
hypertension, indicating that T cells are important in the genesis
of hypertension (40). Matrougui
et al (41) showed that
hypertension is associated with increased numbers of apoptotic
regulatory T cells (Tregs) in the spleen and a reduction in plasma
IL-10 content, and that the transfer of Tregs to hypertensive
animals reduces blood pressure and inhibits the reduction in serum
levels of IL-10. Furthermore, Eller et al (42) indicated that Tregs are key
regulatory cells in the pathogenesis of IR, and that intravenous
transfer of Tregs improves IR in vivo. In the present study,
the frequencies of CD4+CD25+ and
CD4+CD25+Foxp3+ T cells in the
spleen were significantly reduced by a HS CDAA diet in the SHRs,
and these reductions were relatively attenuated by antihypertensive
therapy accompanied by high serum levels of IL-10. Thus, Tregs may
be involved in the pathogenesis of IR in this model of HS-induced
hypertension with steatohepatitis.
MCP-1, which is referred to as chemokine (C-C motif)
ligand (CCL)2, is a potent chemoattractant, which is primarily
secreted by macrophages. CCL2 has also been found to be upregulated
in the livers of animals with high-fat diet-induced NASH (43). Obstfeld et al (44) showed that obesity activates the
hepatocyte expression of CCL2/MCP-1, leading to hepatic recruitment
of CCR2+ myeloid cells, which promote hepatosteatosis.
By contrast, CCL2 deletion in an experimental model of
methionine-choline-deficient diet-induced steatosis did not improve
liver fat accumulation or associated inflammation (45). In the present study, neither
hypertension induced by a HS diet nor antihypertensive therapy
affected hepatic steatosis induced by a CDAA diet (data not shown).
By contrast, our previous study found that long-term hypertension
induced by a HS diet exacerbated hepatic fibrosis induced by a CDAA
diet (15). In the present study,
the hepatic overexpression of MCP-1 was accompanied by an increased
number of CD68+ cells, indicating the presence of
macrophages in the liver. Marra and Tacke (46) also suggested that during the
development of NASH, CCL2 and its receptor are upregulated in the
liver, where they promote macrophage accumulation, inflammation and
fibrosis. Therefore, the present study hypothesized that the
hepatic expression of MCP-1 is associated with hepatic inflammation
and fibrosis rather than hepatic steatosis.
The present study had several limitations. First,
the control rats were fundamentally hypertensive, regardless of
salt concentrations in the diet. This hypertension may have
affected several measurements in the controls, including the levels
of ALT, IL-6 and IL-10, and this may have masked the differences
between the normal and HS groups. Further experiments using other
hypertension models are required to confirm the data. Secondly, the
observed changes in the frequencies of T cell subpopulations in the
spleen appeared to be too small to explain the differences in IL-6
and IL-10 between the CDAA+HS and CDAA+HS+Aml groups. T cell
subpopulations in other tissues, including adipose tissue and the
liver require consideration. Thirdly, antihypertensive therapy did
not decrease the levels of ALT, although these levels were
associated with blood pressure. However, serum levels of ALT do not
always reflect the severity of steatohepatitis induced by MCD diets
in mice (47). The number of
CD68+ cells in the liver and the hepatic expression of
MCP-1 may be more useful markers to evaluate improvements of
steatohepatitis severity in the present study. Finally, the present
study did not identify a direct association between cytokines, IR
and hypertension, and did not investigate long-term hepatic
fibrosis. However, our previous study showed that hypertension
affected hepatic fibrosis, and the present study indicated that
hypertension in the context of steatohepatitis was possibly
associated with IR mediated through cytokine imbalance; these
abnormalities affect the progression of steatohepatitis, which is
associated with hepatic fibrosis. The experimental model used in
the present study is likely to be a useful model of human NASH with
metabolic syndrome.
In conclusion, the present study demonstrated that
rats with HS-induced hypertension developed IR, which may have been
associated with an imbalance of IL-6 and IL-10, suppression of
Tregs in the spleen and hepatic levels of MCP-1. These results may
indicate the importance of cytokines and Tregs in the pathogenesis
of IR in NASH with hypertension, and may lead to novel therapeutic
concepts for the treatment of metabolic syndrome with NASH.
Acknowledgements
The authors would like to thank Yuko Morinaga,
Etsuko Horiguchi and Ayaka Hamabe for their technical assistance.
This study was supported in part by grants from the Ministry of
Education, Culture, Sports, Science and Technology of Japan (grant
no. 23590981) and the Takeda Science Foundation.
References
|
1
|
Oda K, Uto H, Mawatari S and Ido A:
Clinical features of hepatocellular carcinoma associated with
nonalcoholic fatty liver disease: A review of human studies. Clin J
Gastroenterol. 8:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe S, Hashimoto E, Ikejima K, Uto H,
Ono M, Sumida Y, Seike M, Takei Y, Takehara T, Tokushige K, et al:
Evidence-based clinical practice guidelines for nonalcoholic fatty
liver disease/nonalcoholic steatohepatitis. J Gastroenterol.
50:364–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ: The diagnosis and
management of non-alcoholic fatty liver disease: Practice guideline
by the American Association for the Study of Liver Diseases,
American College of Gastroenterology and the American
Gastroenterological Association. Hepatology. 55:2005–2023. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Angulo P: Long-term mortality in
nonalcoholic fatty liver disease: Is liver histology of any
prognostic significance? Hepatology. 51:373–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Charlton MR, Burns JM, Pedersen RA, Watt
KD, Heimbach JK and Dierkhising RA: Frequency and outcomes of liver
transplantation for nonalcoholic steatohepatitis in the United
States. Gastroenterology. 141:1249–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tilg H and Moschen AR: Evolution of
inflammation in nonalcoholic fatty liver disease: The multiple
parallel hits hypothesis. Hepatology. 52:1836–1846. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Than NN and Newsome PN: A concise review
of non-alcoholic fatty liver disease. Atherosclerosis. 239:192–202.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewington S, Clarke R, Qizilbash N, Peto R
and Collins R: Prospective Studies Collaboration: Age-specific
relevance of usual blood pressure to vascular mortality: a
meta-analysis of individual data for one million adults in 61
prospective studies. Lancet. 360:1903–1913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yusuf S, Hawken S, Ounpuu S, Dans T,
Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J and
Lisheng L: INTERHEART Study Investigators: Effect of potentially
modifiable risk factors associated with myocardial infarction in 52
countries (the INTERHEART study): Case-control study. Lancet.
364:937–952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Donnell MJ, Xavier D, Liu L, Zhang H,
Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ,
et al: INTERSTROKE investigators: Risk factors for ischaemic and
intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE
study): A case-control study. Lancet. 376:112–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamabe A, Uto H, Imamura Y, Kusano K,
Mawatari S, Kumagai K, Kure T, Tamai T, Moriuchi A, Sakiyama T, et
al: Impact of cigarette smoking on onset of nonalcoholic fatty
liver disease over a 10-year period. J Gastroenterol. 46:769–778.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marchesini G, Brizi M, Morselli-Labate AM,
Bianchi G, Bugianesi E, McCullough AJ, Forlani G and Melchionda N:
Association of nonalcoholic fatty liver disease with insulin
resistance. Am J Med. 107:450–455. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brea A and Puzo J: Non-alcoholic fatty
liver disease and cardiovascular risk. Int J Cardiol.
167:1109–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donati G, Stagni B, Piscaglia F, Venturoli
N, Morselli-Labate AM, Rasciti L and Bolondi L: Increased
prevalence of fatty liver in arterial hypertensive patients with
normal liver enzymes: Role of insulin resistance. Gut.
53:1020–1023. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arima S, Uto H, Ibusuki R, Kumamoto R,
Tanoue S, Mawatari S, Oda K, Numata M, Fujita H, Oketani M, et al:
Hypertension exacerbates liver injury and hepatic fibrosis induced
by a choline-deficient L-amino acid-defined diet in rats. Int J Mol
Med. 33:68–76. 2014.PubMed/NCBI
|
|
16
|
Yi B, Titze J, Rykova M, Feuerecker M,
Vassilieva G, Nichiporuk I, Schelling G, Morukov B and Choukèr A:
Effects of dietary salt levels on monocytic cells and immune
responses in healthy human subjects: A longitudinal study. Transl
Res. 166:103–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haukeland JW, Damås JK, Konopski Z, Løberg
EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K and
Aukrust P: Systemic inflammation in nonalcoholic fatty liver
disease is characterized by elevated levels of CCL2. J Hepatol.
44:1167–1174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wieckowska A, Papouchado BG, Li Z, Lopez
R, Zein NN and Feldstein AE: Increased hepatic and circulating
interleukin-6 levels in human nonalcoholic steatohepatitis. Am J
Gastroenterol. 103:1372–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cintra DE, Pauli JR, Araújo EP, Moraes JC,
de Souza CT, Milanski M, Morari J, Gambero A, Saad MJ and Velloso
LA: Interleukin-10 is a protective factor against diet-induced
insulin resistance in liver. J Hepatol. 48:628–637. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esposito K, Pontillo A, Giugliano F,
Giugliano G, Marfella R, Nicoletti G and Giugliano D: Association
of low interleukin-10 levels with the metabolic syndrome in obese
women. J Clin Endocrinol Metab. 88:1055–1058. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Braunersreuther V, Viviani GL, Mach F and
Montecucco F: Role of cytokines and chemokines in non-alcoholic
fatty liver disease. World J Gastroenterol. 18:727–735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hui JM, Hodge A, Farrell GC, Kench JG,
Kriketos A and George J: Beyond insulin resistance in NASH:
TNF-alpha or adiponectin? Hepatology. 40:46–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sánchez-Lozada LG, Mu W, Roncal C, Sautin
YY, Abdelmalek M, Reungjui S, Le M, Nakagawa T, Lan HY, Yu X and
Johnson RJ: Comparison of free fructose and glucose to sucrose in
the ability to cause fatty liver. Eur J Nutr. 49:1–9. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Varagic J, Ahmad S, Voncannon JL, Moniwa
N, Simington SW Jr, Brosnihan BK, Gallagher PE, Habibi J, Sowers JR
and Ferrario CM: Nebivolol reduces cardiac angiotensin II,
associated oxidative stress and fibrosis but not arterial pressure
in salt-loaded spontaneously hypertensive rats. J Hypertens.
30:1766–1774. 2012.PubMed/NCBI
|
|
25
|
Jurewicz M, McDermott DH, Sechler JM,
Tinckam K, Takakura A, Carpenter CB, Milford E and Abdi R: Human T
and natural killer cells possess a functional renin-angiotensin
system: Further mechanisms of angiotensin II-induced inflammation.
J Am Soc Nephrol. 18:1093–1102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
González GE, Rhaleb NE, D'Ambrosio MA,
Nakagawa P, Liu Y, Leung P, Dai X, Yang XP, Peterson EL and
Carretero OA: Deletion of interleukin-6 prevents cardiac
inflammation, fibrosis and dysfunction without affecting blood
pressure in angiotensin II-high salt-induced hypertension. J
Hypertens. 33:144–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bassuk SS, Rifai N and Ridker PM:
High-sensitivity C-reactive protein: Clinical importance. Curr
Probl Cardiol. 29:439–493. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ridker PM, Hennekens CH, Buring JE and
Rifai N: C-reactive protein and other markers of inflammation in
the prediction of cardiovascular disease in women. N Engl J Med.
342:836–843. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishida H, Horio T, Suzuki Y, Iwashima Y,
Tokudome T, Yoshihara F, Nakamura S and Kawano Y: Interleukin-6 as
an independent predictor of future cardiovascular events in
high-risk Japanese patients: Comparison with C-reactive protein.
Cytokine. 53:342–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rifai N, Joubran R, Yu H, Asmi M and Jouma
M: Inflammatory markers in men with angiographically documented
coronary heart disease. Clin Chem. 45:1967–1973. 1999.PubMed/NCBI
|
|
31
|
Riquelme A, Arrese M, Soza A, Morales A,
Baudrand R, Pérez-Ayuso RM, González R, Alvarez M, Hernández V,
García-Zattera MJ, et al: Non-alcoholic fatty liver disease and its
association with obesity, insulin resistance and increased serum
levels of C-reactive protein in Hispanics. Liver Int. 29:82–88.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rotter Sopasakis V, Larsson BM, Johansson
A, Holmäng A and Smith U: Short-term infusion of interleukin-6 does
not induce insulin resistance in vivo or impair insulin signalling
in rats. Diabetologia. 47:1879–1887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rotter V, Nagaev I and Smith U:
Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1
adipocytes and is, like IL-8 and tumor necrosis factor-alpha,
overexpressed in human fat cells from insulin-resistant subjects. J
Biol Chem. 278:45777–45784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lagathu C, Bastard JP, Auclair M, Maachi
M, Capeau J and Caron M: Chronic interleukin-6 (IL-6) treatment
increased IL-6 secretion and induced insulin resistance in
adipocyte: Prevention by rosiglitazone. Biochem Biophys Res Commun.
311:372–379. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klover PJ, Clementi AH and Mooney RA:
Interleukin-6 depletion selectively improves hepatic insulin action
in obesity. Endocrinology. 146:3417–3427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klover PJ, Zimmers TA, Koniaris LG and
Mooney RA: Chronic exposure to interleukin-6 causes hepatic insulin
resistance in mice. Diabetes. 52:2784–2789. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meda C: The impact of cytokines and
chemokines on non-alcoholic fatty liver disease (NAFLD). Biotech
Mol Biol Nanomed. 2:15–16. 2014.
|
|
38
|
Kim HJ, Higashimori T, Park SY, Choi H,
Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB and Kim JK:
Differential effects of interleukin-6 and −10 on skeletal muscle
and liver insulin action in vivo. Diabetes. 53:1060–1067. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu
TY, Friedline RH, Kurt-Jones E, Finberg R, Fischer MA, et al:
Interleukin-10 prevents diet-induced insulin resistance by
attenuating macrophage and cytokine response in skeletal muscle.
Diabetes. 58:2525–2535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guzik TJ, Hoch NE, Brown KA, McCann LA,
Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG: Role of
the T cell in the genesis of angiotensin II induced hypertension
and vascular dysfunction. J Exp Med. 204:2449–2460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matrougui K, Abd Elmageed Z, Kassan M,
Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P,
Belmadani S and Partyka M: Natural regulatory T cells control
coronary arteriolar endothelial dysfunction in hypertensive mice.
Am J Pathol. 178:434–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eller K, Kirsch A, Wolf AM, Sopper S,
Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR and Eller P:
Potential role of regulatory T cells in reversing obesity-linked
insulin resistance and diabetic nephropathy. Diabetes.
60:2954–2962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ito M, Suzuki J, Tsujioka S, Sasaki M,
Gomori A, Shirakura T, Hirose H, Ito M, Ishihara A, Iwaasa H and
Kanatani A: Longitudinal analysis of murine steatohepatitis model
induced by chronic exposure to high-fat diet. Hepatol Res.
37:50–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Obstfeld AE, Sugaru E, Thearle M,
Francisco AM, Gayet C, Ginsberg HN, Ables EV and Ferrante AW Jr:
C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment
of myeloid cells that promote obesity-induced hepatic steatosis.
Diabetes. 59:916–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kassel KM, Guo GL, Tawfik O and Luyendyk
JP: Monocyte chemoattractant protein-1 deficiency does not affect
steatosis or inflammation in livers of mice fed a
methionine-choline-deficient diet. Lab Invest. 90:1794–1804. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Marra F and Tacke F: Roles for chemokines
in liver disease. Gastroenterology. 147:577–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Itagaki H, Shimizu K, Morikawa S, Ogawa K
and Ezaki T: Morphological and functional characterization of
non-alcoholic fatty liver disease induced by a
methionine-choline-deficient diet in C57BL/6 mice. Int J Clin Exp
Pathol. 6:2683–2696. 2013.PubMed/NCBI
|