Introduction
Atherosclerosis is the most common cause of
cardiovascular disease and is a dynamic pathological process
(1). Previous studies have focused
on the functions of the vascular intima and media, including
endothelial cells and smooth muscle cells (2,3). An
increasing number of studies are investigating the effect of the
vascular adventitia and adventitial fibroblasts, which are
considered to be the most common cell type of the adventitia.
Studies have shown that adventitial fibroblasts are involved in
inflammatory responses, vascular remodeling, restenosis and the
development of atherosclerotic plaques (4,5). The
activation and migration of adventitial fibroblasts contribute to
the early development of atherosclerosis, during which adventitial
remodeling precedes intimal and medial remodeling, and adventitial
fibroblast are the first to proliferate, differentiate into
myofibroblasts, migrate into the intima and contribute to the
formation of atherosclerotic plaques (6). These proliferating and migrated
adventitial fibroblasts are involved in the synthesis and release
of cytokines, and promote intimal and medial inflammation through
its effects on endothelial cells, smooth muscle cells and
macrophages. Activated adventitial fibroblasts can also transform
into myofibroblasts, synthesize collagen, and lead to extracellular
matrix remodeling and restructuring (7). Therefore, the adventitia theory is
becoming important as a complementary process to the development of
atherosclerosis.
Resveratrol is a plant polyphenolic compound present
in red wine and grapes, which has a wide range of effects,
including antioxidant, immunomodulatory and chemopreventive
effects, in biological systems (8). Resveratrol has been confirmed to have
antitumor and cardioprotective potential, is able to repress cancer
development and is involved in anti-inflammatory and
antiproliferative processes (9).
These beneficial effects of resveratrol are considered to be
associated with the sirtuin 1 (SIRT1) protein (10). Resveratrol is a natural activator
of SIRT1, which is an NAD-dependent protein deacetylase. The SIRT1
pathway regulates processes, including cell apoptosis,
proliferation and life span, through the modulation of several
genes and transcription factors, including p53, the caspase family,
nuclear factor (NF)-κB and the forkhead box O (FOXO) family
(10–12). There is now a focus on the effect
of resveratrol on the cardiovascular system. It has been reported
that resveratrol enhances the expression and activity of
endothelial nitric oxide synthase (eNOS) in human endothelial
cells, downregulates the expression of cycloxygenase-2 and
inducible NOS in macrophages, and inhibits aortic smooth muscle
cell proliferation through the NF-κB pathway (13,14).
These factors synergistically contribute to the
anti-atherosclerotic and cardioprotective effects of resveratrol.
However, there are no reports on the effect of resveratrol on
adventitial fibroblasts, which are also considered to be an
important component in the development of atherosclerosis.
The present study aimed to investigate the effects
of resveratrol on rat adventitial fibroblasts in vitro. The
results showed that resveratrol inhibited cell viability, DNA
synthesis and cell migration, and induced the apoptosis of
adventitial fibroblasts in a concentration-dependent manner. These
effects occurred partially through the SIRT1 pathway. As the
activation of adventitial fibroblasts is an important step in the
early development of atherosclerotic plaques, the
antiproliferative, antimigratory and pro-apoptotic potential of
reseveratrol may be a mechanism underlying of its
anti-atherosclerotic effect.
Materials and methods
Ethics statement
Animal experiments were approved by the Soochow
University Scientific and Animal Ethics Committee (Suzhou, China;
approval no. 20120008) and were in compliance with the Chinese
national regulations on the use of experimental animals. The
procedures for animal experiments were performed in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the US National Institutes of Health (revised in 1996).
Reagents and drugs
SIRT1 antibodies were purchased from Abcam
(Cambridge, MA, USA), The BCA Protein Assay kit, AnnexinV/Propidium
Iodide (PI) Apoptosis Detection kit and EdU Imaging kit were
obtained from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). The Cell Counting Kit-8 (CCK-8) was purchased from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Other
materials and chemicals were purchased from commercial
resources.
Cell culture of adventitial
fibroblasts
The present study used 8-week-old male SPF
Sprague-Dawley rats (n=20). All animals were purchased from the
Laboratory Animal Center of Soochow University. They were
maintained on standard diet and water with a 12 h light/dark cycle
at the Animal Center of the First Affiliated Hospital of Soochow
University. Rats were sacrificed using CO2. Adventitial
fibroblasts were prepared from rat thoracic aorta. The adventitial
fibroblasts were prepared from the rat thoracic aorta. Briefly, the
intima and middle layer of the aorta were scraped off, and the
adventitia was sliced into 1 mm3 pieces, which were cultured in
high glucose Dulbecco's modified Eagle's medium (DMEM) with 10%
fetal bovine serum at 5% CO2 and 37°C. After 24 h, fresh
complete medium was added and the cells were cultured until
adventitial fibroblasts grew out. Fresh complete medium was
replaced every 3 days. The cells were stained with α-actin and Von
Willebrand factor to exclude vascular smooth muscle cells and
vascular endothelial cells. The cells were cultured at 37°C in a
humidified atmosphere containing 5% CO2.
RNA interference
Rat small interfering RNA (si)RNA and control
scrambled siRNA were synthesized by Invitrogen; Thermo Fisher
Scientific, Inc. The adventitial fibroblasts at a density of
1×104 were transfected with 100 nmol/l SIRT1 siRNA
(forward 5′-GAAGUUGACCUCCUCAUUGUdTdT-3′ and reverse
5′-ACAAUGAGGAGGUCAACUUCdTdT-3′) using lipofectamine 2000 according
to manufacturer's protocol. The transfected cells were incubated
for 48 h at 37°C prior to subsequent assays. The cells were divided
into four groups, which comprised adventitial fibroblasts treated
with resveratrol (20 or 80 µmol/l) for 24 h at 37°C with or without
transfection with siRNA targeting SIRT1 (20 µmol/l+siRNA or 80
µmol/l+siRNA).
CCK-8 cell proliferation assay
Cell proliferation was assessed using a CCK-8 assay,
based on the enzymatic reduction of WST-8 in living cells and the
production of a proportional color change. Briefly, 1×104 cells
suspended in 150 µl complete medium were seeded in each well of a
96-well culture plate and incubated at 37°C under 5% CO2
for 24 h. The WST-8 cell proliferation reagent (50 µl) was added
and incubated for 4 h at 5% CO2 and 37°C. The negative
control comprised WST-8 reagent and complete medium with no cells.
The absorbance was measured at 450 nm using a spectrophotometer and
the optical density (OD) was calculated as OD = ODsample
- ODcontrol. Each experiment was performed in triplicate
wells and repeated three times.
EdU DNA synthesis assay
The present study used a Click-iT® EdU
Imaging kit to assess cell DNA synthesis. Briefly, the cells
cultured in 6-well plates were incubated with EdU for 24 h.
Following fixation and permeabilization, 0.5 ml
Click-iT® reaction cocktail was added and incubated for
30 min at room temperature in the dark. Hoechst 33342 was used to
stain the cell nuclei. The cells were observed using a fluorescence
microscope, and nuclei undergoing DNA synthesis were stained red.
The numbers of proliferating nuclei were counted in 10 randomly
selected fields (magnification, ×100) and the average was
calculated. The rate of DNA synthesis was calculated as the number
of proliferating nuclei / 100. Each experiment was performed in
triplicate wells and repeated three times.
Cell apoptosis assay
A cell apoptosis assay was performed using an
apoptosis detection kit. The cells were cultured in 6-well plates
to 70% confluence at a density of 1×105 cells/well.
Following digestion with trypsin and washing twice with PBS, the
cells were labeled with 5 µl Annexin V-fluorescein isothiocyanate
(FITC) and 5 µl PI for 5 min each at room temperature in the dark.
The cell suspensions were analyzed by fluorescence-activated cell
sorting with CellQuest version 3.3 (BD Biosciences, San Jose, CA,
USA) software.
Cell migration assay
A Transwell assay was performed to assess cell
migration. Briefly, freshly isolated cells were pre-washed twice
with serum-free DMEM and 5×104 cells were seeded into the upper
chamber of a Transwell plate. The lower chamber contained complete
medium to induce cell migration. Following incubation for 24 h at
5% CO2 and 37°C, the non-migrated cells were scraped off
with a cotton swab, and the migrated cells were stained purple with
0.1% hexamethylpararosaniline and counted in 10 randomly selected
fields (magnification, ×100) using an inverted microscope. The
migration rate was calculated as follows: Migration rate =
migration number(Treated) / migration
number(Control). Each assay was performed in triplicate
wells and repeated three times.
Western blot analysis
The harvested cells were lysed with RIPA lysis
buffer and protease inhibitor cocktail. Protein concentrations were
quantified using a BCA protein assay kit. The proteins (30 µg per
lane) were separated using 10% SDS-polyacrylamide gel
electrophoresis and transferred onto a PVDF membrane. The membrane
was blocked with Tris-buffered saline-Tween 20 (TBS-T) and 5%
nonfat dried milk for 2 h, and incubated with SIRT1 (1:500; cat.
no. 19A7AB4) primary antibody overnight at 4°C. Following washing
twice with TBS-T and incubation with peroxidase-conjugated
secondary antibodies (1:1,000; A996702 Amyjet Scientific, Co.,
Ltd., Wuhan, China) for 1 h at room temperature, the bands were
detected using an chemiluminescence detection system (Invitrogen;
Thermo Fisher Scientific, Inc.). The band intensities were
quantified using the Photo-Image System (Siemens AG, Erfurt,
Germany).
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent experiments. Statistical
analyses were performed using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). Multiple comparisons were performed using
one-way analysis of variance with Scheffe's post-hoc test when the
distributions were normal, or with the non-parametric
Kruskal-Wallis test with a Dunn post-hoc test when the
distributions were not normal. P<0.05 (two-tailed) was
considered to indicate a statistically significant difference.
Results
Resveratrol inhibits adventitial
fibroblast viability and DNA synthesis
The present study used a CCK-8 assay to detect
alterations in cell viability. The CCK-8 results are expressed as
OD and cell viability was positively correlated with the OD index.
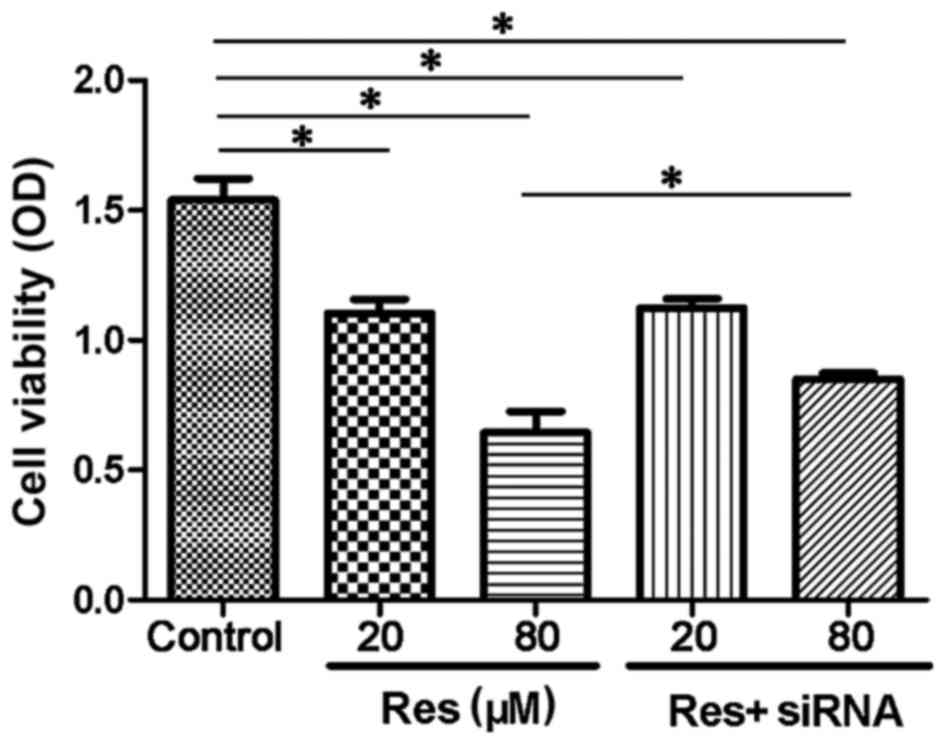
As shown in Fig. 1, resveratrol
inhibited the cell viability of the adventitial fibroblasts in a
concentration-dependent manner. The 20 µmol/l resveratrol group
(1.18±0.15) showed a decreased OD, compared with the control group
(1.56±0.10; P<0.05), and in the 80 µmol/l resveratrol group,
cell viability compared was inhibited further, compared with the
control (0.68±0.16; P<0.05). The cells transfected with siRNA
targeting SIRT1 showed a reversal in cell viability, compared with
the non-transfected cells in the 80 µmol/l+siRNA (0.82±0.02) group
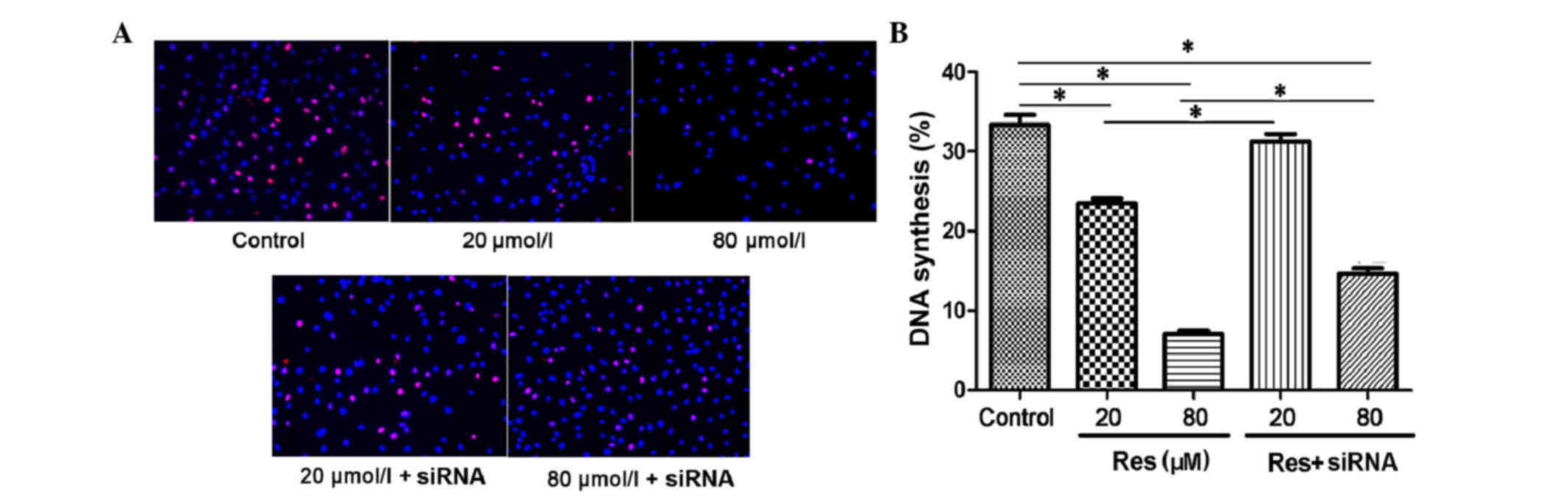
(P<0.05). In the Edu DNA synthesis assay, the proliferating
nuclei were stained red and normal nuclei were blue. As shown in
Fig. 2A, with the increased
concentration of resveratrol, the DNA synthesis of the adventitial
fibroblast was inhibited. The synthesis rates were 33.34 ± 2.85% in
the control, 23.48 ± 1.34% in the 20 µmol/l resveratrol group and
7.04 ± 0.98% in the 80 µmol/l resveratrol group (P<0.05;
Fig. 2B). When transfected with
siRNA, the DNA synthesis inhibition was rescued, with synthesis
rates of 31.28 ± 2.02% in the 20 µmol/l resveratrol + siRNA group
and 14.6 ± 1.57% in the 80 µmol/l resveratrol + siRNA group, which
were higher, compared with that in the non-transfected group
(P<0.05).
High-dose resveratrol induces
adventitial fibroblast apoptosis
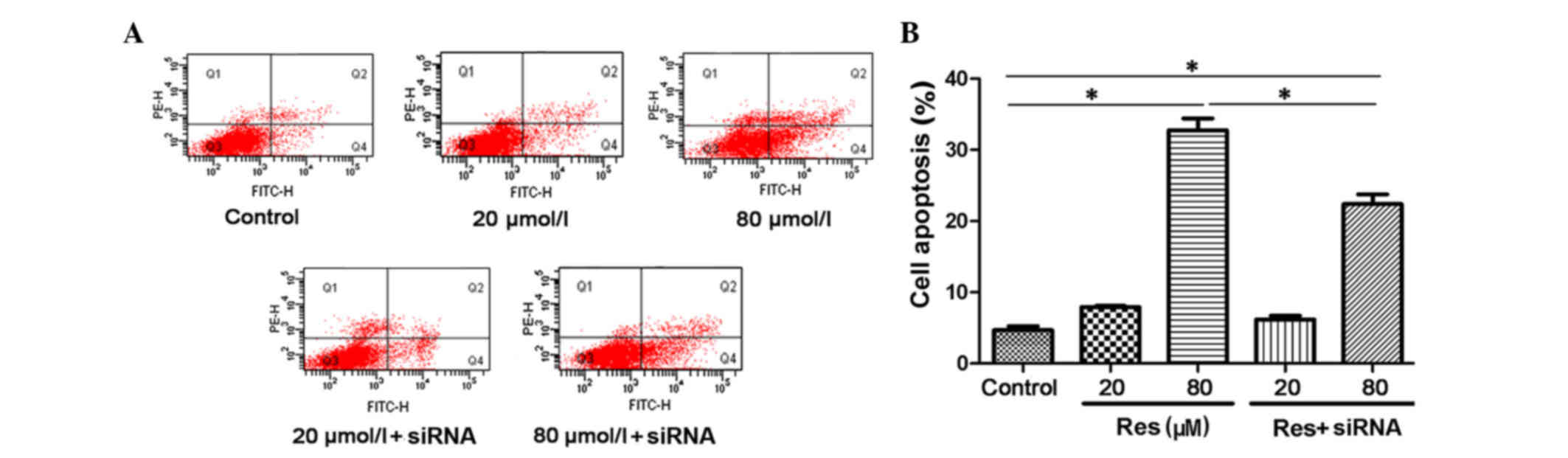
The present study analyzed cell apoptosis using an
AnnexinV/PI immunofluorescent cytometry assay. The proportions of
apoptotic cells are expressed as AnnexinV (FITC)-positive and PI
(phycoerythrin)-negative. As shown in Fig. 3A and B, resveratrol induced cell
apoptosis in a concentration-dependent manner, and this
pro-apoptotic effect was also reversed by siRNA transfection. The
levels of cell apoptosis were 4.7 ± 0.55% in the control, 7.9 ±
0.17% in the 20 µmol/l resveratrol group and 32.73 ± 1.68% in the
80 µmol/l resveratrol group. There was a significant difference
between the 80 µmol/l resveratrol group and the control
(P<0.05). Following siRNA transfection, the cell apoptotic rate
showed a marginal decrease (6.2 ± 0.5%) in the 20 µmol/l
resveratrol + siRNA group. A more marked decrease (22.4 ± 1.36%)
was found in the 80 µmol/l resveratrol+siRNA group, compared with
the corresponding 80 µmol/l resveratrol group (P<0.05). This
suggested that resveratrol induced cell apoptosis through
activation of the SIRT1 pathway.
High-dose resveratrol inhibits
adventitial fibroblast migration
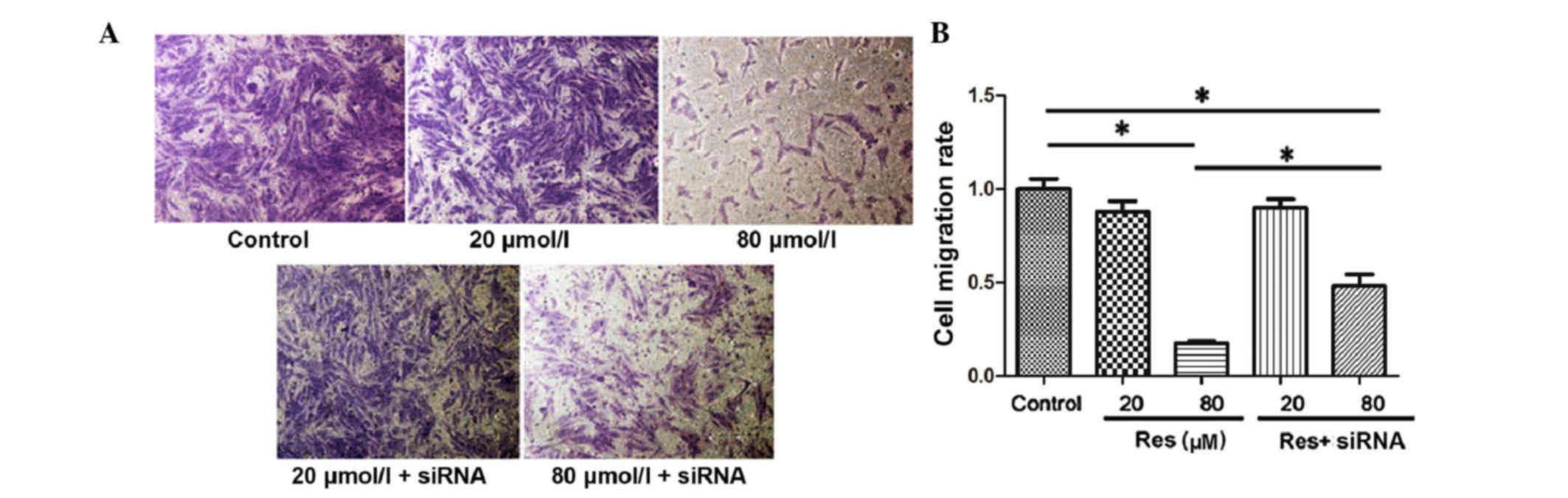
The present study assessed cell migration in
vitro using a Transwell assay and the migrated adventitial
fibroblasts were stained purple (Fig.
4A). By counting the number of migrated cells, it was found
that the migratory ability of the adventitial fibroblasts was
inhibited with the increase of resveratrol concentration. The
migration index was 1.0±0.12 in the control, 0.88±0.13 in the 20
µmol/l resveratrol treatment group and 0.18±0.02 in the 80 µmol/l
resveratrol treatment group. There was a significant difference
between the 80 µmol/l resveratrol treatment group and the control
group (P<0.05). Following inhibition of the SIRT1 pathway with
siRNA, the inhibitory effect of resveratrol on adventitial
fibroblast migration was rescued, and the migration rates were
0.90±0.11 in the 20 µmol/l resveratrol + siRNA group and 0.48±0.14
in the 80 µmol/l resveratrol + siRNA group (80 µmol/l resveratrol,
vs. 80 µmol/l resveratrol+siRNA; P<0.05; Fig. 4B).
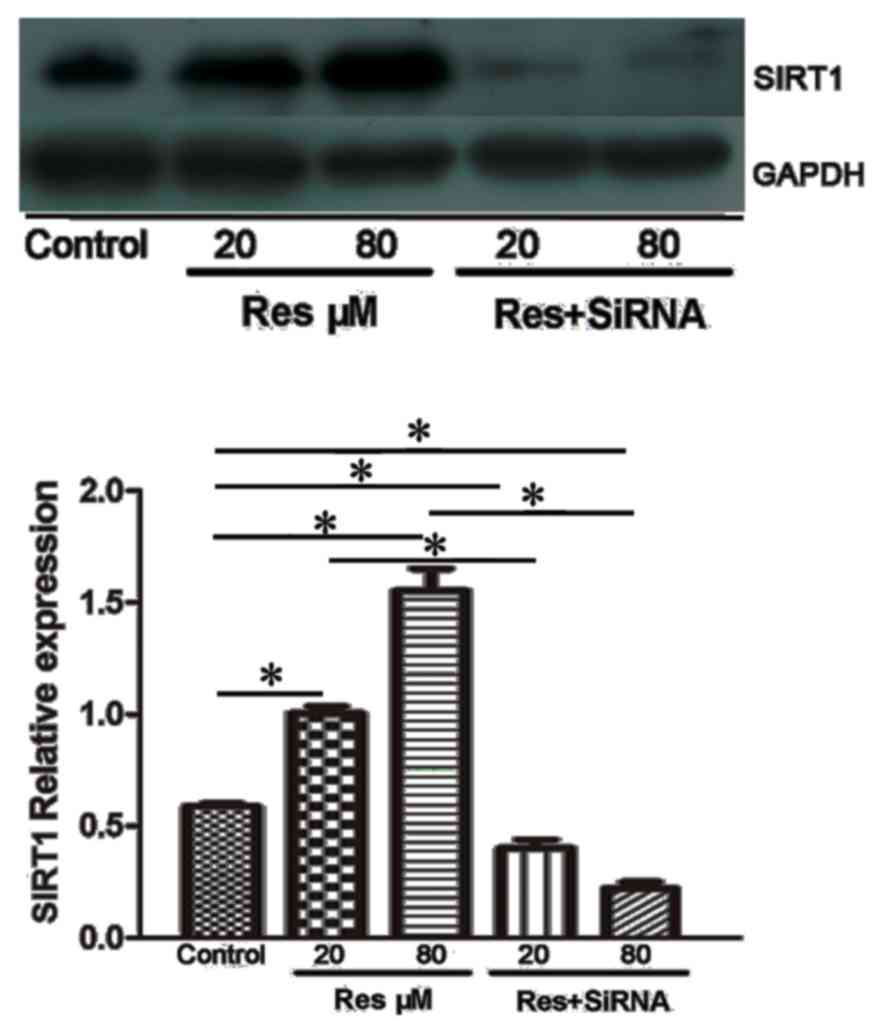
Resveratrol upregulates the expression
of SIRT1
The present study used western blot analysis to
detect alterations in the level of SIRT1. As shown in Fig. 5, the protein expression of SIRT1
was increased following resveratrol treatment. The adjusted protein
expression levels of SIRT1 were 0.59±0.01 in the control, 1.00±0.03
in the 20 µmol/l resveratrol group and 1.55±0.09 in the 80 µmol/l
resveratrol group. Following treatment with SIRT1 siRNA, the
protein expression levels were decreased in the 20 µmol/l + siRNA
group (0.41±0.03), and was significantly different, compared with
that in the 20 µmol/l group (P<0.05). A further decrease in the
protein expression of SIRT1 was found in the 80 µmol/l + siRNA
group, with an expression of 0.22±0.02 (P<0.05, vs. 80 µmol/l +
siRNA group).
Discussion
Increasing evidence has shown that the aorta
adventitia is involved in the development of atherosclerosis and
the process of plaque formation. The adventitia is no longer
determined as a supportive tissue, but is actively involved in the
formation and progression of atherosclerosis (5,15).
Adventitial fibroblasts are the major cell type in the adventitia,
and studies have confirmed that these cells are active in the early
stage of atherosclerosis, proliferating first in plaque formation.
Following proliferation, they differentiate into myofibroblasts and
secrete several inflammatory factors. They also migrate into the
inner layers of the artery wall and affect the kinetics of smooth
muscle cells in the media or endothelial cells in the intima of the
artery wall (16,17). The inflammatory factors secreted by
adventitia fibroblasts inhibit the release of nitric oxide from
endothelial cells, increase the transition of smooth muscle cells
and promote the pathological process of atherosclerosis (18). Therefore, agents or drugs able to
inhibit the proliferation and migration of adventitia fibroblasts
may contribute to the prevention or delay of atherosclerosis
formation and progression.
Resveratrol is a flavor found in grapes, which has
been confirmed to be beneficial to the cardiovascular system as an
anti-atherosclerotic agent (8,9).
Evidence shows that resveratrol is able to inhibit the progression
of atherosclerotic plaques through acting on endothelial cells,
smooth muscle cells or inflammatory cytokines. It weakens the
adherent effect of monocytes to the endothelium and inhibits the
chemotaxis or migration of several inflammatory cells. It can also
inhibit smooth muscle cell proliferation and activation. These
effects may be mediated by activating the AMP-activated protein
kinase and Akt pathways (18,19).
As the adventitia is becoming increasingly recognized as an
important component in the progression of atherosclerosis, the
present study investigated the effect of resveratrol on adventitial
fibroblasts. The results demonstrated that resveratrol inhibited
the proliferation and migration of adventitial fibroblasts, and
induced cell apoptosis in a dose-dependent manner. The EdU assay
showed that resveratrol reduced the DNA synthesis of adventitial
fibroblasts in vitro. As the adventitia and adventitial
fibroblasts are essential in the early stage of atherosclerosis
prior to activation of endothelial cells or smooth muscle cells in
the intima and medial layer of the aorta (2), the results of the present study
suggested that the anti-atherosclerotic effect of resveratrol may
be medicated, at least in part, through the direct inhibition of
cell viability, proliferation and migration, and the induction of
cell apoptosis on adventitial fibroblasts in the adventitia.
The results of the present study showed that the
apparent inhibition of cell survival and induction of cell
apoptosis by resveratrol on adventitial fibroblasts were mediated
by the SIRT1 pathway. Inhibition of the SIRT1 pathway by siRNA
successfully reversed the effects of resveratrol. SIRT1 is an
NAD-dependent protein deacetylase, and the SIRT1 pathway is
essential in the viability, proliferation and differentiation of
various types of cells, including vascular smooth muscle cells and
colon cancer cells (11,19). SIRT1 is able to modulate several
downstream molecules, including the caspase and FOXO families
(13,20). The interaction or balance among
these pathways finally decides the fate of cells. It has been shown
that the activation of SIRT1 in different cells may act in two
ways; one is to protect cells from the apoptosis induced by various
conditions through regulating the expression and activity of the
FOXO family; the other is to induce the activation of the caspase
family and promote cell apoptosis (21,22).
It has been shown that SIRT1 inhibits angiotensin II-induced
vascular smooth muscle cell hypertrophy through modulating
nicotinamide adenine dinucleotide phosphate oxidase 1 and GATA
binding protein 6 (23).
Pretreatment with resveratrol inhibits adipocyte proliferation by
inducing the overexpression of SIRT1 and subsequently reducing the
expression of peroxisome proliferator-activated receptor-γ
(24). The present study showed
that resveratrol treatment upregulated the protein expression of
SIRT1. As resveratrol is a natural activator of SIRT1 and the
overexpression of SIRT1 may further activate caspase 9 (25), the upregulation of SIRT1 was in
correlation with the increased cell apoptosis, decreased cell
viability and altered cell proliferation observed.
This present study focused on alterations in the
cell kinetics of adventitial fibroblasts following resveratrol
treatment in vitro. However, atherosclerosis is a
complicated process and several cell types are involved in this
process, including endothelial cells, smooth muscle cells,
adventitial fibroblasts and monocytes. The interactions among these
cells lead to final plaque formation (3). A previous study showed that
resveratrol has an overall anti-atherosclerotic effect on these
various cell types in vivo (26), therefore, further investigations
aim to investigate the in vitro effect of resveratrol on the
interactions of these cells involved in atherosclerosis.
In conclusion, the present study demonstrated that
resveratrol inhibited cell viability, DNA synthesis and migration,
and induced apoptosis of the adventitial fibroblasts through
activation of the SIRT1 pathway. As adventitial fibroblasts are
important in the development of atherosclerosis, this may be a
mechanism underlying the anti-atherosclerotic effect of
resveratrol.
Acknowledgements
This study was supported by the Youth Science and
Technology of Suzhou Science and Education Project (grant no.
KJXW2013004), the Youth Science Foundation of Jiangsu Province,
China (grant no. BK20140296) and the Science Foundation for Youth
Teacher of Soochow University (grant no. SDY2013A29).
References
|
1
|
Koon CM, Woo KS, Leung PC and Fung KP:
Salviae miltiorrhizae radix and puerariae lobatae radix herbal
formula mediates anti-atherosclerosis by modulating key atherogenic
events both in vascular smooth muscle cells and endothelial cells.
J Ethnopharmacol. 138:175–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tull SP, Anderson SI, Hughan SC, Watson
SP, Nash GB and Rainger GE: Cellular pathology of atherosclerosis:
Smooth muscle cells promote adhesion of platelets to cocultured
endothelial cells. Circ Res. 98:98–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wara AK, Mitsumata M, Yamane T, Kusumi Y
and Yoshida Y: Gene expression in endothelial cells and intimal
smooth muscle cells in atherosclerosis-prone or
atherosclerosis-resistant regions of the human aorta. J Vasc Res.
45:303–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haurani MJ and Pagano PJ: Adventitial
fibroblast reactive oxygen species as autacrine and paracrine
mediators of remodeling: Bellwether for vascular disease?
Cardiovasc Res. 75:679–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu P, Zhang C, Feng JB, Zhao YX, Wang XP,
Yang JM, Zhang MX, Wang XL and Zhang Y: Cross talk among Smad,
MAPK, and integrin signaling pathways enhances adventitial
fibroblast functions activated by transforming growth factor-beta1
and inhibited by Gax. Arterioscler Thromb Vasc Biol. 28:725–731.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai XJ, Chen L, Li L, Feng M, Li X, Zhang
K, Rong YY, Hu XB, Zhang MX, Zhang Y and Zhang M: Adiponectin
inhibits lipopolysaccharide-induced adventitial fibroblast
migration and transition to myofibroblasts via AdipoR1-AMPK-iNOS
pathway. Mol Endocrinol. 24:218–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen WL, Gao PJ, Che ZQ, Ji KD, Yin M, Yan
C, Berk BC and Zhu DL: NAD(P)H oxidase-derived reactive oxygen
species regulate angiotensin-II induced adventitial fibroblast
phenotypic differentiation. Biochem Biophys Res Commun.
339:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruana BF, Lua XQ, Songa J and Zhu HL:
Derivatives of Resveratrol: Potential agents in prevention and
treatment of cardiovascular disease. Curr Med Chem. 19:4175–4183.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomé-Carneiro J, Gonzálvez M, Larrosa M,
Garcia-Almagro FJ, Avilés-Plaza F, Parra S, Yáñez-Gascón MJ,
Ruiz-Ros JA, García-Conesa MT, Tomás-Barberán FA and Espín JC:
Consumption of a grape extract supplement containing resveratrol
decreases oxidized LDL and ApoB in patients undergoing primary
prevention of cardiovascular disease: A triple-blind, 6-month
follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res.
56:810–821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim M, Chung H, Yoon C, Lee E, Kim T, Kwon
M, Lee S, Rhee B and Park J: Increase of INS-1 cell apoptosis under
glucose fluctuation and the involvement of FOXO-SIRT pathway.
Diabetes Res Clin Pract. 98:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peck B, Chen CY, Ho KK, Di Fruscia P,
Myatt SS, Coombes RC, Fuchter MJ, Hsiao CD and Lam EW: SIRT
inhibitors induce cell death and p53 acetylation through targeting
both SIRT1 and SIRT2. Mol Cancer Ther. 9:844–855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho
CT and Pan MH: Xanthigen suppresses preadipocyte differentiation
and adipogenesis through down-regulation of PPARγ and C/EBPs and
modulation of SIRT-1, AMPK, and FoxO pathways. J Agric Food Chem.
60:1094–1101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shakibaei M, Buhrmann C and Mobasheri A:
Resveratrol-mediated SIRT-1 interactions with p300 modulate
receptor activator of NF-kappaB ligand (RANKL) activation of NF-κB
signaling and inhibit osteoclastogenesis in bone-derived cells. J
Biol Chem. 286:11492–11505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simão F, Pagnussat AS, Seo JH, Navaratna
D, Leung W, Lok J, Guo S, Waeber C, Salbego CG and Lo EH:
Pro-angiogenic effects of resveratrol in brain endothelial cells:
Nitric oxide-mediated regulation of vascular endothelial growth
factor and metalloproteinases. J Cereb Blood Flow Metab.
32:884–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Tao J, Zhang J, Tian X, Liu S, Sun
M, Zhang X, Yan C and Han Y: Cellular repressor E1A-stimulated
genes controls phenotypic switching of adventitial fibroblasts by
blocking p38MAPK activation. Atherosclerosis. 225:304–314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Liang C, Liu X, Liao B, Pan X, Ren
Y, Fan M, Li M, He Z, Wu J and Wu Z: AGEs increased migration and
inflammatory responses of adventitial fibroblasts via RAGE, MAPK
and NF-κB pathways. Atherosclerosis. 208:34–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heckenkamp J, Aleksic M, Gawenda M, Breuer
S, Brabender J, Mahdavi A, Aydin F and Brunkwall JS: Modulation of
human adventitial fibroblast function by photodynamic therapy of
collagen matrix. Eur J Vasc Endovasc Surg. 28:651–659. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schreiner CE, Kumerz M, Gesslbauer J,
Schachner D, Joa H, Erker T, Atanasov AG, Heiss EH and Dirsch VM:
Resveratrol blocks Akt activation in angiotensin II- or
EGF-stimulated vascular smooth muscle cells in a redox-independent
manner. Cardiovasc Res. 90:140–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Price NL, Gomes AP, Ling AJ, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Busch F, Mobasheri A, Shayan P, Stahlmann
R and Shakibaei M: Sirt-1 is required for the inhibition of
apoptosis and inflammatory responses in human tenocytes. J Biol
Chem. 287:25770–25781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singh N, Nigam M, Ranjan V, Sharma R,
Balapure AK and Rath SK: Caspase mediated enhanced apoptotic action
of cyclophosphamide- and resveratrol-treated MCF-7 cells. J
Pharmacol Sci. 109:473–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pozo-Guisado E, Merino JM, Mulero-Navarro
S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A and
Fernandez-Salguero PM: Resveratrol-induced apoptosis in MCF-7 human
breast cancer cells involves a caspase-independent mechanism with
downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 115:74–84.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Gao P, Zhang H, Chen H, Zheng W, Lv
X, Xu T, Wei Y, Liu D and Liang C: SIRT1 inhibits angiotensin
II-induced vascular smooth muscle cell hypertrophy. Acta Biochim
Biophys Sin (Shanghai). 43:103–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rahman M, Halade GV, Bhattacharya A and
Fernandes G: The fat-1 transgene in mice increases antioxidant
potential, reduces pro-inflammatory cytokine levels, and enhances
PPAR-gamma and SIRT-1 expression on a calorie restricted diet. Oxid
Med Cell Longev. 2:307–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Wang X and Chen T: Resveratrol
induces mitochondria-mediated AIF and to a lesser extent
caspase-9-dependent apoptosis in human lung adenocarcinoma ASTC-a-1
cells. Mol Cell Biochem. 354:29–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matos RS, Baroncini LA, Précoma LB, Winter
G, Lambach PH, Caron EY, Kaiber F and Précoma DB: Resveratrol
causes antiatherogenic effects in an animal model of
atherosclerosis. Arq Bras Cardiol. 98:136–142. 2012.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|