Introduction
Cardiovascular disease is the leading cause of
mortality in Western countries and ~75% of these cases may be
attributed to atherosclerosis (1).
Unstable atherosclerotic plaque rupture may form occlusive thrombi
that block coronary arteries, which may lead to myocardial
infarction. Currently, no effective therapeutic agents have been
developed to stabilize vulnerable plaques; thus, it is necessary to
develop novel methods and tools to investigate and treat vulnerable
plaques. Gene therapy may be a promising novel strategy for
addressing this clinical problem. Local gene therapy using
adenoviral vectors in the vascular lumen in animal models has been
identified to improve the composition of plaques; however, surgery
is required to administer the treatment (2–4).
Gene intervention aimed at preventing unstable atherosclerotic
plaque rupture appears to be highly effective when a vector that
specifically targets gene expression in the plaque is injected
intravenously. This approach requires determining the appropriate
therapeutic dose to provide localized treatment of the correct
targets. Previous studies have demonstrated that in the
adeno-associated virus 2 (AAV2) platform, the peptides are exposed
on the viral capsid surface, enabling effective receptor binding at
a plaque; therefore, this is a potentially effective targeting
vector (5,6). Atherosclerosis is a chronic disease,
thus, there is a potential need for long-term gene expression.
However, to the best of our knowledge, no previous study has
reported on vectors that specifically target atherosclerotic
plaques at different time points to identify the optimal conditions
for transfection efficiency and safety.
AAV2 is used in various gene therapies due to its
various desirable properties, including in vivo induction of
gene expression for >10 months (7). However, following systemic
administration of AAV2, the vascular cell transfection efficiency
was low (8,9) and a large quantity of the virus was
located in the liver and spleen (10,11).
When AAV2 was compared with other serotypes (12,13),
it was observed that following intravascular injection, the AAV1,
AAV6, AAV8 and AAV9 serotypes passed more efficiently through the
endothelial barrier and were more effective at targeting organ gene
expression (14–18). Among these novel AAV serotypes, AAV
serotype 9 (AAV9) has been identified as an attractive vector based
on its superior performance in transduction of the muscles, heart
and lungs (16,17,19).
Due to its wide range of tissue tropism, particularly in the liver,
when an AAV vector is transfected via the intravascular route, the
liver may act as a large storage location. Tissue-specific
promoters have also been used to reduce unintended gene
intervention; however, their efficiency is lower compared with that
of non-specific promoters, such as the cytomegalovirus (CMV)
promoter (20). Previous studies
have focused on identifying effective AAV serotypes for cardiac or
hepatic gene therapy (16,18,20,21);
however, to the best of our knowledge, there has been no
investigation on AAV9 gene transfer driven by CMV in apolipoprotein
E−/− (ApoE−/−) mice that are prone to form
plaques at different time points.
The most common method for producing recombinant
AAV9 (rAAV9) is by using packaging plasmids in HEK293 cells,
however, the traditional production process is limited by the
difficulty in producing a sufficient number of vectors for
large-scale pre-clinical testing and clinical trials, thereby
hindering the progression of gene therapy (22). Recombinant baculovirus (rBac)-based
systems may produce a large number of AAV vectors (22–24),
thus increasing potential of clinical gene therapy. However, the
biological information regarding rAAV9 vectors produced using an
rBac system remains to be fully elucidated. Thus, the present study
aimed to evaluate whether rAAV9 with the CMV promoter produced
using the rBac system may be systemically transduced into
ApoE−/− mice, that are vulnerable to plaque formation
and to determine the transfection efficiency, safety profile and a
timeline of transduced gene expression.
Materials and methods
Vector design
The AAV9 recombinant vector was purchased from
Virovek (Hayward, CA, USA) and produced using the rBac-based system
in SF9 cells, as previously described (23–25).
rAAV9 vectors were composed of single-stranded DNA containing the
enhanced green fluorescent protein (GFP) gene and driven by the
human cytomegalovirus (CMV) promoter (rAAV9-CMV-GFP). Vector titers
were determined using a quantitative polymerase chain reaction
(qPCR) according to a previously described method (17,26)
with primers corresponding to the CMV enhancer region.
Animals
A total of 40 C57BL/6 and 40 ApoE-/− male mice
(weight, 18–22 g) were bred and housed in a specific pathogen-free
barrier facility at 22–25°C and were purchased from the Peking
University Health Science Center (Beijing, China). Approval for
animal studies was obtained from the Ethics Committee of the First
Affiliated Hospital of Xinjiang Medical University (Urumqi, China).
All the mice used in the present study were aged 8 weeks. The mice
were housed in the Xinjiang Medical University Animal Center with a
12-h light/dark cycle with free access to food and water. C57BL/6
mice were maintained on a normal diet for 16 weeks, and ApoE-/−
mice received a high-fat diet (0.25% cholesterol and 15% cocoa
butter) for 16 weeks. After 16 weeks, 35 C57BL/6 and 35 ApoE-/−
mice were randomly selected for the gene transfection experiments.
Mice were anesthetized with a mixture containing 100 mg/kg
ketamine, 20 mg/kg xylazine and 1.2 mg/kg atropine administered by
intraperitoneal injection. Subsequently, the animals received a
tail vein injection of 100 µl saline containing 5.0×1011
rAAV9-CMV-GFP vector particles. The transduction dose was similar
to that in previous studies, which determined that this dose
induced a specific and efficient transgene expression in the aorta
and in atherosclerotic plaques (27,28).
The remaining 5 C57BL/6 and 5 ApoE-/− mice were injected with 0.09%
saline and served as the control group.
Mice were sacrificed following anesthesia with 100
mg/kg ketamine, 20 mg/kg xylazine and 1.2 mg/kg atropine by
intraperitoneal injection at 14, 21, 28, 35, 60, 90 and 120 days
following virus administration. A total of 5 mice aorta specimens
were acquired at each time point. Prior to sacrifice the mice were
fasted for 12 h. Blood samples were collected by cardiac puncture
at the time of sacrifice and the serum was separated by
centrifugation at 600 × g for 10 min at 4°C. The aorta was
isolated from the aortic arch to the iliac bifurcation. The aorta,
heart, and liver were immediately frozen in liquid nitrogen and
stored at −80°C for further molecular analysis or fresh frozen for
histological studies. The brachiocephalic artery was also removed,
immersed in 4% formaldehyde overnight at 4°C, embedded in optimal
cutting temperature (OCT) compound (Sakura Finetek USA, Inc.,
Torrance, CA, USA), and stored at −80°C.
Quantification of biological
markers
At 14, 21, 28, 35, 60, 90 and 120 days after vector
transduction, blood was collected from the mice via cardiac
puncture for subsequent biochemical testing. Plasma levels of
lactate dehydrogenase (LDH), and creatine kinase (CK) for cardiac
toxicity, aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) for liver toxicity, along with creatinine
and urea nitrogen (BUN) for renal function were determined using an
automatic biochemical analyzer (HITACHI-7600; Hitachi, Ltd., Tokyo,
Japan).
Laser confocal microscopy
The mice were anesthetized and subsequently blood
samples were collected via cardiac puncture from the left ventricle
using a heparin-coated syringe. Subsequently, the left common
carotid artery and the aortic root were excised, immersed in 4%
formaldehyde overnight at 4°C, and embedded in OCT compound. The
entire length of the common carotid artery underwent histological
analysis to ascertain the transfection efficiency of the
rAAV9-CMV-GFP in atherosclerotic plaques. To eliminate background
autofluorescence, Sky Blue 6B (cat. no. C8679; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was used. Frozen sections were
viewed by confocal fluorescence microscopy to identify GFP
expression. In the present study, OCT compound-embedded carotid
artery samples were cross-sectioned into 6-µm thick pieces at 50-µm
intervals. A total of 5 cross-sections from each mouse were
analyzed by confocal fluorescence microscopy. The whole
cross-section was viewed in one field for the quantitative
measurements and the GFP expression percentage values were averaged
(mean ± standard deviation). An automated image analysis system
(Image-Pro Plus version 5.0; Media Cybernetics, Inc., Rockville,
MD, USA) was used for the quantitative measurements. The
GFP-positive area was quantified by computer-assisted color-gated
measurements and the ratio of the positive staining area to the
plaque area was also calculated.
Western blot analysis
Protein extracts from fresh carotid plaques were
separated on 12% SDS-polyacrylamide gels and transferred to
polyvinylidene fluoride membranes. Membranes were blocked with 5%
non-fat milk, then washed with PBS containing 0.1% Tween 20 and
incubated with an appropriate primary antibody at 4°C overnight.
The blots were probed with antibodies against GAPDH (1:1,000;
catalog no. 2118S; Cell Signaling Technology, Inc., Danvers, MA,
USA) and GFP (1:1,000; catalog no. 2956S; Cell Signaling
Technology, Inc.). Following an overnight incubation, the blots
were washed with Tris-buffered saline-Tween-20 and incubated with
an alkaline phosphatase-conjugated secondary antibody at room
temperature for 2 h (catalog no. WP20007; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and then washed again. The
blots were then visualized using diaminobenzidine. Image Lab™
software version 4.0 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used for densitometry. The results were calculated as GFP
gray value/GAPDH gray value.
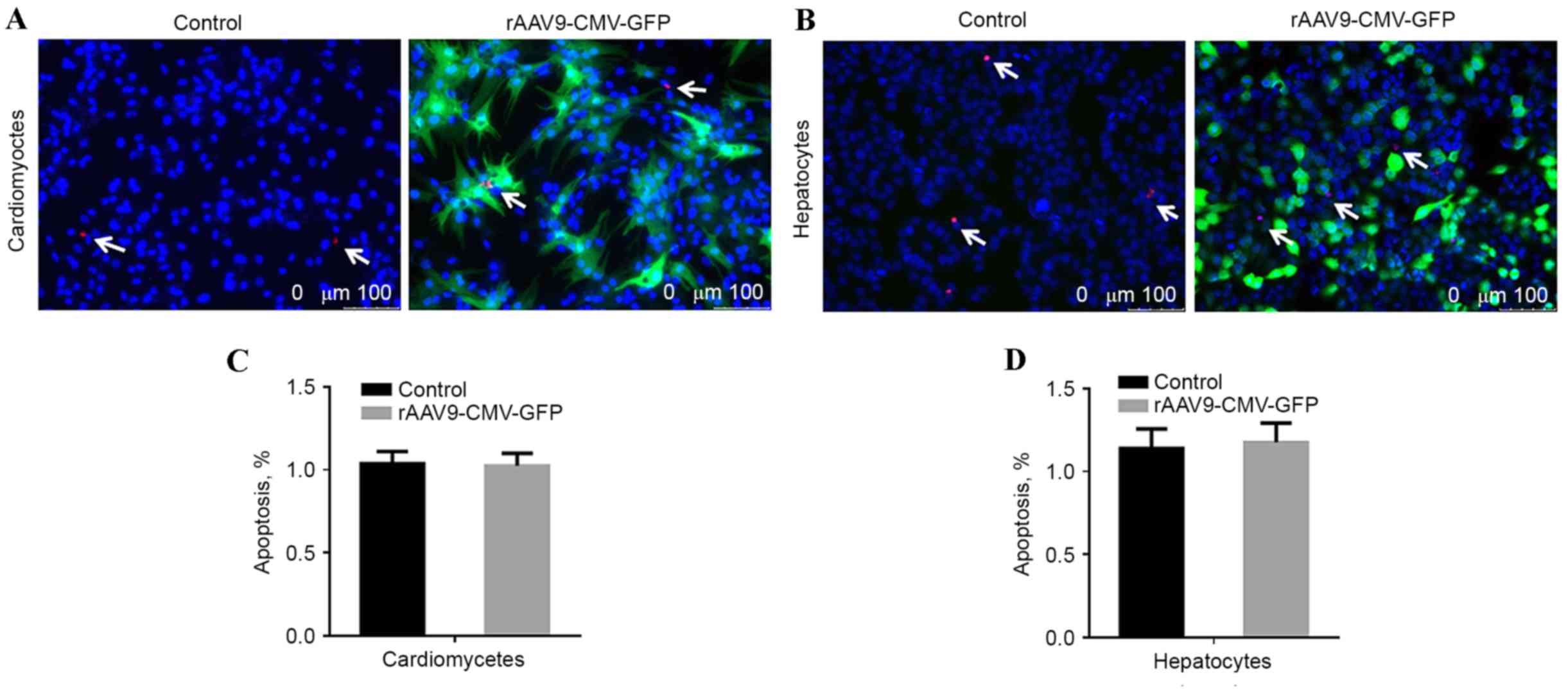
Apoptosis evaluation
To evaluate whether the rAAV9 vector transduction
resulted in increased cytotoxicity, cardiomyocytes and hepatocytes
were seeded at a density of 3×105 cells/well in 12-well plates,
treated with rAAV9-CMV-GFP, and cultured for 4 days prior to a
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) assay. Images were captured using the Leica DM4000 Inverted
fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany)
and the results were analyzed by Image J software version 5.0
(National Institutes of Health, Bethesda, MD, USA). Untreated cells
were used as the control group.
Statistical analysis
Data are presented as the mean ± standard deviation.
Factorial analysis of variance was conducted to analyze the
individual and interactive effects. Multiple comparisons were
analyzed by least significant difference post-hoc tests. All the
analyses were performed using SPSS version 22.0 (IBM SPSS, Armonk,
NY, USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
Quantitative analysis of experimental
model
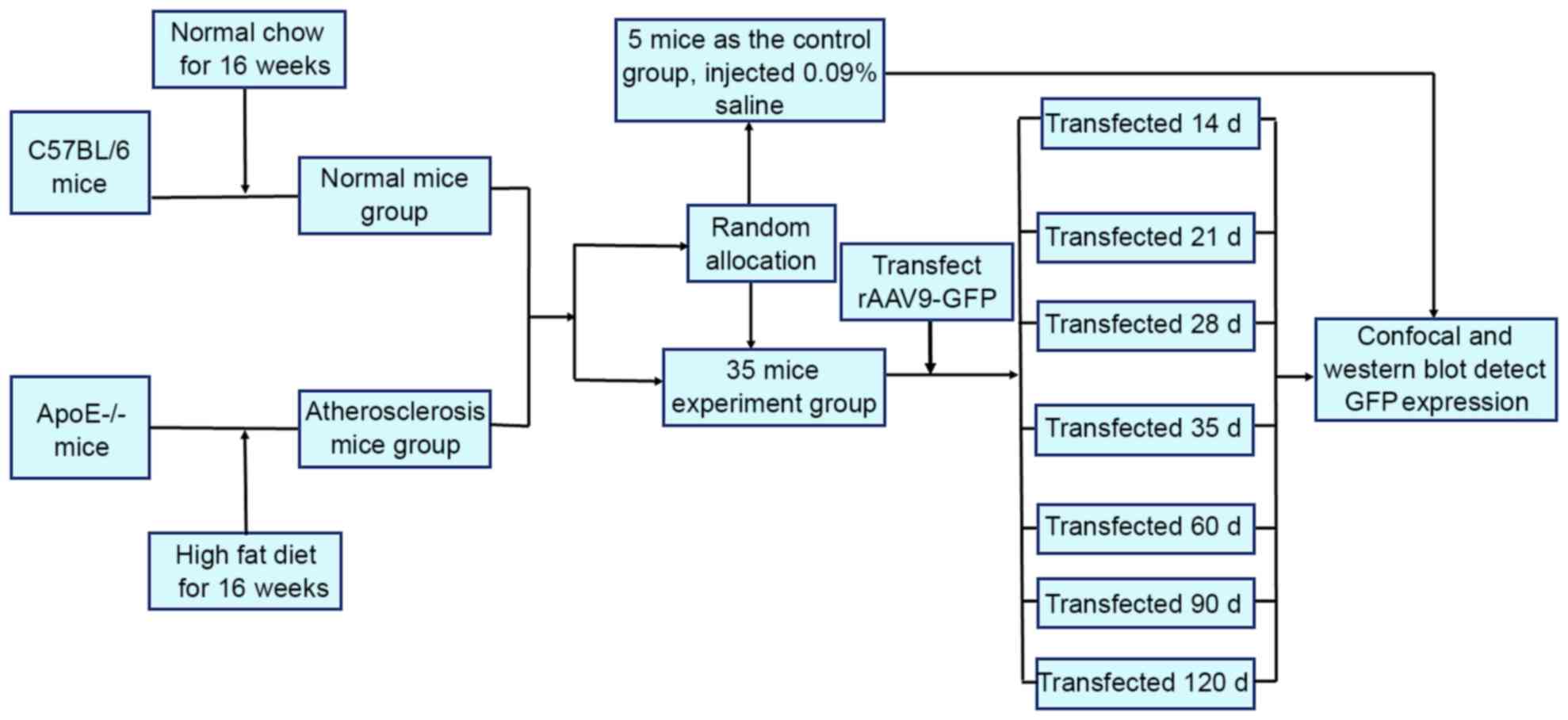
A total of 40 C57BL/6 and 40 ApoE-/− mice were
divided into 8 groups. The experiments were performed without
eliminating any mice, data from all the mice were included in the
analysis. The experimental procedural flow has been presented in
Fig. 1.
Time course of GFP expression in the
aorta of C57BL/6 mice
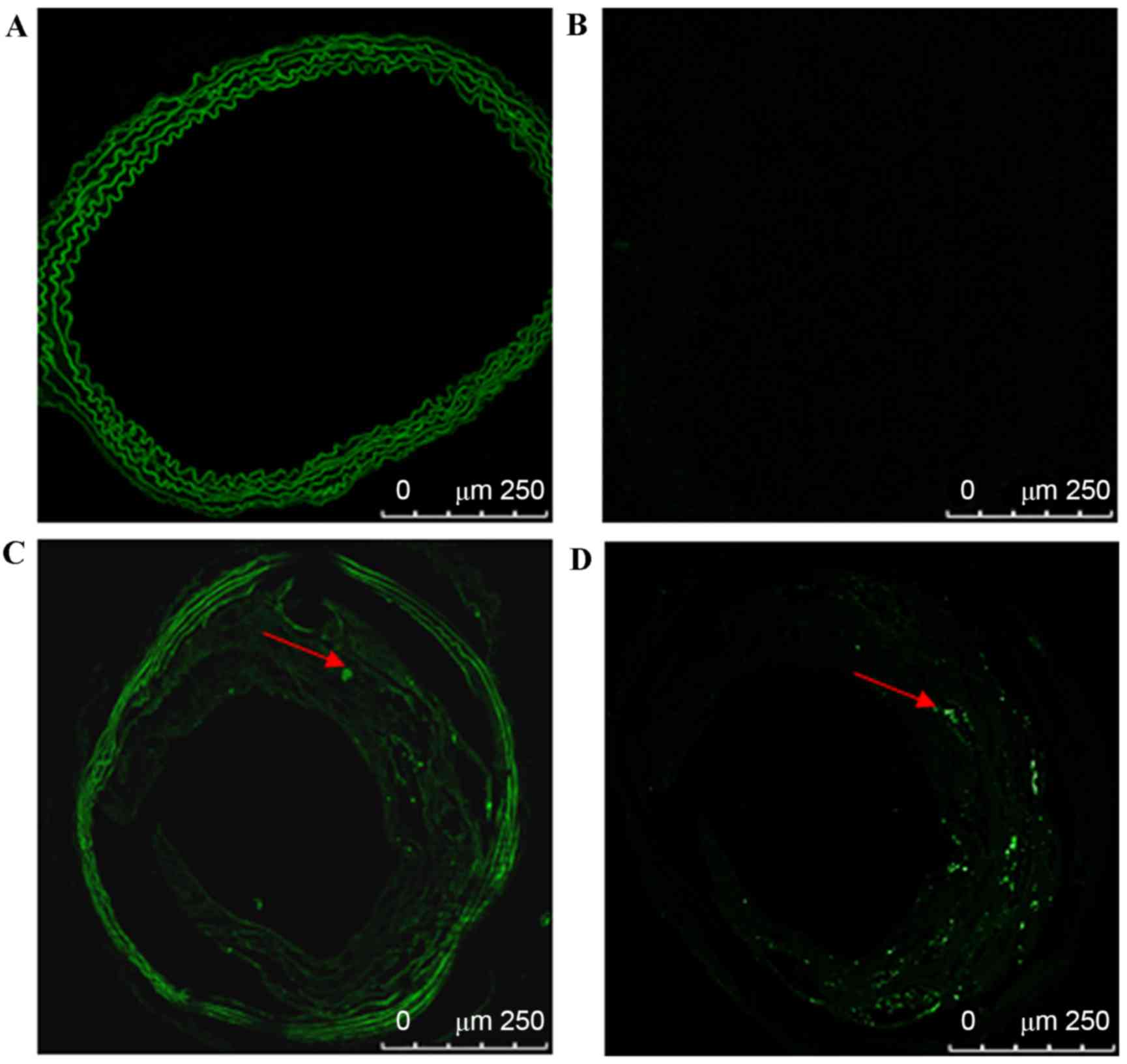
To eliminate background autofluorescence, Sky Blue
6B was used on frozen aorta sections from C57BL/6 and ApoE-/− mice
and vasculature and atherosclerotic plaque autofluorescence were
successfully eliminated. GFP fluorescence in the carotid plaques
was detected at 35 days as presented in Fig. 2.
In C57BL/6 mice, vascular cryosections were viewed
by confocal fluorescence microscopy to identify GFP expression at
different time points. Vascular background autofluorescence was
eliminated by the use of Sky Blue 6B; GFP fluorescence was not
detected in the carotid vasculature at 14, 21, 35, 60, 90, or 120
days after transfection.
Western blot analysis of GFP in the
aorta of C57BL/6 mice
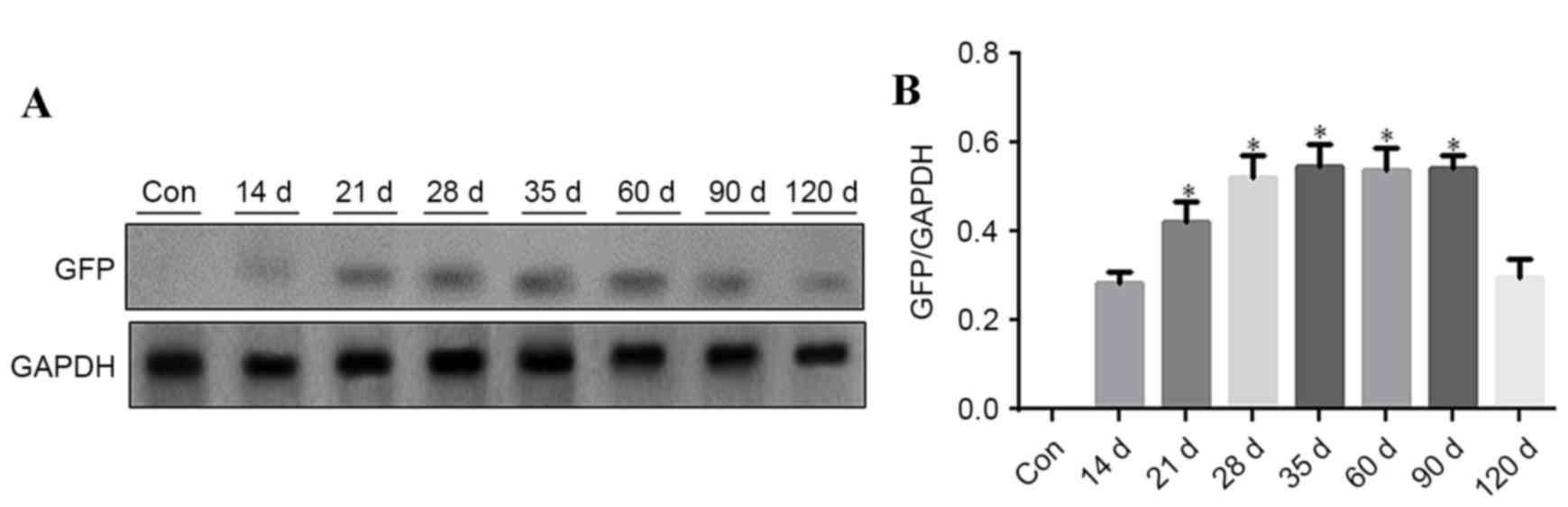
Aorta vasculature tissues were used for western blot
analysis to detect GFP expression at different time points, 5 mice
were analyzed at each time point. GFP was detected at different
time points after rAAV9-GFP transfection. GFP expression level
gradually increased from day 14 onwards, remaining at a
persistently low level, and was reduced at day 120. GFP expression
was significantly increased at 21, 28, 35, 60 and 90 days (Fig. 3; P<0.05 vs. 14 days).
Time course of GFP expression in
aortic plaques in ApoE−/− mice
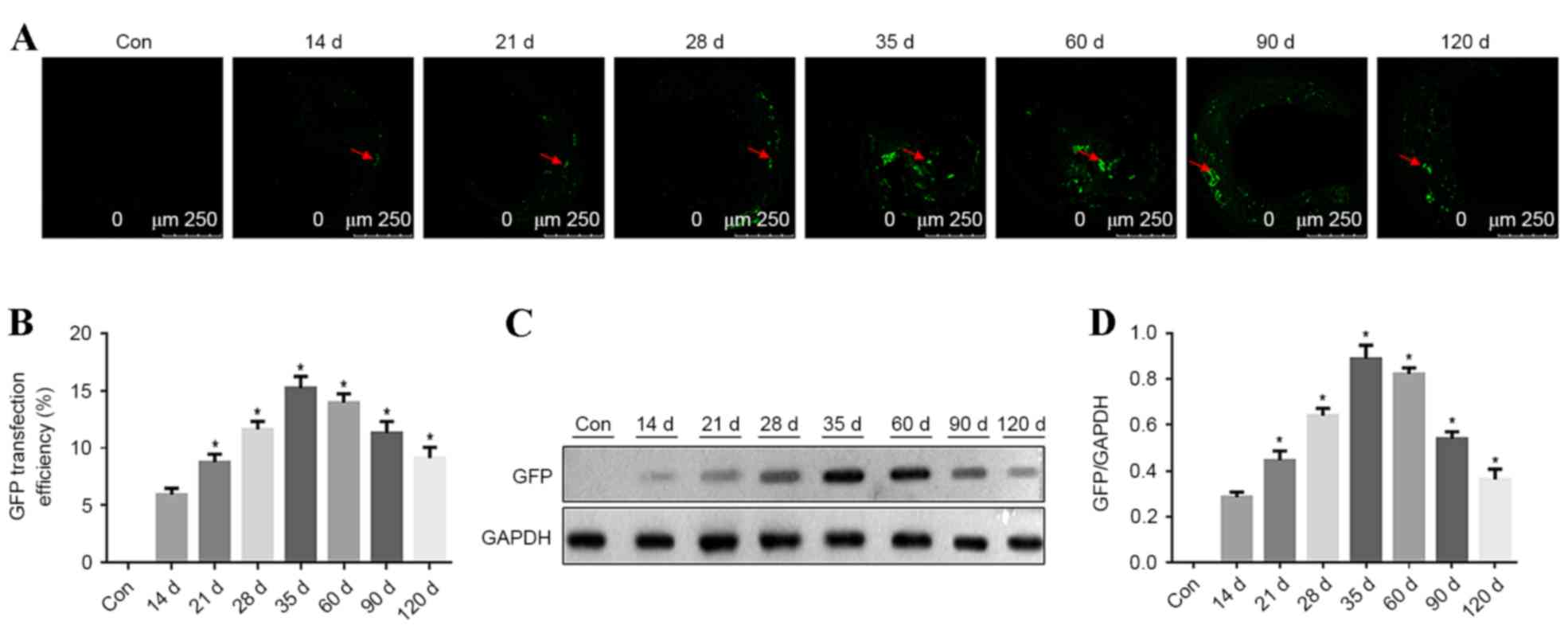
In ApoE-/− mice, the carotid atherosclerotic plaque
cryosections were observed using a confocal fluorescence microscope
to identify GFP expression at different time points. GFP
fluorescence in carotid vascular plaques was detected at 14, 21,
35, 60, 90 and 120 days after transfection. GFP fluorescence in
atherosclerotic plaques steadily increased from 14 days after
transfection. The highest strongest GFP fluorescence was detected
at 35 days after transfection in the atherosclerotic plaques, with
a transfection efficiency of ~15%. Subsequently, the fluorescence
intensity decreased, as presented in Fig. 4A and B (P<0.05 vs. previous time
point). GFP expression in aorta vascular tissues was detected by
western blotting at different time points and 5 mice were analyzed
at each time point. GFP expression was detected at different time
points and it increased with time. GFP expression peaked at 35 days
after transfection. Subsequently, GFP expression was reduced, as
presented in Fig. 4C and D
(P<0.05 vs. previous time point).
Safety analysis
In order to determine the safety of this procedure,
serum levels of LDH, AST, ALT, CK, creatinine and BUN were
determined at different time points in ApoE-/− mice. No significant
difference was identified in the levels of these myocardial enzymes
and markers of liver and kidney function in treated ApoE-/− mice
compared with control mice, as presented in Fig. 5. At 4 days after viral
transfection, cell apoptotic rate was analyzed using TUNEL assay.
No significant difference was identified in the number of
TUNEL-positive cardiomyocytes and hepatic cells compared with
non-transfected control cells, as presented in Fig. 6.
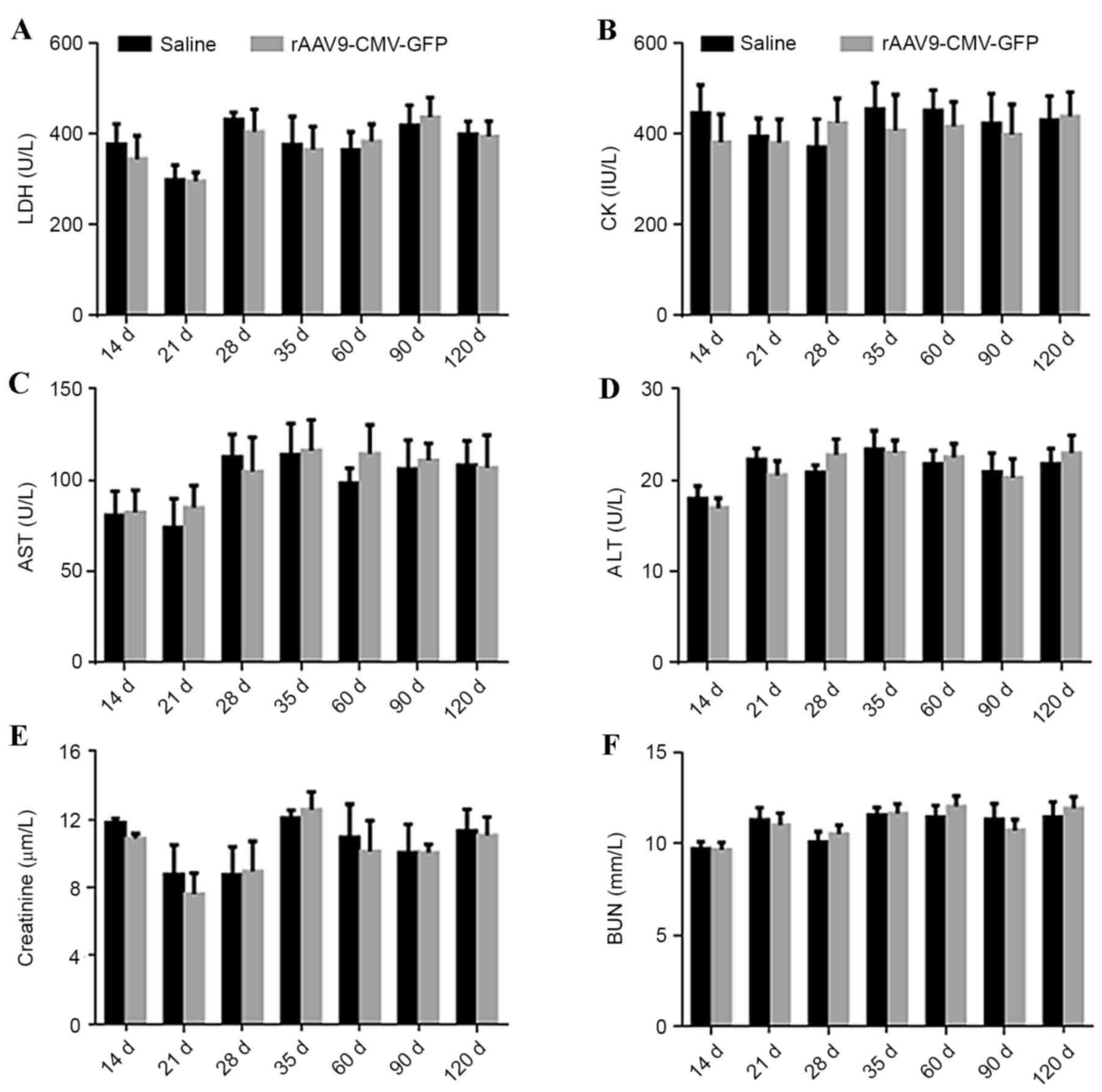 | Figure 5.Evaluation of cardiac and liver damage
and renal function following systemic AAV9 administration in
ApoE−/− mice. Plasma levels of (A) LDH, (B) CK, (C) AST,
(D) ALT, (E) creatinine and (F) BUN from 14 to 120 days following
systemic AAV9-CMV vector administration and normal saline injection
(control) (n=5 per group). LDH, lactate dehydrogenase; CK, creatine
kinase; AST, aspartate aminotransferase; ALT, alanine
aminotransferase; BUN, blood urea nitrogen; rAAV-CMV-GFP,
recombinant adeno-associated virus 9-cytomegalovirus-green
fluorescent protein; ApoE, apolipoprotein E. |
Discussion
The primary challenge of using AAV vectors in
clinical trials is the inability to produce a sufficient quantity
of high purity vectors. The rBac-SF9 system has a 10-fold higher
cost performance compared with the HEK293 system in producing AAV
vectors (24). rAAV9 vectors
produced using the rBac system have been widely used, it is
critical to determine whether vectors produced using this platform
have efficient infectivity, appropriate distribution, long-term
expression and an adequate safety profile. The present study
determined that the rAAV9 viral vectors produced using the rBac
system were effective in vivo and induced enhanced GFP
transgene expression predominantly in ApoE−/− mouse
carotid atherosclerotic plaques for up to 120 days. In addition,
the transfected rAAV9 vectors did not lead to major organ damage,
including cardiomyocyte and hepatic cell injury, and apoptosis.
Sky blue solution was used to eliminate vascular
tissue autofluorescence, which may be detected when localizing the
GFP reporter gene and protein expression by immunofluorescence.
Green immunofluorescence in the C57BL/6 aorta vasculature was low
when the animals were infected with rAAV9-CMV-GFP at a high dose of
5.0×1011 virus genomes/mouse at different time periods.
Additionally, GFP expression levels detected by western blotting
were low. This result is consistent with of a previous study as it
is challenging to transduce the arterial wall using AAV (29). In a previous study, aorta smooth
muscle cells were poorly transduced, changing the route of
administration (from intravenous to intra-arterial injections) did
not increase the AAV9 transfection efficiency in the aorta of
newborn mice (14). Notably, it
was determined that rAAV9 transfection of the mouse aorta was more
efficient in adult mice, highlighting the importance of designing
age-specific gene therapy applications for the aorta. This
age-specific difference in the aorta may due to the fact that that
adult mice have more vascular endothelial cells that can be
infected by rAAV9 compared with newborn mice. Previous studies have
stated hypotheses regarding the underlying mechanisms for the
rate-limiting barriers in systematic rAAV9-mediated gene
transduction (15–18). The present study also determined
that rAAV9-CMV-GFP may be transfected into atherosclerotic lesions,
where it induced persistent gene expression for up to 120 days.
Immunofluorescence staining and western blotting for GFP indicated
peak expression at 35 days, indicating a time-dependent process.
These findings are in agreement with our previous study, which
determined that rAAV9-CMV-GFP may efficiently transfect the heart
for a long time, with peak expression occurring at the same time
point of 35 days (21). This time
delay in peak expression may occur as in order for rAAV9 to mediate
transgene expression, single-stranded DNA should be converted into
double-stranded DNA (25,30). This process may lead to the delayed
expression of genes transfected with rAAV9 compared with other gene
transduction vectors, such as adenovirus and lentivirus (7). Notably, transfection with
rAAV9-CMV-GFP was more challenging in C57/6B mice, whereas the
atherosclerotic lesions in the ApoE−/− mice were
efficiently transfected at higher level and persisted for longer.
The exact molecular mechanism underlying this process remains to be
elucidated. It is possible that the ApoE−/− mouse
lesions contained a greater number of cells, such as smooth muscle
and endothelial cells, and macrophages compared with the
corresponding vasculature in the C57/6B mice. It may also be that
the greater organ and cell tropism of the CMV promoter may lead to
this difference (20). Future
investigations are required to elucidate the mechanism to improve
clinical gene therapy.
The present study also determined that the rAAV9
vector delivery system did not lead to significant cardiac, liver
or kidney damage compared with the control group based on the serum
levels of LDH, CK, AST, ALT, creatinine and BUN detected. No
significant difference was identified in the number of
TUNEL-positive cardiomyocytes and hepatic cells between transfected
and non-transfected control groups. Thus, a high dose of the rAAV9
vector had no marked cellular cytotoxic effects. These findings
indicated that the single-stranded rAAV9-CMV vector may be an
optimal and safe choice for gene therapy of chronic heart disease,
particularly for advanced atherosclerosis. However, the present
study had certain limitations. Initially, no comparisons between
different doses of rAAV9-CMV were made to identify the optimal
dose, the recommended dose reported in a previous study was used
instead. Subsequently, the type of cells transfected by rAAV9-CMV
was not identified, further investigations are required to
determine the transfected cell types to improve targeted gene
therapy.
In conclusion, the present study demonstrated that
the rAAV9 viral vector produced using the rBac system may be
effectively expressed in atherosclerotic plaques. As
atherosclerosis is a chronic condition, long-term gene expression
is required. The current study indicated that this may be achieved
using an rAAV9-based vector. Systemic administration of rAAV9 or
direct transduction of atherosclerotic plaques did not induce major
organ injury and apoptosis. rAAV9 vectors with a CMV promoter may
be used for efficient gene transfer into atherosclerotic plaques in
the future.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81160042).
References
|
1
|
Lewis SJ: Prevention and treatment of
atherosclerosis: A practitioner's guide for 2008. Am J Med. 122(1
Suppl): S38–S50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Nooijer R, Verkleij CJ, von der Thüsen
JH, Jukema JW, van der Wall EE, van Berkel TJ, Baker AH and Biessen
EA: Lesional overexpression of matrix metalloproteinase-9 promotes
intraplaque hemorrhage in advanced lesions but not at earlier
stages of atherogenesis. Arterioscler Thromb Vasc Biol. 26:340–346.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zadelaar AS, von der Thüsen JH, Boesten
LS, Hoeben RC, Kockx MM, Versnel MA, van Berkel TJ, Havekes LM,
Biessen EA and van Vlijmen BJ: Increased vulnerability of
pre-existing atherosclerosis in ApoE-deficient mice following
adenovirus-mediated Fas ligand gene transfer. Atherosclerosis.
183:244–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Von Der Thüsen JH, Kuiper J, Fekkes ML, De
Vos P, Van Berkel TJ and Biessen EA: Attenuation of atherogenesis
by systemic and local adenovirus-mediated gene transfer of
interleukin-10 in LDLr-/− mice. FASEB J. 15:2730–2732.
2001.PubMed/NCBI
|
|
5
|
White K, Büning H, Kritz A, Janicki H,
McVey J, Perabo L, Murphy G, Odenthal M, Work LM, Hallek M, et al:
Engineering adeno-associated virus 2 vectors for targeted gene
delivery to atherosclerotic lesions. Gene Ther. 15:443–451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skubis-Zegadło J, Stachurska A and Małecki
M: Vectrology of adeno-associated viruses (AAV). Med Wieku Rozwoj.
17:202–206. 2013.PubMed/NCBI
|
|
7
|
Pacak CA and Byrne BJ: AAV vectors for
cardiac gene transfer: Experimental tools and clinical
opportunities. Mol Ther. 19:1582–1590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
White SJ, Nicklin SA, Büning H, Brosnan
MJ, Leike K, Papadakis ED, Hallek M and Baker AH: Targeted gene
delivery to vascular tissue in vivo by tropism-modified
adeno-associated virus vectors. Circulation. 109:513–519. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Work LM, Büning H, Hunt E, Nicklin SA,
Denby L, Britton N, Leike K, Odenthal M, Drebber U, Hallek M and
Baker AH: Vascular bed-targeted in vivo gene delivery using
tropism-modified adeno-associated viruses. Mol Ther. 13:683–693.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nathwani AC, Davidoff A, Hanawa H, Zhou
JF, Vanin EF and Nienhuis AW: Factors influencing in vivo
transduction by recombinant adeno-associated viral vectors
expressing the human factor IX cDNA. Blood. 97:1258–1265. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koeberl DD, Alexander IE, Halbert CL,
Russell DW and Miller AD: Persistent expression of human clotting
factor IX from mouse liver after intravenous injection of
adeno-associated virus vectors. Proc Natl Acad Sci USA.
94:1426–1431. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aikawa R, Huggins GS and Snyder RO:
Cardiomyocyte-specific gene expression following recombinant
adeno-associated viral vector transduction. J Biol Chem.
277:18979–18985. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prasad KM, Xu Y, Yang Z, Toufektsian MC,
Berr SS and French BA: Topoisomerase inhibition accelerates gene
expression after adeno-associated virus-mediated gene transfer to
the mammalian heart. Mol Ther. 15:764–771. 2007. View Article : Google Scholar
|
|
14
|
Bostick B, Ghosh A, Yue Y, Long C and Duan
D: Systemic AAV-9 transduction in mice is influenced by animal age
but not by the route of administration. Gene Ther. 14:1605–1609.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gregorevic P, Blankinship MJ, Allen JM,
Crawford RW, Meuse L, Miller DG, Russell DW and Chamberlain JS:
Systemic delivery of genes to striated muscles using
adeno-associated viral vectors. Nat Med. 10:828–834. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inagaki K, Fuess S, Storm TA, Gibson GA,
Mctiernan CF, Kay MA and Nakai H: Robust systemic transduction with
AAV9 vectors in mice: Efficient global cardiac gene transfer
superior to that of AAV8. Mol Ther. 14:45–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pacak CA, Mah CS, Thattaliyath BD, Conlon
TJ, Lewis MA, Cloutier DE, Zolotukhin I, Tarantal AF and Byrne BJ:
Recombinant adeno-associated virus serotype 9 leads to preferential
cardiac transduction in vivo. Circ Res. 99:e3–e9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Zhu T, Qiao C, Zhou L, Wang B,
Zhang J, Chen C, Li J and Xiao X: Adeno-associated virus serotype 8
efficiently delivers genes to muscle and heart. Nat Biotechnol.
23:321–328. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghosh A, Yue Y, Long C, Bostick B and Duan
D: Efficient whole-body transduction with trans-splicing
adeno-associated viral vectors. Mol Ther. 15:750–755. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pacak CA, Sakai Y, Thattaliyath BD, Mah CS
and Byrne BJ: Tissue specific promoters improve specificity of AAV9
mediated transgene expression following intra-vascular gene
delivery in neonatal mice. Genet Vaccines Ther. 6:132008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen BD, He CH, Chen XC, Pan S, Liu F, Ma
X, Li XM, Gai MT, Tao J, Ma YT, et al: Targeting transgene to the
heart and liver with AAV9 by different promoters. Clin Exp
Pharmacol Physiol. 42:1108–1117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Virag T, Cecchini S and Kotin RM:
Producing recombinant adeno-associated virus in foster cells:
Overcoming production limitations using a baculovirus-insect cell
expression strategy. Hum Gene Ther. 20:807–817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H: Intron splicing-mediated
expression of AAV Rep and Cap genes and production of AAV vectors
in insect cells. Mol Ther. 16:924–930. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H: Exploiting the intron-splicing
mechanism of insect cells to produce viral vectors harboring toxic
genes for suicide gene therapy. Mol Ther Nucleic Acids. 1:e572012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geisler A, Jungmann A, Kurreck J, Poller
W, Katus HA, Vetter R, Fechner H and Müller OJ:
microRNA122-regulated transgene expression increases specificity of
cardiac gene transfer upon intravenous delivery of AAV9 vectors.
Gene Ther. 18:199–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo W, Wong S and Bhasin S: AAV-mediated
administration of myostatin pro-peptide mutant in adult Ldlr null
mice reduces diet-induced hepatosteatosis and arteriosclerosis.
PLoS One. 8:e710172013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Foster K, Graham IR, Otto A, Foster H,
Trollet C, Yaworsky PJ, Walsh FS, Bickham D, Curtin NA, Kawar SL,
et al: Adeno-associated virus-8-mediated intravenous transfer of
myostatin propeptide leads to systemic functional improvements of
slow but not fast muscle. Rejuvenation Res. 12:85–94. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baker AH, Kritz A, Work LM and Nicklin SA:
Cell-selective viral gene delivery vectors for the vasculature. Exp
Physiol. 90:27–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Ma HI, Li J, Sun L, Zhang J and
Xiao X: Rapid and highly efficient transduction by double-stranded
adeno-associated virus vectors in vitro and in vivo. Gene Ther.
10:2105–2111. 2003. View Article : Google Scholar : PubMed/NCBI
|