Introduction
Transient cerebral ischemia leads to selective
neuronal damage/death in certain brain regions, including the
hippocampal CA1 region (1,2). In the hippocampal CA1 region,
transient cerebral ischemia-induced neuronal death occurs in
pyramidal neurons several days after ischemia-reperfusion, and this
phenomenon is referred to as ‘delayed neuronal death’ (1). However, the underlying mechanisms
that are associated with ischemia-induced delayed neuronal death
have not yet been fully elucidated (3–5).
Following the onset of transient cerebral ischemia, dysfunction of
cerebral microcirculation occurs, including cerebral blood-brain
barrier (BBB) breakdown and increase in BBB permeability (6–10),
resulting in the extravasation of serum proteins in the
perivascular space and brain parenchyma (6,10,11).
The dysfunction of cerebral microcirculation following ischemic
insult is known to be closely associated with the ischemia-induced
neuro-inflammation, cerebral edema and neuronal injury (12).
Albumin, the most abundant plasma protein, has
multifunctional properties, including maintaining the osmotic
pressure of plasma and specifically binding to low molecular weight
molecules (13). Several studies
have reported that treatment with albumin improves neurological
score and decreases histopathological damage in experimental animal
models of focal and global cerebral ischemia (14–17).
These neuroprotective effects of albumin are thought to be due to
its properties, such as its antioxidant activity, its inhibitory
activity on pathological platelet aggregation, and its role in
regulation of endothelial integrity (18,19).
However, few studies regarding albumin expression and its change in
neurons or glial cells in the hippocampal CA1 region following
transient cerebral ischemia exist to date. Therefore, in the
present study, albumin expression in the hippocampal CA1 region of
the gerbil, a popular animal model of transient cerebral ischemia
(20), was examined following 5
min of transient cerebral ischemia. This suggests that albumin has
the potential to be an attractive target for the treatment of
cerebral ischemia.
Materials and methods
Experimental animals and induction of
transient cerebral ischemia
Male, 6-month old Mongolian gerbils (Meriones
unguiculatus) were obtained from the Experimental Animal Center of
the Kangwon National University (Chuncheon, Republic of Korea) and
were housed according to the animal handling and care guidelines as
in compliance with the current international laws and policies
(Guide for the Care and Use of Laboratory Animals, The National
Academies Press, 8th edition, 2011) (21). The present study was approved by
the Institutional Animal Care and Use Committee of Kangwon National
University (Chuncheon, Korea; approval no. KW-130424-1).
The surgical procedure for transient cerebral
ischemia was performed as previously described (22). In brief, the animals were
anesthetized with a mixture of 2.5% isoflurane in 33% oxygen and
67% nitrous oxide. The complete interruption of blood flow mediated
by the 5 min occlusion of bilateral common carotid arteries was
confirmed by observing the central artery in retinae under an
ophthalmoscope. Normothermic (37±0.5°C) conditions were maintained
prior to, during and following the surgery until the animals
completely recovered from anesthesia. Sham-operated animals were
subjected to the same surgical procedures except that the common
carotid arteries were not occluded.
Cresyl violet (CV) staining and
immunohistochemistry for albumin
Sections were prepared from the sham and
ischemia-operated animals (n=7 at each time-point) at 2 and 4 days
after reperfusion, according to the previously published procedure
(22). In brief, the animals were
perfused transcardially with 4% paraformaldehyde in 0.1 M
phosphate-buffer (pH 7.4). The brain tissues were then serially
sectioned into 30 µm coronal sections and they were then placed
into 6-well plates containing phosphate-buffered saline.
To examine cellular distribution and damage CV
staining was performed as previously described (23). In brief, CV acetate (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was dissolved at 1.0% (w/v), and
glacial acetic acid was added at 0.28% in this solution. The
sections were stained and dehydrated by immersing in serial ethanol
baths.
Albumin immunohistochemistry was carried out
according to our previously published procedure (22). Briefly, sections were incubated
with mouse anti-albumin (catalog no. ab19194; dilution 1:100;
Abcam, Cambridge, MA, USA), exposed to biotinylated goat anti-mouse
immunoglobulin (Ig) G and streptavidin peroxidase complex (Vector
Laboratories, Inc., Burlingame, CA, USA) and visualized with
3,3′-diaminobenzidine. In order to confirm the cell type containing
the albumin immunoreactivity, sections were also processed by
double immunofluorescence staining. In brief, sections were
incubated in a mixture of mouse anti-albumin (dilution 1:50; Abcam)
and rabbit anti-ionized calcium-binding adapter molecule 1 (Iba-1;
an established microglia marker; catalog no. 019–19741; dilution
1:100; Wako Pure Chemical Industries, Ltd., Osaka, Japan), followed
by a mixture of Cy3-conjugated goat anti-mouse IgG (dilution 1:200)
and fluorescein isothiocyanate-conjugated goat anti-rabbit IgG
(dilution 1:200) (both from Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA). The immunoreactions were observed under
a confocal microscope (LSM 510 META NLO; Carl Zeiss AG, Oberkochen,
Germany).
For the analysis of albumin immunoreactivity, six
sections per animal in each group (n=7) were selected with 120 µm
intervals to quantitatively analyze albumin immunoreactivity.
Digital images of the hippocampal CA1 region were captured with an
AxioM1 light microscope (Carl Zeiss AG) equipped with a digital
camera (Axiocam; Carl Zeiss AG). According to our previously
published procedure (22),
semi-quantification of the immunostaining intensities was evaluated
with digital image analysis software (MetaMorph 4.01; Universal
Imaging, Inc., Bedford Hills, NY, USA). The mean intensity of the
albumin immunostaining in each immunoreactive structure was
measured using a 0–255 gray scale system. The level of
immunoreactivity was scored as follows: -, no staining (gray scale
value: ≥200) ±, weakly positive (gray scale value: 150–199); +,
moderate (gray scale value: 100–149); ++, strong (gray scale value:
50–99); or +++, very strong (gray scale value: ≤49). The
immunoreactivity scores were obtained by averaging the measurements
from each animal.
Results
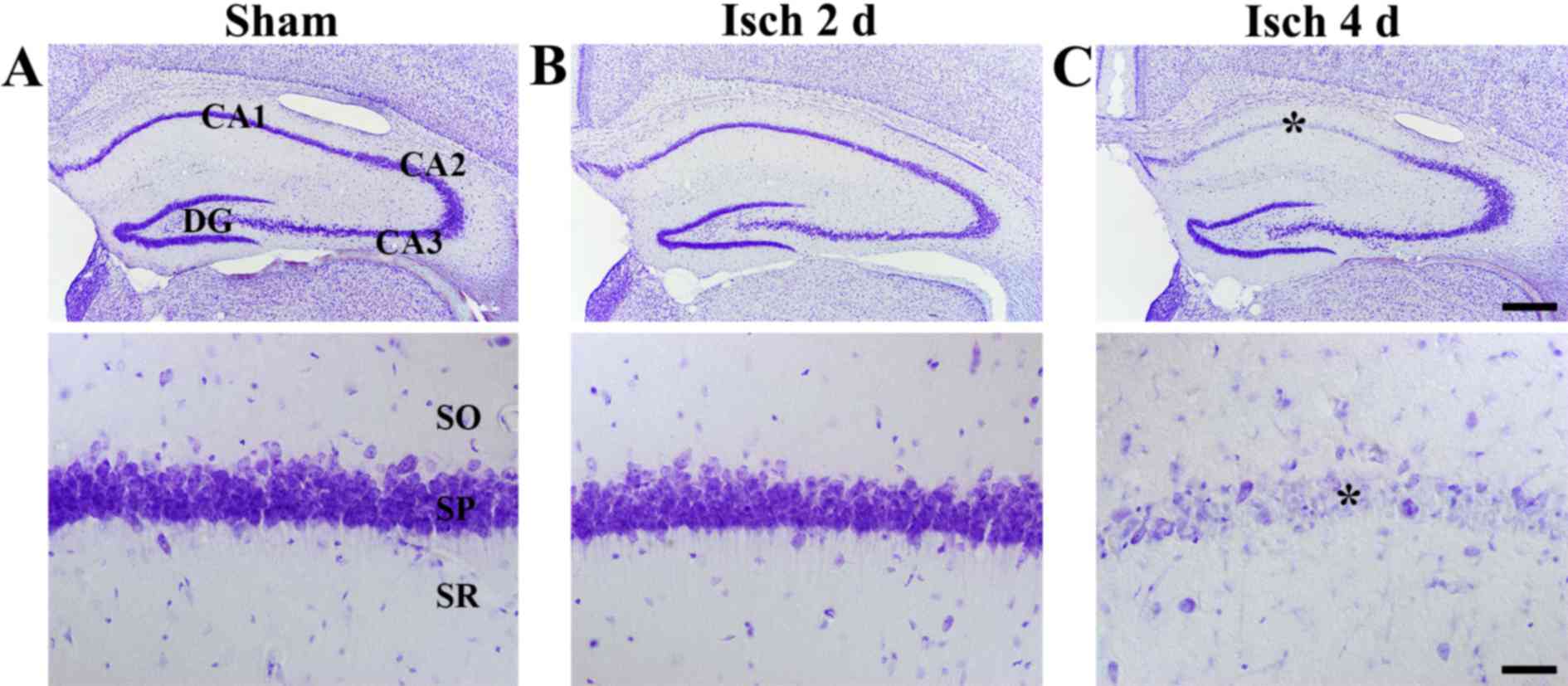
Delayed neuronal death
To examine ischemia-induced delayed neuronal death
in the hippocampus, CV staining was performed. In the sham-operated
group, CV-positive cells were observed in all subregions of the
hippocampus (Fig. 1A). In the
ischemia-operated groups, the distribution pattern of CV-positive
cells was not altered at 2 days following ischemia-reperfusion
compared with the sham-operated group (Fig. 1B). However, at 4 days following
ischemia-reperfusion, a markedly decreased number of neurons in the
stratum pyramidale of the CA1 region were stained with CV compared
with the sham-operated group (Fig.
1C). This effect was evident only in the stratum pyramidale, as
pyramidal neurons in other subregions of the hippocampus were
well-stained with CV in the ischemia-operated and the sham-operated
groups (Fig. 1C).
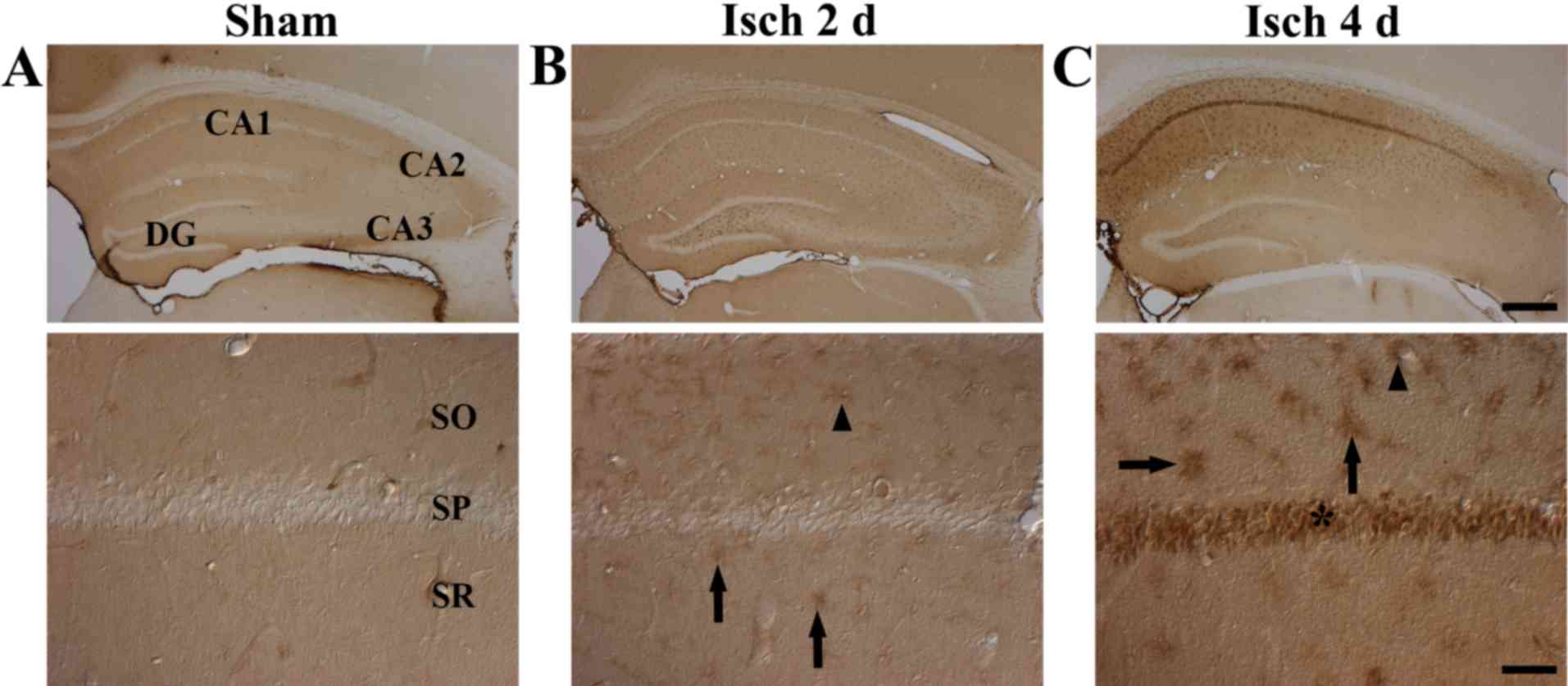
Albumin immunoreactivity
In the CA1 region tissue sections of the
sham-operated group, albumin immunoreactivity was observed only in
blood vessels, and no albumin-immunoreactive cells were observed
(Fig. 2A). However, following
transient cerebral ischemia, albumin immunoreactivity was generally
increased in the tissue sections and observed in cells. At 2 days
after ischemia-reperfusion, albumin immunoreactivity was observed
in microglia-like cells in the stratum oriens and stratum radiatum
of the CA1 region (Table I and
Fig. 2B). At 4 days after
ischemia-reperfusion, albumin immunoreactivity was further
increased in microglia-like cells in the stratum oriens and stratum
radiatum through the CA1 region compared with the sham and the 2
day post-ischemia groups (Table I
and Fig. 2C). Notably, at 4 days
after ischemia-reperfusion, strong albumin immunoreactivity was
also detected in the stratum pyramidale (Table I and Fig. 2C).
 | Table I.Level of albumin immunoreactivity in
microglia of the CA1 hippocampal region following transient
cerebral ischemia. |
Table I.
Level of albumin immunoreactivity in
microglia of the CA1 hippocampal region following transient
cerebral ischemia.
|
| Time after
ischemia/reperfusion |
|---|
|
|
|
|---|
| Region | Sham | 2 days | 4 days |
|---|
| SO | − | ± | +++ |
| SP | − | − | +++ |
| SR | − | ± | +++ |
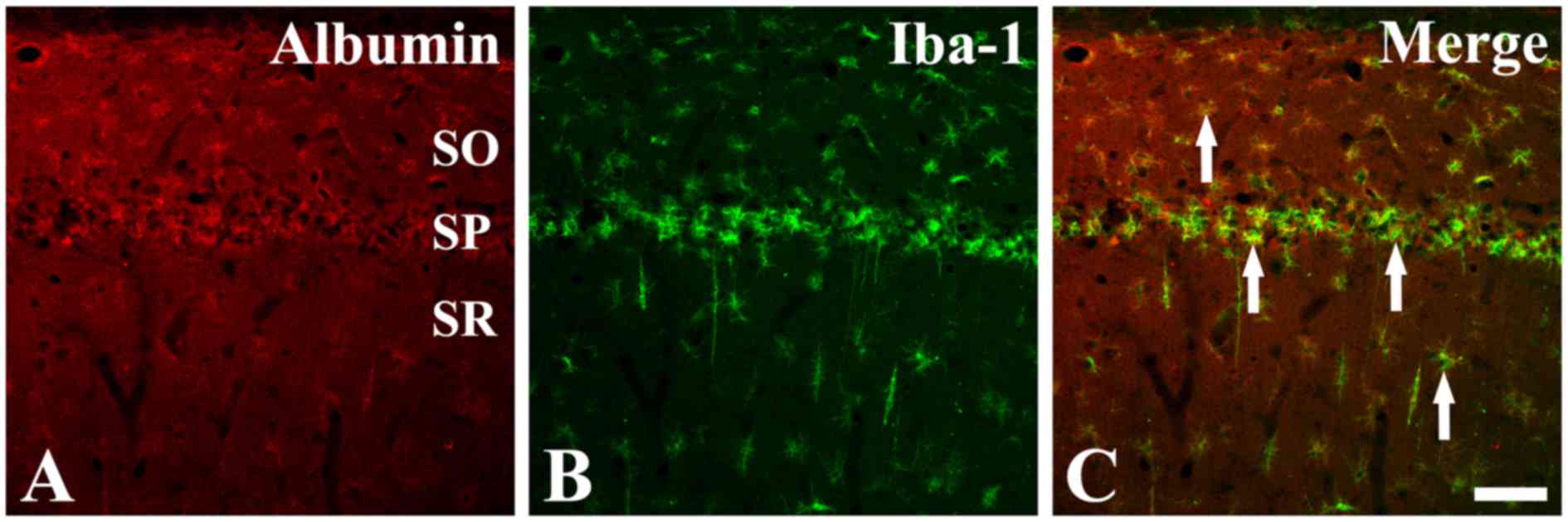
To confirm the identity of the cell type that
exhibited specific positive albumin immunostaining in the CA1
region tissue sections at 4 days post-ischemia, double
immunofluorescence staining was performed for albumin and Iba-1, an
established microglia marker. The results demonstrated that many of
the albumin-immunoreactive cells were colocalized with
Iba-1-positive microglia (Fig. 3),
suggesting that albumin is strongly expressed by microglia in the
hippocampal CA1 region following ischemic insult.
Discussion
In the present study, delayed neuronal death in the
hippocampal CA1 region following transient cerebral ischemia was
examined using CV staining. The results demonstrated that pyramidal
neurons in the CA1 region exhibited a ‘delayed neuronal death’
phenotype at 4 days post-ischemic insult. This result is in
agreement with the results of previously published studies
(1,2,21).
Maeda et al (24) demonstrated that expression of
albumin is observed in the extracellular space of the brain
parenchyma shortly after transient cerebral ischemia and its prompt
accumulation occurred in the periphery of dendrites with neurons,
likely due to the process of degeneration and death. In addition,
it has been reported that BBB breakdown and extravasation of
intravenously injected Evans Blue dye is observed in the forebrain
2 days after ischemic insult, and the extravasation was most severe
at 4–6 days following 5 min of transient cerebral ischemia in
gerbils (6). Based on these
reports, the present study examined albumin immunoreactivity in the
hippocampal CA1 region at 2 and 4 days after transient cerebral
ischemia. The results demonstrated that albumin immunoreactivity
was first observed in Iba-1-immunoreactive microglia near the
microvessels in the CA1 region at 2 days after
ischemia-reperfusion. In addition, albumin immunoreactivity was
further increased in Iba-1-immunoreactive microglia at 4 days after
ischemia-reperfusion, and at this time, strong albumin
immunoreactivity was also observed in the stratum pyramidale. The
present finding is in accordance with previous studies
demonstrating that microglia aggregate in the stratum pyramidale of
the CA1 region at 4 days after transient cerebral ischemia, the
subregion where most of the pyramidal neuron death occurs (23,25).
Albumin is known to induce a unique signaling
cascade in microglia, which triggers microglial proliferation
(26). Alonso et al
(27) reported that extravasated
albumin was taken up by activated microglia following
ultrasound-mediated BBB opening. However, it has been reported that
albumin is synthesized by microglia in the brain as well (28), and that its synthesis and
extracellular secretion enhances microglial activation following
amyloid β protein fragment 1–42 (Aβ1-42) or
lipopolysaccharide-treatment (28). In addition, Byun et al
(29) reported that albumin,
synthesized mainly from activated microglia, is conjugated with
advanced glycation end-product (AGE) to produce a toxic
AGE-albumin, which promotes Aβ polymerization and neuronal death.
In the present study, ischemia-induced activated microglia were
demonstrated to increase albumin immunoreactivity in the CA1
hippocampal region. The underlying mechanism for the increased
albumin immunoreactivity observed in microglia only in the CA1
region following transient cerebral ischemia remains unknown.
However, based on the above-mentioned literature and the our
present study, it can be postulated that albumin in microglia may
actively participate in the process of ‘delayed neuronal death’ of
the CA1 pyramidal neurons following transient cerebral ischemia. By
contrast, albumin is not observed in microglia in striatal
parenchyma following thromboembolic middle cerebral artery
occlusion in rats (30).
Therefore, the expression and function of albumin in microglia may
be different in various animal models of ischemia insults and
should be investigated further.
In summary, the present study demonstrated that
albumin immunoreactivity was newly observed in microglia in the
hippocampal CA1 region following 5 min of transient cerebral
ischemia. These results indicate that transient ischemia-induced
albumin expression in microglia might be associated with
ischemia-induced ‘delayed neuronal death’ in the hippocampal CA1
pyramidal neurons.
Acknowledgements
The present research was supported by the
Bio-Synergy Research Project (grant no. NRF-2015M3A9C4076322) of
the Ministry of Science, ICT and Future Planning through the
National Research Foundation, and by Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Education (grant no. NRF-2014R1A1A2058440).
References
|
1
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirino T and Sano K: Selective
vulnerability in the gerbil hippocampus following transient
ischemia. Acta Neuropathol. 62:201–208. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan PH: Reactive oxygen radicals in
signaling and damage in the ischemic brain. J Cereb Blood Flow
Metab. 21:2–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stoll G, Jander S and Schroeter M:
Inflammation and glial responses in ischemic brain lesions. Prog
Neurobiol. 56:149–171. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Won MH, Kang T, Park S, Jeon G, Kim Y, Seo
JH, Choi E, Chung M and Cho SS: The alterations of
N-Methyl-D-aspartate receptor expressions and oxidative DNA damage
in the CA1 area at the early time after ischemia-reperfusion
insult. Neurosci Lett. 301:139–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kataoka Y, Cui Y, Yamada H, Utsunomiya K,
Niiya H, Yanase H, Nakamura Y, Mitani A, Kataoka K and Watanabe Y:
Neovascularization with blood-brain barrier breakdown in delayed
neuronal death. Biochem Biophys Res Commun. 273:637–641. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ritter LS, Orozco JA, Coull BM, McDonagh
PF and Rosenblum WI: Leukocyte accumulation and hemodynamic changes
in the cerebral microcirculation during early reperfusion after
stroke. Stroke. 31:1153–1161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suchadolskiene O, Pranskunas A, Baliutyte
G, Veikutis V, Dambrauskas Z, Vaitkaitis D and Borutaite V:
Microcirculatory, mitochondrial, and histological changes following
cerebral ischemia in swine. BMC Neurosci. 15:22014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu XS, Ma ZZ, Wang F, Hu BH, Wang CS, Liu
YY, Zhao XR, An LH, Chang X, Liao FL, et al: The antioxidant
Cerebralcare Granule attenuates cerebral microcirculatory
disturbance during ischemia-reperfusion injury. Shock. 32:201–209.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan BY, Pan CS, Mao XW, Yang L, Liu YY,
Yan L, Mu HN, Wang CS, Sun K, Liao FL, et al: Icariside II improves
cerebral microcirculatory disturbance and alleviates hippocampal
injury in gerbils after ischemia-reperfusion. Brain Res.
1573:63–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Z, He B, Behrle BL, Fejleh MP, Cui L,
Paule MG and Greenfield LJ: Ischemia-induced increase in
microvascular phosphodiesterase 4D expression in rat hippocampus
associated with blood brain barrier permeability: Effect of age.
ACS Chem Neurosci. 3:428–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenberg GA and Yang Y: Vasogenic edema
due to tight junction disruption by matrix metalloproteinases in
cerebral ischemia. Neurosurg Focus. 22:E42007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petricoin EF, Belluco C, Araujo RP and
Liotta LA: The blood peptidome: A higher dimension of information
content for cancer biomarker discovery. Nat Rev Cancer. 6:961–967.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Belayev L, Liu Y, Zhao W, Busto R and
Ginsberg MD: Human albumin therapy of acute ischemic stroke: Marked
neuroprotective efficacy at moderate doses and with a broad
therapeutic window. Stroke. 32:553–560. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belayev L, Saul I, Huh PW, Finotti N, Zhao
W, Busto R and Ginsberg MD: Neuroprotective effect of high-dose
albumin therapy against global ischemic brain injury in rats. Brain
Res. 845:107–111. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eady TN, Khoutorova L, Obenaus A,
Mohd-Yusof A, Bazan NG and Belayev L: Docosahexaenoic acid
complexed to albumin provides neuroprotection after experimental
stroke in aged rats. Neurobiol Dis. 62:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao X, Miao W, Li M, Wang M, Ma J, Wang Y,
Miao L and Feng H: Protective effect of albumin on VEGF and brain
edema in acute ischemia in rats. Neurosci Lett. 472:179–183. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belayev L, Pinard E, Nallet H, Seylaz J,
Liu Y, Riyamongkol P, Zhao W, Busto R and Ginsberg MD: Albumin
therapy of transient focal cerebral ischemia: In vivo analysis of
dynamic microvascular responses. Stroke. 33:1077–1084. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Belayev L, Zhao W, Pattany PM, Weaver RG,
Huh PW, Lin B, Busto R and Ginsberg MD: Diffusion-weighted magnetic
resonance imaging confirms marked neuroprotective efficacy of
albumin therapy in focal cerebral ischemia. Stroke. 29:2587–2599.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strosznajder RP, Gadamski R, Czapski GA,
Jesko H and Strosznajder JB: Poly(ADP-ribose) polymerase during
reperfusion after transient forebrain ischemia: Its role in brain
edema and cell death. J Mol Neurosci. 20:61–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Institute of Laboratory Animal Research,
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals, National Research Council: Guide for the Care
and Use of Laboratory Animals. 8th. Washington, DC: National
Academies Press; pp. 2202011
|
|
22
|
Lee CH, Park JH, Cho JH, Ahn JH, Yan BC,
Lee JC, Shin MC, Cheon SH, Cho YS, Cho JH, et al: Changes and
expressions of Redd1 in neurons and glial cells in the gerbil
hippocampus proper following transient global cerebral ischemia. J
Neurol Sci. 344:43–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH, Shin BN, Chen BH, Kim IH, Ahn JH,
Cho JH, Tae HJ, Lee JC, Lee CH, Kim YM, et al: Neuroprotection and
reduced gliosis by atomoxetine pretreatment in a gerbil model of
transient cerebral ischemia. J Neurol Sci. 359:373–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeda M, Akai F, Nishida S and Yanagihara
T: Intracerebral distribution of albumin after transient cerebral
ischemia: Light and electron microscopic immunocytochemical
investigation. Acta Neuropathol. 84:59–66. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CH, Park JH, Yoo KY, Choi JH, Hwang
IK, Ryu PD, Kim DH, Kwon YG, Kim YM and Won MH: Pre- and
post-treatments with escitalopram protect against experimental
ischemic neuronal damage via regulation of BDNF expression and
oxidative stress. Exp Neurol. 229:450–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hooper C, Taylor DL and Pocock JM: Pure
albumin is a potent trigger of calcium signalling and proliferation
in microglia but not macrophages or astrocytes. J Neurochem.
92:1363–1376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alonso A, Reinz E, Fatar M, Hennerici MG
and Meairs S: Clearance of albumin following ultrasound-induced
blood-brain barrier opening is mediated by glial but not neuronal
cells. Brain Res. 1411:9–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn SM, Byun K, Cho K, Kim JY, Yoo JS, Kim
D, Paek SH, Kim SU, Simpson RJ and Lee B: Human microglial cells
synthesize albumin in brain. PLoS One. 3:e28292008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Byun K, Bayarsaikhan E, Kim D, Kim CY,
Mook-Jung I, Paek SH, Kim SU, Yamamoto T, Won MH, Song BJ, et al:
Induction of neuronal death by microglial AGE-albumin: Implications
for Alzheimer's disease. PLoS One. 7:e379172012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lehmann J, Härtig W, Seidel A, Füldner C,
Hobohm C, Grosche J, Krueger M and Michalski D: Inflammatory cell
recruitment after experimental thromboembolic stroke in rats.
Neuroscience. 279:139–154. 2014. View Article : Google Scholar : PubMed/NCBI
|