Macrophages are involved in several pathological
conditions, including severe sepsis, autoimmune disorders, cancer
and low-grade inflammatory disorders, such as metabolic syndrome,
atherosclerosis and asthma. Macrophages are essential in regulating
the activation and resolution of immune responses, and can
influence the progression of a disease (1). Cluster of differentiation (CD)163 is
a monocyte/macrophage-associated antigen that has been identified
as a hemoglobin (Hb) scavenger receptor with anti-inflammatory and
immunoregulatory properties. This surface receptor undergoes
ectodomain shedding, triggered by an inflammatory stimulus,
generating the soluble(s) form, sCD163, in plasma (2). CD163 is a scavenger receptor for the
endocytosis of Hb and haptoglobin (Hp)-Hb complexes (3). It is almost exclusively expressed on
monocytes and macrophages, and participates in the modulation of
inflammatory responses (3,4). sCD163 is a novel marker associated
with states of low-grade inflammation characteristic of conditions
such as diabetes, obesity, liver disease and atherosclerosis
(5).
The proteolytic cleavage of monocyte-bound CD163 by
matrix metalloproteinases (MMPs), which is triggered by exposure to
oxidative stress or an inflammatory stimulus, releases sCD163
(6–8). Oxidative stress pathways, induced by
prostaglandin F2α (PGF2α) and 8-iso-PGF2α, enhance the expression
of tumor necrosis factor (TNF)-α and CD163 (8). Lipopolysaccharide (LPS) can also
increase the levels of sCD163 and TNF-α, via stimulation of a
disintegrin and metalloproteinase metallopeptidase domain 17
(ADAM17), which is known to mediate the shedding of the
extracellular domains of CD163 and TNF-α (9). The significant negative correlation,
which was revealed between membrane CD163 expression and sCD163
levels, suggests that plasma sCD163 may be derived from circulating
monocytes, in addition to being secreted by tissue macrophages
(10). sCD163 is constitutively
being shed from the cell surface into the circulation, and it is
stable and easily detectable in serum. The present review focuses
on examining the role of sCD163 in various inflammatory disorders,
including inflammatory disorders of the airways, and specifically
in the pathogenesis of asthma.
Classically activated M1 macrophages are the first
line of defense against bacterial infections and obtain energy
through glycolysis. Cell-surface markers of classically activated
macrophages are not well defined; however, CD40 is predominantly
used (11,12). Conversely, alternatively activated
M2 macrophages, which are CD163+ and CD206+,
are involved in tissue repair and wound healing, and use oxidative
metabolism to fuel their long-term functions.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) and
interferons (IFNs) can enhance the macrophage lineage, and modulate
macrophage differentiation and function. M1 macrophages can be
produced in vitro by culture and subsequent differentiation
of human peripheral blood monocytes. The cytokines M-CSF,
interleukin (IL)-4 and IL-10 stimulate monocyte differentiation
into M2 macrophages (13). M1
macrophages secrete proinflammatory cytokines, such as IL-12 and
TNF-α, and also have antigen-presenting capacity and promote Th1
immune responses.
Conversely, M2 macrophages secrete anti-inflammatory
mediators, such as IL-10, and have poor antigen-presenting
capabilities and stimulate the generation of regulatory T cells
(13–15). The activation of M2 macrophages is
primarily triggered by T helper (Th) 2 cytokines, such as IL-4,
IL-13 and IL-10, as well as anti-inflammatory mediators, such as
glucocorticoids (16).
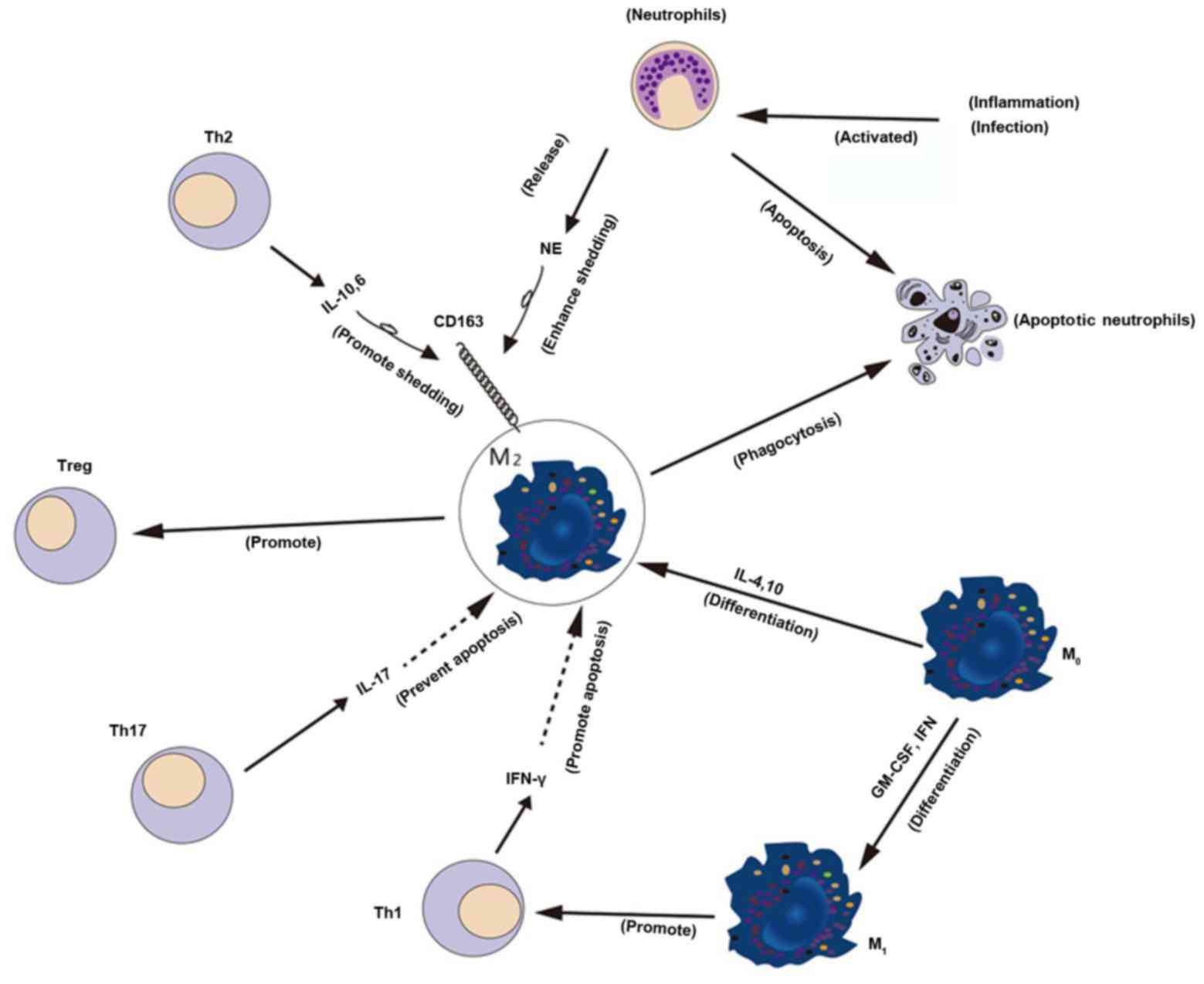
CD163+ M2 macrophages reduce M1 populations through the
release of anti-inflammatory cytokines, such as IL-10. Macrophage
mannose receptor (MRC)-1, IL-13, IL-1 receptor antagonist (IL-1RA)
and CD163 serve important roles in M2 differentiation (17). Monocyte-derived macrophages,
classically activated via IFN-γ priming and LPS stimulation,
demonstrate a decreased CD163 expression; however, the alternative
activation route, involving IL-4/IL-13 priming, does not affect the
expression of CD163 and calprotectin on macrophages (18). The presence of IFN-γ, indicative of
Th1 inflammation, or a prolonged exposure to IL-4, promotes
apoptosis of macrophages and suppresses M2 differentiation, which
leads to a reduction in the clearance of apoptotic neutrophils,
increased accumulation of apoptotic cells and persistent
inflammation (19). Conversely, in
the presence of IL-17, indicative of a Th17 response, macrophage
apoptosis is prevented and M2 differentiation is stimulated, which
ensures that apoptotic neutrophils are cleared efficiently and
anti-inflammatory conditions are restored (Fig. 1) (19). Following IL-4 or IL-13 stimulation,
M2 macrophages derived from peripheral blood mononuclear cells
exhibit markedly increased mRNA expression levels of thymus
activation regulating chemokine (CCL) 11, CCL17, CCL24 and CCL26,
and the production of CCL17 and CCL24 is also potentiated (20).
CD163 is a 130-kDa, type I transmembrane protein,
which belongs to class B of the cysteine-rich scavenger receptor
family, and was first identified in 1987 (21). The expression of CD163 on
circulating monocytes and most tissue macrophages is constitutive
and/or induced by some stimuli (22). CD163 has been reported to bind
human pathogenic bacteria (10,23)
and TNF-α-like weak inducer of apoptosis (TWEAK) (24). Using western blot analysis of CD163
variants, a panel of 10 monoclonal antibodies was mapped to
scavenger receptor cysteine-rich (SRCR) domains 1, 3, 4, 6, 7 and 9
(25). Four of the SRCR domains of
CD163 (domains 2, 3, 7 and 9) have conserved consensus motifs for
Ca2+ binding, whereas domain 5 has a
potentially/semi-conserved Ca2+ binding site. The other
four SRCR domains have at least one non-conservative mutation of an
essential residue in the consensus Ca2+ binding
sequences (26).
Only the two antibodies targeting SRCR domain 3 can
effectively inhibit ligand binding. This is an exposed domain and a
critical factor regulating the Ca2+-sensitive coupling
of Hp-Hb complexes (25). Since
CD163 is a scavenger receptor on the surface of macrophages, its
extracellular region, consisting of nine SRCR domains, can be
stimulated by inflammation or other stimuli, resulting in the
release of its soluble form, sCD163, in the plasma (22,25).
Ligands of Toll-like receptors (TLR) 2, 4 and 5 can stimulate
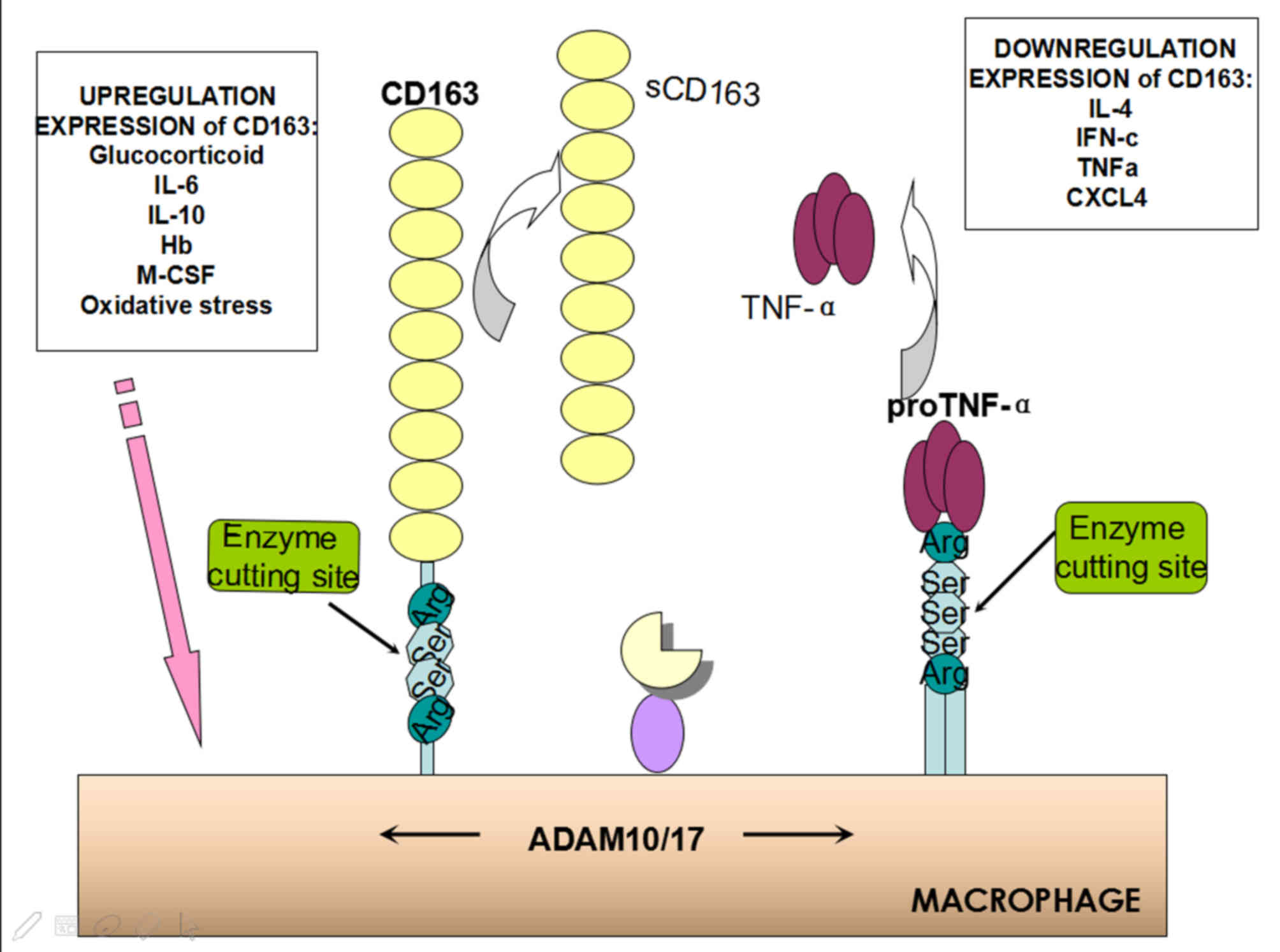
ectodomain shedding of CD163, thereby releasing sCD163 (3). CD163 and pro-TNF-α are transmembrane
proteins subjected to hydrolytic cleavage by the
inflammation-responsive proteases ADAM17 (23,27)
and ADAM10 (23) from the monocyte
surface. This results in the release of sCD163 and bioactive TNF-α
in the circulation. A sequence comparison of their juxtamembrane
region identified similar palindromic sequences in human CD163
(1044Arg-Ser-Ser-Arg) and pro-TNF-α (78Arg-Ser-Ser-Ser-Arg)
(Fig. 2) (27).
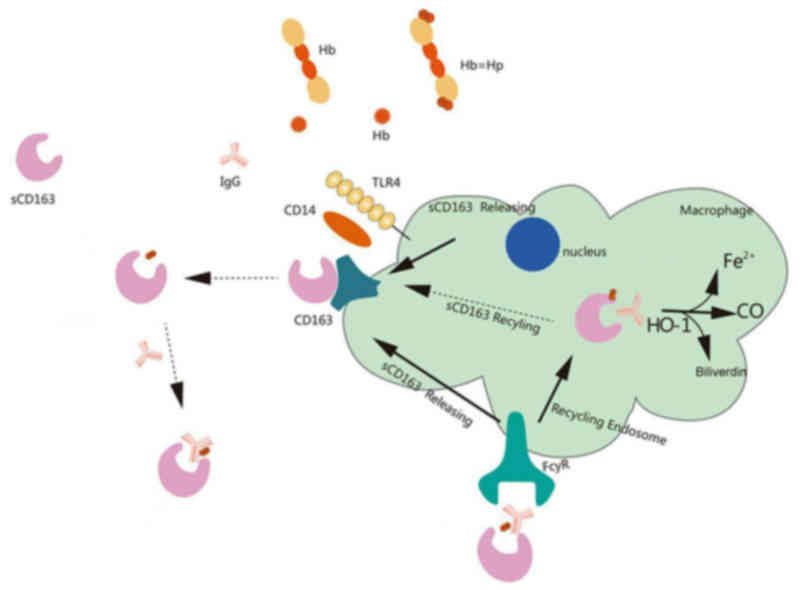
sCD163 and immunoglobulin G interact with the free
Hb in plasma, leading to the endocytosis of the sCD163-Hb-IgG
complex via the Fcγ receptor (FcγR) into monocytes. The endocytosed
sCD163 is recycled to restore the homeostasis of CD163 on the
monocyte membrane, whereas the internalized Hb is catabolized
(28). Paracrine transactivation
of endothelial cells is mediated by the shed sCD163, which
detoxifies and clears residual Hb. Circulating sCD163 only weakly
competes with membrane CD163 for the uptake of Hp-Hb complexes, and
Hp-Hb saturation of sCD163 in serum can only be achieved with a
large surplus of Hp-Hb complex. These findings indicated that the
Hp-Hb complex may be harder to dissociate from the membrane form of
CD163 (Fig. 3) (29).
CD163 is expressed only on cells of the
monocytic-macrophage lineage, and its expression increases as
monocytes mature into macrophages. CD163 expression is particularly
high on macrophages in the liver (Kupffer cells), red pulp of the
spleen, lungs and bone marrow (21). sCD163 is a marker of activated
macrophages (30). Following an
inflammatory stimulus or oxidative stress, sCD163 is released from
the cell surface by proteolytic cleavage of monocyte-bound CD163
through the action of MMPs (6–8) and
after LPS stimulation (22,31),
whereas proinflammatory cytokines, such as TNF, reduce CD163
expression (3). Furthermore, TLR7
levels have been associated with concentrations of IL-10, IL-1RA
and CD163 (32).
Elevated sCD163 serum levels are currently the most
specific marker for distinguishing bacterial infections, such as
brucellosis, or those caused by Staphylococcus aureus and
Haemophilus influenzae (41,42),
from non-bacterial infections, based on previously described
results comparing lumbar puncture with composite reference
standards (43). sCD163 expression
is correlated with levels of IL-6 (21,41),
IL-10 and IL-8 (44), but not with
LPS-binding protein, procalcitonin (PCT) or C-reactive protein
(CRP) levels (41). Plasma levels
of CRP, PCT and sCD163 are increased in patients with bacterial
infections (45). CRP and PCT are
also valuable diagnostic tools and can be used as markers of
bacterial infections. In patients with sepsis, sCD163 levels were
significantly lower than in patients with severe sepsis; however,
sCD163 levels in both groups were considerably increased compared
with in the control group (46,47).
Furthermore, higher sCD163 levels were reported in patients with
sepsis who succumbed compared with in surviving patients (46–48).
MRC and sCD163 expression is markedly increased in septic patients
compared with in non-septic patients and healthy controls (48). Increases in serum sCD163 levels
were delayed in animals that were infected with virulent strains of
Haemophilus parasuis (44).
sCD163 serum levels are elevated in patients with
acute and chronic liver diseases. In patients with cirrhosis,
sCD163 concentration is ~3 times higher compared with in healthy
controls (49,50). In addition, although sCD163 is
linearly associated with the pressure gradient in the portal vein,
its concentration remained unaltered after a transjugular
intrahepatic portosystemic shunt procedure (51). High sCD163 serum levels are
considered an independent risk factor for variceal/gastrointestinal
bleeding, portal hypertension and mortality in patients with
cirrhosis (49,52,53).
High serum sCD163 concentrations have been reported during acute
liver damage, but are lower in acute hepatitis; however, in both
conditions, they are higher than those reported in patients with
chronic hepatitis (12,26,30).
Hepatitis B infection is characterized by higher serum sCD163
levels when compared with hepatitis C, particularly when
accompanied by liver fibrosis (40,54),
whereas higher serum sCD163 concentrations have been associated
with higher mortality (55,56).
sCD163 is also associated with obesity, insulin
resistance, and the development of type 2 diabetes (57–59).
In patients with type 2 diabetes, sCD163 appears strongly
associated with known risk factors, such as physical inactivity,
body mass index, elevated CRP levels and triglyceride content
(60). High serum sCD163 levels
have been associated with complications in patients with type 2 and
type 1 diabetes mellitus (59,61);
conversely, levels of soluble TWEAK, a cytokine that regulates
inflammation, angiogenesis and tissue remodeling, follow an
opposite trend (61). Serum sCD163
levels are significantly higher in obese patients compared with in
lean patients, whereas efferocytosis by M2 macrophages appears to
be impaired in obese patients (61–63).
A low-fat diet reduced the levels of sCD163 (64,65);
however, a 12-week exercise program had no such effect (66).
sCD163 has been associated with arterial
inflammation, non-calcified plaque formation, perivascular fat
accumulation and carotid atherosclerosis (67,68).
Neutrophil elastase has been demonstrated to promote CD163
shedding, and CD163 expression on the surfaces of macrophages was
decreased, resulting in impaired of Hb clearance by macrophages.
These effects may be correlated with acute coronary syndrome and
stable angina pectoris, and may increase the risk of myocardial
infarction (69). It has been
demonstrated that CD163 can bind and neutralize TWEAK (70), whereas sCD163 functions as a decoy
receptor for TWEAK. An imbalance between TWEAK and CD163 could
reflect the progression of atherosclerosis. Furthermore, the
CD163/TWEAK plasma ratio may have potential as a biomarker of
atherosclerosis in asymptomatic individuals (71). Substantially elevated sCD163/sTWEAK
ratios have been reported in patients with critical limb ischemia
and peripheral artery disease (72,73).
Serum sCD163 levels were estimated in patients with
systemic sclerosis (SSc) as an indicator of disease deterioration,
pulmonary fibrosis and pulmonary hypertension (4,6,74,75).
sCD163 levels and the sCD163/sTWEAK ratio were significantly
increased in patients with SSc compared with in controls. Elevated
plasma sCD163 and an increased sCD163/sTWEAK ratio were associated
with a lower risk of digital ulcers in patients with SSc (70).
Early rheumatoid arthritis (RA) patients have
significantly increased sCD163 plasma levels, which are reduced
following treatment. Therefore it may be hypothesized that sCD163
is implicated in RA activity, although an association between
sCD163 and disease activity has yet to be demonstrated (37,76).
sCD163 shed from resident tissue macrophages were abundant in
inflamed synovium (77). In
addition, sCD163 levels have been correlated with the Systemic
Lupus Erythematosus Disease Activity Index (78). Patients with elevated serum sCD163
levels exhibit significantly higher rates of anti-double-strand-DNA
antibodies (79).
Macrophages serve key roles in tumor development and
invasion in several types of human cancer, and sCD163 is a marker
of alternatively activated M2 macrophages. High sCD163
concentrations have been detected in hepatocellular carcinoma
(80), ovarian cancer (81,82),
T cell lymphoma (83) and multiple
myeloma (84). Furthermore,
elevated sCD163 concentrations are associated with a poor prognosis
in patients with cancer (40,81–83).
In conclusion, sCD163 levels in infection, liver
disease, autoimmune disorders, metabolic disease and cancer are
elevated, whereas the clinical significance of this elevation
varies among the various diseases (Table I).
Macrophages have a central role in the regulation
and efficiency of the immune response, and participate in innate
and adaptive immunity. Macrophages exert important functions in
autoimmune disorders, including RA, Crohn's disease, psoriasis,
sarcoidosis and atherosclerosis. M2 macrophages are associated with
responses to anti-inflammatory stimuli and tissue remodeling
(88). Since they participate in
tissue repair and in the restoration of lung microenvironment
homeostasis, M2 macrophages may serve a major role in asthma
(89). Macrophages represent the
majority of immune cells present in lungs under physiological
conditions and serve to dictate the innate defense mechanisms of
the airways. Pulmonary macrophage populations are heterogeneous and
demonstrate notable plasticity, due to variations in their origin,
tissue residency and environmental influences (90). In mice with moderately severe
asthma, the population of M1 macrophages is elevated and negatively
correlated with the population of M2 (CD163+)
macrophages. Decreased numbers of M2-like macrophages are reported
after HDM exposure, and they are negatively correlated with the
number of M1 macrophages (88). In
addition, macrophages have been implicated in the pathogenesis of
chronic obstructive pulmonary disease (COPD). Ex-smokers with COPD
have a higher percentage of CD163+ macrophages in
bronchoalveolar lavage (BAL) than current smokers. Furthermore, the
percentage of CD163+ M2 macrophages is higher in BAL
than in sputum (91). In ovalbumin
(OVA)-sensitized mice, exposure to the airborne particulate matter
PM2.5 caused a slight increase in the number of neutrophils and
macrophages (92). The balance
between macrophage phenotypes fluctuates, depending on the severity
of allergic airway inflammation (88). This balance is regulated by
cytokines, such as IL-13, which is a typical pro-M2-Th2 cytokine
that has been linked to allergic diseases and asthma. MicroRNA
(miR)-155 may also be involved in regulation of the M1/M2 balance
via modulating the effects of IL-13. miR-155 directly targets the
IL-13RA1 gene and reduces the protein levels of IL-13RA1, thus
preventing the activation of the signal transducer and activator of
transcription (STAT) 6 (93).
Serum amyloid P (SAP) inhibits the generation of M2
markers, such as arginase and the chitinase Ym-1, through an
FcγR-dependent mechanism in cultured macrophages. This effect has
been correlated with a decrease in STAT6 phosphorylation in
SAP-treated M2 macrophages (94).
Type 2 cytokines, i.e. IL-4 and IL-13, can drive the
differentiation of macrophages into M2 macrophages. This population
of macrophages is associated with allergic inflammation (95). Monocytes co-cultured with
regulatory T cells display typical features of alternatively
activated macrophages, including upregulated expression of CD206
(macrophage mannose receptor) and CD163, and increased production
of CCL18 (96). OVA-sensitized and
challenged mice exhibit a significant increase in white blood
cells, eosinophilia, mucus accumulation and goblet cell
hyperplasia, which were correlated with increased expression of
genes associated with alternatively activated M2 macrophages, such
as arginase 1, Ym-1, Ym-2, resistin like-α, and
eosinophil-associated, ribonuclease A family member 11. The
expression of other genes associated with asthma, including
FcγRIIb, MMP-14, CCL-8, CCL-17 (20,97),
ADAM-8, lymphotoxin β receptor 1 (LTβR1), aquaporin-9 and IL-7R, is
also upregulated in bronchoalveolar macrophages isolated from
OVA-sensitized/challenged mice compared with in macrophages from
healthy controls (97).
CD163 participates in inflammatory responses and may
contribute to connective tissue remodeling. CD163 may function as a
pulmonary defense element, as suggested by its local expression in
the lungs, and its secretion during lung infection and as part of
inflammatory respiratory responses (98). Cell surface expression of CD163 on
alveolar macrophages is reduced in human subjects with asthma,
which suggests that CD163 may participate in the regulation of
airway inflammatory responses in the lung (85). In addition, sCD163 is inversely
associated with predicted forced expiratory volume in 1 sec in
patients with asthma (9) and COPD,
particularly in those with severe disease (94). During Dermatophagoides
pteronyssinus (Dp)-induced bronchoconstriction, alterations in
monocyte CD163 expression and sCD163 were negatively correlated
with fractional exhaled nitric oxide concentrations (99). Asthma in obese adults has been
associated with impaired macrophage efferocytosis. This impairment
is associated with altered monocyte programming, impaired response
to glucocorticoids and systemic oxidative stress (62). Obese asthmatic children exhibit
increased sCD163 expression, in addition to sex-specific macrophage
activation, which may impair asthma control and lung function
(9). Furthermore, sCD163
concentration in sputum is significantly higher in patients with
allergic asthma compared with in controls. Treatment with inhaled
corticosteroids results in a significant increase in sCD163
concentrations in sputum (33).
Macrophages isolated from sputum samples from patients with asthma
demonstrate significantly higher CCL17 and lower CD163 mRNA
expression levels compared with macrophages from healthy subjects
(100). CD163+
alveolar macrophages were decreased in patients with asthma
(85), whereas sputum sCD163
levels were increased (33). This
inverse relationship between surface and soluble CD163 has already
been described (44). Therefore,
we speculate that airway inflammation and some inflammatory
mediators induce alveolar macrophages to release CD163 from the
cell surface in patients with asthma, and sCD163 participates in
the airway inflammatory response, and the phagocytosis of
CD163+ M2 macrophages is impaired in asthma.
Macrophages serve a key role in the regulation of
immunity and tissue remodeling. CD163, which is a transmembrane
scavenger receptor found on the surface of macrophages, is released
in the circulation in its soluble form, sCD163, via cleavage by
MMPs following oxidative stress or inflammatory stimuli. sCD163 is
involved in the pathogenesis of autoimmune diseases,
atherosclerosis, diabetes and cancer. Bronchial asthma is
characterized by nonspecific inflammation of the airways, and the
alternatively activated CD163+ M2 macrophages have a key
role in this pathological condition. Through phagocytosis and the
subsequent release of biologically active substances, neutrophils
participate in defense mechanisms of the airways. After completing
their mission, neutrophils undergo apoptosis. Macrophages
effectively eliminate apoptotic neutrophils, a process critical in
suppressing acute inflammation and restoring homeostasis.
Neutrophil elastase has been revealed to enhance CD163 shedding,
and sCD163 is a marker of macrophage activation. Neutrophil
elastase serves as a neutrophil activation marker, which suggests
that macrophage activation is associated with the activation of
neutrophils. A Th1/Th2 imbalance has been suggested as an indicator
of the pathogenesis of asthma. Th2 cytokines, such as IL-4, IL-13
and IL-10, can influence M2 macrophage activation. IL-10 and IL-6
promote sCD163 shedding from M2 macrophages, whereas release of
Th17 and IL-17 can inhibit the apoptosis of CD163+ M2
macrophages. In addition, M2 macrophages are associated with T
lymphocytes. IL-4 and IL-13 can stimulate eosinophil activation.
sCD163, a marker of M2 macrophages, is associated with the
eosinophil count through several cytokines. Furthermore, sCD163 is
associated with body mass index in patients with asthma, and the
concentration of sCD163 in plasma or induced sputum is inversely
correlated to predicted forced expiratory volume in 1 sec.
Therefore, investigating the role of sCD163 may contribute to
elucidating the underlying molecular mechanisms of asthma, and may
represent a promising target for the development of effective
therapeutic agents for the treatment of asthma.
|
1
|
Becker M, De Bastiani MA, Parisi MM, Guma
FT, Markoski MM, Castro MA, Kaplan MH, Barbé-Tuana FM and Klamt F:
Integrated transcriptomics establish macrophage polarization
signatures and have potential applications for clinical health and
disease. Sci Rep. 5:133512015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vishwanath P, Prashant A, Nataraj SM,
Kotekar N and Doddamani P: Can soluble CD163 predict outcome of
patients with acute respiratory distress from mechanical
ventilation? A pilot study. Indian J Crit Care Med. 17:355–358.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buechler C, Eisinger K and Krautbauer S:
Diagnostic and prognostic potential of the macrophage specific
receptor CD163 in inflammatory diseases. Inflamm Allergy Drug
Targets. 12:391–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bielecki M, Kowal K, Lapinska A,
Chyczewski L and Kowal-Bielecka O: Increased release of soluble
CD163 by the peripheral blood mononuclear cells is associated with
worse prognosis in patients with systemic sclerosis. Adv Med Sci.
58:126–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomsen HH, Møller HJ, Trolle C, Groth KA,
Skakkebæk A, Bojesen A, Høst C and Gravholt CH: The macrophage
low-grade inflammation marker sCD163 is modulated by exogenous sex
steroids. Endocr Connect. 2:216–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimizu K, Ogawa F, Yoshizaki A, Akiyama
Y, Kuwatsuka Y, Okazaki S, Tomita H, Takenaka M and Sato S:
Increased serum levels of soluble CD163 in patients with
scleroderma. Clin Rheumatol. 31:1059–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabriek BO, Møller HJ, Vloet RP, van
Winsen LM, Hanemaaijer R, Teunissen CE, Uitdehaag BM, van den Berg
TK and Dijkstra CD: Proteolytic shedding of the macrophage
scavenger receptor CD163 in multiple sclerosis. J Neuroimmunol.
187:179–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Timmermann M and Högger P: Oxidative
stress and 8-iso-prostaglandin F (2alpha) induce ectodomain
shedding of CD163 and release of tumor necrosis factor-alpha from
human monocytes. Free Radic Biol Med. 39:98–107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Periyalil HA, Wood LG, Scott HA, Jensen ME
and Gibson PG: Macrophage activation, age and sex effects of
immunometabolism in obese asthma. Eur Respir J. 45:388–395. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davis BH and Zarev PV: Human monocyte
CD163 expression inversely correlates with soluble CD163 plasma
levels. Cytometry B Clin Cytom. 63:16–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Etzerodt A, Maniecki MB, Møller K, Møller
HJ and Moestrup SK: Tumor necrosis factor α-converting enzyme
(TACE/ADAM17) mediates ectodomain shedding of the scavenger
receptor CD163. J Leukoc Biol. 88:1201–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Møller HJ, Aerts H, Grønbaek H, Peterslund
NA, Petersen Hyltoft P, Hornung N, Rejnmark L, Jabbarpour E and
Moestrup SK: Soluble CD163: A marker molecule for
monocyte/macrophage activity in disease. Scand J Clin Lab Invest
Suppl. 237:29–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verreck FA, de Boer T, Langenberg DM, van
der Zanden L and Ottenhoff TH: Phenotypic and functional profiling
of human proinflammatory type-1 and anti-inflammatory type-2
macrophages in response to microbial antigens and IFN-gamma- and
CD40L-mediated costimulation. J Leukoc Biol. 79:285–293. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savage ND, de Boer T, Walburg KV, Joosten
SA, van Meijgaarden K, Geluk A and Ottenhoff TH: Human
anti-inflammatory macrophages induce
Foxp3+GITR+CD25+ regulatory T
cells, which suppress via membrane-bound TGFbeta-1. J Immunol.
181:2220–2226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu W, Roos A, Schlagwein N, Woltman AM,
Daha MR and van Kooten C: IL-10-producing macrophages
preferentially clear early apoptotic cells. Blood. 107:4930–4937.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmieder A, Schledzewski K, Michel J,
Schönhaar K, Morias Y, Bosschaerts T, Van den Bossche J, Dorny P,
Sauer A, Sticht C, et al: The CD20 homolog Ms4a8a integrates pro-
and anti-inflammatory signals in novel M2-like macrophages and is
expressed in parasite infection. Eur J Immunol. 42:2971–2982. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paulus P, Holfeld J, Urbschat A, Mutlak H,
Ockelmann PA, Tacke S, Zacharowski K, Reissig C, Stay D and
Scheller B: Prednisolone as preservation additive prevents from
ischemia reperfusion injury in a rat model of orthotopic lung
transplantation. PLoS One. 8:e732982013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duvel A, Frank C, Schnapper A, Schuberth
HJ and Sipka A: Classically or alternatively activated bovine
monocyte-derived macrophages in vitro do not resemble
CD163/Calprotectin biased macrophage populations in the teat.
Innate Immun. 18:886–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zizzo G and Cohen PL: IL-17 stimulates
differentiation of human anti-inflammatory macrophages and
phagocytosis of apoptotic neutrophils in response to IL-10 and
glucocorticoids. J Immunol. 190:5237–5246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujimura T, Kambayashi Y, Furudate S,
Kakizaki A and Aiba S: A possible mechanism in the recruitment of
eosinophils and Th2 cells through CD163(+) M2 macrophages in the
lesional skin of eosinophilic cellulitis. Eur J Dermatol.
24:180–185. 2014.PubMed/NCBI
|
|
21
|
Onofre G, Koláčková M, Jankovičová K and
Krejsek J: Scavenger receptor CD163 and its biological functions.
Acta Medica (Hradec Kralove). 52:57–61. 2009. View Article : Google Scholar
|
|
22
|
Sulahian TH, Pioli PA, Wardwell K and
Guyre PM: Cross-linking of FcgammaR triggers shedding of the
hemoglobin-haptoglobin scavenger receptor CD163. J Leukoc Biol.
76:271–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kneidl J, Loffler B, Erat MC, Kalinka J,
Peters G, Roth J and Barczyk K: Soluble CD163 promotes recognition,
phagocytosis and killing of Staphylococcus aureus via binding of
specific fibronectin peptides. Cell Microbiol. 14:914–936. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Knudsen TB, Gustafson P, Kronborg G,
Kristiansen TB, Moestrup SK, Nielsen JO, Gomes V, Aaby P, Lisse I,
Møller HJ and Eugen-Olsen J: Predictive value of soluble
haemoglobin scavenger receptor CD163 serum levels for survival in
verified tuberculosis patients. Clin Microbiol Infect. 11:730–735.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madsen M, Møller HJ, Nielsen MJ, Jacobsen
C, Graversen JH, van den Berg T and Moestrup SK: Molecular
characterization of the haptoglobin.hemoglobin receptor CD163.
Ligand binding properties of the scavenger receptor cysteine-rich
domain region. J Biol Chem. 279:51561–51567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maniecki MB, Etzerodt A, Moestrup SK,
Møller HJ and Graversen JH: Comparative assessment of the
recognition of domain-specific CD163 monoclonal antibodies in human
monocytes explains wide discrepancy in reported levels of cellular
surface CD163 expression. Immunobiology. 216:882–890. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Etzerodt A, Rasmussen MR, Svendsen P,
Chalaris A, Schwarz J, Galea I, Møller HJ and Moestrup SK:
Structural basis for inflammation-driven shedding of CD163
ectodomain and tumor necrosis factor-α in macrophages. J Biol Chem.
289:778–788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Subramanian K, Du R, Tan NS, Ho B and Ding
JL: CD163 and IgG codefend against cytotoxic hemoglobin via
autocrine and paracrine mechanisms. J Immunol. 190:5267–5278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Møller HJ, Nielsen MJ, Maniecki MB, Madsen
M and Moestrup SK: Soluble macrophage-derived CD163: A homogenous
ectodomain protein with a dissociable haptoglobin-hemoglobin
binding. Immunobiology. 215:406–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Craig DG, Lee P, Pryde EA, Hayes PC and
Simpson KJ: Serum neopterin and soluble CD163 as markers of
macrophage activation in paracetamol (acetaminophen)-induced human
acute liver injury. Aliment Pharmacol Ther. 38:1395–1404. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hintz KA, Rassias AJ, Wardwell K, Moss ML,
Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP and Guyre
PM: Endotoxin induces rapid metalloproteinase-mediated shedding
followed by up-regulation of the monocyte hemoglobin scavenger
receptor CD163. J Leukoc Biol. 72:711–717. 2002.PubMed/NCBI
|
|
32
|
Salagianni M, Galani IE, Lundberg AM,
Davos CH, Varela A, Gavriil A, Lyytikäinen LP, Lehtimäki T, Sigala
F, Folkersen L, et al: Toll-like receptor 7 protects from
atherosclerosis by constraining ‘inflammatory’ macrophage
activation. Circulation. 126:952–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kowal K, Moniuszko M and Bodzenta-Lukaszyk
A: The effect of inhaled corticosteroids on the concentration of
soluble CD163 in induced sputum of allergic asthma patients. J
Investig Allergol Clin Immunol. 24:49–55. 2014.PubMed/NCBI
|
|
34
|
Goldstein JI, Goldstein KA, Wardwell K,
Fahrner SL, Goonan KE, Cheney MD, Yeager MP and Guyre PM: Increase
in plasma and surface CD163 levels in patients undergoing coronary
artery bypass graft surgery. Atherosclerosis. 170:325–332. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hogger P and Sorg C: Soluble CD163
inhibits phorbol ester-induced lymphocyte proliferation. Biochem
Biophys Res Commun. 288:841–843. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Funding M, Vorum H, Nexø E, Moestrup SK,
Ehlers N and Møller HJ: Soluble CD163 and interleukin-6 are
increased in aqueous humour from patients with endothelial
rejection of corneal grafts. Acta Ophthalmol Scand. 83:234–239.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jude C, Dejica D, Samasca G, Balacescu L
and Balacescu O: Soluble CD163 serum levels are elevated and
correlated with IL-12 and CXCL10 in patients with long-standing
rheumatoid arthritis. Rheumatol Int. 33:1031–1037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kneidl J, Mysore V, Geraci J, Tuchscherr
L, Löffler B, Holzinger D, Roth J and Barczyk-Kahlert K: Soluble
CD163 masks fibronectin-binding protein A-mediated inflammatory
activation of Staphylococcus aureus infected monocytes. Cell
Microbiol. 16:364–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kusi KA, Gyan BA, Goka BQ, Dodoo D,
Obeng-Adjei G, Troye-Blomberg M, Akanmori BD and Adjimani JP:
Levels of soluble CD163 and severity of malaria in children in
Ghana. Clin Vaccine Immunol. 15:1456–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andersen ES, Rødgaard-Hansen S, Moessner
B, Christensen PB, Møller HJ and Weis N: Macrophage-related serum
biomarkers soluble CD163 (sCD163) and soluble mannose receptor
(sMR) to differentiate mild liver fibrosis from cirrhosis in
patients with chronic hepatitis C: A pilot study. Eur J Clin
Microbiol Infect Dis. 33:117–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gaini S, Pedersen SS, Koldkaer OG,
Pedersen C, Moestrup SK and Møller HJ: New immunological serum
markers in bacteraemia: Anti-inflammatory soluble CD163, but not
proinflammatory high mobility group-box 1 protein, is related to
prognosis. Clin Exp Immunol. 151:423–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ayarci AO, Yilmaz E, Sigirli D, Budak F,
Göral G and Oral HB: Diagnostic value of serum concentrations of
high-mobility group-box protein 1 and soluble hemoglobin scavenger
receptor in brucellosis. Microbiol Immunol. 57:150–158. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Knudsen TB, Larsen K, Kristiansen TB,
Møller HJ, Tvede M, Eugen-Olsen J and Kronborg G: Diagnostic value
of soluble CD163 serum levels in patients suspected of meningitis:
Comparison with CRP and procalcitonin. Scand J Infect Dis.
39:542–553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Costa-Hurtado M, Olvera A,
Martinez-Moliner V, Galofré-Milà N, Martínez P, Dominguez J and
Aragon V: Changes in macrophage phenotype after infection of pigs
with Haemophilus parasuis strains with different levels of
virulence. Infect Immun. 81:2327–2333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Carrol ED, Mankhambo LA, Jeffers G, Parker
D, Guiver M, Newland P and Banda DL: IPD Study Group, Molyneux EM,
Heyderman RS: The diagnostic and prognostic accuracy of five
markers of serious bacterial infection in Malawian children with
signs of severe infection. PLoS One. 4:e66212009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cui Y, Zhang YC, Rong QF and Zhu Y:
Changes and significance of soluble CD163 in sepsis and severe
sepsis in children. Zhonghua Er Ke Za Zhi. 50:653–656. 2012.(In
Chinese). PubMed/NCBI
|
|
47
|
Su L, Feng L, Liu C, Jiang Z, Li M, Xiao
K, Yan P, Jia Y, Feng D and Xie L: Diagnostic value of urine sCD163
levels for sepsis and relevant acute kidney injury: A prospective
study. BMC Nephrol. 13:1232012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kjaergaard AG, Rodgaard-Hansen S, Dige A,
Krog J, Møller HJ and Tønnesen E: Monocyte expression and soluble
levels of the haemoglobin receptor (CD163/sCD163) and the mannose
receptor (MR/sMR) in septic and critically ill non-septic ICU
patients. PLoS One. 9:e923312014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gronbaek H, Sandahl TD, Mortensen C,
Vilstrup H, Møller HJ and Møller S: Soluble CD163, a marker of
Kupffer cell activation, is related to portal hypertension in
patients with liver cirrhosis. Aliment Pharmacol Ther. 36:173–180.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang YY, Huang YT, Tsai TH, Hou MC, Lee
FY, Lee SD and Lin HC: Kupffer cell depletion attenuates
leptin-mediated methoxamine-stimulated portal perfusion pressure
and thromboxane A2 release in a rodent model of NASH-cirrhosis.
Clin Sci (Lond). 123:669–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bover LC, Cardo-Vila M, Kuniyasu A, Sun J,
Rangel R, Takeya M, Aggarwal BB, Arap W and Pasqualini R: A
previously unrecognized protein-protein interaction between TWEAK
and CD163: Potential biological implications. J Immunol.
178:8183–8194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Waidmann O, Brunner F, Herrmann E, Zeuzem
S, Piiper A and Kronenberger B: Macrophage activation is a
prognostic parameter for variceal bleeding and overall survival in
patients with liver cirrhosis. J Hepatol. 58:956–961. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang YY, Hou MC, Lin MW, Chen PH, Liao WC,
Chu CJ and Lin HC: Combined platelet count with sCD163 and genetic
variants optimizes esophageal varices prediction in cirrhotic
patients. J Gastroenterol Hepatol. 28:112–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kazankov K, Barrera F, Møller HJ, Bibby
BM, Vilstrup H, George J and Grønbaek H: Soluble CD163, a
macrophage activation marker, is independently associated with
fibrosis in patients with chronic viral hepatitis B and C.
Hepatology. 60:521–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ye H, Wang LY, Zhao J and Wang K:
Increased CD163 expression is associated with acute-on-chronic
hepatitis B liver failure. World J Gastroenterol. 19:2818–2825.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Møller HJ, Grønbaek H, Schiødt FV,
Holland-Fischer P, Schilsky M, Munoz S, Hassanein T and Lee WM:
U.S. Acute Liver Failure Study Group: Soluble CD163 from activated
macrophages predicts mortality in acute liver failure. J Hepatol.
47:671–676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Parkner T, Sorensen LP, Nielsen AR,
Fischer CP, Bibby BM, Nielsen S, Pedersen BK and Møller HJ: Soluble
CD163: A biomarker linking macrophages and insulin resistance.
Diabetologia. 55:1856–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zanni MV, Burdo TH, Makimura H, Williams
KC and Grinspoon SK: Relationship between monocyte/macrophage
activation marker soluble CD163 and insulin resistance in obese and
normal-weight subjects. Clin Endocrinol (Oxf). 77:385–390. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Diaz-López A, Chacón MR, Bulló M,
Maymó-Masip E, Martínez-González MA, Estruch R, Vendrell J, Basora
J, Díez-Espino J, Covas MI and Salas-Salvadó J: Serum sTWEAK
concentrations and risk of developing type 2 diabetes in a high
cardiovascular risk population: A nested case-control study. J Clin
Endocrinol Metab. 98:3482–3490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Møller HJ, Frikke-Schmidt R, Moestrup SK,
Nordestgaard BG and Tybjaerg-Hansen A: Serum soluble CD163 predicts
risk of type 2 diabetes in the general population. Clin Chem.
57:291–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Llauradó G, González-Clemente JM,
Maymó-Masip E, Subías D, Vendrell J and Chacón MR: Serum levels of
TWEAK and scavenger receptor CD163 in type 1 diabetes mellitus:
Relationship with cardiovascular risk factors. A case-control
study. PLoS One. 7:e439192012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fernandez-Boyanapalli R, Goleva E,
Kolakowski C, Min E, Day B, Leung DY, Riches DW, Bratton DL and
Sutherland ER: Obesity impairs apoptotic cell clearance in asthma.
J Allergy Clin Immunol. 131:1041–1047, 1047.e1-e3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shakeri-Manesch S, Zeyda M, Huber J,
Ludvik B, Prager G and Stulnig TM: Diminished upregulation of
visceral adipose heme oxygenase-1 correlates with waist-to-hip
ratio and insulin resistance. Int J Obes (Lond). 33:1257–1264.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Al-Daghri NM, Al-Attas OS, Bindahman LS,
Alokail MS, Alkharfy KM, Draz HM, Yakout S, McTernan PG, Sabico S
and Chrousos GP: Soluble CD163 is associated with body mass index
and blood pressure in hypertensive obese Saudi patients. Eur J Clin
Invest. 42:1221–1226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kračmerová J, Rossmeislová L, Kováčová Z,
Klimčáková E, Polák J, Tencerová M, Mališová L, Štich V, Langin D
and Šiklová M: Soluble CD163 is associated with CD163 mRNA
expression in adipose tissue and with insulin sensitivity in
steady-state condition but not in response to calorie restriction.
J Clin Endocrinol Metab. 99:E528–E535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fjeldborg K, Christiansen T, Bennetzen M,
Møller JH, Pedersen SB and Richelsen B: The macrophage-specific
serum marker, soluble CD163, is increased in obesity and reduced
after dietary-induced weight loss. Obesity (Silver Spring).
21:2437–2443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Longenecker CT, Jiang Y, Yun CH, Debanne
S, Funderburg NT, Lederman MM, Storer N, Labbato DE, Bezerra HG and
McComsey GA: Perivascular fat, inflammation, and cardiovascular
risk in HIV-infected patients on antiretroviral therapy. Int J
Cardiol. 168:4039–4045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Knudsen A, Møller HJ, Katzenstein TL,
Gerstoft J, Obel N, Kronborg G, Benfield T, Kjaer A and Lebech AM:
Soluble CD163 does not predict first-time myocardial infarction in
patients infected with human immunodeficiency virus: A nested
case-control study. BMC Infect Dis. 13:2302013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Levy AP, Purushothaman KR, Levy NS,
Purushothaman M, Strauss M, Asleh R, Marsh S, Cohen O, Moestrup SK,
Moller HJ, et al: Downregulation of the hemoglobin scavenger
receptor in individuals with diabetes and the Hp 2–2 genotype:
Implications for the response to intraplaque hemorrhage and plaque
vulnerability. Circ Res. 101:106–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kowal-Bielecka O, Bielecki M, Guiducci S,
Trzcinska-Butkiewicz B, Michalska-Jakubus M, Matucci-Cerinic M,
Brzosko M, Krasowska D, Chyczewski L and Kowal K: High serum
sCD163/sTWEAK ratio is associated with lower risk of digital ulcers
but more severe skin disease in patients with systemic sclerosis.
Arthritis Res Ther. 15:R692013. View
Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shaked I, Hanna DB, Gleißner C, Marsh B,
Plants J, Tracy D, Anastos K, Cohen M, Golub ET, Karim R, et al:
Macrophage inflammatory markers are associated with subclinical
carotid artery disease in women with human immunodeficiency virus
or hepatitis C virus infection. Arterioscler Thromb Vasc Biol.
34:1085–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Urbonaviciene G, Martin-Ventura JL,
Lindholt JS, Urbonavicius S, Moreno JA, Egido J and Blanco-Colio
LM: Impact of soluble TWEAK and CD163/TWEAK ratio on long-term
cardiovascular mortality in patients with peripheral arterial
disease. Atherosclerosis. 219:892–899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Moreno JA, Dejouvencel T, Labreuche J,
Smadja DM, Dussiot M, Martin-Ventura JL, Egido J, Gaussem P,
Emmerich J, Michel JB, et al: Peripheral artery disease is
associated with a high CD163/TWEAK plasma ratio. Arterioscler
Thromb Vasc Biol. 30:1253–1262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Moreno JA, Ortega-Gómez A, Delbosc S,
Beaufort N, Sorbets E, Louedec L, Esposito-Farèse M, Tubach F,
Nicoletti A, Steg PG, et al: In vitro and in vivo evidence for the
role of elastase shedding of CD163 in human atherothrombosis. Eur
Heart J. 33:252–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nakayama W, Jinnin M, Makino K, Kajihara
I, Makino T, Fukushima S, Inoue Y and Ihn H: Serum levels of
soluble CD163 in patients with systemic sclerosis. Rheumatol Int.
32:403–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Greisen SR, Møller HJ, Stengaard-Pedersen
K, Hetland ML, Hørslev-Petersen K, Jørgensen A, Hvid M and Deleuran
B: Soluble macrophage-derived CD163 is a marker of disease activity
and progression in early rheumatoid arthritis. Clin Exp Rheumatol.
29:689–692. 2011.PubMed/NCBI
|
|
77
|
De Rycke L, Baeten D, Foell D, Kruithof E,
Veys EM, Roth J and De Keyser F: Differential expression and
response to anti-TNFalpha treatment of infiltrating versus resident
tissue macrophage subsets in autoimmune arthritis. J Pathol.
206:17–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zizzo G, Guerrieri J, Dittman LM, Merri JT
and Cohen PL: Circulating levels of soluble MER in lupus reflect
M2c activation of monocytes/macrophages, autoantibody specificities
and disease activity. Arthritis Res Ther. 15:R2122013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nakayama W, Jinnin M, Makino K, Kajihara
I, Makino T, Fukushima S, Sakai K, Inoue Y and Ihn H: CD163
expression is increased in the involved skin and sera of patients
with systemic lupus erythematosus. Eur J Dermatol. 22:512–517.
2012.PubMed/NCBI
|
|
80
|
Waidmann O, Köberle V, Bettinger D, Trojan
J, Zeuzem S, Schultheiß M, Kronenberger B and Piiper A: Diagnostic
and prognostic significance of cell death and macrophage activation
markers in patients with hepatocellular carcinoma. J Hepatol.
59:769–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lim R, Lappas M, Riley C, Borregaard N,
Moller HJ, Ahmed N and Rice GE: Investigation of human cationic
antimicrobial protein-18 (hCAP-18), lactoferrin and CD163 as
potential biomarkers for ovarian cancer. J Ovarian Res. 6:52013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
No JH, Moon JM, Kim K and Kim YB:
Prognostic significance of serum soluble CD163 level in patients
with epithelial ovarian cancer. Gynecol Obstet Invest. 75:263–267.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sugaya M, Miyagaki T, Ohmatsu H, Suga H,
Kai H, Kamata M, Fujita H, Asano Y, Tada Y, Kadono T, et al:
Association of the numbers of CD163(+) cells in lesional skin and
serum levels of soluble CD163 with disease progression of cutaneous
T cell lymphoma. J Dermatol Sci. 68:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Andersen MN, Abildgaard N, Maniecki MB,
Møller HJ and Andersen NF: Monocyte/macrophage-derived soluble
CD163: A novel biomarker in multiple myeloma. Eur J Haematol.
93:41–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dai C, Yao X, Gordon EM, Barochia A,
Cuento RA, Kaler M, Meyer KS, Keeran KJ, Nugent GZ, Jeffries KR, et
al: A CCL24-dependent pathway augments eosinophilic airway
inflammation in house dust mite-challenged Cd163 mice. Mucosal
Immunol. 9:702–717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Akahori H, Karmali V, Polavarapu R, Lyle
AN, Weiss D, Shin E, Husain A, Naqvi N, Van Dam R, Habib A, et al:
CD163 interacts with TWEAK to regulate tissue regeneration after
ischaemic injury. Nat Commun. 6:77922015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Etzerodt A, Kjolby M, Nielsen MJ, Maniecki
M, Svendsen P and Moestrup SK: Plasma clearance of hemoglobin and
haptoglobin in mice and effect of CD163 gene targeting disruption.
Antioxid Redox Signal. 18:2254–2263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Draijer C, Robbe P, Boorsma CE, Hylkema MN
and Melgert BN: Characterization of macrophage phenotypes in three
murine models of house-dust-mite-induced asthma. Mediators Inflamm.
2013:6320492013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu YC, Zou XB, Chai YF and Yao YM:
Macrophage polarization in inflammatory diseases. Int J Biol Sci.
10:520–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Byrne AJ, Mathie SA, Gregory LG and Lloyd
CM: Pulmonary macrophages: Key players in the innate defence of the
airways. Thorax. 70:1189–1196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kunz LI, Lapperre TS, Snoeck-Stroband JB,
Budulac SE, Timens W, van Wijngaarden S, Schrumpf JA, Rabe KF,
Postma DS, Sterk PJ, et al: Smoking status and anti-inflammatory
macrophages in bronchoalveolar lavage and induced sputum in COPD.
Respir Res. 12:342011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang X, Zhong W, Meng Q, Lin Q, Fang C,
Huang X, Li C, Huang Y and Tan J: Ambient PM2.5 exposure
exacerbates severity of allergic asthma in previously sensitized
mice. J Asthma. 52:785–794. 2015.PubMed/NCBI
|
|
93
|
Martinez-Nunez RT, Louafi F and
Sanchez-Elsner T: The interleukin 13 (IL-13) pathway in human
macrophages is modulated by microRNA-155 via direct targeting of
interleukin 13 receptor alpha1 (IL13Ralpha1). J Biol Chem.
286:1786–1794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Moreira AP, Cavassani KA, Hullinger R,
Rosada RS, Fong DJ, Murray L, Hesson DP and Hogaboam CM: Serum
amyloid P attenuates M2 macrophage activation and protects against
fungal spore-induced allergic airway disease. J Allergy Clin
Immunol. 126:712–721.e7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Han H, Headley MB, Xu W, Comeau MR, Zhou B
and Ziegler SF: Thymic stromal lymphopoietin amplifies the
differentiation of alternatively activated macrophages. J Immunol.
190:904–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hamzaoui A, Ammar J and Hamzaoui K:
Regulatory T cells in induced sputum of asthmatic children:
Association with inflammatory cytokines. Multidiscip Respir Med.
5:22–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Siddiqui S, Secor ER Jr and Silbart LK:
Broncho-alveolar macrophages express chemokines associated with
leukocyte migration in a mouse model of asthma. Cell Immunol.
281:159–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Abdullah M, Kahler D, Vock C, Reiling N,
Kugler C, Drömann D, Rupp J, Hauber HP, Fehrenbach H, Zabel P, et
al: Pulmonary haptoglobin and CD163 are functional immunoregulatory
elements in the human lung. Respiration. 83:61–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Axelsson J, Møller HJ, Witasp A, Qureshi
AR, Carrero JJ, Heimbürger O, Bárány P, Alvestrand A, Lindholm B,
Moestrup SK and Stenvinkel P: Changes in fat mass correlate with
changes in soluble sCD163, a marker of mature macrophages, in
patients with CKD. Am J Kidney Dis. 48:916–925. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Staples KJ, Hinks TS, Ward JA, Gunn V,
Smith C and Djukanović R: Phenotypic characterization of lung
macrophages in asthmatic patients: Overexpression of CCL17. J
Allergy Clin Immunol. 130:1404–1412.e7. 2012. View Article : Google Scholar : PubMed/NCBI
|