Introduction
Diabetes mellitus (DM) is a metabolic disease in
patients with high blood glucose over prolonged period (1). It has been well documented as an
urgent worldwide health issue present in a wide age range, from
children to adults (2). Diabetes
leads to numerous complications associated with the heart,
including ischemic heart disease, peripheral vascular disease, and
cerebrovascular disease (3). Type
1 DM is caused by the autoimmune destruction of the pancreatic
β-cells resulting in reduced insulin production (4). Chronically, microvascular and
macrovascular complications are the major reasons for increasing
morbidity and mortality in patients with diabetes. In developed
countries, cardiovascular disease is the main complication causing
death resulted from diabetes (5,6).
Patients with type 1 DM face a two to four times
higher mortality rate than non-diabetic people (7–10).
The main risk factor raising the mortality and morbidity of type 1
DM is cardiovascular complications. Their impact has become clearer
and more pernicious to patients with DM (7,10).
Coronary atherosclerosis appears in the majority of the diabetes
population, with the mortality of up to 20% diabetic subjects
caused by myocardial infarction (9). Normoglycemia is the key to attenuate
the microvascular and neurological complications of type 1
diabetes. There are many methods of maintaining normal blood sugar
levels, including treatment with insulin focus to a therapy area by
external pumps, blood glucose monitoring and physical exercise.
However, there is an increasing interest in using natural foods as
herbal treatments, including as diabetes therapeutics (11–14).
The pharmacological properties of herbal remedies, including the
amelioration of insulin sensitivity, the promotion of insulin
secretion, the increase of glucose uptake by adipose and muscle
tissues, the inhibition of sugar absorption in intestines and the
generation of sugar by hepatocytes, have been demonstrated in
several studies (15,16). Herbal and traditional Chinese
medicine have also exhibited a strong impact on treatment of
cardiovascular complications in diabetes (17). Sesamum indicum has been used
as a nutrient-rich food with medicinal functions for a many years;
it is also known as a precious herb. Sesamin is the bioactive
compound that is extracted in high amounts from Sesamum
indicum. Sesamin has been used in pharmaceuticals to perform
various valuable functions (18).
Numerous studies have demonstrated that sesamin provides
considerable support to human health by improving fat metabolism,
anti-oxidant activity, hypolipidemic activity, reducing
cholesterol, preventing inflammation and enhancing the potency of
vitamin E (18–21). However, whether sesamin can
regulate diabetes or the cardiac disease network is remains largely
unknown.
High-throughput microarray technology is the most
powerful tool to analyze gene expression on a global scale,
offering measurement of the expression of a huge number of genes.
With the rapid expansion of microarray datasets, high-throughput
microarray technology has become more popular and more accessible
from the Gene Expression Omnibus (GEO) (22), under the management of National
Center for Biotechnology Information. Expression data from the GEO
(series accession no. GSE42693) was selected (23) for analyzing the potential signal
transduction pathways in sesamin-treated rats compared with a group
treated with a controlled diet. Previous studies have also
demonstrated that sesamin can regulate fatty acid oxidation in
rats, while other studies have reported that sesamin can recover
left ventricular remodeling in spontaneously hypertensive rats
(24). However, the association
between diabetes-induced cardiac hypertrophy and sesamin is remains
largely unknown.
Sesamin has been revealed to reduce the effects of
oxidative stress and nitric oxide synthesis on β-cell destruction
(25). However, to the best of our
knowledge, there has been no previous research on the effect of
sesamin on cardiac dysfunction in type 1 diabetes. Thus, the
current study was a comparative analysis of how sesamin affected
diabetes-induced cardiac dysfunction. Based on the bioinformatics
analysis within the present study, the microarray network data
demonstrated that the cardiac hypertrophy pathway was an important
pathway in the sesamin-treated group. Therefore, the present study
aimed to investigate the cardioprotective effect of sesamin in rats
with type 1 diabetes induced by streptozotocin (STZ).
Materials and methods
Bioinformatics analysis
Previous research reported that the decline in
responsiveness of diabetes-associated heart disease induced by STZ
is caused via β-adrenoreceptor (AR) agonists as a consequent of
decrease of β1-AR and the increase in β3-AR expression (26). A report also demonstrated that the
maximal left ventricular developed pressure (LVDP) is increased by
the effect of β3-AR agonists in STZ-induced diabetic rats (24). However, the details of the
association between sesamin and diabetic-induced cardiac
hypertrophy remain. Thus, the GEO database (http://www.ncbi.nlm.nih.gov/geo/; series accession no.
GSE42693) was used to analyze the potential pathways that are
stimulated in sesamin-treated rats (23).
From the GSE42693 dataset, male Sprague Dawley (SD)
rats with type 1 DM were treated with 0.2% sesamin in their diet,
whereas the control rats were provided with diet free of sesamin
for a period of 15 days. RNA extracted from the rats was subjected
to microarray analysis. A robust multichip average algorithm was
applied to analyze the raw data correction and normalization, and
its signal was calculated for gene-level probe set summaries with
the Affymetrix Expression Console Version 1.3.1.187 (http://www.affymetrix.com/). Then the fold change and
expression level between the sesamin-treated group and the control
group were also calculated. MetaCore (GeneGo, Inc., St. Joseph, MI,
USA) was employed to investigate the signal transduction pathways
regulating the sesamin-treated group by analyzing gene ontology
molecular functions and GeneGo pathways. MetaCore received the data
input and created biological links with associated processes. In
order to compare the average expression level of rats treated with
sesamin (n=5) and the control (n=5), the upper quartile of highest
expressed genes (7,774 genes) with significant expression
differences was uploaded to the MetaCore software. From the
analysis, the cardiac hypertrophy pathway was considered to be a
crucial pathway in the sesamin-treated group. P<0.05 was
considered to indicate a statistically significant difference.
Sesamin preparation and reagents
Pure sesamin compound was purchased from ChemBio Co.
(Taipei, Taiwan), a glucose kit was purchased from Randox
Laboratories Ltd. (County Antrim, UK) and STZ was purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
STZ-induced type I diabetes rat
model
A total of 30 male, pathogen and disease-free
Sprague-Dawley rats (4-week-old; weight, 100–120 g) from the
National Laboratory Animal Center (Taipei, Taiwan), were used in
the current study. The rats were raised under standard experimental
conditions with a 12 h light/12 h dark cycle at 23–25°C. Animals
were fed ad libitum with free access to water. All animal
experiments were approved by the Institutional Animal Care and Use
Committee or Panel (IACUC/IACUP) of Chinese Culture University
(Taipei, Taiwan). The diabetes rat model was created in 10 rats by
STZ injection following a standard protocol of the Animal Models of
Diabetic Complications Consortium (27). STZ was diluted in sodium citrate
buffer solution (pH 4.5). The rats were fasted for 4 h before the
STZ injection and then were anesthetized using 2% isoflurane mixed
with 2l/min compressed oxygen (KYS Technology, Taipei, Taiwan). An
appropriate amount of the STZ solution (50 mg/kg) was injected
subcutaneously in the abdomen area, repeating one injection each
day for five consecutive days. Each rat was supplied with 10%
sucrose water following each injection, was allowed to awaken and
was placed back in the cage. The rats were tested for sufficient
levels of hyperglycemia and only rats with blood glucose level
>200 mg/dl (5 rats) were selected for further
experimentation.
Sesamin treatment in STZ-induced
diabetic rats
A total of 5 groups of experiments with 5 rats of
each were used in this study (control, model diabetes, and sesamin
treatment (3 groups). Sesamin (dissolved in olive oil) oral
administration was performed after the STZ-treated rats were
randomly divided into three groups that received different sesamin
concentrations as follows: Low concentration (50 mg/kg body
weight), medium concentration (100 mg/kg body weight), and high
concentration (200 mg/kg body weight). The experimental process was
performed for 4 weeks (oral administration of sesamin to rats was
performed once per day), and after each 7-day period of treatment
with sesamin, blood samples were collected in order to determine
the blood glucose levels. Body weight, heart rate (HR) and blood
pressure (BP) were also measured, and echocardiogram was
performed.
Blood glucose measurement
The rats were fasted for 4 h before the blood sample
collections on day 0, 7, 14, 21 and 28 of the sesamin
administration process. Serum was separated for the measurement of
the blood glucose, using commercial kits according to the
manufacturer's protocols (Randox Laboratories, Ltd., County Antrim,
UK).
Blood pressure and electrocardiogram
measurements
BP was measured by inserting two catheters into the
femoral artery. For BP, the custom catheter (polyurethane; 1 French
size) was inserted to the femoral artery. The waveforms delivering
information about LVDP were visualized on a computer monitor.
The electrocardiogram was measured by using three
lead vectors, one connected to the chest, one connected to the
armpit, and the third connected to the femoral part of the rat. All
results were recorded and analyzed by the LabChart 7 software
(ADInstruments, Ltd., Dunedin, New Zealand).
Histopathological assay
Rats were scarified by anesthetizing with 2%
isoflurane (KYS Technology, Taipei, Taiwan) mixed with 2l/min of
compressed oxygen and followed by decapitalization. The hearts were
collected and stored in 10% formalin at 4°C for subsequent paraffin
embedding. Sections (5 µm) of each tissue were stained with
hematoxylin and eosin (H&E) staining and were observed for
histopathological alterations under a light microscope.
Data collection and analysis
Heart rate measurements, electrocardiography
performance and blood pressure recording was performed using
cardiovascular continuous monitoring software (PowerLab/4SP;
ADInstruments, Ltd.). Values for hemodynamic measures were derived
by a 1 KHz sampling rate.
Statistical analysis
All results are presented as the mean ± standard
deviation. Statistical analysis was evaluated by one-way analysis
of variance followed by the Student-Newman-Keuls test with SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
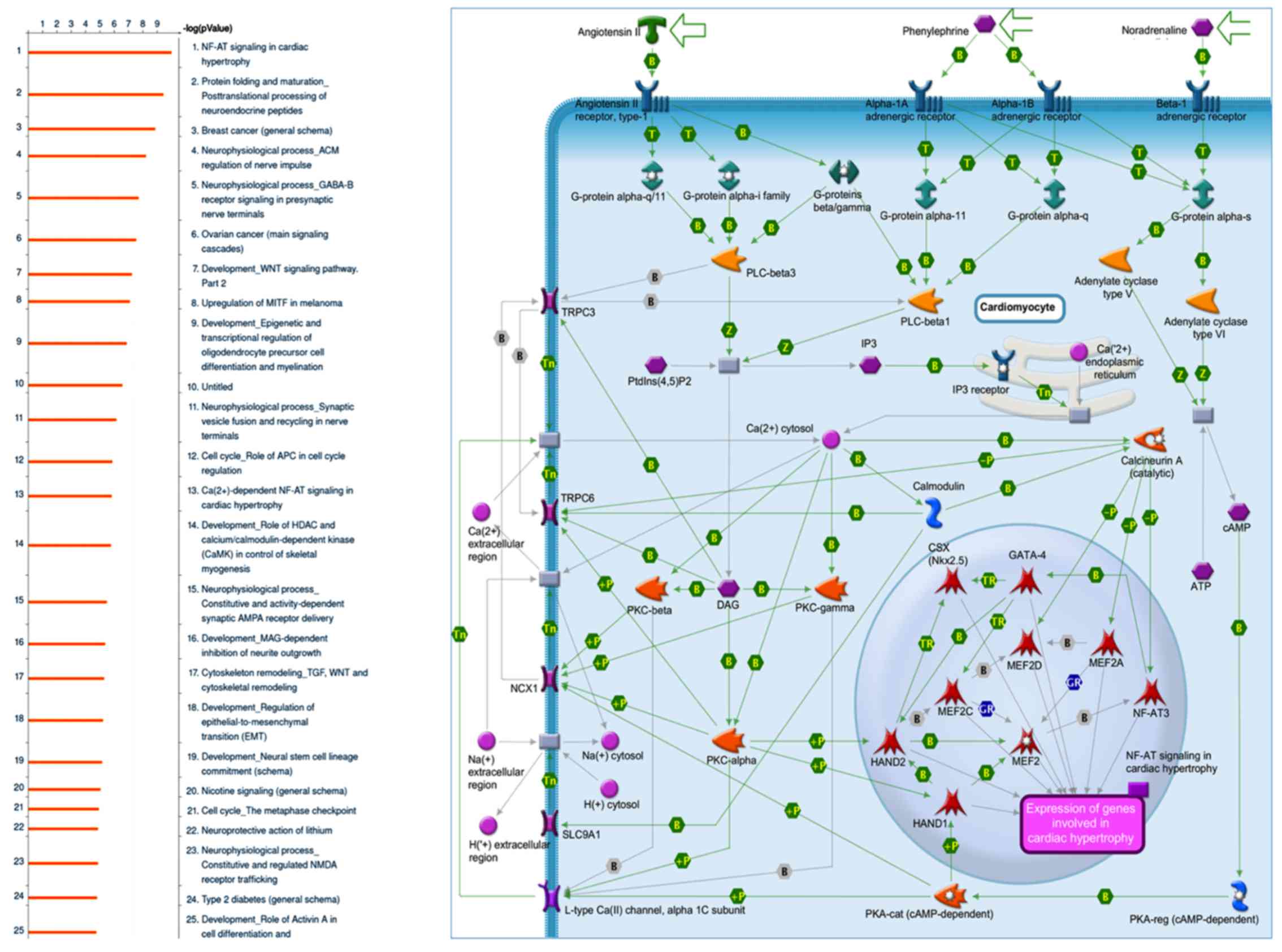
Cardiac hypertrophy pathways are
modulated by sesamin treatment
MetaCore GeneGo software was used to identify genes
with different expression levels in sesamin-treated and control
samples. MetaCore software employs an exclusive, manually selected
and organized database comprising of protein-protein, protein-DNA,
protein compound interactions, and their metabolic and signaling
pathways. There were 7,774 genes with significant expression
differences between the sesamin-treated (n=5) and the control
diet-treated rats (n=5). MetaCore pathway analysis of these two
groups indicated that most significant signaling pathway for
upregulated genes in sesamin-treated rats was cardiac hypertrophy
signaling (P=1.148E-10 and FDR=9.945E-8).
Cardiac hypertrophy is a compensatory mechanism of
the heart to maintain stable cardiac output throughout different
medical states. The outcome of this chronic process results in
pathological cardiac growth and is correlated with increased
morbidity and mortality. Myocyte enhancer factor 2 (MEF2) family
proteins (MEF2A, MEF2C, MEF2D) are involved as intermediate signal
responses of the cardiac transcriptional program (26,28,29).
Numerous genes serve roles in the cardiac hypertrophy process,
including α-myosin (α-MHC) and β-MHC, troponin I cardiac and
troponin T cardiac, via a system of transcription factors and
co-factors (GATA binding protein 4, NK2 homeobox 5, calmodulin
binding transcription activator 2, MEF2, heart and neural crest
derivatives expressed 1 (HAND1), HAND2, CREB binding protein and
E1A binding protein p300) incorporating with nuclear factor of
activated T-cells (NF-AT) signaling (30–32).
The bioinformatics results suggest that sesamin may participate in
the cardiac hypertrophy pathway via NF-AT signaling (Fig. 1). However, currently, the detailed
mechanism of how sesamin affects cardiac hypertrophy is still
obscured and demands more investigation in animal model.
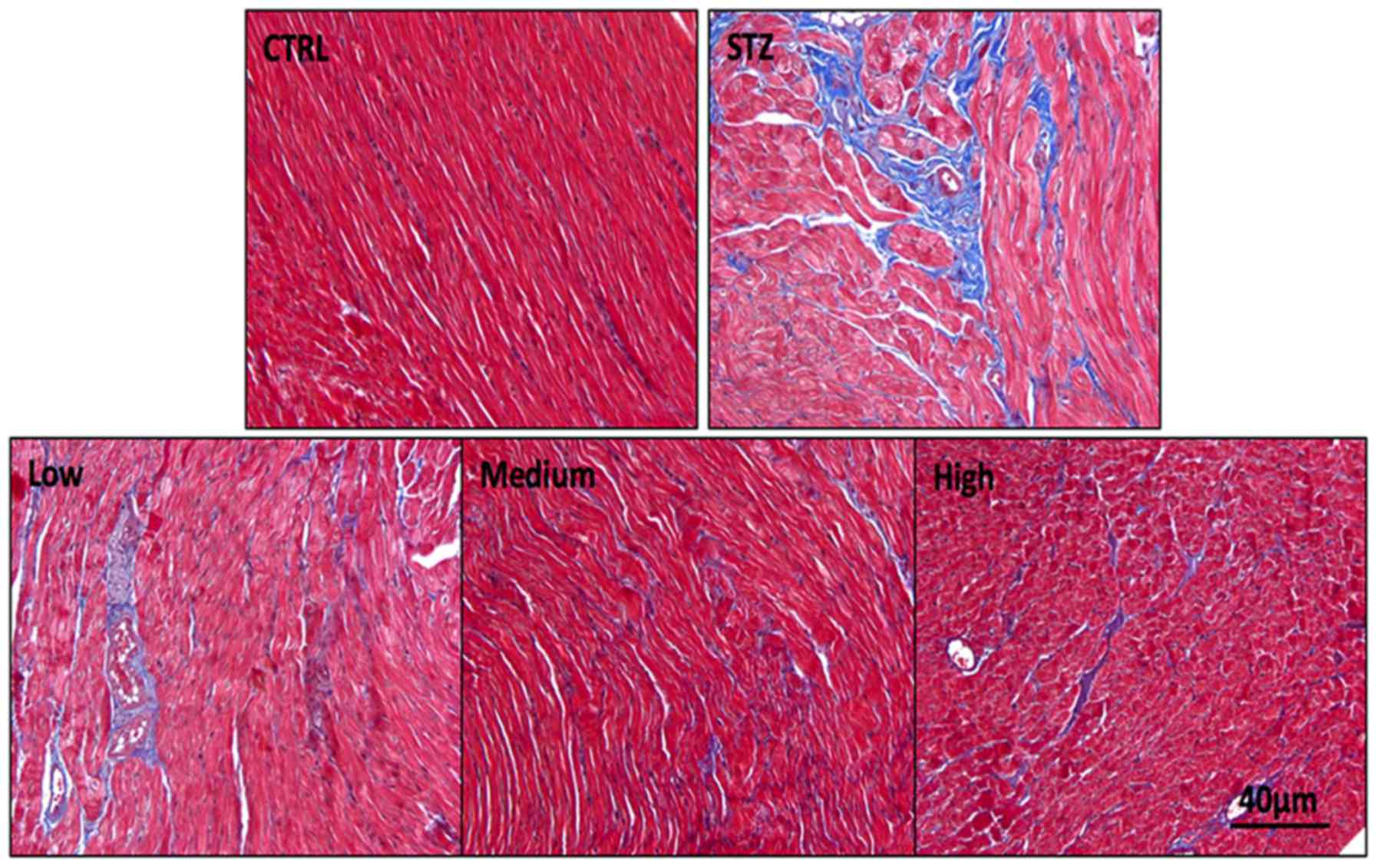
Histology of the cardiac tissue
Results from H&E staining demonstrated that the
nuclei in the control group were oval, single and observed at the
center of the cardiomyocytes (Fig.
2). However, deformation in the shapes and sizes of nuclei was
observed in the diabetes group. In addition, the cardiac myofiber
pattern in this group spread irregularly relative to the control
group. An increase in protein degradation may lead to this
observation. Compared with the diabetic group, serious histological
changes in the sesamin oral administration group were reduced.
Among the treatment groups, the medium-dose group improved features
compared with the diabetes group. Massive fibroblast infiltration
in the left ventricle was observed in the diabetes group. Treatment
with different doses of sesamin induced different changes in the
diabetes rats. In the low-dose group, the cardiomyocytes
degenerated, and segmentation was observed after 4 weeks of sesamin
administration. Even mature fibrocyte infiltration was observed in
this group. In the high-dose group, the staining indicated
degenerated, vacuolated and dissociated myocardiac fibers in the
focal region of the left ventricle. By contrast, the heart tissue
improved greatly after 4 weeks of oral treatment with the medium
sesamin dose.
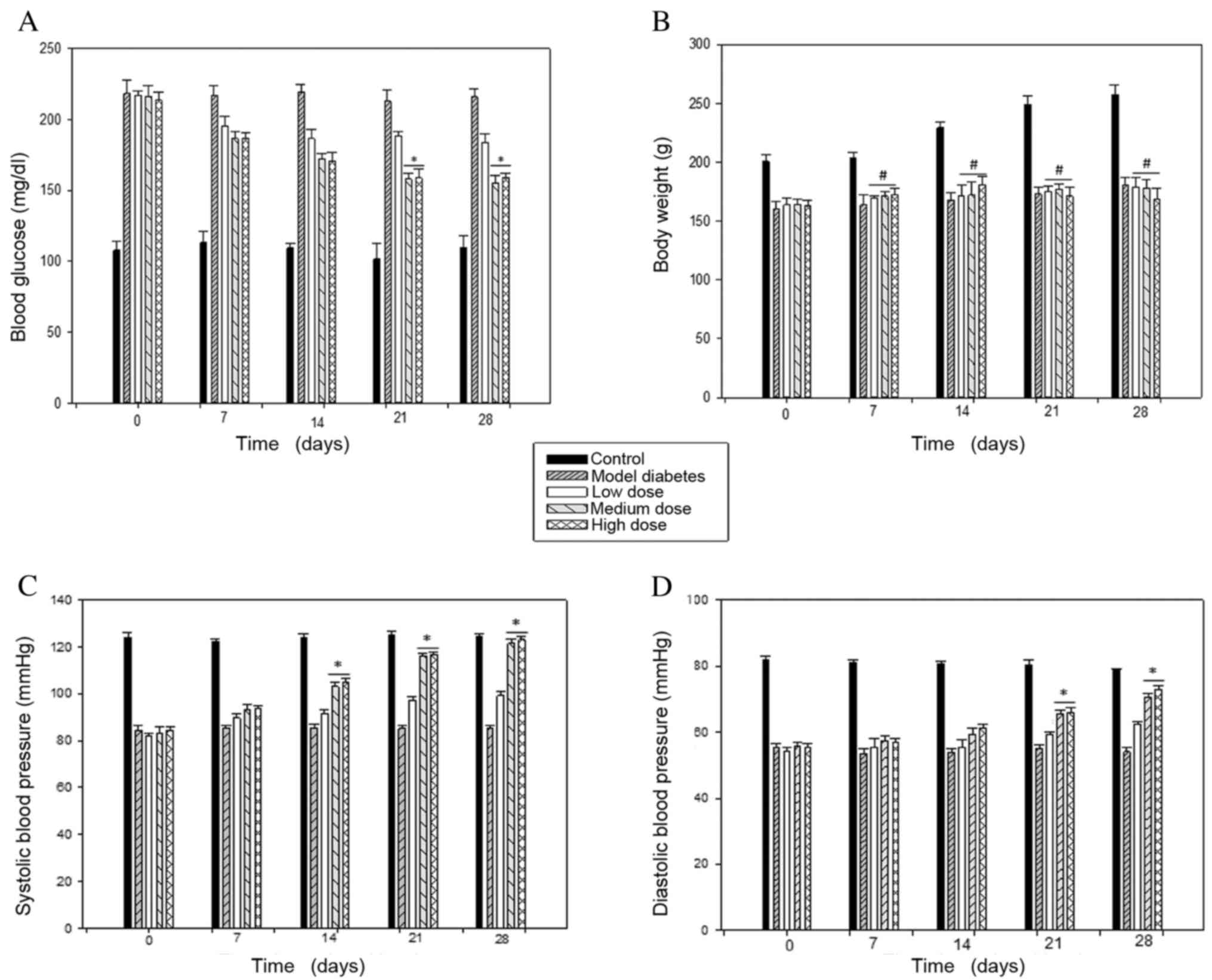
Effect of sesamin on blood glucose and
body weight
Following STZ injection, SD rats exhibited high
glucose blood level (>200 mg/dl). At day 28th of the treatment,
The STZ-treated rats exhibited a reduction in body weight
(160.2±6.49 g, n=5) and an increase in blood glucose (220.47±6.19
mg/dl, n=5) compared with the age-matched controls, with 200.4±6.34
g and 107.81±6.69 mg/dl, respectively (Fig. 3A). However, following oral sesamin
administration, there was significant glycemic amelioration
compared with the diabetes group by 17% in the low dose group, by
30% in the medium dose group and by 26% in the high dose group.
Body weight was increased compared with the diabetes group by 17%
in the low dose group, 18% in the medium dose group, and 20% in the
high dose group at 4 weeks (Fig.
3B). The body weight was increased in the sesamin-treated
groups compared with the diabetes group, however, for the groups
administered medium and high doses of sesamin, blood glucose levels
were reduced further than in the low dose group.
Effect of sesamin on blood
pressure
After STZ-induction, at day 28th of the treatment,
the systolic and diastolic blood pressure in the diabetic subjects
(85.46±0.92 and 55.59±1.06 mmHg) was markedly reduced compared with
the control subjects (123.12±1.54 and 81.90±1.09 mmHg, n=5)
(Fig. 3C). Following sesamin
consumption, blood pressure was significantly increased in
STZ-induced rats, particularly in the groups administered medium
and high doses of sesamin, with the low-dose group from
82.28±0.98/54.34±0.99 mmHg to 99.15±1.59/62.49±0.82 mmHg, the
medium-dose group from 83.74±0.87 to
121.47±1.09/55.96±1.67/70.67±1.05, and the high-dose group from
84.53±1.19/55.48±1.16 mmHg to 123.14±1.21/72.99±1.14 mmHg (Fig. 3D).
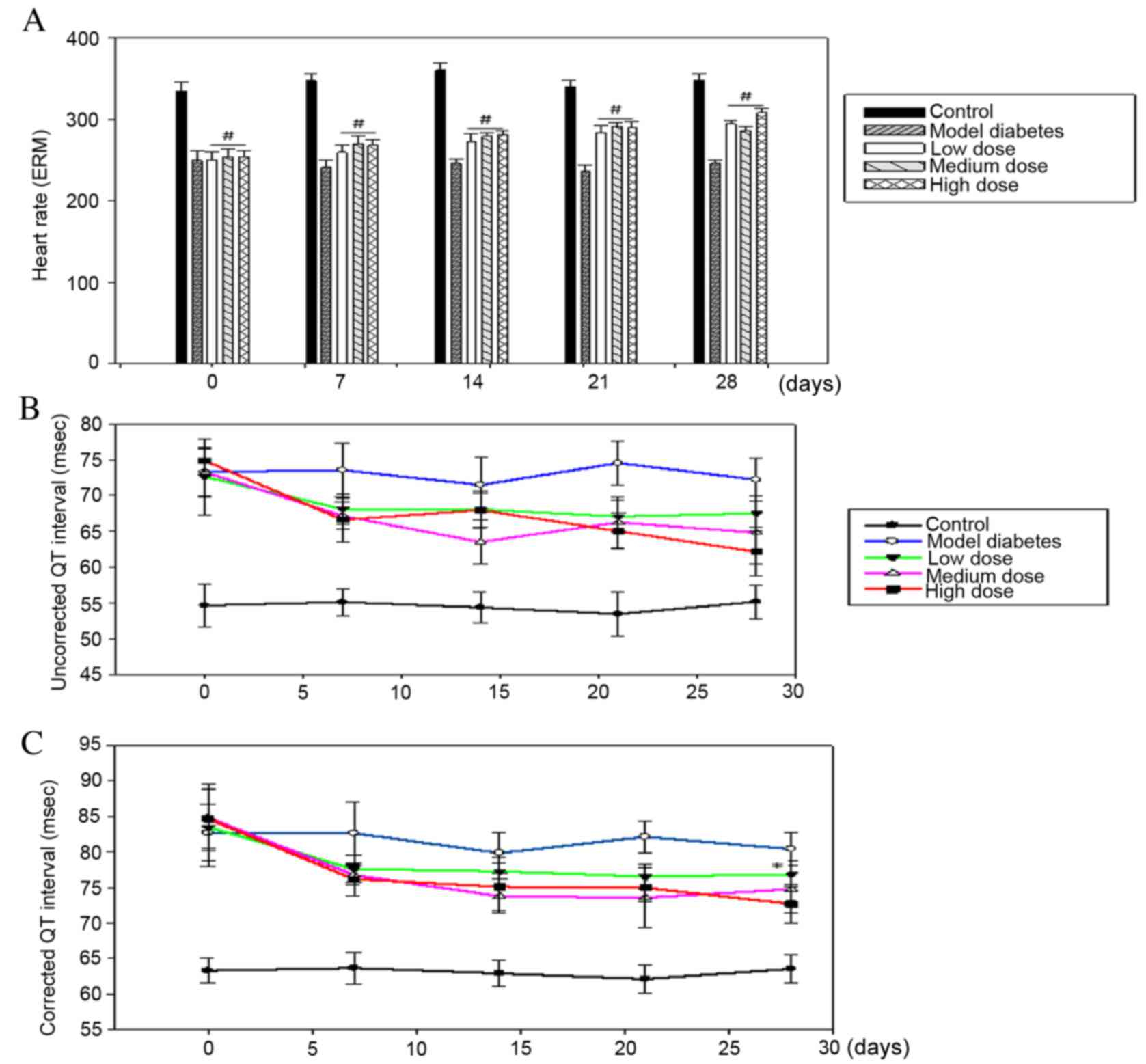
Effect of sesamin on heart rate
The heart rate in rats injected with STZ declined by
~1.33-fold compared with the healthy subjects (333.6±10.21 vs.
249.4±12.17 BPM). The heart rate was significantly increased
following treatment with sesamin compared with the diabetes group,
particularly in the high-dose sesamin group at day 28th of the
treatment (255±9.61 vs. 308±5.65 BPM; Fig. 4A).
The effect of sesamin on QT
interval
The uncorrected QT interval was prolonged in
STZ-treated rats compared with the control group at day 28th of the
treatment (73.29±3.31 vs. 54.71±2.18 msec). Following sesamin
treatment, the QT interval dramatically recovered by 7.4, 11.5 and
15.2% at day 28th of the treatment with the low-dose, medium-dose
and high-dose group, respectively, compared with the STZ-treated
rats (Fig. 4B). Fridericia's
formula was used to correct the QT interval by adjusting to an R-R
interval. Corrected QT intervals were 82.67±3.99 and. 63.28±1.77
msec in STZ-treated rats and control rats at day 28th of the
treatment, respectively. There was a corrected QT interval of 8, 12
and 14% in the low-dose, medium-dose and high-dose groups at day
28th of the treatment, respectively (Fig. 4C).
Discussion
Cardiac fibrosis and pressure dysfunction in
patients with diabetes were reported in a previous study (33). Diabetic cardiomyopathy in rats with
type 2 diabetes causes an increase in heart weight and in the
nuclei size in cardiomyocytes. However, in rats with type 1
diabetes, studies by Cosyns et al (34) and Thent et al (35) demonstrated the same result, where
the disarray in cardiac myofibers and changes in nuclei size led to
a decrease in the cardiomyocytes, which caused cardiac atrophy
(34,35). Cardiac atrophy may be due to an
insulin deficiency, leading to a decline in protein synthesis and
serious protein degradation, which affects the function of the
mitochondria and leads to the damage of myocardial myofibers
(36). Other causes that can lead
to atrophy of the heart include an increase in hyperosmolarity in
hyperglycemic conditions, an increase of collagen bundles, and a
rise in the left ventricle contraction; all of these may lead to
the changing of nuclei sizes and the shapes of cardiomyocytes
(37). Sesame contains large
amounts of antioxidants such as flavonoids and vitamin C and E.
Sesamin extracted from Sesamum indicum has anti-inflammatory
effects, supports vitamin E absorption, and reduces β
cell-destroying factors, therefore, increasing the amount of
insulin, ameliorating hyperglycemia, and increasing protein
synthesis (38). Through the
anti-inflammatory effects of sesamin, the myocardial injury also
somewhat improved. It reduced the contraction of the left
ventricle, decreasing the size and changing the shape of the nuclei
of myocardial cells, thus, improving the disorder in the myofiber
arrangement (39). In the current
study, the results of H&E staining demonstrated that structural
cardiomyocytes in rats with diabetes induced by STZ were damaged.
The order links among the myofibers were broken, and the nuclei of
cardiac muscle cells were deformed and fragmented in the diabetic
group. However, in the treatment groups, the disordered
associations were markedly minimized, particularly in the
medium-dose group. Findings of myocardial injury were also reduced.
But these injuries were still visible in the low and high sesamin
concentration groups.
The combined consumption of sesame oil and
glibenclamide reduces blood glucose levels significantly (36% with
treatment compared with no treatment), and it also decreases total
plasma cholesterol; this significantly improves the condition of
patients with diabetic disease (40). An STZ-induced diabetic heart has
been revealed exhibit decreased responsiveness via β-AR agonists,
in which reduced β1-AR and increased β3-AR expression are important
factors (26). In addition, a
β3-AR agonist also raised the maximal LVDP responses in rats with
STZ-induced diabetes (41). The
data of the current study demonstrated that following STZ
injection, the body weight and blood glucose level of rats declined
rapidly and markedly, which has also been described in a previous
study (42). This is explained by
the destruction of β cells induced by STZ (43). In a previous study, significant
improvements in the blood sugar levels and body weight of rats were
observed (44). However, there was
no marked change in the body weight of diabetes rats treated with
sesamin relative to model diabetes rat in the current study.
Following oral administration of sesamin, the blood
glucose level of rats was significantly reduced compared with
STZ-induced rats, particularly at the medium and high sesamin
concentrations. This demonstrates that sesamin has a marked
hypoglycemic effect; the previous studies also reported a similar
outcome (45,46). However, the results in Fig. 1 indicate that using the correct
sesamin concentration is critical because the reduction in blood
glucose levels is was not significant at the low sesamin
concentration.
The data of the present study demonstrated that
STZ-induced diabetes results in a decrease in blood pressure and
that sesamin treatment can restore blood pressure in diabetic rats.
Following oral sesamin administration, the blood pressure was
restored significantly. This is reflected in the recovery of the
diastolic and systolic blood pressure. Previous studies reported a
significant attenuation of vascular dysfunction following treatment
with sesamin (44). Possibly the
recovery of the blood vessels in mice with sesamin treatment led to
the recovery of the blood pressure. Endothelial cells are involved
in the restoration of blood vessel walls. Due to these cells, the
improvement of the blood vessels via the sesamin treatment was
considerably increased (44).
Another study demonstrated that sesamin had an
effect on the protection of β cells following STZ-induction. The
administration of sesamin reversed the STZ-induced cells viability
reduction. Increasing the oxidative stressors and nitride oxide
synthesis by sesamin led to reduced β cells destruction (41). In rats with STZ-induced diabetes,
the heart rate was decreased in the present study, which is
consistent with what has been revealed in previous studies
(47,48). Hyperglycemia appears to be the
predominant cause of this clinical abnormality (slow heart rate)
(48). Also, the effect of STZ on
repolarization and the action potential may have led to heart rate
reduction (44). An increased
heart rate in patients with type 1 diabetes is closely associated
with cardiac autonomic neuropathy, thus, leading to many abnormal
problems, including high blood pressure and poor blood supply
(47). Due to the effect of
sesamin treatment, the β cells were significantly restored, causing
reduced glycemia and leading to a recovered heart rate in a
previous report (38). Improving
blood pressure is involved in the effect of sesamin that led to the
heart rate recovery in STZ-treated animals (44).
QT interval measurement can predict sudden cardiac
death in certain diseases, including hypertension (49), chronic heart failure (50,51)
and peripheral vascular disease (52). QT interval is a good factor to
predict cardiac malfunction and state in patients with diabetes
(53,54). A previous study reported a
significant reduction of the QT interval in subjects with type 1
diabetes; however, when adjusted by RR intervals, the difference
was abolished (55). The previous
study also reported a prolonged QT interval (by ~35%) (56). The correction of the QT interval
was computed by Fridericia's formulation:
QTcF=QTRR3
The formulation used the cube root of RR (53). This difference was maintained in
QTcF (prolonged~30%). This may explain by the decline in heart rate
affected by high blood sugar and high blood pressure. This
difference was maintained in the corrected QT interval in the
current study.
Following oral administration of sesamin, the QT
interval and QTcF were markedly improved, by 15.2 and 14% at day
28th of treatment, respectively, in the high-dose group. This
suggests that sesamin has a positive effect in restoring the QT
interval in an STZ-induced type 1 diabetes rat. The restoration of
blood pressure and β cells (38,44)
reduced the blood glucose levels, leading to a reduction in the
heart rate and substantial improvement of the QT interval. The
beneficial effects of sesamin have been demonstrated through
various previous studies (57–62).
The data of the current revealed that sesamin has a positive role
in improving cardiovascular dysfunction in rats with STZ-induced
type 1 diabetes.
In conclusion, cardiovascular dysfunction has caused
more mortality among patients than any other complications of type
1 diabetes. The oral administration of sesamin for 4 weeks
significantly ameliorated the changes to heart rate, blood pressure
and QT interval in STZ-induced type 1 diabetes. This indicates that
sesamin has a potential therapeutic effect in reducing
cardiovascular complications in diabetic subjects.
Acknowledgments
Computational analyses and data mining were
performed using the system provided by the Bioinformatics Core at
the National Cheng Kung University (Tainan, Taiwan), supported by
the National Science Council Taiwan. The study was supported by the
National Science Council Taiwan of the Executive Yuan (grant nos.
NSC 101-2320-B-034-001 and 104-2320-B-034 −003) and the Ministry of
Science and Technology, Taiwan (grant nos. 104-2917-I-006-002,
MOST103-2325-B006-012 and 104-2917-I-006-002).
References
|
1
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: Diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Organization WH: Diabetes programme: Facts
and figures. http://www/who.int/diabetes/facts/world_figures/en/Accessed.
December 17–1990.-1999, 2007.
|
|
3
|
UK prospective diabetes study (UKPDS).
VIII. Study design, progress and performance. Diabetologia.
34:877–890. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Atkinson MA and Maclaren NK: The
pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med.
331:1428–1436. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buowari OY: Diabetes mellitus in
developing countries and case series. INTECH Open Access Publisher;
2013
|
|
6
|
Laing SP, Swerdlow AJ, Slater SD, Burden
AC, Morris A, Waugh NR, Gatling W, Bingley PJ and Patterson CC:
Mortality from heart disease in a cohort of 23,000 patients with
insulin-treated diabetes. Diabetologia. 46:760–765. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dahl-Jørgensen K, Larsen JR and Hanssen
KF: Atherosclerosis in childhood and adolescent type 1 diabetes:
Early disease, early treatment? Diabetologia. 48:1445–1453. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duca L, Sippl R and Snell-Bergeon JK: Is
the risk and nature of CVD the same in type 1 and type 2 diabetes?
Curr Diab Rep. 13:350–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahgoub MA and Abd-Elfattah AS: Diabetes
mellitus and cardiac function. Mol Cell Biochem. 180:59–64. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marcovecchio ML, Tossavainen PH and Dunger
DB: Prevention and treatment of microvascular disease in childhood
type 1 diabetes. Br Med Bull. 94:145–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong YJ, Kim N, Lee K, Sonn Hee C, Lee Eun
J, Tae Kim S, Ho Baeg I and Lee KM: Korean red ginseng (Panax
ginseng) ameliorates type 1 diabetes and restores immune cell
compartments. J Ethnopharmacol. 144:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watanabe K, Matsuura K, Gao P,
Hottenbacher L, Tokunaga H, Nishimura K, Imazu Y, Reissenweber H
and Witt CM: Traditional Japanese Kampo medicine: Clinical research
between modernity and traditional medicine-the state of research
and methodological suggestions for the future. Evid Based
Complement Alternat Med. 2011:5138422011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikemoto T, Sugimoto K, Takita M, Shimoda
M, Noguchi H, Naziruddin B, Levy MF, Shimada M and Matsumoto S:
Japanese herbal medicine TJ-48 prevents autoimmune diabetes in NOD
mice. Am J Chin Med. 39:743–756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li WL, Zheng HC, Bukuru J and De Kimpe N:
Natural medicines used in the traditional Chinese medical system
for therapy of diabetes mellitus. J Ethnopharmacol. 92:1–21. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li GQ, Kam A, Wong KH, Zhou X, Omar EA,
Alqahtani A, Li KM, Razmovski-Naumovski V and Chan K: Herbal
medicines for the management of diabetes. Adv Exp Med Biol.
771:396–413. 2012.PubMed/NCBI
|
|
16
|
Cui TH and Li YY: Treatment of type 2
diabetes mellitus oral Chinese patent medicine literature metrology
analysis. Zhongguo Zhong Yao Za Zhi. 37:2649–2652. 2012.(In
Chinese). PubMed/NCBI
|
|
17
|
Ceylan-Isik AF, Fliethman RM, Wold LE and
Ren J: Herbal and traditional Chinese medicine for the treatment of
cardiovascular complications in diabetes mellitus. Curr Diabetes
Rev. 4:320–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeng K and Hou R: Sesamin and sesamolin:
Nature's therapeutic lignans. Current Enzyme Inhibition. 1:11–20.
2005. View Article : Google Scholar
|
|
19
|
Kiso Y: Antioxidative roles of sesamin, a
functional lignan in sesame seed, and it's effect on lipid- and
alcohol-metabolism in the liver: A DNA microarray study.
Biofactors. 21:191–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogawa H, Sasagawa S, Murakami T and
Yoshizumi H: Sesame lignans modulate cholesterol metabolism in the
stroke-prone spontaneously hypertensive rat. Clin Exp Pharmacol
Physiol Suppl. 22:(Suppl). S310–S312. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong X, Yang JR, Guo LQ, Xiong Y, Wu XQ,
Huang K and Zhou Y: Sesamin improves endothelial dysfunction in
renovascular hypertensive rats fed with a high-fat, high-sucrose
diet. Eur J Pharmacol. 620:84–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edgar R, Domrachev M and Lash AE: Gene
Expression Omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ide T, Lim JS, Odbayar TO and Nakashima Y:
Comparative study of sesame lignans (sesamin, episesamin and
sesamolin) affecting gene expression profile and fatty acid
oxidation in rat liver. J Nutr Sci Vitaminol (Tokyo). 55:31–43.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li WX, Kong X, Zhang JX and Yang JR:
Long-term intake of sesamin improves left ventricular remodelling
in spontaneously hypertensive rats. Food Funct. 4:453–460. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei H, Han J, Wang Q, Guo S, Sun H and
Zhang X: Effects of sesamin on streptozotocin (STZ)-induced NIT-1
pancreatic β-cell damage. Int J Mol Sci. 13:16961–16970. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dinçer UD, Bidasee KR, Güner S, Tay A,
Özçelikay AT and Altan VM: The effect of diabetes on expression of
beta1-, beta2-, and beta3-adrenoreceptors in rat hearts. Diabetes.
50:455–461. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolb H: Mouse models of insulin dependent
diabetes: Low-dose streptozocin-induced diabetes and nonobese
diabetic (NOD) mice. Diabetes Metab Rev. 3:751–778. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okatan EN, Tuncay E, Hafez G and Turan B:
Profiling of cardiac β-adrenoceptor subtypes in the cardiac left
ventricle of rats with metabolic syndrome: Comparison with
streptozotocin-induced diabetic rats. Can J Physiol Pharmacol.
93:517–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kasler HG, Victoria J, Duramad O and
Winoto A: ERK5 is a novel type of mitogen-activated protein kinase
containing a transcriptional activation domain. Mol Cell Biol.
20:8382–8389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu J, Gong NL, Bodi I, Aronow BJ, Backx PH
and Molkentin JD: Myocyte enhancer factors 2A and 2C induce dilated
cardiomyopathy in transgenic mice. J Biol Chem. 281:9152–9162.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morin S, Pozzulo G, Robitaille L, Cross J
and Nemer M: MEF2-dependent recruitment of the HAND1 transcription
factor results in synergistic activation of target promoters. J
Biol Chem. 280:32272–32278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhavsar PK, Dellow KA, Yacoub MH, Brand NJ
and Barton PJ: Identification of cis-acting DNA elements required
for expression of the human cardiac troponin I gene promoter. J Mol
Cell Cardiol. 32:95–108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hayat SA, Patel B, Khattar RS and Malik
RA: Diabetic cardiomyopathy: Mechanisms, diagnosis and treatment.
Clin Sci (Lond). 107:539–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cosyns B, Droogmans S, Weytjens C,
Lahoutte T, Van Camp G, Schoors D and Franken PR: Effect of
streptozotocin-induced diabetes on left ventricular function in
adult rats: An in vivo Pinhole Gated SPECT study. Cardiovasc
Diabetol. 6:302007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thent ZC, Lin TS, Das S and Zakaria Z:
Histological changes in the heart and the proximal aorta in
experimental diabetic rats fed with Piper sarmentsoum. Afr J Tradit
Complement Altern Med. 9:396–404. 2012.PubMed/NCBI
|
|
36
|
Nemoto O, Kawaguchi M, Yaoita H, Miyake K,
Maehara K and Maruyama Y: Left ventricular dysfunction and
remodeling in streptozotocin-induced diabetic rats. Circ J.
70:327–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Malone JI, Schocken DD, Morrison AD and
Gilbert-Barness E: Diabetic cardiomyopathy and carnitine
deficiency. J Diabetes Complications. 13:86–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lei H, Han J, Wang Q, Guo S, Sun H and
Zhang X: Effects of sesamin on streptozotocin (STZ)-induced NIT-1
pancreatic beta-cell damage. Int J Mol Sci. 13:16961–16970. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Utsunomiya T, Shimada M, Rikimaru T,
Hasegawa H, Yamashita Y, Hamatsu T, Yamasaki M, Kaku S, Yamada K
and Sugimachi K: Antioxidant and anti-inflammatory effects of a
diet supplemented with sesamin on hepatic ischemia-reperfusion
injury in rats. Hepatogastroenterology. 50:1609–1613.
2003.PubMed/NCBI
|
|
40
|
Sankar D, Ali A, Sambandam G and Rao R:
Sesame oil exhibits synergistic effect with anti-diabetic
medication in patients with type 2 diabetes mellitus. Clin Nutr.
30:351–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okatan EN, Tuncay E, Hafez G and Turan B:
Profiling of cardiac β-adrenoceptor subtypes in the cardiac left
ventricle of rats with metabolic syndrome: Comparison with
streptozotocin-induced diabetic rats. Can J Physiol Pharmacol.
93:517–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Howarth FC, Jacobson M, Shafiullah M and
Adeghate E: Long-term effects of streptozotocin-induced diabetes on
the electrocardiogram, physical activity and body temperature in
rats. Exp Physiol. 90:827–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akbarzadeh A, Norouzian D, Mehrabi MR,
Jamshidi SH, Farhangi A, Verdi AA, Mofidian SM and Rad BL:
Induction of diabetes by Streptozotocin in rats. Indian J Clin
Biochem. 22:60–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baluchnejadmojarad T, Roghani M, Nadoushan
Jalali MR, Mahdavi Vaez MR, Kalalian-Moghaddam H, Roghani-Dehkordi
F, Dariani S and Raoufi S: The sesame lignan sesamin attenuates
vascular dysfunction in streptozotocin diabetic rats: Involvement
of nitric oxide and oxidative stress. Eur J Pharmacol. 698:316–321.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hong L, Yi W, Liangliang C, Juncheng H,
Qin W and Xiaoxiang Z: Hypoglycaemic and hypolipidaemic activities
of sesamin from sesame meal and its ability to ameliorate insulin
resistance in KK-Ay mice. J Sci Food Agric. 93:1833–1838. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sankar D, Ali A, Sambandam G and Rao R:
Sesame oil exhibits synergistic effect with anti-diabetic
medication in patients with type 2 diabetes mellitus. Clin Nutr.
30:351–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Turker Y, Aslantas Y, Aydin Y, Demirin H,
Kutlucan A, Tibilli H, Turker Y and Ozhan H: Heart rate variability
and heart rate recovery in patients with type 1 diabetes mellitus.
Acta Cardiol. 68:145–150. 2013.PubMed/NCBI
|
|
48
|
Jaiswal M, Urbina EM, Wadwa RP, Talton JW,
D'Agostino RB Jr, Hamman RF, Fingerlin TE, Daniels S, Marcovina SM,
Dolan LM and Dabelea D: Reduced heart rate variability among youth
with type 1 diabetes: The SEARCH CVD study. Diabetes Care.
36:157–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sahu P, Lim PO, Rana BS and Struthers AD:
QT dispersion in medicine: Electrophysiological holy grail or
fool's gold? QJM. 93:425–431. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Barr CS, Naas A, Freeman M, Lang CC and
Struthers AD: QT dispersion and sudden unexpected death in chronic
heart failure. Lancet. 343:327–329. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fu GS, Meissner A and Simon R:
Repolarization dispersion and sudden cardiac death in patients with
impaired left ventricular function. Eur Heart J. 18:281–289. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Darbar D, Luck J, Davidson N, Pringle T,
Main G, McNeill G and Struthers AD: Sensitivity and specificity of
QTc dispersion for identification of risk of cardiac death in
patients with peripheral vascular disease. BMJ. 312:874–879. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Naas AA, Davidson NC, Thompson C, Cummings
F, Ogston SA, Jung RT, Newton RW and Struthers AD: QT and QTc
dispersion are accurate predictors of cardiac death in newly
diagnosed non-insulin dependent diabetes: Cohort study. BMJ.
316:745–746. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rana BS, Lim PO, Naas AA, Ogston SA,
Newton RW, Jung RT, Morris AD and Struthers AD: QT interval
abnormalities are often present at diagnosis in diabetes and are
better predictors of cardiac death than ankle brachial pressure
index and autonomic function tests. Heart. 91:44–50. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Howarth FC, Adeghate E and Jacobson M:
Heart rate and QT interval in streptozotocin-induced diabetic rat.
J Med Sci. 2:108–118. 2009.
|
|
56
|
Fridericia LS: The duration of systole in
an electrocardiogram in normal humans and in patients with heart
disease. 1920. Ann Noninvasive Electrocardiol. 8:343–351. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fukuda Y, Osawa T, Namiki M and Ozaki T:
Studies on antioxidative substances in sesame seed. Agric Biol
Chem. 49:301–306. 1985. View Article : Google Scholar
|
|
58
|
Ying D and Yin G: Extraction of
Antioxidant from Sesame Cake and Its Activity Study. Food Science.
2:2007.
|
|
59
|
Bian HL: Effect of sesamin on immune
function and proliferating cell nuclear antigen in S180
sarcoma-bearing mice. China J Mod Med. 18:3411–3417. 2008.
|
|
60
|
Wei YJ, Bian HL and Zhao CF: Inhibitory
effect of sesamin on tumor growth and mechanism in H (22)
hepatoma-bearing mice. Shandong Med J. 19:25–28. 2008.
|
|
61
|
Matsumura Y, Kita S, Tanida Y, Taguchi Y,
Morimoto S, Akimoto K and Tanaka T: Antihypertensive effect of
sesamin. III. Protection against development and maintenance of
hypertension in stroke-prone spontaneously hypertensive rats. Biol
Pharm Bull. 21:469–473. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tsuruoka N, Kidokoro A, Matsumoto I, Abe K
and Kiso Y: Modulating effect of sesamin, a functional lignan in
sesame seeds, on the transcription levels of lipid- and
alcohol-metabolizing enzymes in rat liver: A DNA microarray study.
Biosci Biotechnol Biochem. 69:179–188. 2005. View Article : Google Scholar : PubMed/NCBI
|