Introduction
Chronic obstructive pulmonary disease (COPD), a
chronic inflammatory disease involving the airways and lung
parenchyma (1), is currently a
primary cause of disability and death worldwide (2,3).
COPD is characterized by various clinical conditions, including
emphysema and chronic bronchitis (4). It has been reported that airway
inflammation serves a significant role in COPD, although the
precise role remains unclear (5).
The inflammatory process has been demonstrated to be involved in
the complex pathogenesis of COPD (6), and the inflammatory response in COPD
is associated with increased airway smooth muscle (ASM) cell
hyperplasia, which results in increased ASM mass and small airway
remodeling (7). As a result of
airway wall remodeling in patients with COPD, ASM cells may produce
inflammatory mediators (8). Thus,
ASM cells may be a novel therapeutic target for the treatment of
inflammation and airway remodeling in COPD patients.
Mortality rates and lung function have previously
been improved by glucagon-like peptide 1 (GLP-1) in a model of
experimental obstructive lung disease in female mice (9). GLP-1, a potent glucoincretin hormone,
is secreted by intestinal L-cells (10). GLP-1 increases insulin secretion
and production via its receptor (GLP-1R) in pancreatic β-cells
(11). GLP-1 additionally exerts
various effects outside the pancreas, including regulation of food
intake and appetite (12),
inhibition of gastrointestinal motility and secretion (13) and a cardioprotective effect
(14,15). GLP-1 acts via GLP-1R on various
types of target cells (11,16);
GLP-1R is expressed in the pancreatic islets, heart, brain, kidney,
gastrointestinal tract and lungs (15,17,18).
GLP-1 serves a role in inflammation and may inhibit
lipopolysaccharide (LPS)-induced cytokine secretion in various
types of cells, including peritoneal macrophages and cortical
astrocytes (19–21). However, although GLP-1R serves a
role in obstructive lung disease, its exact underlying mechanisms
remain unclear.
Hu et al (22) demonstrated that G protein-coupled
receptor 119 (GPR119) significantly increased the expression levels
of adenosine triphosphate-binding cassette, subfamily A, member 1
(ABCA1) via GLP-1R, and ABCA1 expression levels were markedly
reduced by small interfering (si)RNA-mediated silencing of GLP-1R
in a THP-1 macrophage cell line. ABCA1 is a member of a highly
conserved transmembrane transport protein family, and is expressed
in various mammalian tissues and cells (23,24).
ABCA1 serves a crucial role in pulmonary diseases and abnormalities
(25), and ABCA1 overexpression in
mouse vascular endothelial cells has been associated with
ovalbumin-induced neutrophilic airway inflammation (26,27).
Therefore, the present study hypothesized that GLP-1R may serve an
important role in COPD, and that ABCA1 is involved in the effects
of GLP-1R. ASM cells were isolated from patients with COPD and a
GLP-1R-overexpressing cell model was established to investigate the
function and underlying mechanisms of GLP-1R. The present study
provided evidence that GLP-1R is associated with ASM cell
proliferation and the secretion of inflammatory cytokines.
Materials and methods
Subjects
The present study was approved by the Ethics
Committee of the Zhujiang Hospital, Southern Medical University
(Guangzhou, China). Written informed consent was obtained from each
subject. Healthy controls (n=8) and patients with COPD (n=12) were
recruited (Table I) from the
Department of Respiratory Diseases of Zhujiang Hospital.
 | Table I.Characteristics of subjects. |
Table I.
Characteristics of subjects.
| Characteristic | Healthy subjects
(n=8) | COPD patients
(n=12) |
|---|
| Age (years) | 44.6±4.15 |
65.41±2.748a |
| Gender
(male:female) | 5:3 | 4:6 |
| Duration of COPD
(years) | NA | 16.35±2.28 |
| FEV1 (%
predicted) | 103.9±4.08 |
58.49±6.91a |
| FVC (%
predicted) | 109.7±6.1 | 98.54±4.35 |
| FEV1/FVC ratio
(%) | 86.39±3.24 |
50.37±7.11a |
ASM cell culture and transfection
ASM cells were isolated from bronchial biopsy
specimens according to a method described previously (28). Isolated human ASM cells from eight
healthy donors and twelve COPD donors were used at passage 3–4.
Cells were maintained in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 20 µg/ml streptomycin, 4 mM L-glutamine, 2.5
µg/ml amphotericin B, 20 U/l penicillin and 10% fetal bovine serum
(FBS; Invitrogen; Thermo Fisher Scientific, Inc.). Human GLP-1R
cDNA was purchased from OriGene Technologies, Inc. (Rockville, MD,
USA) and GLP-1R cDNA was inserted into the HindIII/EcoRI site of
pcDNA3.1 (Invitrogen; Thermo Fisher Scientific, Inc.) to form
pcDNA3.1-GLP-1R. Cells were transfected with pcDNA3.1-GLP-1R or
pcDNA3.1 vectors using Lipofectamine 2000™ (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
protocol.
MTT assay
ASM cell proliferation was determined by MTT assay
as described previously (29). A
total of 72 h following transfection, 5 mg/ml MTT was added to
cells, which were subsequently incubated for 4 h. Dimethyl
sulfoxide (150 µl) was added to dissolve the insoluble purple
formazan. The colorimetric method is based on the reduction of the
soluble tetrazolium dye MTT to its insoluble formazan. Absorbance
was measured at a wavelength of 490 nm using a microplate
reader.
Cell migration assay
ASM cell migration was measured using Transwell cell
culture chambers (8.0-µm pore size; EMD Millipore, Billerica, MA,
USA) as described previously (30). ASM cells were transfected with
pcDNA301-GLP-1R or GLP-1R siRNA. Cells were trypsinized and seeded
into the upper chamber of the Transwell insert (1×105 cells/well)
and medium containing 10% FBS was added to the lower chamber. After
24 h of incubation, Giemsa dye was used to stain migrated cells for
observation. Migrated cells were quantified under a light
microscope (magnification, ×200) (31).
Enzyme-linked immunosorbent assays
(ELISA)
The culture supernatants of ASM cells were collected
and centrifugation was performed for 10 min at 1,500 × g and 4°C to
remove particles and polymers. Interleukin (IL)-6 and -8, tumor
necrosis factor (TNF)-α, and granulocyte-macrophage
colony-stimulating factor (GM-CSF) cytokine levels in ASM cell
culture supernatants were measured using commercial ELISA kits
(catalog nos. D6050, D8000C, DTA00C and DGM00, respectively;
R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted from ASM cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA (5 µg) was used to reverse transcribe
first-strand cDNA using a High-Capacity cDNA Archive kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). GLP-1R and ABCA1
transcripts were quantified using SsoFast™
EvaGreen® Supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer's protocol and the
amplification was performed using a ABI PRISM 7900HT sequence
detection system (Applied Biosciences; Thermo Fisher Scientific,
Inc.). The following PCR conditions were performed in this assay:
95°C for 10 min; followed by 40 cycles of 95°C for 30 sec, 55°C for
10 sec and 72°C for 45 sec, followed by a final elongation step at
72°C for 5 min. The following primers were used: GLP-1R,
5′-TCAAGGTCAACGGCTTATTAGTGAA-3′ (forward) and
5′-CCCAAGTGATGCAAGCAGAG-3′ (reverse); ABCA1,
5′-AACAGTTTGTGGCCCTTTTG-3′ (forward) and 5′-AGTTCCAGGCTGGGGTACTT-3′
(reverse); β-actin, 5′-TCATGAAGTGTGACGTTGACATCCGT-3′ (forward) and
5′-CTTAGAAGCATTTGCGGTGCACGATG-3′ (reverse). Results were calculated
using the 2−ΔΔCq method, and are expressed as fold
change compared with control values following normalization against
β-actin (32).
Western blotting
Protein expression levels of GLP-1R and ABCA1 were
measured by western blotting. Proteins were isolated from ASM cells
using radioimmunoprecipitation assay lysis buffer (Invitrogen;
Thermo Fisher Scientific, Inc.), subsequently, protein (40 µg per
lane) was separated by 10% SDS-PAGE and subsequently transferred to
a polyvinylidene difluoride membrane. Membranes were incubated with
rabbit polyclonal anti-GLP-1R (1:1,000; 1 µg/ml; catalog no.
ab39072), mouse monoclonal anti-ABCA1 (1:500; catalog no. ab18180)
or mouse monoclonal anti-β-actin (1:5,000; 1 mg/ml; catalog no.
ab8226) antibodies (Abcam, Cambridge, MA, USA) overnight at 4°C.
Subsequently, the membrane was probed with horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:3,000; catalog no.
ab6721; Abcam Cambridge, MA, USA) or rabbit anti-mouse IgG
(1:2,000; catalog no. ab6728; Abcam.) for 1 h at room temperature.
Protein bands were developed with an Enhanced Chemiluminescence
detection kit (GE Healthcare Life Sciences, Chalfont, UK), and
visualized and quantified using Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
RNA silencing
GLP-1R- and ABCA1-silenced cells were generated
using Lipofectamine 2000 reagent according to the manufacturer's
protocol. For cells transfected with pcDNA3.1-GLP-1R vector + ABCA1
or mock siRNA, co-transfection was performed. GLP-1R and control
siRNAs were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The sequences of the siRNA oligomers for GLP-1R were as
follows: Forward, 5′-UCAUCAAGCUGUUUACAGATT-3′ and reverse,
5′-UCUGUAAACAGCUUGAUGAAG-3′. The scrambled siRNA sequences of
GLP-1R were as follows: Forward, 5′-UGUGGAUGACUGAGUACCUGA-3′ and
reverse, 5′-UCAGGUACUCAGUCAUCCACA-3′. ABCA1 siRNA was purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA; catalog no.
sc-41136). The sequences of the siRNA oligomers for ABCA1 were as
follows: Forward, 5′-GAACCUCACUUAGAAGAUU-3′ and reverse,
5′-UUCUUGGAGUGAAAGUCUUCU-3′. The scrambled siRNA sequences used for
ABCA1 were the same as scrambled GLP-1R siRNA sequences.
Statistical analysis
All statistical analyses were performed using SPSS
software version 13.0 (SPSS, Chicago, IL, USA). Data are expressed
as the mean ± standard error. Differences between two groups were
determined by the unpaired Student's t-test, and differences
between multiple groups were determined by one-way analysis of
variance followed by Fisher's least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were performed in quadruplicate and
repeated three times.
Results
GLP-1R mRNA and protein expression
levels are reduced in ASM cells from COPD patients
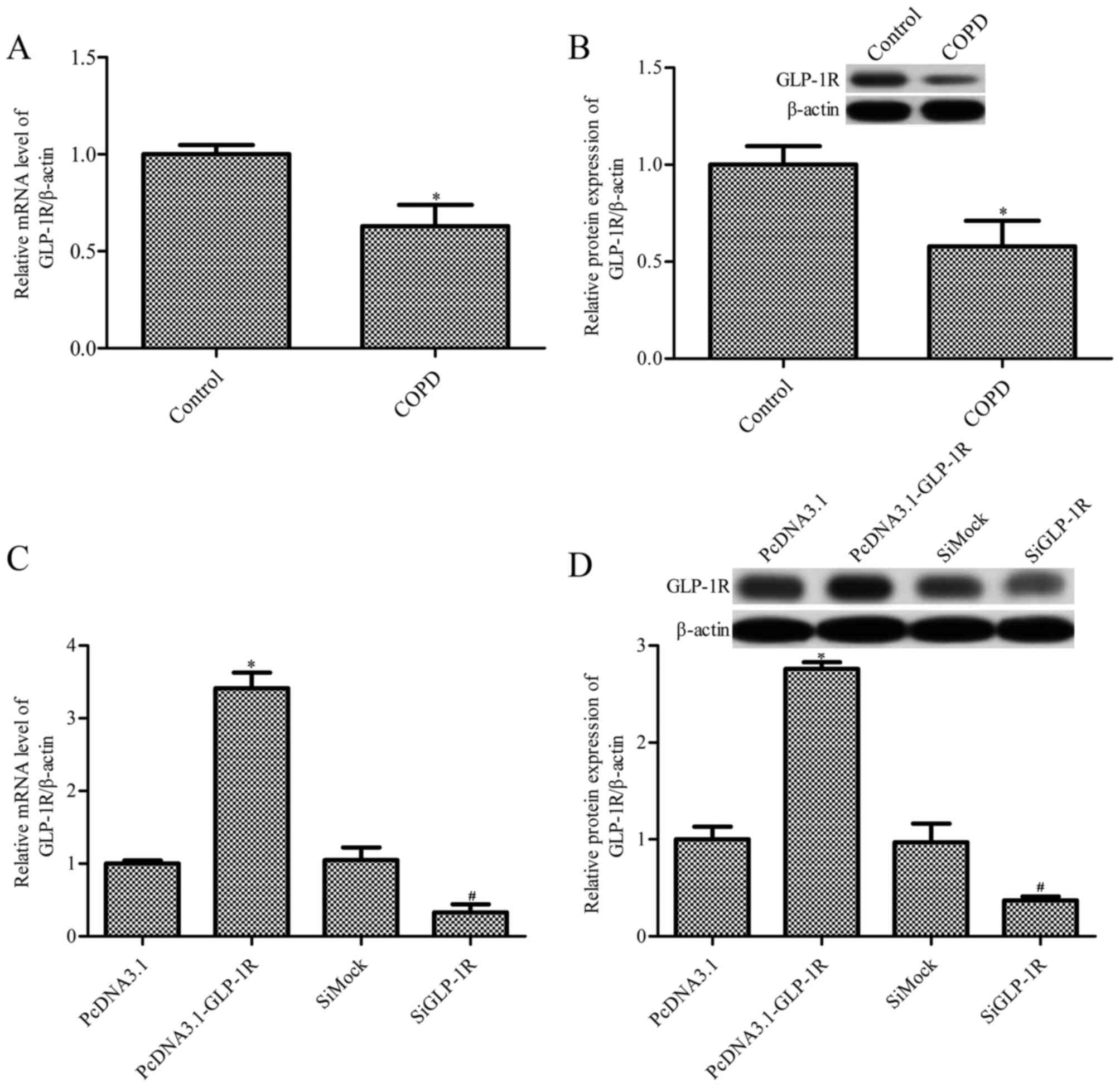
The present study examined GLP-1R mRNA expression
levels in ASM cells obtained from patients with COPD and healthy
controls (Table I) by RT-qPCR.
GLP-1R mRNA expression levels were significantly decreased in ASM
cells from patients with COPD, compared with healthy control cells
(P<0.05; Fig. 1A). GLP-1R
protein expression levels demonstrated the same effect, as revealed
by western blot analysis (P<0.05; Fig. 1B). To further investigate the
effects of GLP-1R in ASM cells from COPD patients, overexpression
or silencing of GLP-1R in ASM cells was performed. As revealed by
RT-qPCR (Fig. 1C) and western blot
analysis (Fig. 1D), the mRNA and
protein expression levels of GLP-1R were significantly increased in
ASM cells transfected with pcDNA3.1-Glp-1R for 72 h, and
significantly decreased in those transfected with GLP-1R siRNA for
48 h. The mRNA and protein expression levels of GLP-1R were
additionally measured in ASM cells transfected with pcDNA3.1-GLP-1R
or GLP-1R siRNA for 24, 48, 72 or 96 h. The results indicated that
the mRNA and protein expression levels of GLP-1R were significantly
increased in ASM cells transfected with pcDNA3.1-Glp-1R, and
significantly decreased in those transfected with GLP-1R siRNA
(data not shown).
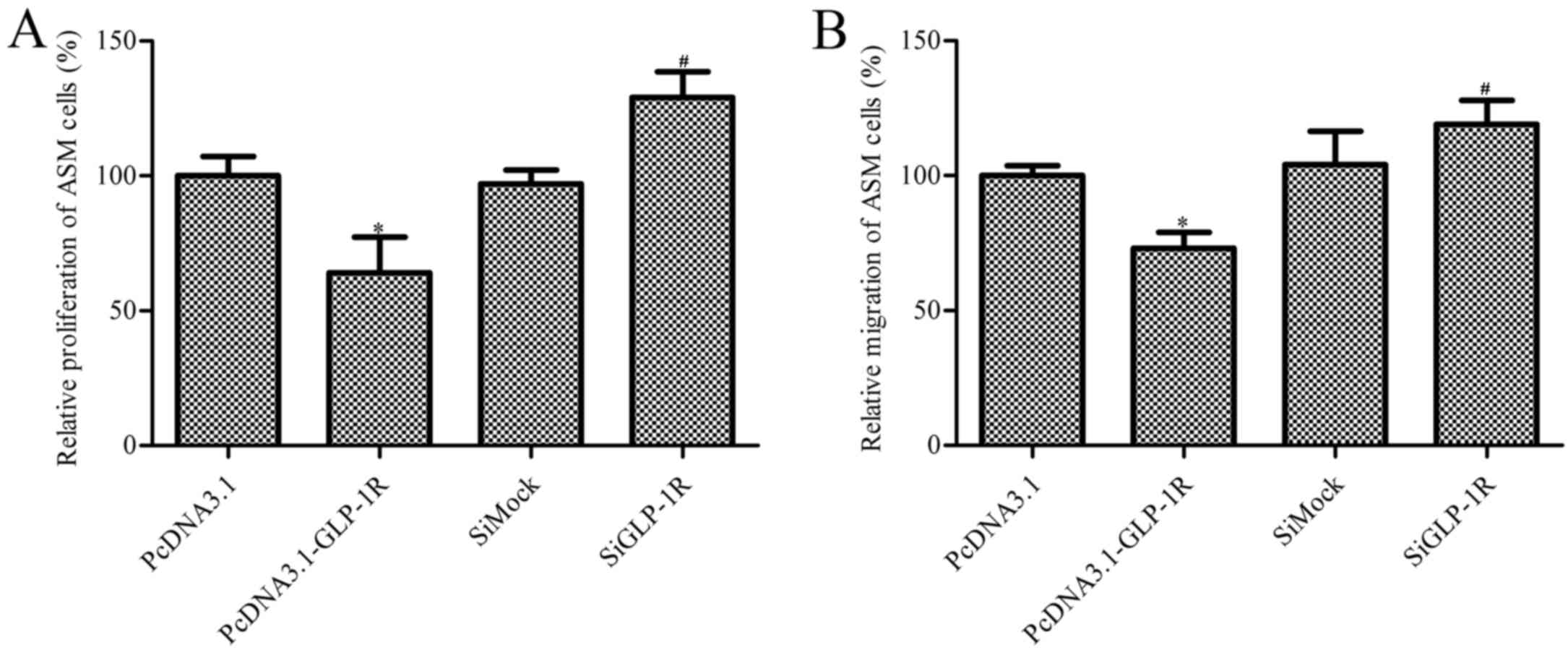
Effect of GLP-1R overexpression on ASM
cell proliferation and migration
ASM cells from COPD patients demonstrated reduced
proliferation following GLP-1R overexpression compared with the
pcDNA3.1 empty-plasmid group Additionally, ASM cells transfected
with GLP-1R siRNA exhibited increased proliferation by 32% compared
with the siMock group (Fig. 2A).
ASM cell migration was subsequently determined, and the results
were similar to the proliferation study. GLP-1R overexpression
suppressed the migration of ASM cells, whereas silencing increased
ASM cell migration (Fig. 2B).
These data indicated that GLP-1R overexpression reduces cell
proliferation and migration, and therefore may serve a role in
airway remodeling in COPD patients.
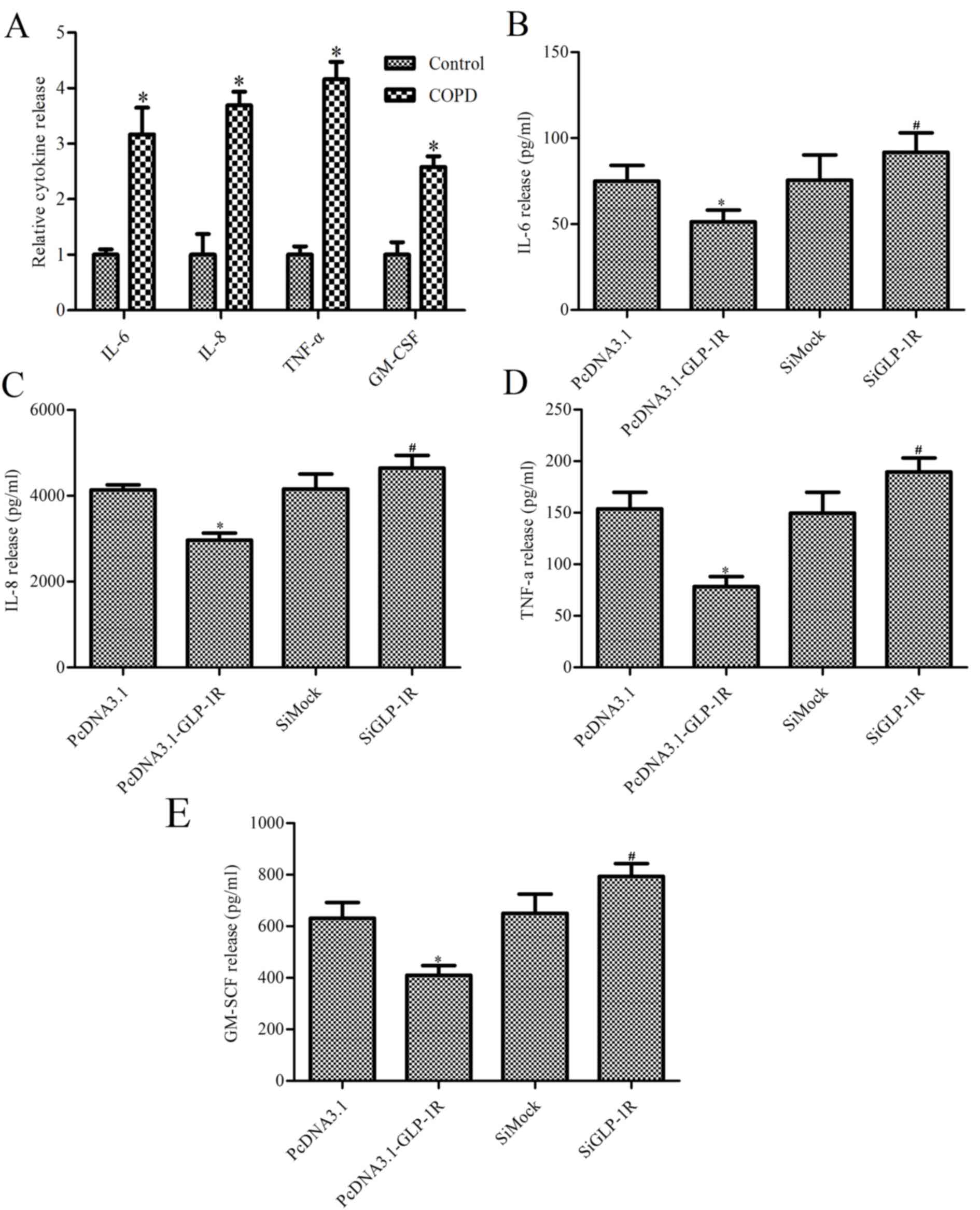
Effect of GLP-1R overexpression on
IL-6, IL-8, TNF-α and GM-CSF release
To determine whether GLP-1R overexpression mediates
ASM cell inflammatory cytokine release, the levels of IL-6, IL-8,
TNF-α and GM-CSF were measured. Cultured ASM cells isolated from
patients with COPD released increased levels of IL-6, IL-8, TNF-α
and GM-CSF compared with ASM cells from healthy control subjects
(P<0.05; Fig. 3A). In addition,
GLP-1R overexpression inhibited the release of IL-6, IL-8, TNF-α
and GM-CSF, whereas its ablation increased the release of these
inflammatory cytokines (Fig.
3B-E).
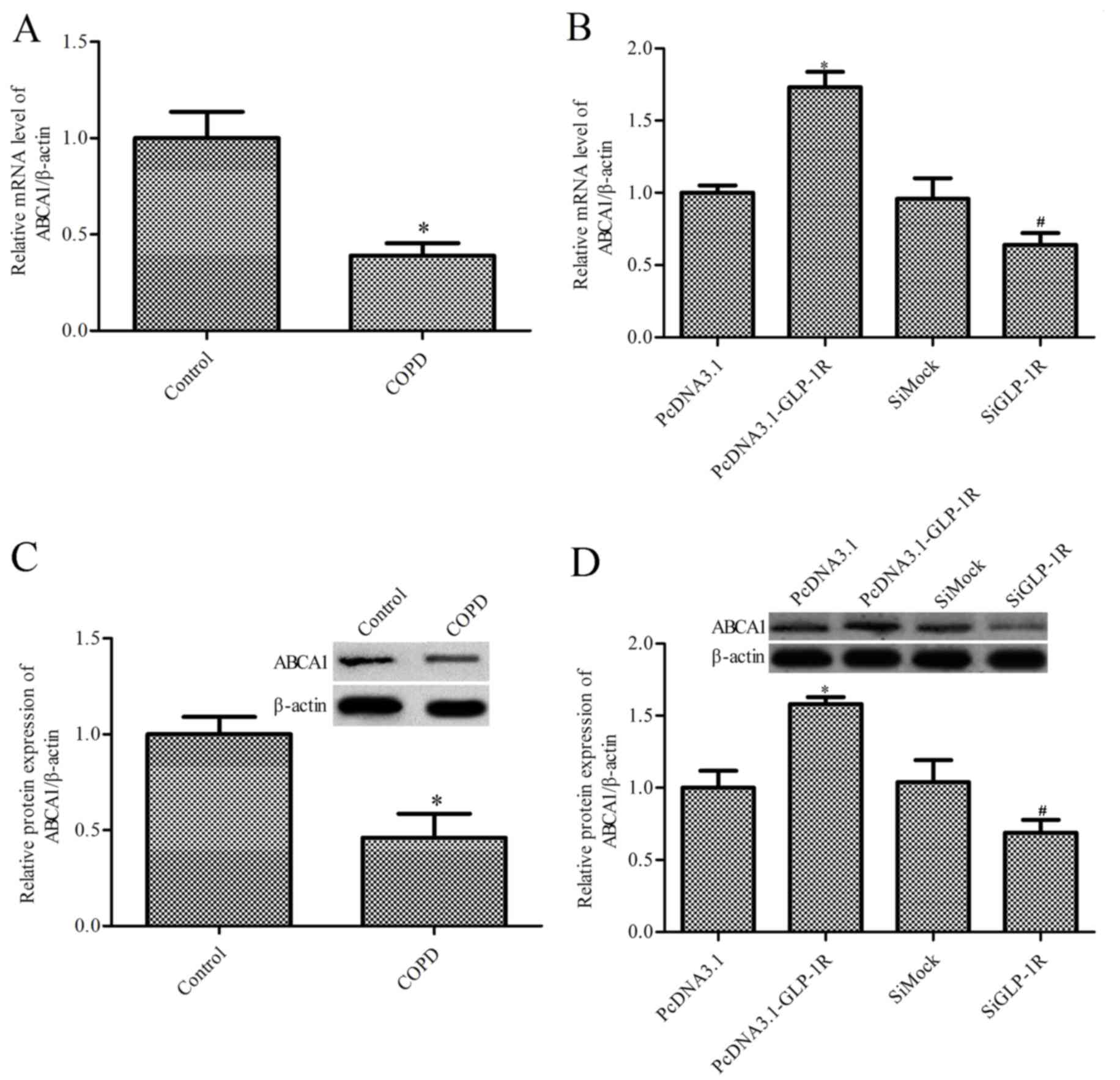
GLP-1R overexpression markedly
upregulates ABCA1 expression levels
GLP-1R may upregulate the expression levels of
ABCA1, and previous studies have indicated that ABCA1 serves an
important role in airway inflammation (23,27).
Thus, the present study aimed to determine the effects of GLP-1R on
ABCA1 expression levels. RT -qPCR and western blotting were
performed to assess the expression levels of ABCA1 in ASM cells
obtained from patients with COPD and healthy control subjects. mRNA
(Fig. 4A) and protein (Fig. 4B) expression levels of ABCA1 were
reduced in ASM cells from COPD patients compared with the healthy
control subjects (P<0.05). Furthermore, GLP-1R overexpression
increased the expression levels of ABCA1 in ASM cells obtained from
patients with COPD, whereas GLP-1R siRNA decreased ABCA1 expression
levels (P<0.05; Fig. 4C and
D).
GLP-1R-mediated cell proliferation,
migration and cytokine release is dependent on ABCA1
expression
The above results suggested that GLP-1R increases
the expression levels of ABCA1, and it was therefore hypothesized
that the effects of GLP-1R on ASM cell proliferation, migration and
cytokine release are dependent on altered ABCA1 expression levels.
To further investigate the interaction between ABCA1 and GLP-1R in
ASM cells obtained from patients with COPD, ABCA1-silenced ASM
cells were established. As presented in Fig. 5A, ABCA1 siRNA markedly inhibited
the mRNA expression levels of ABCA1. Treatment with pcDNA3.1-GLP-1R
significantly inhibited proliferation (Fig. 5B) and migration (Fig. 5C) of ASM cells from COPD patients,
whereas this effect was ameliorated by co-transfection with ABCA1
siRNA (P<0.05). IL-6, IL-8, TNF-α and GM-CSF levels were
additionally measured, and the results indicated that ABCA1 siRNA
reversed the GLP-1R overexpression-induced decrease in IL-6, IL-8,
TNF-α and GM-CSF expression levels (P<0.05; Fig. 5D).
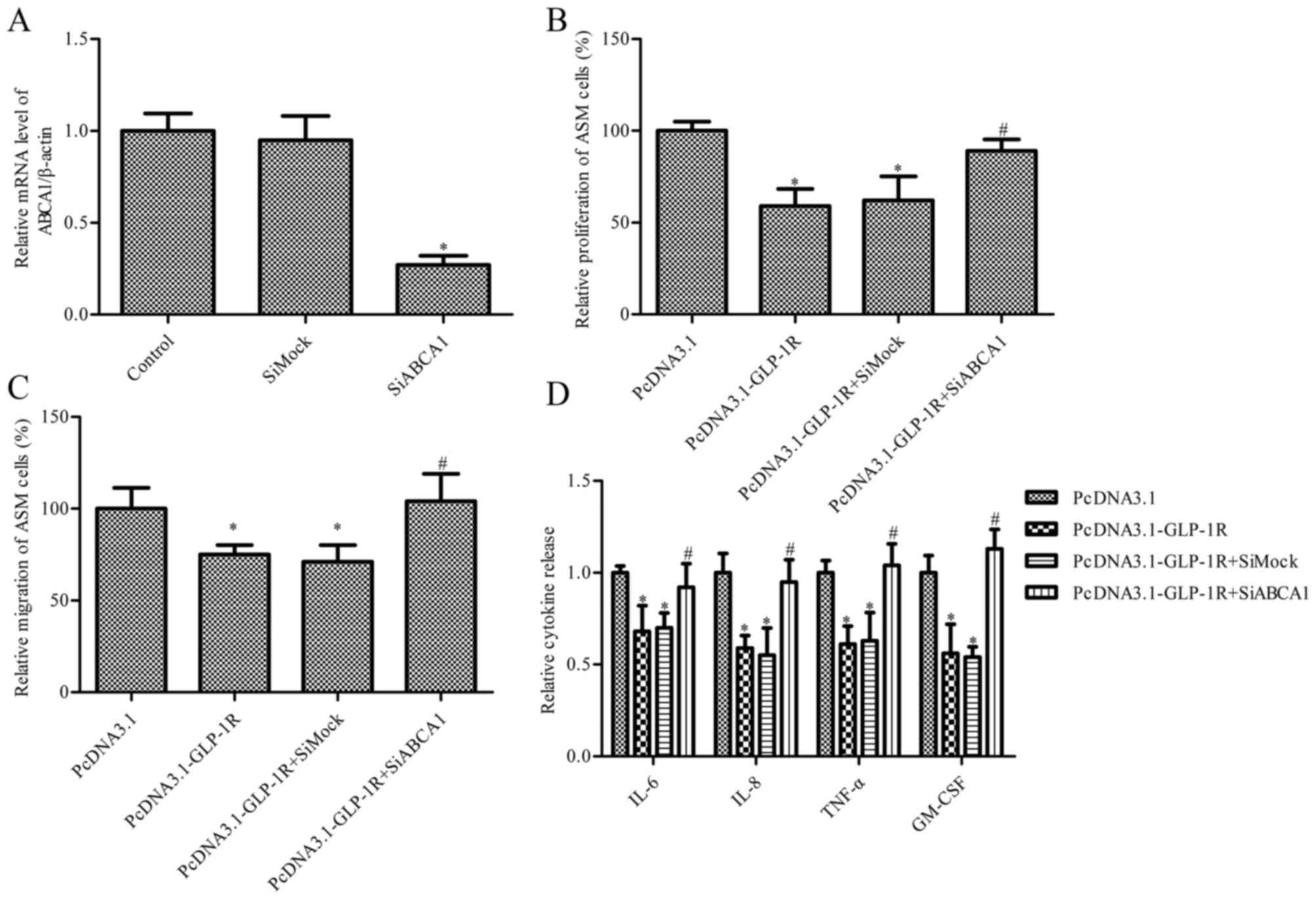 | Figure 5.Effect of ABCA1 silencing on
GLP-1R-mediated ASM cell proliferation, migration and cytokine
release. Cells from COPD patients were transfected with
pcDNA3.1-GLP-1R and ABCA1 siRNA. (A) mRNA expression levels of
ABCA1; β-actin served as an internal control. *P<0.05 vs.
control. (B) An MTT assay was used to measure ASM cell
proliferation. (C) Cell migration was measured using a Transwell
assay. (D) The levels of IL-6, IL-8, TNF-α and GM-CSF secreted by
ASM cells obtained from COPD patients were detected by ELISA. Data
are presented as the mean ± standard error. *P<0.05 vs. pcDNA3.1
group and #P<0.05 vs. pcDNA3.1-Glp-1R group + siMock.
si, small interfering; GLP-1R, glucagon-like peptide 1 receptor;
ASM, airway smooth muscle; COPD, chronic obstructive pulmonary
disorder; ABCA1, adenosine triphosphate-binding cassette, subfamily
A, member 1; IL, interleukin; TNF-α, tumor necrosis factor-α;
GM-CSF, granulocyte-macrophage colony-stimulating factor. |
Discussion
The present study demonstrated decreased expression
levels of GLP-1R and ABCA1 in ASM cells from patients with COPD.
Furthermore, overexpression of GLP-1R suppressed proliferation and
cytokine release by ASM cells from patients with COPD via increased
ABCA1 expression levels.
GLP-1 serves a cardioprotective role in humans
following myocardial infarction (MI) and in animal models of MI
(14). Liu et al (33) demonstrated that chronic treatment
with GLP-1 or AC3174 improves glucose metabolism, fluid balance and
respiratory efficiency in a rat model of congestive heart failure,
compared with vehicle control animals. GLP-1Rs are known to serve
an important role in models of experimental obstructive lung
disease in female mice (9); GLP-1R
agonists were demonstrated to reduce mortality rates and improve
lung function, and may have therapeutic potential for the treatment
of obstructive pulmonary diseases (9). However, the specific function and
underlying mechanisms of action remain unclear. Consistent with
this study, the present study revealed that GLP-1R serves a crucial
role in COPD in vitro. GLP-1R overexpression significantly
suppressed proliferation and cytokine release by ASM cells from
patients with COPD, which may serve roles in airway remodeling and
inflammation.
Notably, COPD is analogous to asthma in that the two
diseases are associated with chronic inflammation and smooth muscle
hyperplasia. ASM cells may mediate immune modulation and
inflammation in the airway via secretion of inflammatory mediators
and cytokines (34). Furthermore,
ASM cell hyperplasia contributes to an increase in ASM mass and
results in small airway remodeling in COPD (7). Adenosine triphosphate-binding
cassette transporters, including ABCA1, are known to be important
in the pathogenesis of ASM cells. ABCA1 may serve a critical role
in airway diseases, including asthma, via dysregulation of
cholesterol homeostasis (35).
Although the function of ABCA1 has been well characterized in ASM
cells, its role in COPD remains unclear.
The ABCA1 gene has previously been
demonstrated to be a target of liver X receptors (LXRs) (7,26,33);
macrophage expression of ABCA1 and intestinal cholesterol
absorption was abolished in LXR-null mice. Mostafa et al
(36) revealed that GLP-1 may
modulate ABCA1 expression levels in adipocytes via an
LXR-α-mediated process. Hu et al (22) demonstrated that GLP-1R-silencing
markedly inhibits ABCA1 expression in THP-1 macrophages. Consistent
with these studies, the present study revealed that GLP-1R
overexpression promoted ABCA1 expression levels, whereas GLP-1R
silencing inhibited its expression levels. Using siRNA knockdown
approaches, ABCA1 was demonstrated to be necessary for
GLP-1R-induced cell proliferation, migration and cytokine release
inhibition in ASM cells.
In conclusion, the present study revealed that
overexpression of GLP-1R significantly reduces proliferation,
migration and cytokine release in ASM cells from COPD patients;
this involved a significant increase in ABCA1 expression levels.
This provided evidence to suggest that GLP-1R may be a potential
therapeutic target for the treatment of COPD.
Glossary
Abbreviations
Abbreviations:
|
GLP-1
|
glucagon-like peptide-1
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
ASM
|
airway smooth muscle
|
|
ABCA1
|
adenosine triphosphate-binding
cassette, subfamily A, member 1
|
|
LPS
|
lipopolysaccharide
|
References
|
1
|
Tangedal S, Aanerud M, Persson LJ,
Brokstad KA, Bakke PS and Eagan TM: Comparison of inflammatory
markers in induced and spontaneous sputum in a cohort of COPD
patients. Respir Res. 15:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niewoehner DE: Clinical practice.
Outpatient management of severe COPD. N Engl J Med. 362:1407–1416.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogelmeier C, Hederer B, Glaab T, Schmidt
H, Rutten-van Mölken MP, Beeh KM, Rabe KF and Fabbri LM: POET-COPD
Investigators: Tiotropium versus salmeterol for the prevention of
exacerbations of COPD. N Engl J Med. 364:1093–1103. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang MJ, Yoon CM, Kim BH, Lee CM, Zhou Y,
Sauler M, Homer R, Dhamija A, Boffa D, West AP, et al: Suppression
of NLRX1 in chronic obstructive pulmonary disease. J Clin Invest.
125:2458–2462. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brusasco V, Crimi E and Pellegrino R:
Airway inflammation in COPD: Friend or foe? Am J Respir Crit Care
Med. 176:425–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogawa E, Elliott WM, Hughes F, Eichholtz
TJ, Hogg JC and Hayashi S: Latent adenoviral infection induces
production of growth factors relevant to airway remodeling in COPD.
Am J Physiol Lung Cell Mol Physiol. 286:L189–L197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michaeloudes C, Wiegman C, Kirkham P,
Chung KF and Adcock I: Mitochondrial reactive oxygen species (ROS)
mediate proliferation and cytokine release in airway smooth muscle
cells of patients with COPD. European Respir J. 44:P38452014.
|
|
8
|
Chung KF: The role of airway smooth muscle
in the pathogenesis of airway wall remodeling in chronic
obstructive pulmonary disease. Proc Am Thorac Soc. 2:347–354,
371–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viby NE, Isidor MS, Buggeskov KB, Poulsen
SS, Hansen JB and Kissow H: Glucagon-like peptide-1 (GLP-1) reduces
mortality and improves lung function in a model of experimental
obstructive lung disease in female mice. Endocrinology.
154:4503–4511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Drucker DJ: Glucagon-like peptides.
Diabetes. 47:159–169. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou Y, Ernst SA, Heidenreich K and
Williams JA: Glucagon-like peptide-1 receptor is present in
pancreatic acinar cells and regulates amylase secretion through
cyclic AMP. Am J Physiol Gastrointest Liver Physiol. 310:G26–G33.
2016.PubMed/NCBI
|
|
12
|
Tang-Christensen M, Vrang N and Larsen PJ:
Glucagon-like peptide 1(7–36) amide's central inhibition of feeding
and peripheral inhibition of drinking are abolished by neonatal
monosodium glutamate treatment. Diabetes. 47:530–537. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nauck MA, Niedereichholz U, Ettler R,
Holst JJ, Orskov C, Ritzel R and Schmiegel WH: Glucagon-like
peptide 1 inhibition of gastric emptying outweighs its
insulinotropic effects in healthy humans. Am J Physiol.
273:E981–E988. 1997.PubMed/NCBI
|
|
14
|
Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM,
Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M and Drucker
DJ: GLP-1R agonist liraglutide activates cytoprotective pathways
and improves outcomes after experimental myocardial infarction in
mice. Diabetes. 58:975–983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS,
Drucker DJ and Husain M: Cardioprotective and vasodilatory actions
of glucagon-like peptide 1 receptor are mediated through both
glucagon-like peptide 1 receptor-dependent and -independent
pathways. Circulation. 117:2340–2350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dillon JS, Tanizawa Y, Wheeler MB, Leng
XH, Ligon BB, Rabin DU, Yoo-Warren H, Permutt MA and Boyd AE III:
Cloning and functional expression of the human glucagon-like
peptide-1 (GLP-1) receptor. Endocrinology. 133:1907–1910. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bullock BP, Heller RS and Habener JF:
Tissue distribution of messenger ribonucleic acid encoding the rat
glucagon-like peptide-1 receptor. Endocrinology. 137:2968–2978.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campos RV, Lee YC and Drucker DJ:
Divergent tissue-specific and developmental expression of receptors
for glucagon and glucagon-like peptide-1 in the mouse.
Endocrinology. 134:2156–2164. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arakawa M, Mita T, Azuma K, Ebato C, Goto
H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R and Watada H:
Inhibition of monocyte adhesion to endothelial cells and
attenuation of atherosclerotic lesion by a glucagon-like peptide-1
receptor agonist, exendin-4. Diabetes. 59:1030–1037. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwai T, Ito S, Tanimitsu K, Udagawa S and
Oka J: Glucagon-like peptide-1 inhibits LPS-induced IL-1beta
production in cultured rat astrocytes. Neurosci Res. 55:352–360.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ku HC, Chen WP and Su MJ: GLP-1 signaling
preserves cardiac function in endotoxemic Fischer 344 and
DPP4-deficient rats. Naunyn-Schmiedeberg's Arch Pharmacol.
382:463–474. 2010. View Article : Google Scholar
|
|
22
|
Hu YW, Yang JY, Ma X, Chen ZP, Hu YR, Zhao
JY, Li SF, Qiu YR, Lu JB, Wang YC, et al: A lincRNA-
DYNLRB2-2/GPR119/GLP-1R/ABCA1-dependent signal transduction pathway
is essential for the regulation of cholesterol homeostasis. J Lipid
Res. 55:681–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Langmann T, Klucken J, Reil M, Liebisch G,
Luciani MF, Chimini G, Kaminski WE and Schmitz G: Molecular cloning
of the human ATP-binding cassette transporter 1 (hABC1): Evidence
for sterol-dependent regulation in macrophages. Biochem Biophys Res
Commun. 257:29–33. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bortnick AE, Rothblat GH, Stoudt G, Hoppe
KL, Royer LJ, McNeish J and Francone OL: The correlation of
ATP-binding cassette 1 mRNA levels with cholesterol efflux from
various cell lines. J Biol Chem. 275:28634–28640. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bates SR, Tao JQ, Collins HL, Francone OL
and Rothblat GH: Pulmonary abnormalities due to ABCA1 deficiency in
mice. Am J Physiol Lung Cell Mol Physiol. 289:L980–L989. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai C, Boris V, Yao X, Meyer K, Karen K,
Nugent GJ, Qu X, Yu ZX, Remaley A and Levine SJ: Expression Of
Human Abca1 In Mouse Vascular Endothelial Cells Attenuates
Ovalbumin-Induced Neutrophilic Airway Inflammation. Am J Respir
Crit Care Med. 185:A56372012.
|
|
27
|
Dai C, Yao X, Vaisman B, Brenner T, Meyer
KS, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, et al: ATP-binding
cassette transporter 1 attenuates ovalbumin-induced neutrophilic
airway inflammation. Am J Respir Cell Mol Biol. 51:626–636. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Michaeloudes C, Sukkar MB, Khorasani NM,
Bhavsar PK and Chung KF: TGF-β regulates Nox4, MnSOD, and catalase
expression, and IL-6 release in airway smooth muscle cells. Am J
Physiol Lung Cell Mol Physiol. 300:L295–L304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sturrock A, Huecksteadt TP, Norman K,
Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR and Kennedy
TP: Nox4 mediates TGF-beta1-induced retinoblastoma protein
phosphorylation, proliferation, and hypertrophy in human airway
smooth muscle cells. Am J Physiol Lung Cell Mol Physiol.
292:L1543–L1555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL,
Lin JP, Ma YS, Wu CC and Chung JG: Curcumin inhibits the migration
and invasion of human A549 lung cancer cells through the inhibition
of matrix metalloproteinase-2 and-9 and Vascular Endothelial Growth
Factor (VEGF). Cancer Lett. 285:127–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu SC, Kuo CL, Lin JP, Lee JH, Lin CC, Su
CC, Yang MD and Chung JG: Crude extracts of Euchresta formosana
radix inhibit invasion and migration of human hepatocellular
carcinoma cells. Anticancer Res. 27:2377–2384. 2007.PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Q, Anderson C, Broyde A, Polizzi C,
Fernandez R, Baron A and Parkes DG: Glucagon-like peptide-1 and the
exenatide analogue AC3174 improve cardiac function, cardiac
remodeling, and survival in rats with chronic heart failure.
Cardiovasc Diabetol. 9:762010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lazaar AL and Panettieri RA Jr: Airway
smooth muscle as an immunomodulatory cell: A new target for
pharmacotherapy? Curr Opin Pharmacol. 1:259–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Delvecchio CJ, Bilan P, Nair P and Capone
JP: LXR-induced reverse cholesterol transport in human airway
smooth muscle is mediated exclusively by ABCA1. Am J Physiol Lung
Cell Mol Physiol. 295:L949–L957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mostafa AM, Hamdy NM, El-Mesallamy HO and
Abdel-Rahman SZ: Glucagon-like peptide 1 (GLP-1)-based therapy
upregulate LXR-ABCA1/ABCG1 cascade in adipocytes. Biochem Biophys
Res Commun. 468:900–905. 2015. View Article : Google Scholar : PubMed/NCBI
|