Introduction
Cervical cancer is the fourth most common malignancy
in women globally (1,2). Due to increased human papillomavirus
infection rates, there were 450,000 new cases of cervical cancer
worldwide and ~20,000 women died from cervical cancer in 2014
(3,4). In China, ~131,500 new cases are
diagnosed each year, accounting for ~1/3 of the world's new cases
(5,6). With improvements in diagnosis and
treatment, the incidence and mortality of cervical cancer has
significantly reduced, but has become more prevalent in younger age
categories, with the number of patients <35 years old with
cervical cancer significantly increased (7). The Pacific region has been
particularly affected by increased rates of cervical cancer, with
current age standardized incidence rates ranging from 8.2 to 50.7
per 100,000 women per year, and age standardized mortality rates
ranging from 2.7 to 23.9 per 100,000 women per year (8).
Surgery or radiation therapy are the main treatments
for cervical cancers, and traditional methods of treatment achieve
higher cure rates for cervical cancer patients with early diagnosis
(9). The overall 5-year survival
rate is ~70%, and for patients without lymph node metastasis the
5-year survival rate is 80–90% (10). Surgical treatment is mainly used
for patients with early-stage cervical cancer, but for patients
with advanced cervical cancer, surgical treatment is usually
combined with radiotherapy and chemotherapy (7). Chemotherapeutic treatment primarily
comprises platinum based antineoplastic drugs, such as cisplatin
and carboplatin, which are important anticancer drugs for
gynecological malignancies (10,11).
Although cisplatin and carboplatin are both platinum-based, their
ability to kill tumor cells and the side effects they elicit in
normal tissues differ significantly (11). Thus, they cannot be substituted for
each other in clinical applications. Currently, cisplatin is used
more commonly than carboplatin, but cisplatin has greater side
effects and increased toxicity, particularly renal toxicity
(12). However, the greatest
obstacle is resistance to chemotherapeutic drugs in patients with
cervical cancer (13). Enhanced
drug sensitivity and reversed drug resistance in cervical cancer
cells is, therefore, an important area of research.
High mobility group box 1 (HMGB1) is a highly
conserved chromosomal protein that acts as a DNA chaperone
(14). It was first discovered in
the calf thymus, and named according to its high electrophoretic
mobility in polyacrylamide gels (15). HMGB1 is reported to influence
transcription of cytokines, chemokines, and growth factors and
works as a prototypical damage-associated molecular pattern in
initiating and perpetuating inflammatory responses (16). It is involved in regulating
multiple signaling pathways, including inflammation, genome
stability, cell survival, metastasis, cell apoptosis and, in
particular, cell autophagy (17).
Previous studies have revealed a paradoxical dual effect of
autophagy in cancer development and progression. Lipidated
microtubule associated protein 1 light chain 3 (LC3)-II is a
reliable marker for autophagy (18,19).
p62 is a selective autophagy substrate that recognizes
ubiquitinated proteins to the autophagosome for degradation. HMGB1
was previously thought to be an oncogene, promoting cell growth and
metastasis of cancers (15).
However, the associations between HMGB1 expression and
chemoresistance, and the underlying molecular mechanisms, were not
entirely elucidated. Chemotherapy drugs including doxorubicin,
cisplatin and methotrexate induce HMGB1 upregulation in human
osteosarcoma cells, and knockdown of HMGB1 successfully restored
chemosensitivity to osteosarcoma cells in vivo and in
vitro (20).
Xie et al (21) also demonstrated that HMGB1 gene
silencing can enhance the sensitivity of K562/A02 drug resistant
leukemia cells to doxorubicin and reverse cell resistance to
doxorubicin. However, the molecular mechanisms of HMGB1-associated
drug resistance in cervical cancer cells remained unclear. In the
present study, in order to explore the relationship between HMGB1
expression and chemotherapy drug resistance, the
cisplatin-sensitive HeLa cells and cisplatin-resistant HeLa/DDP
cells were used as models.
Materials and methods
Cell lines and reagents
Human cervical carcinoma HeLa cells and
cisplatin-resistant HeLa cells (HeLa/DDP) were obtained from
Shenglong Biological Corporation (Shanghai, China). Cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml
ampicillin and 50 µg/ml streptomycin at 37°C in a humidified
atmosphere of 95% air and 5% CO2. Cisplatin, ethyl
pyruvate (EP) and MTT were obtained from Sigma Aldrich, Merck
Millipore (Darmstadt, Germany). Recombinant human (rh) HMGB1/Fc was
obtained from Sino Biological Inc. (Beijing, China; cat. no.
10326-H01H). The human HMGB1 short hairpin (sh) RNAs (cat. no.
TG316576) and negative control (NC) shRNA (cat. no. TR30013) were
obtained from OriGene Technologies, Inc. (Rockville, MD, USA).
Antibodies
β-actin antibody was purchased from TransBionovo
Co., Ltd. (Beijing, China; cat. no. HC201; 1:1,000). Antibodies for
caspase-3 (cat. no. 9662), cleaved (c)-caspase-3 (cat. no. 9661),
poly ADP ribose polymerase (PARP; cat. no. 9542), c-PARP (cat. no.
9541), extracellular signal-regulated kinase 1/2 (ERK1/2; cat. no.
9102) and phosphorylated (p)-ERK1/2 (cat. no. 8544) were obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA) and all
diluted 1:1,000. β-Tubulin antibody (H-235), a rabbit polyclonal
IgG provided at 200 µg/ml (cat. no. sc-9104; 1:1,000), was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Anti-lamin B1 (cat. no. ab16048; 1:1,000) and anti-HMGB1 (cat. no.
ab18256; 1:1,000), both rabbit polyclonal antibodies, were obtained
from Abcam (Cambridge, UK). LC3B/MAP1LC3B antibody (cat. no.
NB100-2220; 1:1,000) was purchased from Novus Biologicals, LLC
(Littleton, CO, USA).
Cell transfection
HeLa, SiHa and HeLa/DDP cells were plated into
48-well plates (5×105 cells/well) and incubated for 6 h
prior to transfection with HMGB1 shRNA or NC shRNA using
Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific Inc.) according to the manufacturer's protocol. The
cells were then cultured for the indicated times before being
subjected to MTT assays or western blotting analysis.
Annexin V-fluorescein isothiocyanate
(FITC) dual staining analysis
Annexin V-FITC apoptosis detection kit (catalog no.
ab14085) was obtained from Abcam; 2×105 cells were
trypsinized into a single cell suspension. Resuspended cells were
incubated with Annexin V-FITC (0.1 µg/µl) for 15 min in the dark on
ice. Propidium iodide (0.05 µg/µl) was then added and used as a
counterstain to discriminate between necrotic and apoptotic cells.
Fluorescence-activated cell sorting (FACS) analysis was then
performed and data was analyzed using FlowJo 10 software (FlowJo,
LLC, Ashland, OR, USA).
Preparation of subcellular fractions
and western blot analysis
HeLa cells and SiHa cells (2×105
cells/well) were plated into 48-well plates. Six hours later, the
cells were treated with 10 µg/ml of cisplatin for 24 and 48 h,
respectively. Cytosolic extracts and nuclear extracts were prepared
using a Nuclear and Cytoplasmic Protein Extraction kit (catalog no.
P0028; Beyotime, Institute of Biotechnology, Shanghai, China)
according to the manufacturer's protocols. Protein concentrations
of the extracts were measured by bicinchoninic acid assay (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts (15 µg) of the
proteins were loaded and subjected to 10% SDS-PAGE and transferred
onto nitrocellulose membranes. The membrane was blocked with 5%
bovine serum albumin (Sigma-Aldrich; Merck Millipore) in
Tris-buffered saline-0.1% Tween-20 (TBST) buffer for 40 min at room
temperature. Membranes were then incubated with the indicated
primary antibodies overnight at 4°C and secondary antibodies for 40
min at room temperature. The membranes were washed three times in
each step for 5 min with TBST buffer. The bands were then
visualized using Pierce ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.). The bands were captured and analyzed with
ImageJ software (National Institutes of Health, Bethesda, MD, USA)
and the expression of HMGB1 was normalized to β-actin.
MTT assay
The tumor inhibition rates of cisplatin and EP on
HeLa cells and HeLa/DPP cells were detected by MTT assay. The
cervical cancer cells were plated into 96-well plates
(2×104 cells/well) and cultured for 6 h in DMEM. They
were subsequently treated with the 10 µg/ml cisplatin and
stimulated for 24, 48 and 72 h, respectively. MTT (5 mg/ml) was
added into the medium and 150 µl DMSO was used to dissolve the
formazan crystals. The 96-well plates were read on a microplate
reader at a test wavelength of 490 nm (A490).
The growth inhibition rate was calculated as
follows: growth inhibition rate (%) = (average A490 of
the control group - average A490 value of the
experimental group)/average A490 value of the control
group × 100%.
Statistical analysis
All of the data were analyzed by SPSS 19.0 software
(SPSS Inc., Chicago, IL, USA) and are presented as the mean ±
standard deviations. P<0.05 was considered to indicate a
statistically significant difference.
Results
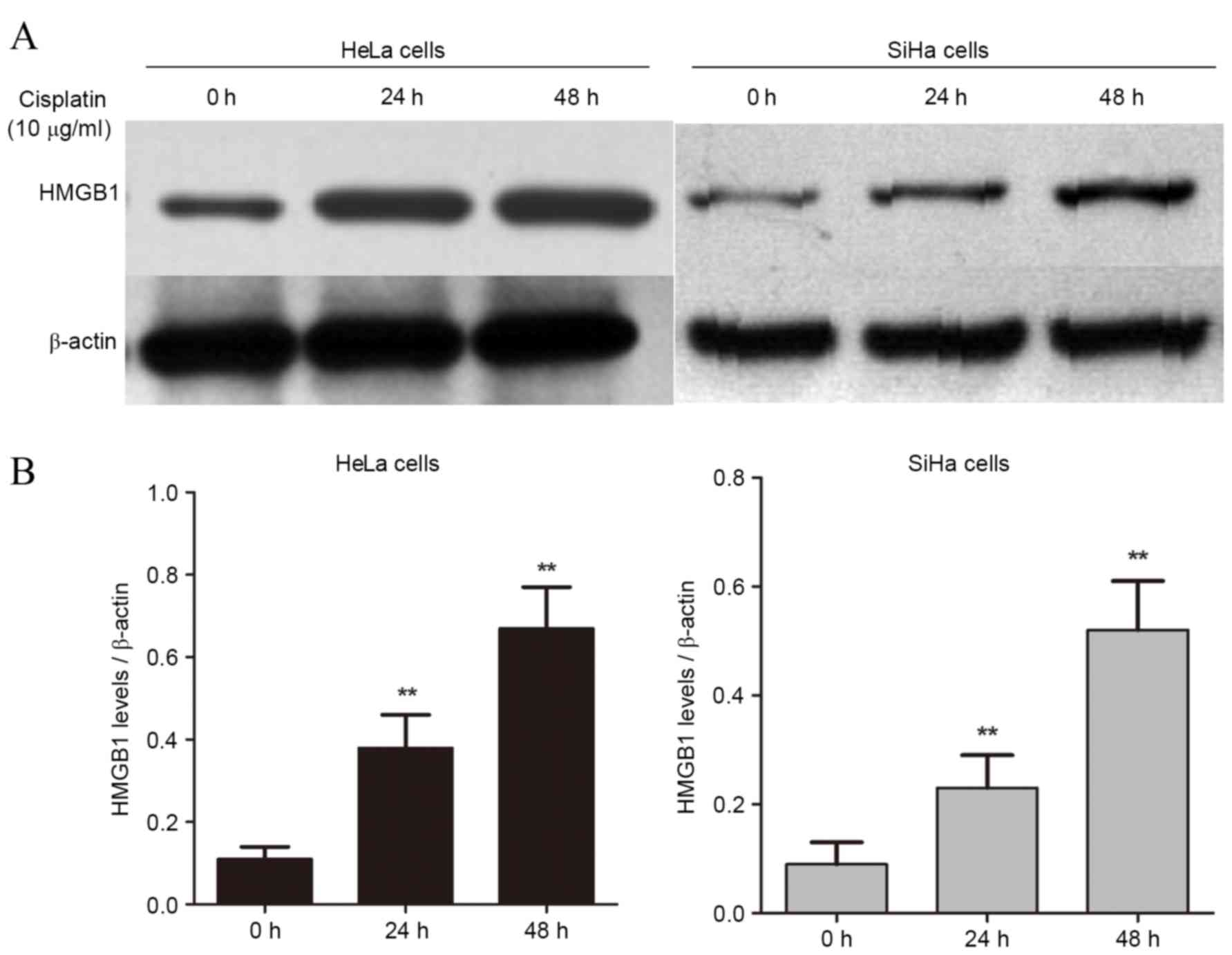
Cisplatin treatment increases the
protein expression levels of HMGB1 in a time-dependent manner
HMGB1 is a nucleoprotein that is associated with
cancer progression (22–24). To examine whether HMGB1 is involved
in drug resistance in cervical cancer cells, two
cisplatin-sensitive cell lines, HeLa and SiHa, were treated with 10
µg/ml of cisplatin for 24 and 48 h. Expression of HMGB1 was
significantly increased compared with untreated cells in at 24 and
48 h in both HeLa (P=0.032 and P=0.014, respectively; Fig. 1A) and SiHa cells (P=0.038 and
P=0.011, respectively; Fig. 1B).
These findings suggest that cisplatin-induced HMGB1 expression in
cervical cancers may be involved in drug resistance.
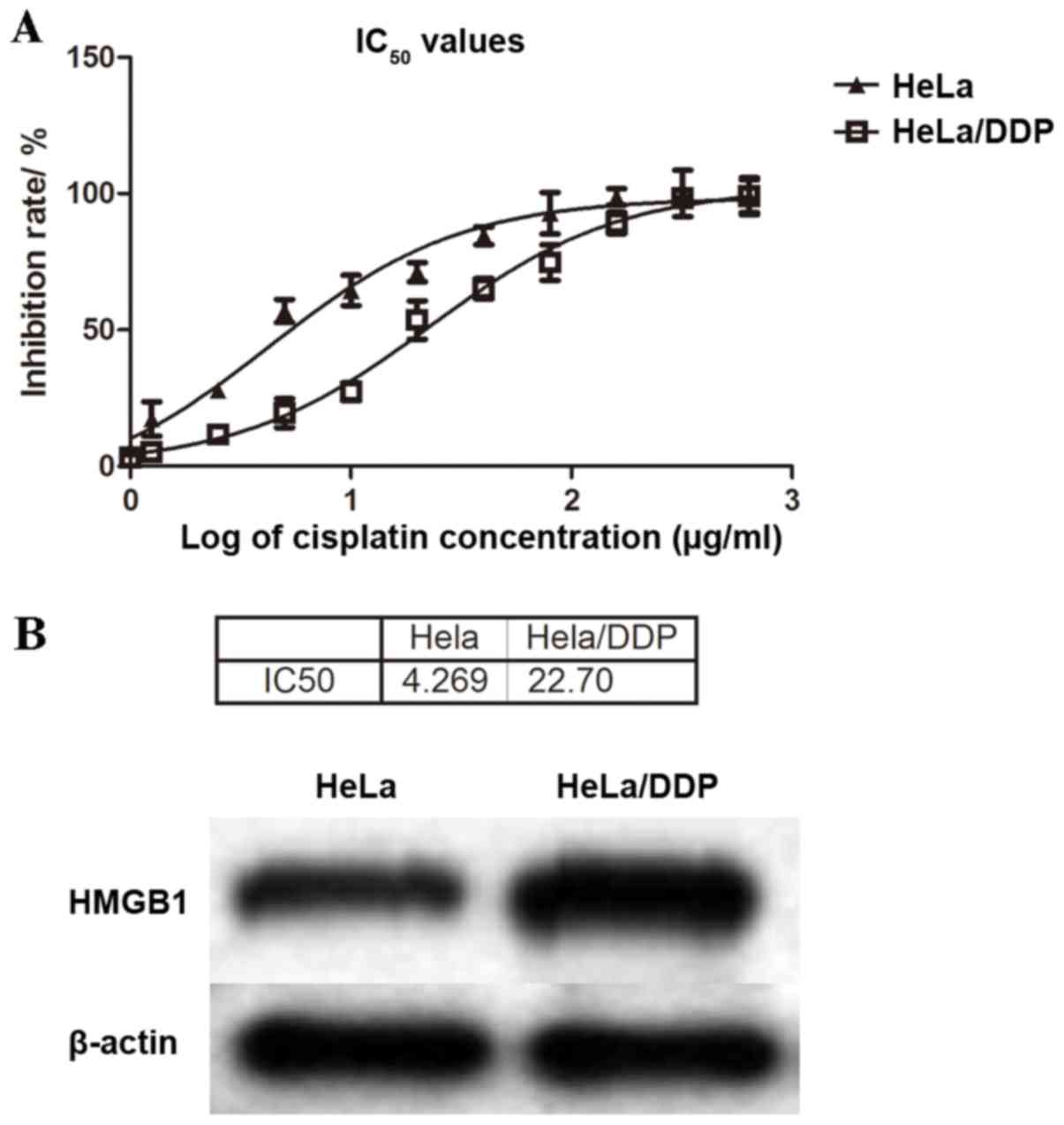
Higher levels of HMGB1 are detected in
a cisplatin-resistant subline of cervical cancer cells
The cervical cancer cell line HeLa and its
cisplatin-resistant subline, HeLa/DDP were used as models. MTT
assays were performed to examine the levels of cisplatin resistance
in these cell lines. The cisplatin-sensitive HeLa and
cisplatin-resistant HeLa/DDP cells were treated with increasing
concentrations of cisplatin for 48 h, and half maximal inhibitory
concentration (IC50) values were calculated as 4.27 and
22.70 µg/ml, respectively (Fig.
2A). The IC50 value in the HeLa/DDP cell line was
~5.3-fold that in the HeLa cell line, confirming that HeLa/DDP had
higher resistance to cisplatin than HeLa cells (Fig. 2A).
HMGB1 protein expression levels were detected by
western blotting analysis in both HeLa and HeLa/DDP cells. HeLa/DDP
cells exhibited higher protein expression levels of HMGB1 than
cisplatin-sensitive HeLa cells (Fig.
2B), which suggested that increased HMGB1 expression was
associated with cisplatin drug resistance in HeLa cervical cancer
cells.
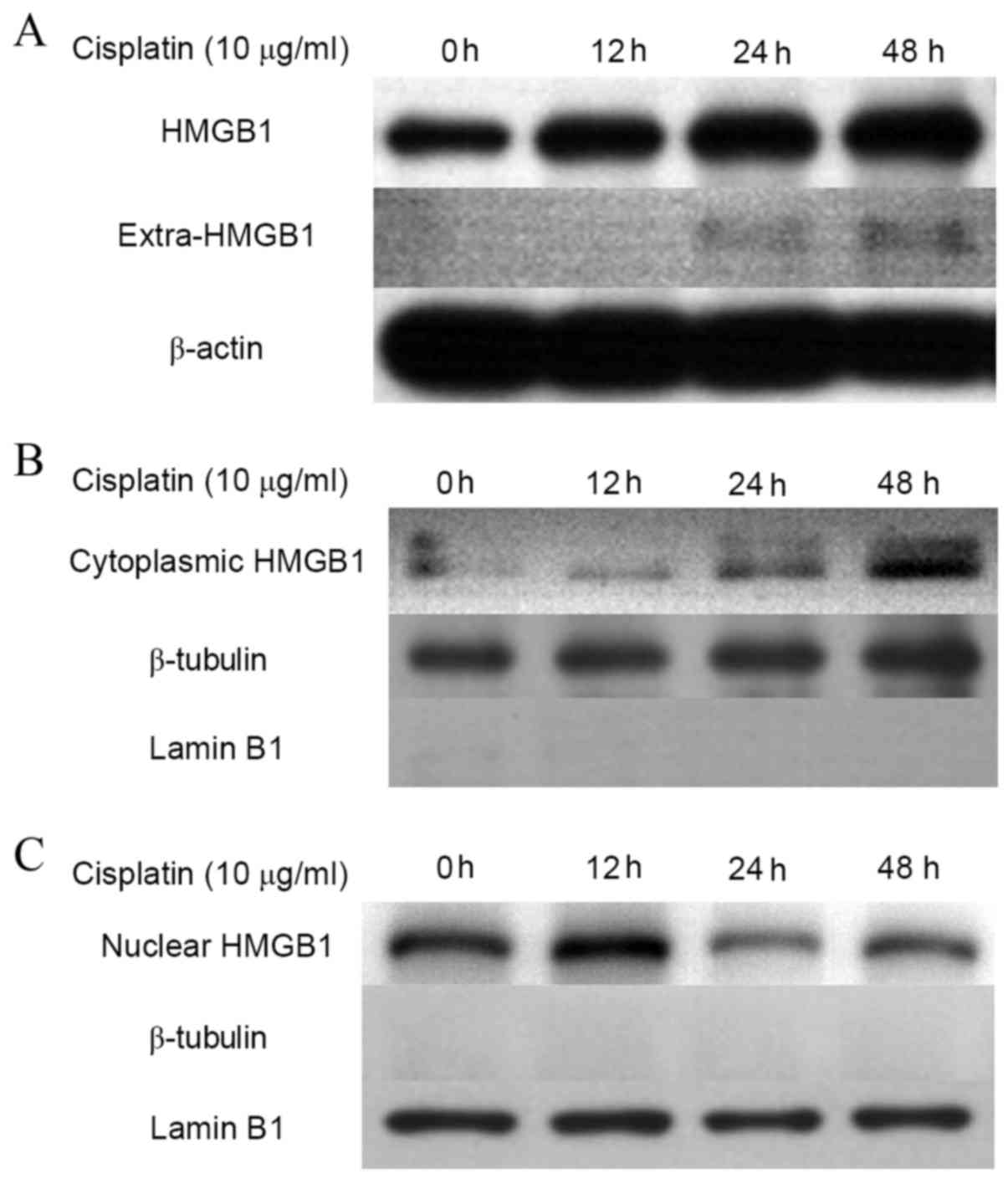
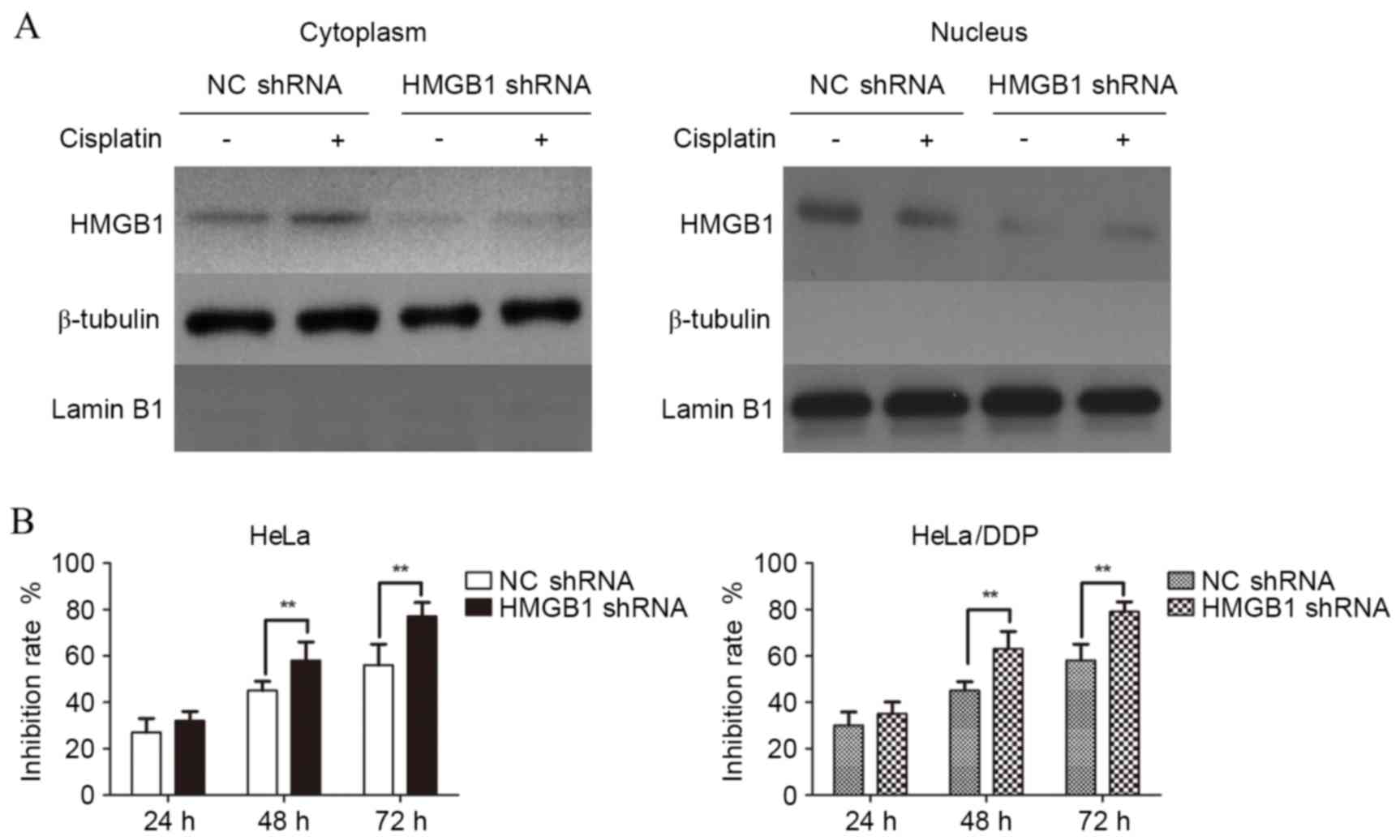
Association of cytoplasmic location of
HMGB1 and cisplatin resistance in cervical cancer cells
Under normal conditions, HMGB1 is a non-histone
nuclear protein (25,26). To establish whether the location of
HMGB1 was altered in drug resistant cancer cells, protein in the
cytoplasm and nucleus was extracted from cisplatin-treated HeLa
cells, and HMGB1 expression was detected in cytoplasmic and nuclear
fractions by western blotting (Fig.
3). Protein expression levels of HMGB1 in HeLa cells increased
in a time dependent manner over the course of 48 h (Fig. 3A). In addition, cytoplasmic HMGB1
increased with time when treated with cisplatin (Fig. 3B), while the levels of HMGB1 in the
nucleus decreased (Fig. 3C). This
suggests that HMGB1 translocation from the nucleus to cytoplasm may
be associated with cisplatin resistance.
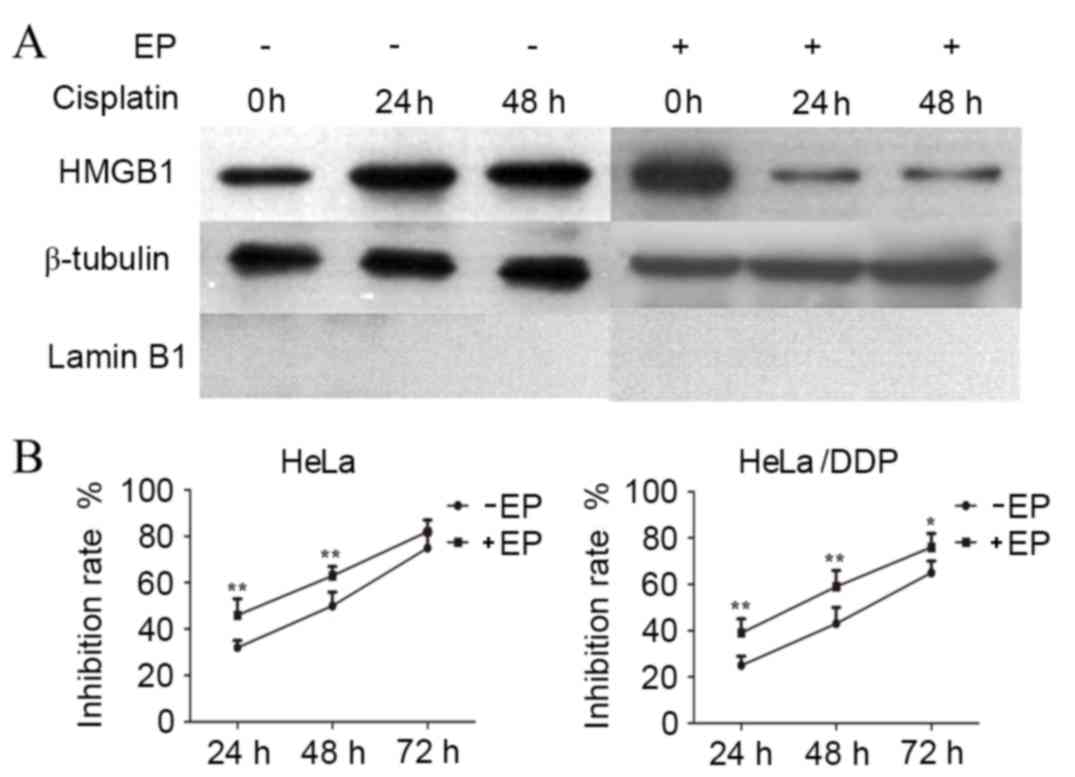
Ethyl pyruvate (EP) inhibits the
cytoplasmic translocation of HMGB1 and suppresses the proliferation
of cervical cancer cells
To further investigate the involvement of HMGB1
translocation in drug resistance a pharmacological inhibitor of
HMGB1 cytoplasmic translocation, EP, was used to treat HeLa cells.
EP treatment alongside cisplatin reduced the levels of cytoplasmic
HMGB1 in cisplatin-treated HeLa cells at 24 and 48 h (Fig. 4A). Notably, the growth inhibition
rate in EP-treated HeLa and HeLa/DDP cells was significantly
increased compared with cells without EP treatment (Fig. 4B), suggesting EP may significantly
inhibit translocation of HMGB1 into the cytoplasm and suppressed
the proliferation of cervical cancer cells.
Interference with endogenous HMGB1
expression inhibits the proliferation of HeLa cells exposed to
cisplatin
RNA interference technology was used to interfere
with the expression of endogenous HMGB1. As demonstrated in
Fig. 5A, the expression of HMGB1
was reduced in both the cytoplasm and nucleus of HeLa cells
transfected with HMGB1 shRNA compared with NC shRNA. MTT assays
were then used to detect the effects of interference with HMGB1 on
the drug resistance of cisplatin in HeLa cells. As demonstrated in
Fig. 5B, the growth inhibition
rate was significantly higher in HMGB1 shRNA transfected,
cisplatin-treated HeLa cells, compared with NC shRNA transfected,
cisplatin-treated HeLa cells at 48 and 72 h (P=0.009 and P=0.009;
Fig. 5B, HeLa cells). This effect
was also observed in HeLa/DPP cells at 48 and 72 h (P=0.009 and
P=0.007; Fig. 5B; HeLa/DPP cells).
Therefore, HeLa and HeLa/DPP cells transfected with HMGB1 shRNA
demonstrated reduced proliferation.
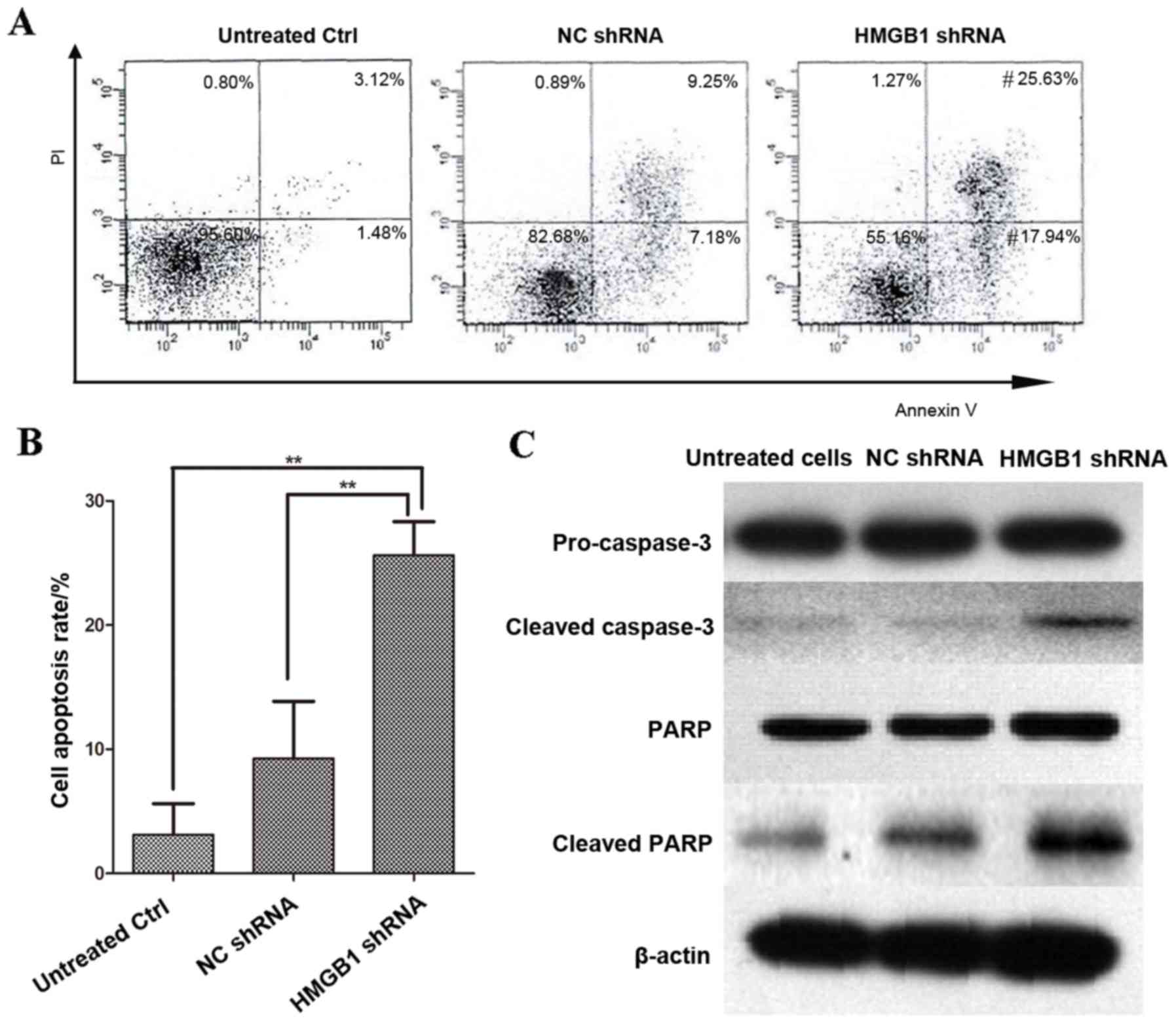
Transfection with HMGB1 in HeLa cells
induces cell apoptosis in cervical cancer cells
The cell apoptosis rate was determined by FACS
analysis in HeLa cells transfected with HMGB1 shRNA and NC shRNA,
and then treated with 10 µg/ml cisplatin for 48 h. FACS analysis
revealed that the apoptosis rate in HMGB1 shRNA transfected HeLa
cells was significantly greater than in NC shRNA transfected HeLa
cells (P=0.003; Fig. 6A). In
addition, the presence of caspase-3, the executor of cell
apoptosis, and its substrate PARP, were detected by western
blotting analysis in cisplatin-treated HMGB1 shRNA/NC shRNA
transfected HeLa cells, and in untreated/untransfected cells
(Fig. 6B). The levels of cleaved,
and therefore activated, caspase-3 and PARP were higher in HMGB1
shRNA transfected HeLa cells than in untreated/untransfected cells
and cisplatin-treated NC shRNA cells (Fig. 6B).
Cell autophagy induced by
extracellular HMGB1 treatment is mediated by the ERK1/2
pathway
Compared with FBS-treated cells, the levels of
LC3-II in HeLa cells treated with rhHMGB1 were increased, while
nucleoporin p62 levels were reduced (Fig. 7A). This suggests that
administration of rhHMGB1 resulted in degradation of p62 and
induction of cell autophagy. Furthermore, treatment with rhHMGB1
increased the phosphorylation of ERK1/2 (Fig. 7A), which results in activation of
the MEK/ERK1/2 signaling pathway. By contrast, interference with
endogenous expression of HMGB1 using HMGB1 shRNA, did not affect
levels of p-ERK1/2, LC3-II and p62 compared with NC shRNA
transfected cells (Fig. 7B).
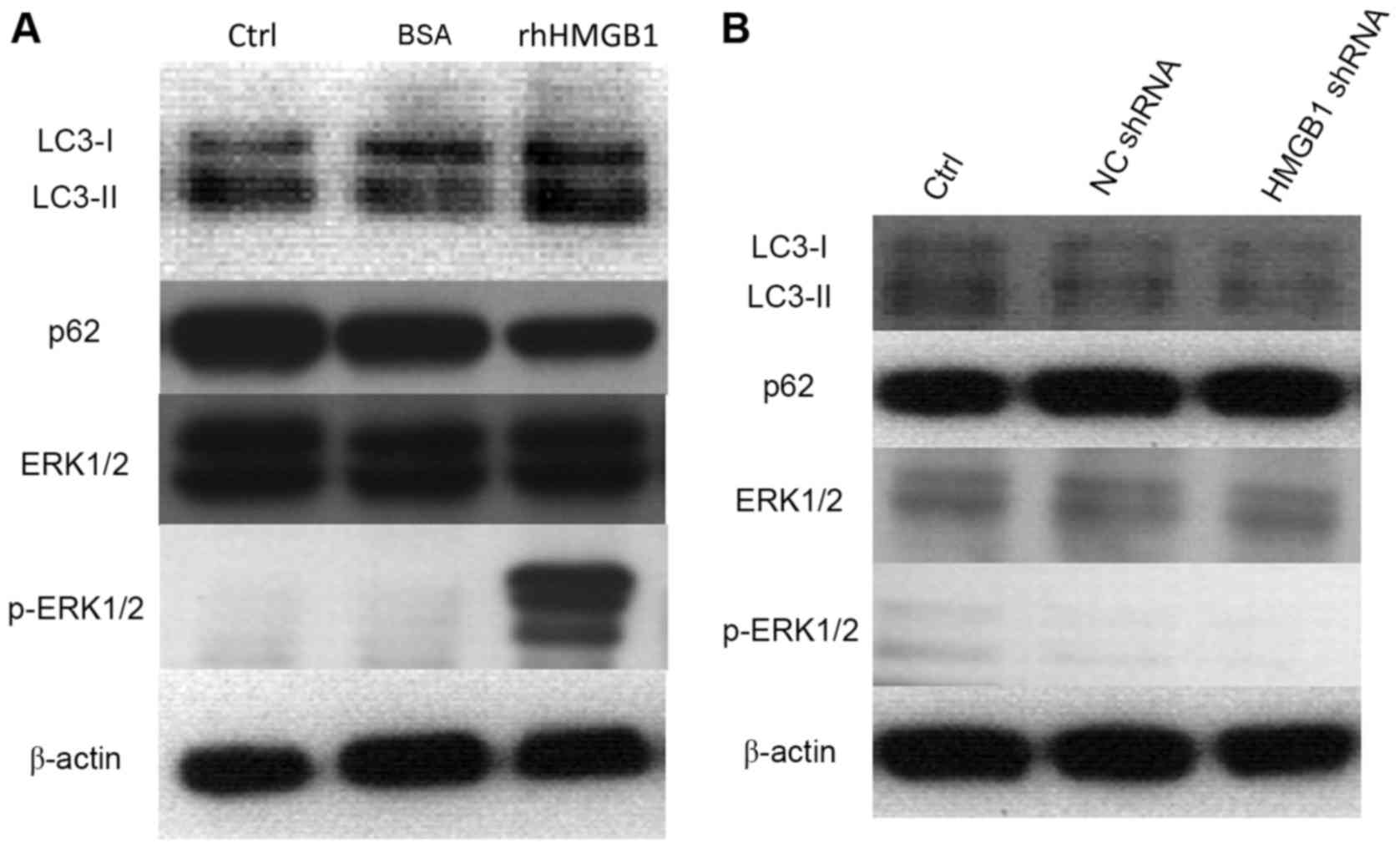 | Figure 7.Cell autophagy induced by HMGB1
treatment in HeLa cells is mediated by the ERK1/2 pathway. Western
blot analysis of LC3-I, LC3-II, p62, ERK1/2 and p-ERK1/2 protein
levels was performed in (A) 24 h serum-starved HeLa cells, treated
with 100 µg/ml rhHMGB1 or 100 µg/ml BSA for 48 h, and (B) HeLa
cells transfected with NC shRNA and HMGB1 shRNA. β-actin was used
as a loading control. HMGB1, high mobility group box protein 1;
ERK1/2, extracellular signal-related kinases 1 and 2; LC3,
microtubule associated protein 1 light chain 3; shRNA, short
hairpin RNA; NC, negative control; p62, nucleoporin p62; p,
phosphorylated; BSA, bovine serum albumin; rh, recombinant
human. |
Discussion
Previous studies into cervical cancer treatments
have focused on prevention methods, including HPV DNA testing, HPV
vaccination and Pap smear issues (27,28).
However, resistance to chemotherapeutic drugs remains a major
obstacle in the treatment of cervical cancer. Investigations into
the mechanisms of drug resistance in human cervical cancer cell
lines, and methods of reversing resistance, have been explored by
numerous researchers, but the mechanisms are yet to be clearly
elucidated.
In the present study, it was revealed that levels of
HMGB1 in cervical cancer cells significantly increased as a result
of cisplatin treatment, in a time-dependent manner. Protein
expression levels of HMGB1 were also demonstrated to be
significantly higher in cisplatin-resistant HeLa/DDP than in
cisplatin-sensitive HeLa cells, suggesting that increased levels of
HMGB1 contribute to cisplatin resistance in cervical cancer cells.
Furthermore, HMGB1 levels were upregulated as the cisplatin
treatment time increased. Clinical samples of cervical cancers will
therefore be collected in a future study to determine whether HMGB1
levels in the serum or tissues could be used as a diagnostic marker
for cervical cancers.
Induction of autophagy has been observed to
contribute to drug resistance in tumor cells (20). Chen et al (29) reported that abrogation of autophagy
could restore lapatinib sensitivity to ErbB2 receptor (HER2)
tyrosine kinase-positive breast cancers. In human esophageal
cancers with acquired resistance to cisplatin, induction of
autophagy has been demonstrated to be accompanied by the
suppression of mechanistic target of rapamycin complex 1 (mTORC1)
activity, which promotes cell autophagy, while inhibition of
autophagy re-sensitizes cancer cells to cisplatin (30). In the present study, it was
demonstrated that cisplatin treatment induces HMGB1 expression in
HeLa cells, and that increased HMGB1 is associated with increased
cisplatin resistance and results in induction of autophagy. In
addition, inhibition of HMGB1 expression promotes apoptosis in
cisplatin-treated HeLa cells. These findings demonstrate that HMGB1
may be an important factor in the development of chemoresistance
and may represent a new target for the treatment of human cervical
cancers.
Acknowledgements
The present study was supported by the Sichuan
Provincial Department of Science and Technology (grant no.
14JC0135) and Luzhou Municipal Science and Technology Bureau (grant
no. 2014-S-35).
References
|
1
|
Boutas I, Sofoudis C, Kalampokas E,
Anastasopoulos C, Kalampokas T and Salakos N: Fertility
preservation in women with early stage cervical cancer. Review of
the literature. Eur J Gynaecol Oncol. 35:373–377. 2014.PubMed/NCBI
|
|
2
|
Arimoto T, Kawana K, Adachi K, Ikeda Y,
Nagasaka K, Tsuruga T, Yamashita A, Oda K, Ishikawa M, Kasamatsu T,
et al: Minimization of curative surgery for treatment of early
cervical cancer: A review. Jpn J Clin Oncol. 45:611–616. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang QT, Zhong M, Gao YF, Huang LP, Huang
Q, Wang W, Wang ZJ and Yu YH: Can HPV vaccine have other health
benefits more than cancer prevention? A systematic review of
association between cervical HPV infection and preterm birth. J
Clin Virol. 61:321–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mann L, Foley KL, Tanner AE, Sun CJ and
Rhodes SD: Increasing cervical cancer screening among us
hispanics/latinas: A qualitative systematic review. J Cancer Educ.
30:374–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang J, Pang H, Liu B, Nasca PC, Zhang B,
Wu Y, Han W, Gates M, Lu T, Zou X, et al: Effects of active,
passive, and combined smoking on cervical cancer mortality: A
nationwide proportional mortality study in Chinese urban women.
Cancer Causes Control. 26:983–991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maguire R, Kotronoulas G, Simpson M and
Paterson C: A systematic review of the supportive care needs of
women living with and beyond cervical cancer. Gynecol Oncol.
136:478–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27:e432016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Obel J, Souares Y, Hoy D, Baravilala W,
Garland SM, Kjaer SK and Roth A: A systematic review of cervical
cancer incidence and mortality in the Pacific Region. Asian Pac J
Cancer Prev. 15:9433–9437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gadducci A, Tana R, Cosio S and Cionini L:
Treatment options in recurrent cervical cancer (Review). Oncol
Lett. 1:3–11. 2010.PubMed/NCBI
|
|
10
|
Smits RM, Zusterzeel PL and Bekkers RL:
Pretreatment retroperitoneal para-aortic lymph node staging in
advanced cervical cancer: A review. Int J Gynecol Cancer.
24:973–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lorusso D, Petrelli F, Coinu A,
Raspagliesi F and Barni S: A systematic review comparing cisplatin
and carboplatin plus paclitaxel-based chemotherapy for recurrent or
metastatic cervical cancer. Gynecol Oncol. 133:117–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turan T, Yildirim BA, Tulunay G, Boran N,
Yıldız F and Köse MF: Experience in stage IB2 cervical cancer and
review of treatment. J Turk Ger Gynecol Assoc. 11:27–37.
2010.PubMed/NCBI
|
|
13
|
Sun R, Jiang B, Qi H, Zhang X, Yang J,
Duan J, Li Y and Li G: SOX4 contributes to the progression of
cervical cancer and the resistance to the chemotherapeutic drug
through ABCG2. Cell Death Dis. 6:e19902015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andersson U, Erlandsson-Harris H, Yang H
and Tracey KJ: HMGB1 as a DNA-binding cytokine. J Leukoc Biol.
72:1084–1091. 2002.PubMed/NCBI
|
|
15
|
Kang R, Zhang Q, Zeh HJ III, Lotze MT and
Tang D: HMGB1 in cancer: Good, bad, or both? Clin Cancer Res.
19:4046–4057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lolmede K, Campana L, Vezzoli M, Bosurgi
L, Tonlorenzi R, Clementi E, Bianchi ME, Cossu G, Manfredi AA,
Brunelli S and Rovere-Querini P: Inflammatory and alternatively
activated human macrophages attract vessel-associated stem cells,
relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc
Biol. 85:779–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szénási T, Kénesi E, Nagy A, Molnár A,
Bálint BL, Zvara Á, Csabai Z, Deák F, Boros Oláh B, Mátés L, et al:
Hmgb1 can facilitate activation of the matrilin-1 gene promoter by
Sox9 and L-Sox5/Sox6 in early steps of chondrogenesis. Biochim
Biophys Acta. 1829:1075–1091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galluzzi L, Pietrocola F, Bravo-San Pedro
JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J,
Gewirtz DA, Karantza V, et al: Autophagy in malignant
transformation and cancer progression. EMBO J. 34:856–880. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berardi DE, Campodónico PB, Díaz Bessone
MI, Urtreger AJ and Todaro LB: Autophagy: Friend or foe in breast
cancer development, progression and treatment. Int J Breast Cancer.
2011:5950922011.PubMed/NCBI
|
|
20
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie M, Kang R, Yu Y, Zhu S, He YL, Xu WQ,
Tang DL and Cao LZ: Enhancive effect of HMGB1 gene silence on
adriamycin-induced apoptosis in K562/A02 drug resistance leukemia
cells. Zhonghua Xue Ye Xue Za Zhi. 29:549–552. 2008.(In Chinese).
PubMed/NCBI
|
|
22
|
Suren D, Yıldırım M, Demirpençe Ö, Kaya V,
Alikanoğlu AS, Bülbüller N, Yıldız M and Sezer C: The role of high
mobility group box 1 (HMGB1) in colorectal cancer. Med Sci Monit.
20:530–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gnanasekar M, Kalyanasundaram R, Zheng G,
Chen A, Bosland MC and Kajdacsy-Balla A: HMGB1: A Promising
therapeutic target for prostate cancer. Prostate Cancer.
2013:1571032013.PubMed/NCBI
|
|
24
|
Zhang X, Wang H and Wang J: Expression of
HMGB1 and NF-κB p65 and its significance in non-small cell lung
cancer. Contemp Oncol (Pozn). 17:350–355. 2013.PubMed/NCBI
|
|
25
|
Ohmori H, Luo Y and Kuniyasu H:
Non-histone nuclear factor HMGB1 as a therapeutic target in
colorectal cancer. Expert Opin Ther Targets. 15:183–193. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee H, Shin N, Song M, Kang UB, Yeom J,
Lee C, Ahn YH, Yoo JS, Paik YK and Kim H: Analysis of nuclear high
mobility group box 1 (HMGB1)-binding proteins in colon cancer
cells: Clustering with proteins involved in secretion and
extranuclear function. J Proteome Res. 9:4661–4670. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bukowska-Durawa A and Luszczynska A:
Cervical cancer screening and psychosocial barriers perceived by
patients. A systematic review. Contemp Oncol (Pozn). 18:153–159.
2014.PubMed/NCBI
|
|
28
|
Kavallaris A, Zygouris D, Dafopoulos A,
Kalogiannidis I and Terzakis E: Nerve sparing radical hysterectomy
in early stage cervical cancer. Latest developments and review of
the literature. Eur J Gynaecol Oncol. 36:5–9. 2015.PubMed/NCBI
|
|
29
|
Chen S, Li X, Feng J, Chang Y, Wang Z and
Wen A: Autophagy facilitates the Lapatinib resistance of HER2
positive breast cancer cells. Med Hypotheses. 77:206–208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu L, Gu C, Zhong D, Shi L, Kong Y, Zhou Z
and Liu S: Induction of autophagy counteracts the anticancer effect
of cisplatin in human esophageal cancer cells with acquired drug
resistance. Cancer Lett. 355:34–45. 2014. View Article : Google Scholar : PubMed/NCBI
|