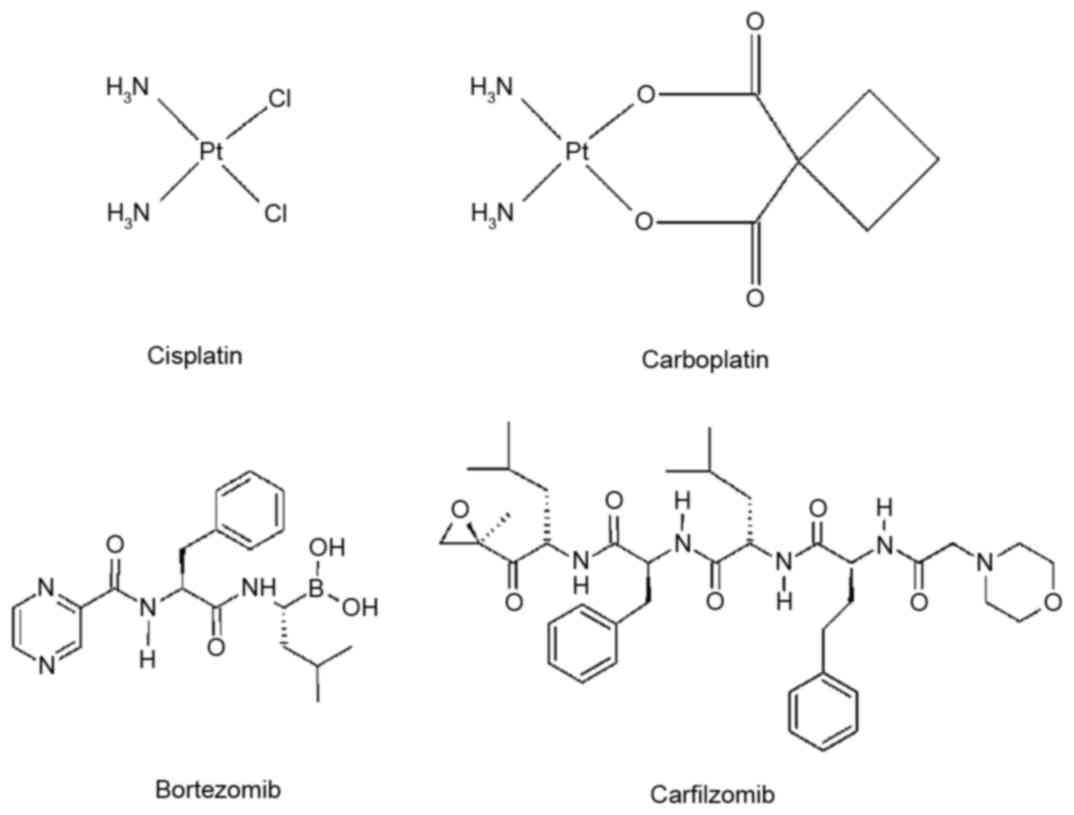
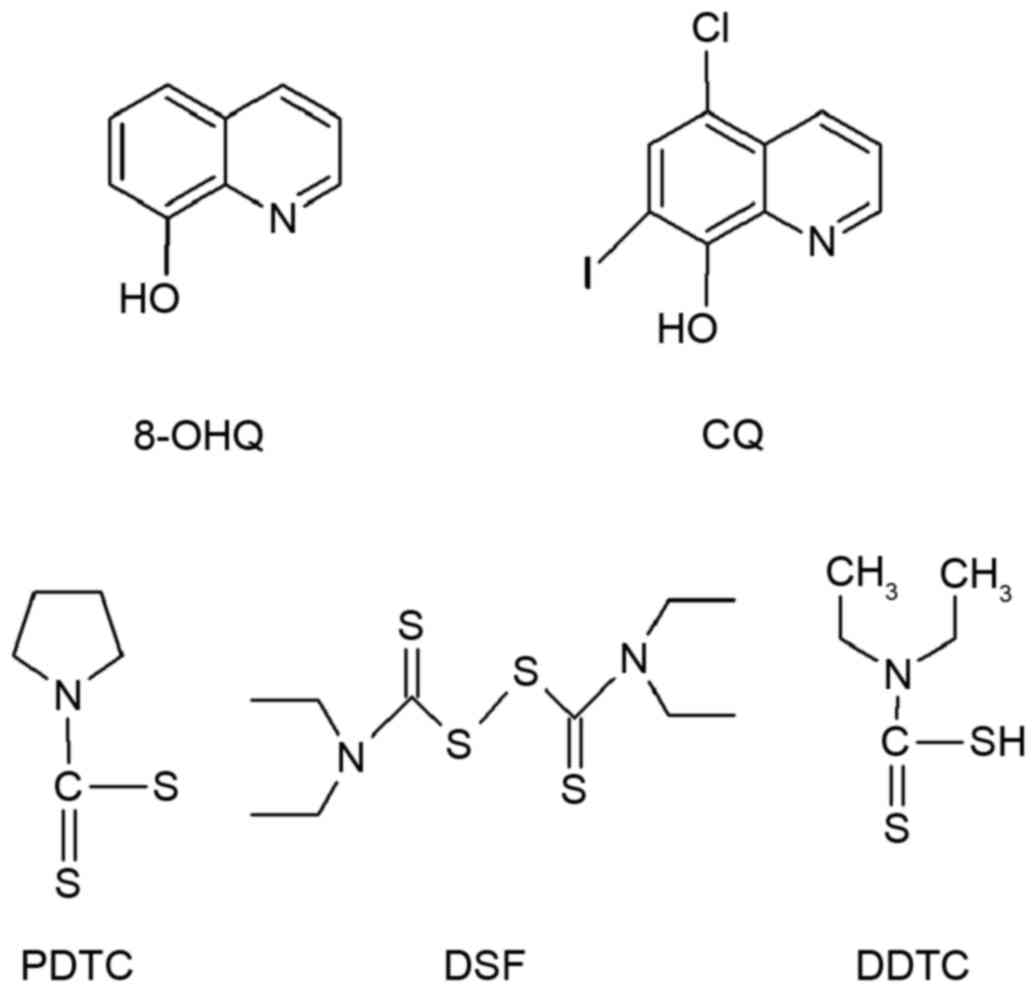
Introduction
Metal ions are required for the maintenance of
numerous critical functions in living organisms and have been used
to treat various human diseases (1–4).
Platinum-based drugs, including cisplatin and carboplatin (Fig. 1) have been developed as effective
anticancer drugs used in chemotherapy of various solid human tumors
(5). In addition to platinum-based
drugs, complexes of several other metals, including copper (Cu),
zinc, gold, palladium and gallium, have demonstrated promising
antitumor activities in vitro and in vivo in cancer
therapy (6–9). The disulfiram (DSF)-Cu and clioquinol
(CQ)-Cu complexes are being investigated as potential therapeutics
for human cancer. Previous results have indicated that these
complexes exhibit potent proteasome-inhibitory and
apoptosis-inducing activities when the Cu was transported into
cancer cells; however, they are minimally toxic toward normal cells
(10–12). The use of metal complexes in the
pharmaceutical industry is increasing, thus leading to the
development of metal-based complexes as potential anticancer
agents.
It has previously been demonstrated that the
ubiquitin-proteasome pathway (UPP) is important in controlling the
expression, activity and location of various cellular proteins
(13). Ciechanover, Hershko and
Rose were awarded the Nobel Prize in Chemistry in 2004 for its
discovery (14,15). The UPP is responsible for
ubiquitination and proteasomal degradation, which regulates cell
cycle progression, signal transduction, differentiation,
proliferation and apoptosis (16).
Tagged proteins are degraded by the 26S proteasome, which is
localized in the nucleus and cytosol of cells. The 26S proteasome
is a multicatalytic enzyme complex, which consists of a 20S
catalytic core and two 19S regulatory complexes (17–19).
The 20S proteasome consists of four stacked heptameric ring
structures, which are composed of two different types of subunits:
α and β (20). The outer two rings
in the stack each consist of seven α subunits, which allow unfolded
proteins to enter the 20S core, whereas β subunits in the inner two
rings primarily contain three distinct catalytic subunits: β1, β2
and β5, which are responsible for caspase or peptidyl-glutamyl
peptide-hydrolyzing-like, trypsin-like and chymotrypsin (CT)-like
activity, respectively (21).
Therefore, it has been suggested that proteasomal activity
contributes to the pathological development of numerous diseases,
including inflammation, neurodegeneration and cancer (22). Notably, it has previously been
demonstrated that inhibition of the CT-like activity is associated
with induction of tumor cell apoptosis programs (3,23,24).
Selective proteasome inhibition in cancer cells and
induction of apoptosis have been regarded as potential anticancer
strategies (25). Various
proteasome inhibitors have been applied in preclinical experiments
and clinical studies as novel anticancer agents (18). Bortezomib (Velcade; Fig. 1) and carfilzomib (Kyprolis;
Fig. 1), to the best of our
knowledge, are the only proteasome inhibitors that have been
granted approval by the US Food and Drug Administration, in 2003
and 2012, respectively (26,27).
Bortezomib is a reversible inhibitor of the 26S proteasome, which
has been used clinically for the treatment of multiple myeloma (MM)
and mantle cell lymphoma (28).
Carfilzomib is a second-generation proteasome inhibitor, which is
primarily used for the treatment of patients with MM following
treatment with bortezomib and an immunomodulatory agent (29,30).
Proteasome inhibitors have been demonstrated to be highly effective
against several cancers in preclinical and clinical trials;
however, resistance (2) and
toxicity (31–33) have emerged as limiting factors of
the continued clinical use of these drugs. A pharmaceutical aim for
the future is to continue to develop a novel generation of
proteasome inhibitors with far less toxicity and a broader spectrum
of activity. Various metal complexes, including gold, Cu and zinc
have been investigated as potential anticancer drugs, due to the
success of platinum-based anticancer therapy. Metal-based complexes
are currently under investigation due to their ability to exert
potent antiproliferative effects by targeting the UPP, resulting in
novel opportunities in cancer therapy (23). The present review focuses on recent
advances in the development of metal complexes as proteasome
inhibitors, with emphasis on Cu complexes for cancer treatment.
Cu and Cu-based complexes
Cu is an essential trace element in all living
organisms, which is important in the process of internal oxidation
and reduction. All animals, including humans, require a finite
amount of Cu for survival and normal physiological function. Cu is
important in maintaining the correct functionality of proteins and
enzymes. Numerous key enzymes require the participation and
activation of Cu to affect metabolic processes in organisms
(34,35). Cu levels in serum and tissue have
been demonstrated to be significantly greater in various human
tumors, including breast (36),
prostate (37), colon (37), lung (38) and brain cancers (39). Kuo et al (36) reported that serum and tissue Cu
levels in breast cancer patients were markedly higher than levels
in the control group. The serum Cu concentration was 1,252.20
µg•l−1 in the malignant group, followed by the benign
group and the control group with the concentrations of 1,038.93 and
964.95 µg•l−1, respectively. Furthermore, a significant
difference between normal and malignant tissues in the malignant
group was been demonstrated with concentrations 6.13 and 11.3
µg•g−1, respectively. A similar result has also been
observed in other types of cancer (37–39).
Recent studies have demonstrated that Cu is
associated with angiogenesis (40), which is important in the
proliferation, invasion and metastasis of tumor cells (41,42).
Therefore, based on the biological function of Cu in tumor
progression, inhibition of angiogenesis by reducing the content of
Cu in vivo may be developed as a novel strategy in cancer
therapy (4,43). Daniel et al (44) demonstrated that Cu complexes
inhibited the activity of the 26S proteasome in vitro and
in vivo. Various chelators, including 8-hydroxyquinoline,
(8-OHQ), dithiocarbamate and clioquinol (CQ), may react with Cu
salts and form complexes that act as potent proteasome inhibitors
and apoptosis inducers in cultured human cancer cells. Further
studies revealed that Cu complexes demonstrated an inhibitory
effect against proteasomes in vitro or in vivo
(45–47).
8-OHQ and CQ
Daniel et al (44) synthesized and tested the anticancer
activity of Cu complexes, including [Cu (8-OHQ)2] (8-OHQ
is presented in Fig. 2). The Cu
(8-OHQ) 2 complex was revealed to be potent, transient,
proteasome inhibitors capable of inducing apoptosis in human
leukemia cells, but not in non-transformed, immortalized human
natural cells under the same conditions. Further experimental
studies demonstrated that the inhibition of CT-like proteasome
activity and apoptotic induction do not result from Cu-mediated
oxidative damage to proteins, but from the formation of a
proteasome inhibitor inside the tumor cell (43).
CQ (5-chloro-7-iodo-8-hydroxyquinoline; Fig. 2) is an analog of 8-OHQ that has
been used to treat Alzheimer's disease in preclinical and clinical
trials (48–51). CQ has been demonstrated to bind Cu
and form novel Cu complexes, which possess proteasome-inhibitory
activities and induce apoptosis in cancer cells (44). Cu-chloride (CuCl2) has
the ability to directly inhibit CT-like activity of purified 20S
proteasome, with a half maximal inhibitory concentration value of
3–5.3 µM (44,45); however, it does not enter cells. CQ
alone did not inhibit the CT-like activity of the proteasome;
however, the combination of CQ and CuCl2 resulted in
different effects in the same experiment and demonstrated selective
inhibitory effects on CT-like activity, but not caspase- or
trypsin-like activities (44,45).
Subsequently, it was hypothesized that targeting highly elevated Cu
in cancer cells and tissues, in conjunction with treatment with
novel compounds including CQ, may result in the formation of
tumor-specific proteasome inhibitors that possess potential for
cancer therapy.
Chen et al (11) investigated the underlying molecular
mechanism of CQ in human prostate cancer cells and xenografts.
Inhibition of proteasomal CT-like activity, suppression of androgen
receptor (AR) protein expression and induction of cell apoptosis
was observed following CQ binding with Cu. CQ was subsequently
administered to mice bearing C4-2B xenografts, and potently
inhibited the tumor growth via in vivo proteasome inhibition
and apoptotic induction. The study suggested that CQ is capable of
targeting the tumor proteasome in vivo depending on the
presence of Cu, and subsequently leads to the formation of an AR
inhibitor and apoptosis inducer, which is responsible for the
observed anti-prostate tumor effect (11,46).
In 2009, to further understand the molecular mechanism of CQ and
8-OHQ-mediated antitumor activity, Barrea et al (47) conducted a human prostate tumor
xenograft experiment. Elemental mapping and the chemical status of
Cu in tumor and normal tissues collected from the same mice were
then measured. The copper in normal tissue and tumor tissue existed
predominantly in the form of Cu (I). Following treatment with CQ,
cellular copper could interact with CQ and convert Cu (I) to Cu
(II), and thus, Cu (II) content increased significantly in tumor
tissue (47). This conversion of
Cu (I) to Cu (II) in tumor tissue is associated with CQ-induced
proteasome inhibition. Zhai et al (12) revealed that 8-OHQ or CQ, but not
their analogs, may bind to Cu salt and transport the Cu complex
into human breast cancer cells or interact with cellular Cu to form
a complex and consequently result in proteasome inhibition,
growth-suppression and apoptotic induction (12). However, further studies are
required to verify the use of 8-OHQ or CQ in clinical trials as
potential anticancer agents.
Dithiocarbamates
Dithiocarbamates, which are a class of metal
chelating reagents, interact with metal ions and form metal
complexes that have been applied as a class of potential agents to
target the UPP in cancer treatment. Pyrrolidine dithiocarbamate
(PDTC; Fig. 2) is the first member
of the dithiocarbamate family, which has been revealed to bind Cu,
inhibit the cancer-specific proteasome and induce cellular
apoptosis in human breast and prostate cancer. However, PDTC alone
failed to exhibit similar results in cultured cells (45,52).
Yu et al (53) and Wang et al (54) synthesized a series of PDTC
analogues with substitutions to the pyrrolidine ring, and studied
the associations between structure and activity. Following the
formation of Cu complexes, it was observed that the size and
polarity of the ring within PDTC affected its activity. When the
pyrrolidine ring was substituted with larger and more polar groups
in the analogues, the effect on proteasome-inhibitory potencies of
the formed Cu complexes was significantly decreased (53). Furthermore, novel Cu (II) complexes
of PDTC analogues had less aldehyde dehydrogenase (ALDH) inhibition
activity and inhibited the CT-like activity of the proteasome in
human breast cancer cells (54).
Disulfiram (DSF; Fig. 2) was
subsequently revealed to have antitumor activities when binding
with Cu and forming a complex. DSF is an irreversible ALDH
inhibitor, which has been clinically used for the treatment of
alcoholism (55).
Chen et al (10) tested the effects of DSF and a
DSF-Cu mixture in cultured breast cancer cells on proteasome
inhibition and apoptotic induction. Cell death was observed
following treatment with the DSF-Cu complex, however not with DSF
alone. Conversely, following an alteration of the Cu concentration
in human breast cancer cells to the Cu levels present in the
patient, DSF alone resulted in a similar biological activity as
observed with the DSF-Cu mixture in cultured cancer cells. These
results demonstrated that once DSF entered the tumor cells, it was
able to react with endogenous Cu, inhibit the proteasome and induce
apoptosis in Cu-enriched MDA-MB-231 human breast cancer cells.
This previous finding was further verified following
treatment of mice bearing MDA-MB-231 tumor xenografts with DSF. The
results indicated that DSF inhibited the tumor growth of mice via
proteasome-inhibitory activity (10). Notably, based on these preclinical
studies of DSF, the use of DSF in human cancer treatment has been
investigated in clinical trials. In addition,
diethyldithiocarbamate (DDTC; Fig.
2) is a member of the dithiocarbamate family and a potent
chelator of Cu. DDTC is a synthetic immunomodulator that has
undergone clinical trials in patients with human immunodeficiency
virus-1 infection; DDTC resulted in a significant delay in disease
progression to acquired immune deficiency syndrome (56,57).
Following binding with Cu, DDTC-Cu (II) (58) and DDTC-Cu (I) (59) complexes significantly inhibited
proteasomal CT-like activity and induced apoptosis in breast,
prostate and pancreatic cell lines. Furthermore, DDTC-Cu (II)
complex inhibited the expression of AR protein in prostate cancer
cells and estrogen receptor proteins in breast cancer cells
(58). However, increased p27 and
decreased nuclear factor-κB expression was detected with DDTC-Cu
(I) treatment in patients with pancreatic cancer (59). The presence of Cu (I) and Cu (II)
results in the formation of DDTC-Cu (I) and DDTC-Cu (II) in
vivo, which may subsequently lead to anticancer activity. These
results suggested that increased Cu in human cancer cells and
tissues may be used as a novel targeting method for cancer
therapy.
Schiff base Cu complexes
The Schiff base is a functional group, which
includes a double bond between carbon and nitrogen, which may be
synthesized by condensation of an aliphatic or aromatic amine and a
carbonyl compound. Schiff bases and their metal complexes have been
used widely in medicine, catalysis, corrosion protection and
analytical chemistry, due to their physiological properties. Schiff
bases and their metal complexes have previously been demonstrated
to exhibit potent antibacterial and antitumor activity (60–66).
Schiff base Cu complexes have been investigated as
potential anticancer drugs that target the UPP. However, the
specific structure of the complex is associated with its anticancer
activity. The synthesis of a series of Schiff bases of
quinoline-2-carboxaldehyde (QC) and their Cu complexes (QC-Cu;
Fig. 3) was previously reported
(61), and these complexes were
revealed to exhibit significant antiproliferative activity against
PC-3 and LNCaP prostate cancer cell lines.
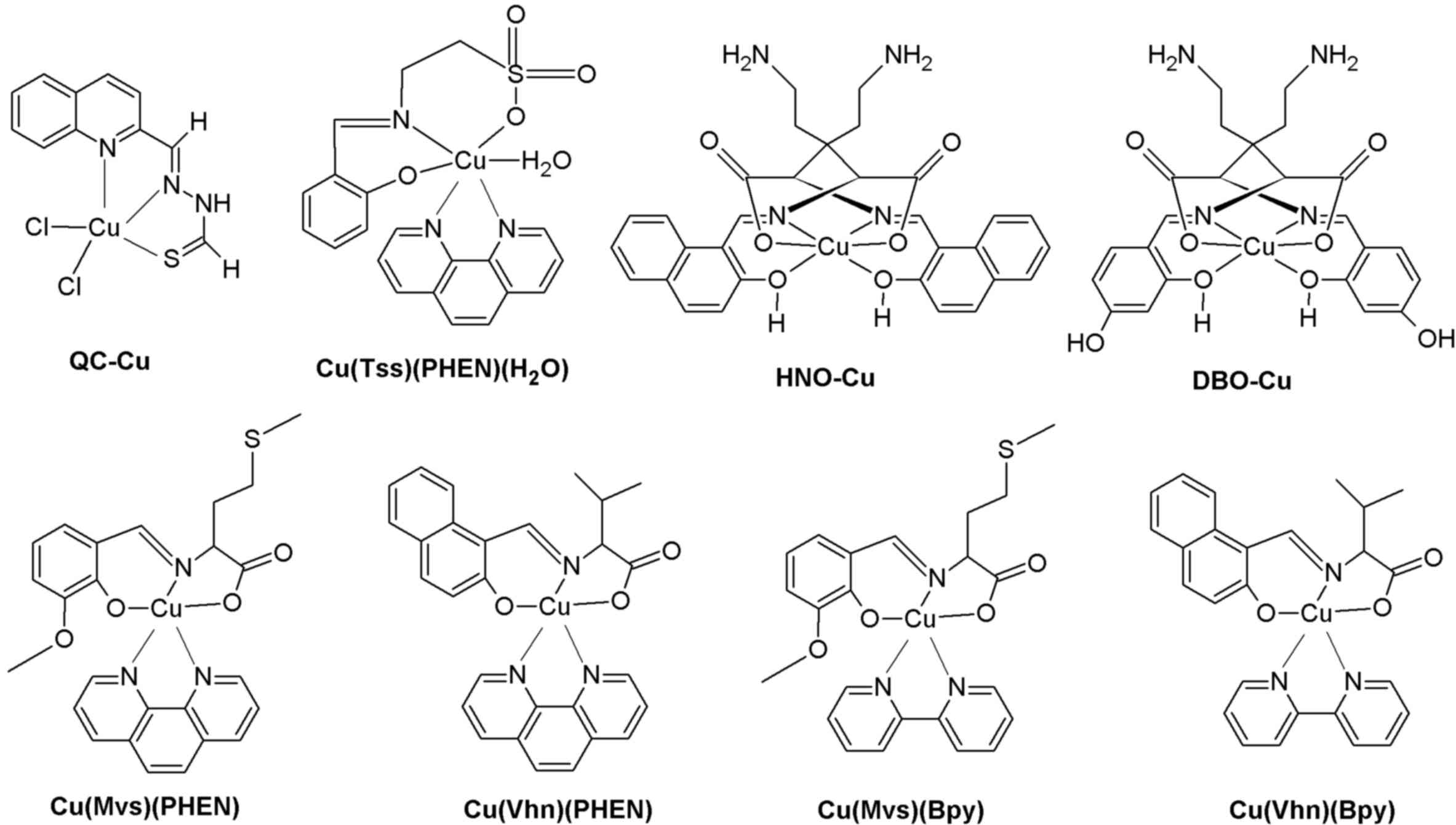 | Figure 3.Chemical structures of Schiff base
copper complexes: QC-Cu, Cu(Tss)(PHEN)(H2O), HNO-Cu,
DBO-Cu, Cu(Mvs)(PHEN), Cu(Vhn)(PHEN), Cu(Mvs)(Bpy), Cu(Vhn)(Bpy).
QC, quinoline-2-carboxaldehyde; Cu, copper; Tss, taurine salicylic
Schiff-base; HNO, 2-hydroxy-1-naphthaldehyde-L-ornithine; DBO,
2,4-dihydroxybenzaldehyde-L-ornithine; PHEN, 1,10-phenanthroline;
Bpy, 2,2′-bipyridine; Mvs, L-methionine-o-vanillin Schiff base;
Vhn, valine-2-hydroxy-1-naphthaldehyde Schiff base. |
Notably, the biological activity and function of
these complexes is affected by the nature of the side chains at
position C2. An introduction of a thiocarbonyl group at the C2
position in the quinoline moiety upon Cu complexation, demonstrated
the greatest cytotoxic activity. Furthermore, Schiff base Cu
complexes are capable of inducing apoptosis via the inhibition of
CT-like proteasome activity, however not via oxidative stress in
LNCaP prostate cancer cells (61).
1,10-phenanthroline (PHEN) is an important metal
chelator with a planar structure. Various metal complexes
containing PHEN and Schiff bases possess anticancer activity. These
include the taurine Schiff base copper complex [Cu (Tss) (PHEN)
(H2O); where Tss is taurine salicylic Schiff-base;
Fig. 3], which potently inhibits
the activity of the proteasome and induces apoptosis in MDA-MB-231
human breast cancer and Jurkat T leukemia cells (67). This conclusion is consistent with
information presented in another report (68). A more recent study has presented
the construction of four novel amino acid Schiff base Cu complexes
[Cu (Mvs)(PHEN), Cu(Vhn)(PHEN), Cu(Mvs)(Bpy) and Cu(Vhn)(Bpy);
where Mvs and Vhn are L-methionine-o-vanillin Schiff base and
valine-2-hydroxy-1-naphthaldehyde Schiff base, respectively;
Fig. 3], which contain PHEN or
2,2′-bipyridine (Bpy) as the second ligand (68). Cytotoxicity and antiproliferation
studies of these Cu complexes against MDA-MB-231 or MCF-7 human
breast cancer cells, and PC-3 prostate cancer cells demonstrated
different effects. Notably, Cu complexes with PHEN as the second
ligand were able to inhibit cell growth, proteasome activity and
induce cell death; however, the compounds with Bpy as the second
ligand did not. The presence of PHEN as part of the complex has
therefore been demonstrated to be important in determining
cytotoxic activity. The preliminary docking analysis explained why
various activities are exhibited between the two substances. The
complex Cu(Mvs) (PHEN) possesses important intermolecular
interactions with the enzymatic pocket and fits exactly to the
CT-like binding pocket, thus leading to the reversible inhibition
of activity; however, Cu(Mvs)(Bpy) does not exhibit this property
(68).
Schiff base Cu complexes HNO-Cu (HNO,
2-hydroxy-1-naphthaldehyde-L-ornithine; Fig. 3) and DBO-Cu (DBO,
2,4-dihydroxybenzaldehyde-L-ornithine; Fig. 3) are novel Cu-containing complexes
that have been synthesized and compared for their abilities to
inhibit the proliferation and induce apoptosis of MDA-MB-231 breast
cancer and LNCaP human prostate cancer cells. HNO-Cu suppressed the
proliferation in a dose-dependent manner, resulting in 95%
inhibition of cell proliferation at 60 µM; however, DBO-Cu did not
induce significant inhibition of cell proliferation in the cell
lines (69). The anticancer
activity is therefore dependent on the specific structure of the
complex.
Further Cu complexes
A series of Cu complexes: [Cu(Dpmp)Cl], [Cu(Dpmp)
OAc], and [Cu(HDpmp)(Dpmp)]OAc [where Dpmp is
2,4-diiodo-6-((pyridine-2-ylmethylamino)methyl)phenol; Fig. 4] have recently been reported to
demonstrate anticancer activity in vitro against human
prostate cancer and leukemia cells (70). Distinctive stoichiometries resulted
in three compounds exhibiting different cytotoxicity, and the 1:1
metal-to-ligand complexes were more effective than the 1:2 species.
Furthermore, it is necessary for the Cu (II) ion to interact with
the ligand that acts as a carrier to enable crossing of the cell
membrane. Once the compound has reached the proteasome of the tumor
cell, the metal center of the complex may coordinate to
identifiable amino acids, which enable Cu-N, Cu-S or Cu-O bonds to
form, thus resulting in proteasome inhibition. Consequently, the
active form of the Cu complex binds to the N-terminal threonine
residue of the CT active center in the 20S proteasome (70).
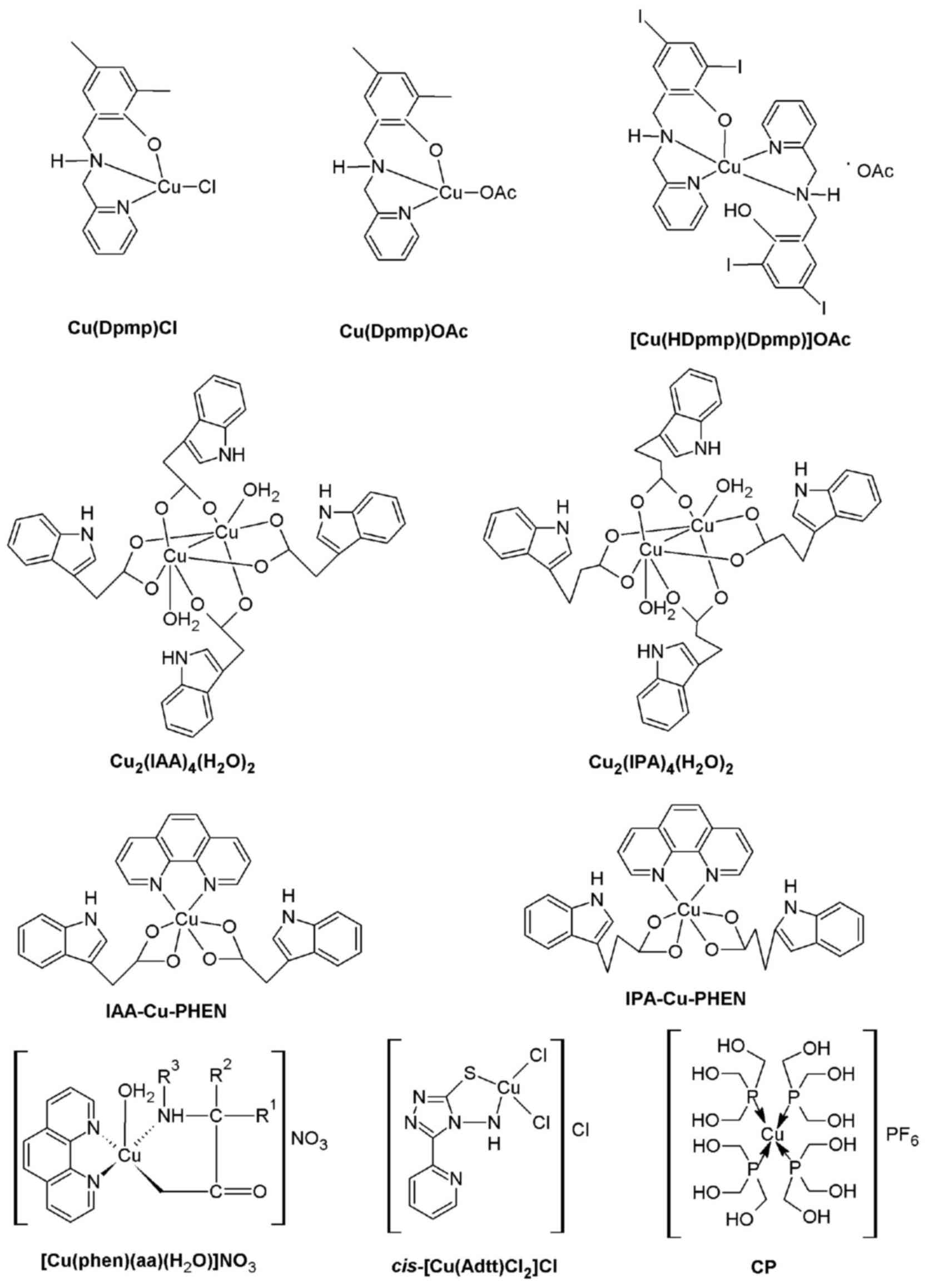 | Figure 4.Chemical structures of further copper
complexes: Cu(Dpmp)Cl, Cu(Dpmp)OAc, [Cu(HDpmp)(Dpmp)]OAc,
Cu(IAA)4(H2O)2,
Cu(IPA)4(H2O)2, IAA-Cu-PHEN,
IPA-Cu-PHEN, [Cu(PHEN)(aa)(H2O)]NO3,
cis-[Cu(Adtt)Cl2]Cl and CP. Cu, copper; Dpmp,
2,4-diiodo-6-((pyridine-2-ylmethylamino)methyl)phenol; IAA,
3-indole acetic acid; IPA, 3-indole propionic acid; PHEN,
1,10-phenanthroline; CP, [Cu(thp)4][PF6];
thp, tri(hydroxymethyl)phosphine; aa, methylated glycine,
DL-alanine, aarcosine or 2,2-dimethyglycine; Adtt,
4-amino-1,4-dihidro-3-(2-pyridyl)-5-thioxo-1,2,4-triazole. |
To further investigate the association between
biological activity and Cu complex structure, two types of Cu
complex: Dinuclear
(Cu2(IAA)4(H2O)2 and
Cu2(IPA)4(H2O)2; where
IAA and IPA are 3-indole acetic acid and 3-indole propionic acid,
respectively; Fig. 4) and ternary
(IAA-Cu-PHEN and IPA-Cu-PHEN; Fig.
4) complexes were synthesized, and the effects on cell
proliferation and the ability to inhibit the activity of the
proteasome were measured (71). It
was observed that ternary complexes binding with PHEN as the second
ligand were more efficient in carrying Cu into cancer cells
compared with dinuclear complexes, and these complexes consequently
resulted in deactivation of the proteasome in PC-3 human prostate
cancer cells. In addition, the ternary complexes selectively
inhibited the activity of the proteasome and induced apoptosis in
tumor cells. The study further supported the hypothesis that
carrying Cu into tumor cells to directly interact with and/or
inhibit the proteasome may be applied as a potential anticancer
strategy (71).
It has recently been reported that
[Cu(PHEN)(aa)(H2O)]NO3 (where aa=methylated
glycine, DL-alanine, arcosine or 2,2-dimethyglycine; Fig. 4) has been synthesized (72) and demonstrated the ability to
induce tumor cell apoptosis via reactive oxygen species generation
and proteasome inhibition (73,74).
However, these particular complexes did not inhibit CT-like
activity of the 20S proteasome in MDA-MB-231 breast tumor cells.
However, a higher intensity and greater accumulation of
ubiquitinated proteins was observed when cells were treated with
the above complexes (10 µM). Therefore, proteasome inhibition by
these complexes may involve the 19S regulatory cap of the 26S
proteasome or the ubiquitination step (74).
It has previously been demonstrated that a Cu(II)
thioxotriazole complex, cis-[Cu (Adtt) Cl2]Cl
[where Adtt is
4-amino-1,4-dihidro-3-(2-pyridyl)-5-thioxo-1,2,4-triazole]
(75,76) and a phosphine Cu(I) complex,
[Cu(thp)4] [PF6] (CP; where thp is tri
(hydroxymethyl)phosphine; Fig. 4),
exhibited significantly higher cytotoxic activities compared with
cisplatin (77). The cytotoxic
effects of cis-[Cu (Adtt) Cl2]Cl and CP were
associated with accumulation of ubiquitinated proteins and
inhibition of the UPP in human cancer cells (76,77).
The efficacy of CP was >40-fold compared with that of cisplatin
in human colon carcinoma cell lines, and therefore demonstrated a
notable ability to induce apoptosis. Furthermore, it was able to
overcome multi-drug resistance (78). The proteasome-inhibitory activity
of the majority of Cu complexes was primarily studied and optimized
in accordance with the capability to specifically block the CT-like
active sites. However, CP was able to inhibit all three catalytic
sites of the human 26S proteasome and therefore induce paraptotic
cell death (78) via the
activation of endoplasmic reticulum stress signaling, which was
consistent with inhibition of the UPP and induction of the unfolded
protein response (76,77).
Bortolozzi et al (79) revealed that no mitochondrial
involvement was demonstrated in the cell death process when
leukemia cell lines were treated with CP. However, the activation
of caspase-12, −9, −3 and −7 was observed, indicating that cell
death occurred in a caspase-dependent manner. Furthermore, the 20S
proteasomal chymotrypsin-like activity decreased, ubiquitinated
proteins accumulated and endoplasmic reticulum stress increased
markedly when CP was present. Notably, CP induced endoplasmic
reticulum stress and cell apoptosis in B-acute lymphoblastic
leukemia primary cells and synergistically acted with different
chemotherapeutic drugs in the treatment of RS4;11 and SEM cell
lines.
The combination of pyrithione (PT) and
CuCl2 (CuPT) may induce the accumulation of
ubiquitinated proteins in cancer cells (80). Furthermore, the mechanism of CuPT
may be different compared with that exhibited by the proteasome
inhibitor bortezomib. CuPT may also inhibit ubiquitin c-terminal
hydrolase L5 and ubiquitin specific peptidase 14 activities, which
are associated with the 19S regulatory particles. Therefore, CuPT
may inhibit the UPP via targeting proteasome-specific
deubiquitinases and 20S proteasome peptidases; an inhibition that
may have significant effects in the process of CuPT-mediated
cytotoxicity (80).
Conclusions and perspectives
Cu is a critical component in cellular metabolism
that has been demonstrated to be present at high levels in sera and
tissues in various types of human cancer (36,37).
It has recently been demonstrated that Cu and its coordination
complexes may exhibit potential as tumor-specific proteasome
inhibitors and apoptosis inducers. Two main forms of Cu chelating
compound were investigated for their anticancer properties, and
were revealed to exhibit proteasome-inhibitory activities. The
prominent classes of metal chelating compounds, dithiocarbamates
and 8-OHQs, may result in tumor proteasome inhibition and cell
death by targeting elevated levels of tumor-associated Cu. However,
the underlying molecular mechanisms of synthesized Cu complexes may
differ depending on the mixture of Cu and small molecular ligands.
The synthetic Cu complexes inhibit the proteasome by directly
interacting with it, thus inhibiting its activity, or by triggering
oxidation and deactivation of the cellular proteasome resulting in
proteasome inactivation (41,81).
Cu-based compounds have demonstrated positive
results in preclinical studies and clinical trials; however, the
exact underlying molecular mechanism remains to be elucidated and
Cu-based compounds are not currently marketed as antitumor agents.
Cu is essential for Cu complexes to act as proteasome inhibitors
and apoptosis inducers; therefore, efforts to develop novel
inhibitors based on Cu and associated ligands are of primary
interest. There are at least three important strategies for the
selection of ligands of Cu-based compounds during the process of
novel metal-based proteasome inhibitor design. Firstly, the
selection of various metal chelators, including DSF and CQ, which
have been previously approved for the treatment of diseases;
secondly, the synthesis of novel Cu complexes with bioactive
natural compounds as ligands, including indole derivatives,
pyridine derivatives and Schiff bases; finally, the synthesis of Cu
complexes with compounds that are able to bind effectively to Cu,
exhibiting coplanarity/planar structure.
In conclusion, safe and effective metal complexes
are required for the development of novel Cu-based inhibitors. In
addition, Cu complexes may be developed into potent proteasome
inhibitors that target the UPP and therefore may be used to treat
human cancer.
Acknowledgements
The present study was supported by the Project of
Shandong Province Higher Educational Science and Technology Program
(grant no. J15LC22 to Z.Z.), the Projects of Medical and Health
Technology Development Program in Shandong Province (grant no.
2015WS0413 to Z.Z.) and the Doctoral Foundation of Jining Medical
University (grant no. JY14QD06 to Z.Z).
Glossary
Abbreviations
Abbreviations:
|
Cu
|
copper
|
|
DSF
|
disulfiram
|
|
PDTC
|
pyrrolidine dithiocarbomate
|
|
DDTC
|
diethyldithiocarbomate
|
|
CQ
|
clioquinol
|
|
8-OHQ
|
8-hydroxyquinoline
|
|
PHEN
|
1,10-phenanthroline
|
|
PT
|
pyrithione
|
|
UPP
|
ubiquitin-proteasome pathway
|
|
CT
|
chymotrypsin
|
|
AR
|
androgen receptor
|
|
ALDH
|
aldehyde dehydrogenase
|
References
|
1
|
Guo Z and Sadler PJ: Metals in medicine.
Angew Chem Int Edit. 38:1512–1531. 1999. View Article : Google Scholar
|
|
2
|
Schmitt SM, Frezza M and Dou QP: New
applications of old metal-binding drugs in the treatment of human
cancer. Front Biosci (Schol Ed.). 4:375–391. 2012.PubMed/NCBI
|
|
3
|
Verani CN: Metal complexes as inhibitors
of the 26S proteasome in tumor cells. J Inorg Biochem. 106:59–67.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen D, Milacic V, Frezza M and Dou QP:
Metal Complexes, their cellular targets and potential for cancer
therapy. Curr Pharm Des. 15:777–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang CX and Lippard SJ: New metal
complexes as potential therapeutics. Curr Opin Chem Biol.
7:481–499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skrott Z and Cvek B:
Diethyldithiocarbamate complex with copper: The mechanism of action
in cancer cells. Mini Rev Med Chem. 12:1184–1192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berners-Price SJ and Filipovska A: Gold
compounds as therapeutic agents for human diseases. Metallomics.
3:863–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fregona D, Giovagnini L, Ronconi L,
Marzano C, Trevisan A, Sitran S, Biondi B and Bordin F: Pt(II) and
Pd(II) derivatives of ter-butylsarcosinedithiocarbamate. Synthesis,
chemical and biological characterization and in vitro
nephrotoxicity. J Inorg Biochem. 93:181–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen D, Frezza M, Shakya R, Cui QC,
Milacic V, Verani CN and Dou QP: Inhibition of the proteasome
activity by gallium(III) complexes contributes to their anti
prostate tumor effects. Cancer Res. 67:9258–9265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen D, Cui QC, Yang H and Dou QP:
Disulfiram, a clinically used anti-alcoholism drug and
copper-binding agent, induces apoptotic cell death in breast cancer
cultures and xenografts via inhibition of the proteasome activity.
Cancer Res. 66:10425–10433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen D, Cui QC, Yang H, Barrea RA, Sarkar
FH, Sheng S, Yan B, Reddy GP and Dou QP: Clioquinol, a therapeutic
agent for Alzheimer's disease, has proteasome-inhibitory, androgen
receptor-suppressing, apoptosis-inducing, and antitumor activities
in human prostate cancer cells and xenografts. Cancer Res.
67:1636–1644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhai S, Yang L, Cui QC, Sun Y, Dou QP and
Yan B: Tumor cellular proteasome inhibition and growth suppression
by 8-hydroxyquinoline and clioquinol requires their capabilities to
bind copper and transport copper into cells. J Biol Inorg Chem.
15:259–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Kitagaki J, Wang H, Hou DX and
Perantoni AO: Targeting the ubiquitin-proteasome system for cancer
therapy. Cancer Sci. 100:24–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciehanover A, Hod Y and Hershko A: A
heat-stable polypeptide component of an ATP-dependent proteolytic
system from reticulocytes. Biochem Biophys Res Commun.
81:1100–1105. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hershko A, Ciechanover A, Heller H, Haas
AL and Rose IA: Proposed role of ATP in protein breakdown:
Conjugation of protein with multiple chains of the polypeptide of
ATP-dependent proteolysis. Proc Natl Acad Sci USA. 77:1783–1786.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nalepa G, Rolfe M and Harper JW: Drug
discovery in the ubiquitin-proteasome system. Nat. Rev Drug Discov.
5:596–613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adams J: The proteasome: A suitable
antineoplastic target. Nat Rev Cancer. 4:349–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frezza M, Schmitt S and Dou QP: Targeting
the ubiquitin-proteasome pathway: An emerging concept in cancer
therapy. Curr Top Med Chem. 11:2888–2905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wójcik C and DeMartino GN: Intracellular
localization of proteasomes. Int J Biochem Cell Biol. 35:579–2589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nandi D, Tahiliani P, Kumar A and Chandu
D: The ubiquitin-proteasome system. J Biosci. 31:137–155. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Groll M, Heinemeyer W, Jäger S, Ullrich T,
Bochtler M, Wolf DH and Huber R: The catalytic sites of 20S
proteasomes and their role in subunit maturation: A mutational and
crystallographic study. Proc Natl Acad Sci USA. 96:10976–10983.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Via L Dalla, Nardon C and Fregona D:
Targeting the ubiquitin-proteasome pathway with inorganic compounds
to fight cancer: A challenge for the future. Future Med Chem.
4:525–543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
An B, Goldfarb RH, Siman R and Dou QP:
Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective
function and selectively accumulate the cyclin-dependent kinase
inhibitor p27 and induce apoptosis in transformed, but not normal,
human fibroblasts. Cell Death Differ. 5:1062–1075. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rajkumar SV, Richardson PG, Hideshima T
and Anderson KC: Proteasome inhibition as a novel therapeutic
target in human cancer. J Clin Oncol. 23:630–639. 2005.PubMed/NCBI
|
|
25
|
Orlowski RZ and Kuhn DJ: Proteasome
inhibitors in cancer therapy: Lessons from the first decade. Clin
Cancer Res. 14:1649–1657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kane RC, Bross PF, Farrell AT and Pazdur
R: Velcade: U.S. FDA approval for the treatment of multiple myeloma
progressing on prior therapy. Oncologist. 8:508–513. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Potts BC, Albitar MX, Anderson KC,
Baritaki S, Berkers C, Bonavida B, Chandra J, Chauhan D, Cusack JC
Jr, Fenical W, et al: Marizomib, a proteasome inhibitor for all
seasons: Preclinical profile and a framework for clinical trials.
Curr Cancer Drug Tar. 11:254–284. 2011. View Article : Google Scholar
|
|
28
|
Kane RC, Dagher R, Farrell A, Ko CW,
Sridhara R, Justice R and Pazdur R: Bortezomib for the treatment of
mantle cell lymphoma. Clin Cancer Res. 13:5291–5294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuhn DJ, Orlowski RZ and Bjorklund C:
Second generation proteasome inhibitors: Carfilzomib and
immunoproteasome-specific inhibitors (IPSIs). Curr Cancer Drug
Targets. 11:285–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuhn DJ, Chen Q, Voorhees PM, Strader JS,
Shenk KD, Sun CM, Demo SD, Bennett MK, Leeuwen FW, Chanan-Khan AA
and Orlowski RZ: Potent activity of carfilzomib, a novel,
irreversible inhibitor of the ubiquitin-proteasome pathway, against
preclinical models of multiple myeloma. Blood. 110:3281–3290. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kupperman E, Lee EC, Cao Y, Bannerman B,
Fitzgerald M, Berger A, Yu J, Yang Y, Hales P, Bruzzese F, et al:
Evaluation of the proteasome inhibitor MLN9708 in preclinical
models of human cancer. Cancer Res. 70:1970–1980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee EC, Fitzgerald M, Bannerman B, Donelan
J, Bano K, Terkelsen J, Bradley DP, Subakan O, Silva MD, Liu R, et
al: Antitumor activity of the investigational proteasome inhibitor
MLN9708 in mouse models of B-cell and plasma cell malignancies.
Clin Cancer Res. 17:7313–7323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sanchez E, Li M, Steinberg JA, Wang C,
Shen J, Bonavida B, Li ZW, Chen H and Berenson JR: The proteasome
inhibitor CEP-18770 enhances the anti-myeloma activity of
bortezomib and melphalan. Br J Haematol. 148:569–581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Labbe S and Thiele DJ: Pipes and wiring:
The regulation of copper uptake and distribution in yeast. Trends
Microbiol. 7:500–505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tapiero H, Townsend DM and Tew KD: Trace
elements in human physiology and pathology. Copper. Biomed
Pharmacother. 57:386–398. 2003. View Article : Google Scholar
|
|
36
|
Kuo HW, Chen SF, Wu CC, Chen DR and Lee
JH: Serum and tissue trace elements in patients with breast cancer
in Taiwan. Biol Trace Elem Res. 89:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nayak SB, Bhat VR, Upadhyay D and Udupa
SL: Copper and ceruloplasmin status in serum of prostate and colon
cancer patients. Indian J Physiol Pharmacol. 47:108–110.
2003.PubMed/NCBI
|
|
38
|
Diez M, Arroyo M, Cerdàn FJ, Muñoz M,
Martin MA and Balibrea JL: Serum and tissue trace metal levels in
lung cancer. Oncology. 46:230–234. 1989.PubMed/NCBI
|
|
39
|
Turecký L, Kalina P, Uhlíková E, Námerová
S and Krizko J: Serum ceruloplasmin and copper levels in patients
with primary brain tumors. Klin Wochenschr. 62:187–189. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Finney L, Vogt S, Fukai T and Glesne D:
Copper and angiogenesis: Unravelling a relationship key to cancer
progression. Clin Exp Pharmacol Physiol. 36:88–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fox SB, Gasparini G and Harris AL:
Angiogenesis: Pathological, prognostic, and growth-factor pathways
and their link to trial design and anticancer drugs. Lancet Oncol.
2:278–289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rau KM, Huang CC, Chiu TJ, Chen YY, Lu CC,
Liu CT, Pei SN and Wei YC: Neovascularization evaluated by CD105
correlates well with prognostic factors in breast cancers. Exp Ther
Med. 4:231–236. 2012.PubMed/NCBI
|
|
43
|
Daniel KG, Harbach RH, Guida WC and Dou
QP: Copper storage diseases: Menkes, Wilson's, and cancer. Front
Biosci. 9:2652–2662. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Daniel KG, Gupta P, Harbach RH, Guida WC
and Dou QP: Organic copper complexes as a new class of proteasome
inhibitors and apoptosis inducers in human cancer cells. Biochem
Pharmacol. 67:1139–1151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Daniel KG, Chen D, Orlu S, Cui QC, Miller
FR and Dou QP: Clioquinol and pyrrolidine dithiocarbamate complex
with copper to form proteasome inhibitors and apoptosis inducers in
human breast cancer cells. Breast Cancer Res. 7:R897–R908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Daniel KG, Chen D, Yan B and Dou QP:
Copper-binding compounds as proteasome inhibitors and apoptosis
inducers in human cancer. Front Biosci. 12:135–144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Barrea RA, Chen D, Irving TC and Dou QP:
Synchrotron X-ray imaging reveals a correlation of tumor copper
speciation with clioquinol's anticancer activity. J Cell Biochem.
108:96–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ritchie CW, Bush AI and Masters CL:
Metal-protein attenuating compounds and Alzheimer's disease. Expert
Opin Investig Drugs. 13:1585–1592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ritchie CW, Bush AI, Mackinnon A,
Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX,
Tammer A, et al: Metal-protein attenuation with
iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid
deposition and toxicity in Alzheimer disease: A pilot phase 2
clinical trial. Arch Neurol. 60:1685–1691. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Barcia E, Salama A, Fernández-Carballido A
and Negro S: Protective effects of clioquinol on human
neuronal-like cells: A new formulation of clioquinol-loaded PLGA
microspheres for Alzheimer's disease. J Drug Target. 19:637–646.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mao F, Yan J, Li J, Jia X, Miao H, Sun Y,
Huang L and Li X: New multi-target-directed small molecules against
Alzheimer's disease: A combination of resveratrol and clioquinol.
Org Biomol Chem. 12:5936–5944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen D, Peng FY, Cui QC, Daniel KG, Orlu
S, Liu JG and Dou QP: Inhibition of prostate cancer cellular
proteasome activity by a pyrrolidine dithiocarbamate-copper complex
is associated with suppression of proliferation and induction of
apoptosis. Front Biosci. 10:2932–2939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu Z, Wang F, Milacic V, Li X, Cui QC,
Zhang B, Yan B and Dou QP: Evaluation of copper-dependent
proteasome-inhibitory and apoptosis-inducing activities of novel
pyrrolidine dithiocarbamate analogues. Int J Mol Med. 20:919–925.
2007.PubMed/NCBI
|
|
54
|
Wang F, Zhai S, Liu X, Li L, Wu S, Dou QP
and Yan B: A novel dithiocarbamate analogue with potentially
decreased ALDH inhibition has copper-dependent
proteasome-inhibitory and apoptosis-inducing activity in human
breast cancer cells. Cancer Lett. 300:87–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Meyer RE: Prospects for a rational
pharmacotherapy of alcoholism. J Clin Psychiatry. 50:403–412.
1989.PubMed/NCBI
|
|
56
|
Reisinger EC, Kern P, Ernst M, Bock P,
Flad HD and Dietrich M: Inhibition of HIV progression by
dithiocarb. German DTC Study Group. Lancet. 335:679–682. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hersh EM, Brewton G, Abrams D, Bartlett J,
Galpin J, Gill P, Gorter R, Gottlieb M, Jonikas JJ and Landesman S:
Ditiocarb sodium (diethyldithiocarbamate) therapy in patients with
symptomatic HIV infection and AIDS. A randomized, double-blind,
placebo-controlled, multicenter study. JAMA. 265:1538–1544. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pang H, Chen D, Cui QC and Dou QP: Sodium
diethyldithiocarbamate, an AIDS progression inhibitor and a
copper-binding compound, has proteasome-inhibitory and
apoptosis-inducing activities in cancer cells. Int J Mol Med.
19:809–816. 2007.PubMed/NCBI
|
|
59
|
Han J, Li L, Yue X, Chang J, Shi W and Hua
Y: A binuclear complex constituted by diethyldithiocarbamate and
copper(I) functions as a proteasome activity inhibitor in
pancreatic cancer cultures and xenografts. Toxicol Appl Pharmacol.
273:477–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cerchiaro G, Aquilano K, Filomeni G,
Rotilio G, Ciriolo MR and Ferreira AM: Isatin-Schiff base
copper(II) complexes and their influence on cellular viability. J
Inorg Biochem. 99:1433–1440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Adsule S, Barve V, Chen D, Ahmed F, Dou
QP, Padhye S and Sarkar FH: Novel Schiff base copper complexes of
quinoline-2 carboxyaldehyde as proteasome inhibitors in human
prostate cancer cells. J Med Chem. 49:7242–7246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xiao Y, Bi C, Fan Y, Cui C, Zhang X and
Dou QP: L-glutamine Schiff base copper complex as a proteasome
inhibitor and an apoptosis inducer in human cancer cells. Int J
Oncol. 33:1073–1079. 2008.PubMed/NCBI
|
|
63
|
Zhong X, Yi J, Sun J, Wei HL, Liu WS and
Yu KB: Synthesis and crystal structure of some transition metal
complexes with a novel bis-Schiff base ligand and their antitumor
activities. Eur J Med Chem. 41:1090–1092. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Creaven BS, Devereux M, Karcz D, Kellett
A, McCann M, Noble A and Walsh M: Copper(II) complexes of
coumarin-derived Schiff bases and their anti-Candida activity. J
Inorg Biochem. 103:1196–1203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Creaven BS, Czeglédi E, Devereux M, Enyedy
ÉA, Foltyn-Arfa Kia A, Karcz D, Kellett A, McClean S, Nagy NV,
Noble A, et al: Biological activity and coordination modes of
copper(II) complexes of Schiff base-derived coumarin ligands.
Dalton Trans. 39:10854–10865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Duff B, Thangella VR, Creaven BS, Walsh M
and Egan DA: Anti-cancer activity and mutagenic potential of novel
copper(II) quinolinone Schiff base complexes in hepatocarcinoma
cells. Eur J Pharmacol. 689:45–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang X, Bi CF, Fan Y, Cui Q, Chen D, Xiao
Y and Dou QP: Induction of tumor cell apoptosis by taurine Schiff
base copper complex is associated with the inhibition of
proteasomal activity. Int J Mol Med. 22:677–682. 2008.PubMed/NCBI
|
|
68
|
Zuo J, Bi C, Fan Y, Buac D, Nardon C,
Daniel KG and Dou QP: Cellular and computational studies of
proteasome inhibition and apoptosis induction in human cancer cells
by amino acid Schiff base-copper complexes. J Inorg Biochem.
118:83–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang Z, Bi CF, Fan YH, Zhang N, Deshmukh
R, Yan X, Lv X, Zhang P, Zhang X and Dou QP: L-Ornithine Schiff
base-copper and -cadmium complexes as new proteasome inhibitors and
apoptosis inducers in human cancer cells. J Biol Inorg Chem.
20:109–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hindo SS, Frezza M, Tomco D, Heeg MJ,
Hryhorczuk L, McGarvey BR, Dou QP and Verani CN: Metals in
anticancer therapy: Copper(II) complexes as inhibitors of the 20S
proteasome. Eur J Med Chem. 44:4353–4361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang Z, Bi C, Schmitt SM, Fan Y, Dong L,
Zuo J and Dou QP: 1,10-Phenanthroline promotes copper complexes
into tumor cells and induces apoptosis by inhibiting the proteasome
activity. J Biol Inorg Chem. 17:1257–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang S and Zhou J: Ternary copper(II)
complex of 1,10-phenanthroline and glycine: Crystal structure and
interaction with DNA. J Coord Chem. 61:2488–2498. 2008. View Article : Google Scholar
|
|
73
|
Seng HL, Wang WS, Kong SM, Ong HK Alan,
Win YF, Abd Rahman RN Raja, Chikira M, Leong WK, Ahmad M, Khoo AS
and Ng CH: Biological and cytoselective anticancer properties of
copper(II)-polypyridyl complexes modulated by auxiliary methylated
glycine ligand. Biometals. 125:1061–1681. 2012.
|
|
74
|
Ng CH, Kong SM, Tiong YL, Maah MJ, Sukram
N, Ahmade M and Khoo AB: Selective anticancer copper(II)-mixed
ligand complexes: Targeting of ROS and proteasomes. Metallomics.
6:892–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dallavalle F, Gaccioli F, Franchi-Gazzola
R, Lanfranchi M, Marchiò L, Pellinghelli MA and Tegoni M:
Synthesis, molecular structure, solution equilibrium, and
antiproliferative activity of thioxotriazoline and thioxotriazole
complexes of copper II and palladium II. J Inorg Biochem.
92:95–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tardito S, Isella C, Medico E, Marchiò L,
Bevilacqua E, Hatzoglou M, Bussolati O and Franchi-Gazzola R: The
thioxotriazole copper(II) complex A0 induces endoplasmic reticulum
stress and paraptotic death in human cancer cells. J Biol Chem.
284:24306–24319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gandin V, Pellei M, Tisato F, Porchia M,
Santini C and Marzano C: A novel copper complex induces paraptosis
in colon cancer cells via the activation of ER stress signaling. J
Cell Mol Med. 16:142–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Marzano C, Gandin V, Pellei M, Colavito D,
Papini G, Lobbia GG, Del Giudice E, Porchia M, Tisato F and Santini
C: In vitro antitumor activity of the water soluble copper(I)
complexes bearing the tris(hydroxymethyl)phosphine ligand. J Med
Chem. 51:798–808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bortolozzi R, Viola G, Porcù E, Consolaro
F, Marzano C, Pellei M, Gandin V and Basso G: A novel copper(I)
complex induces ER-stress-mediated apoptosis and sensitizes B-acute
lymphoblastic leukemia cells to chemotherapeutic agents.
Oncotarget. 5:5978–5991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu N, Liu C, Li X, Liao S, Song W, Yang
C, Zhao C, Huang H, Guan L, Zhang P, et al: A novel proteasome
inhibitor suppresses tumor growth via targeting both 19S proteasome
deubiquitinases and 20S proteolytic peptidases. Sci Rep.
4:52402014.PubMed/NCBI
|
|
81
|
Gourley M and Williamson JS: Angiogenesis:
New targets for the development of anticancer chemotherapies. Curr
Pharm Des. 6:417–439. 2000. View Article : Google Scholar : PubMed/NCBI
|