Introduction
Alzheimer's disease (AD) represents the most common
cause of dementia in the aged population, and is characterized by
the degradation of memory and cognitive function (1). AD is characterized by the aggregation
of extracellular senile plaques, which are comprised of amyloid
β(Aβ) precursor protein and intracellular neurofibrillary tangles
(2,3). The mechanism underlying the pathology
of AD remains to be fully elucidated, however, it has been
suggested that the mitochondrial pathway may be involved in the
induction of cell apoptosis (1–3).
Studies have shown that increased oxygen consumption, excess
concentrations of polyunsaturated fatty acids and reduced
quantities of antioxidants in the brain render it sensitive to
oxidative stress (4,5). Aβ generates free radicals or causes
neuronal death during oxidative stress (6,7). The
brain tissues of patients with AD have shown higher concentrations
of nitric oxide species and oxidized proteins (8,9). In
addition, the peroxidation of membrane lipids has been reported in
the brain tissues of patients with AD (10,11).
Therefore, the screening of natural antioxidant products may lead
to the identification of potential molecules for the treatment of
AD.
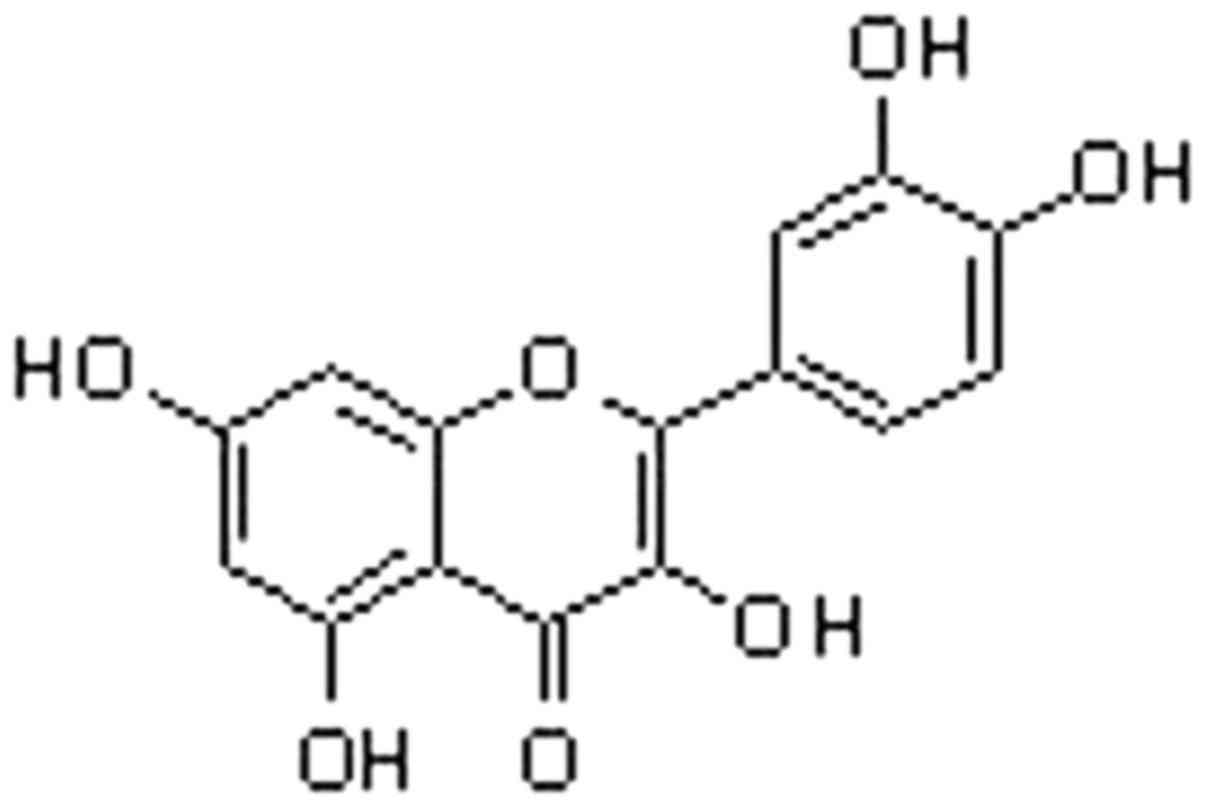
Quercetin (3,3′,4′,5,7-pentahydroxyflavone; Fig. 1), a member of the flavonoid family
is a well-known antioxidant (12).
Due to their structural features, flavonoids possess the promising
ability to transfer electrons to free radicals, induce antioxidant
enzyme activation and suppress oxidative stress (13). The presence of a catechol group and
an -OH group in quercetin imparts improved ability to act as an
antioxidant agent. The presence of these structural features in
quercetin enables it to act as a hydrogen donor for quenching free
radicals (14). Quercetin has been
shown to possess a wide range of biological activities, which
include antioxidant, antifibrotic and antiinflammatory activities.
Previous in vitro studies have demonstrated that quercetin
exhibits the ability to quench oxygen free radicals, suppress the
peroxidation of membrane lipids and alter the redox status of
glutathione (15,16). Quercetin-induced improvements in
the inflammatory processes have been found to be associated with
the generation of inducible nitric oxide synthase and tumor
necrosis factor-α (17). In
addition, quercetin suppresses the proliferation of keloid
fibroblasts, generation of collagen and reduction in keloid through
the inhibition of transforming growth factor-β/small mothers
against decapentaplegic signaling pathway (18). The present study investigated the
role of quercetin in protecting against neuronal cell death, and in
the inhibition of Aβ peptide-induced degradation of cognition and
memory loss. The results revealed that quercetin treatment
prevented neuronal cell death, and inhibited the degradation of
cognition and memory loss induced by Aβ. Therefore, quercetin is of
therapeutic importance for the treatment of neurodegenerative
diseases, including AD.
Materials and methods
Chemicals and materials
Quercetin, Aβ1-42 and fetal bovine serum (FBS) were
obtained from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) was purchased from Bachem (Bubendorf, Switzerland). Dimethyl
sulfoxide and other common chemicals were obtained from
Sigma-Aldrich (Merck Millipore).
Cell culture
The PC12 rat pheochromocytoma cell line was
purchased from the Japanese Collection of Research Bioresources
(Shinjuku, Japan). The cells were cultured in minimum essential
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% FBS and 1% penicillin/streptomycin. The cell
cultures were maintained in a CO2 incubator at 37°C with
a controlled humidified atmosphere of 95% air and 5%
CO2.
1,1-diphenyl-2-picrylhydrazyl
(DPPH)
The DPPH• radical scavenging activity assay is
widely used to evaluate the antioxidant activity of compounds. The
ability of a compound to interact with the stable DPPH free radical
indicates its capacity to quench free radicals. Quercetin treatment
exhibits antiradical activity by inhibiting DPPH radicals. For
investigating the radical scavenging activities, 10 mM quercetin in
methyl alcohol was further diluted using methyl alcohol to obtain
5, 10, 20, 50 and 100 µM solutions. To each of the solutions of
quercetin, 0.25 mM DPPH (Santa Cruz Biotechnology, Inc.) was added,
followed by incubation for 45 min at room temperature. The optical
density of each of the solutions was then measured using a
spectrophotometer at 519 nm. The control used was pure ethyl
alcohol and its absorbance was denoted as A0, whereas the
absorbance of the sample solution was denoted asAq. The scavenging
activity of quercetin was calculated using the following equation:
Scavenging activity (%) = [(A0-Aq)/A0] × 100.
Cell viability assay
The effects on cell viability and the
neuroprotective effect exhibited by quercetin were determined using
an MTT assay. For this purpose, cells at a density of 2×104 cells
per well were distributed onto 96-well plates and cultured
overnight. The media were then removed and the cells were exposed
to either saline as a control, selegiline (10 µM) as a positive
control or different quercetin (10–100 µM) and incubated for 48 h
at 37°C. Following incubation, MTT solution was added to each of
the wells and the plates were incubated for 2 h at 37°C. Dimethyl
sulfoxide was added to the wells for dissolution of the formazan
crystals, which had formed. A microplate reader (Model 550; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to measure the
absorbance at 540 nm. For each of the analyses, three replicate
wells were used.
Animals
Male ICR mice (n=25; 6–8 weeks old; weight, 18–20 g)
were purchased from the Experimental Animal Center of Sichuan
University (Chengdu, China). The mice were acclimatized to the
laboratory environment 1 week prior to commencement of the
experiments, and were housed in a room with a 12 h light and dark
cycle at a temperature of 25°C. The animals had access to standard
food and water ad libitum. All animal experimental procedures were
performed following approval from the Laboratory Animal Care
Committee of Sichuan Province (Sichuan, China).
Treatment strategy
The animals were randomly assigned into five groups,
each containing five animals: i) control, ii) Aβ treatment, iii)
quercetin 50 mg/kg body weight followed by Aβ treatment, iv)
quercetin 100 mg/kg body weight followed by Aβ treatment; v)
selegiline 3 mg/kg body weight followed by Aβ treatment. Quercetin
was dissolved in tap water and administered orally every day for
one month, at doses of 50 or 100 mg/kg/body weight. Aβ1-42 (20
mg/kg/body weight) was administered to mice through injection
following quercetin pretreatment.
Y-maze test
For determination of the role of quercetin on
spatial working memory following 4 days of Aβ injections, a Y-maze
test was performed. The instrument consisted of a maze, which was
35 cm in length, 16 cm in height and 12 cm in width. The instrument
has arms, which are projected symmetrically. The mice were placed
in one of the arms and allowed to enter the other arms for 10 min.
During this period, the number of arms mice entered was calculated,
from which the percentage alternation was calculated as follows:
Alternation (%) = [(number of alternations)/(total arm entries-2)]
× 100.
Passive avoidance test
The determination of the role of quercetin on
learning and memory following 6 days of Aβ injection was performed
using a passive avoidance test. The instrument consists of a double
compartment step-through passive avoidance apparatus (Model
PACS-30; Columbus Instruments International, Columbus, OH, USA).
Internally, the instrument had two similar chambers (24×16×16 cm).
One of the chambers contained a bulb for lighting, whereas the
second chamber was covered with black film. The mice from the lit
chamber were allowed to enter the dark chamber by the raising of a
door, where they received a 1 sec foot shock during the training
period. Training consisted of two 5-day sessions, with 4 trials per
session. The intersession gap was 2 h and inter-trial gap was 30
sec. Following the completion of training, the latency period taken
to enter the dark chamber for each mouse was recorded.
Determination of acute oral
toxicity
To determine the acute oral toxicity induced by
quercetin treatment in the mice, a lethality assay was used.
Briefly, the mice were divided into five groups of five mice. The
mice in the control group received normal saline and carboxymethyl
cellulose (CMC). The mice in the treatment groups were administered
with saline, CMC and quercetin in DMSO at concentrations of 50, 80
and 100 mg/kg body weight. The animals were examined following the
administration of the doses for 100 h. For calculation of the acute
toxic dose (mg/kg body weight), resulting in death of 50%of the
mice (LD50) was determined using the Sigma plot version
12 program (Sigma-Aldrich).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Student's t-test was used for statistical comparisons.
All statistical tests were calculated using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant differences.
Results
Effect of quercetin treatment onPC12
cell toxicity
The primary factor examined in determining the
anti-oxidant nature of a compound is its scavenging activity for
oxygen radicals (19). The results
revealed that quercetin was potentially involved in the inhibition
of DPPH radical activity. It reduced the radical activity of DPPH
by 76.5%. To investigate the effect of quercetin on the viability
of PC12 cells, an MTT assay was used. The results showed that
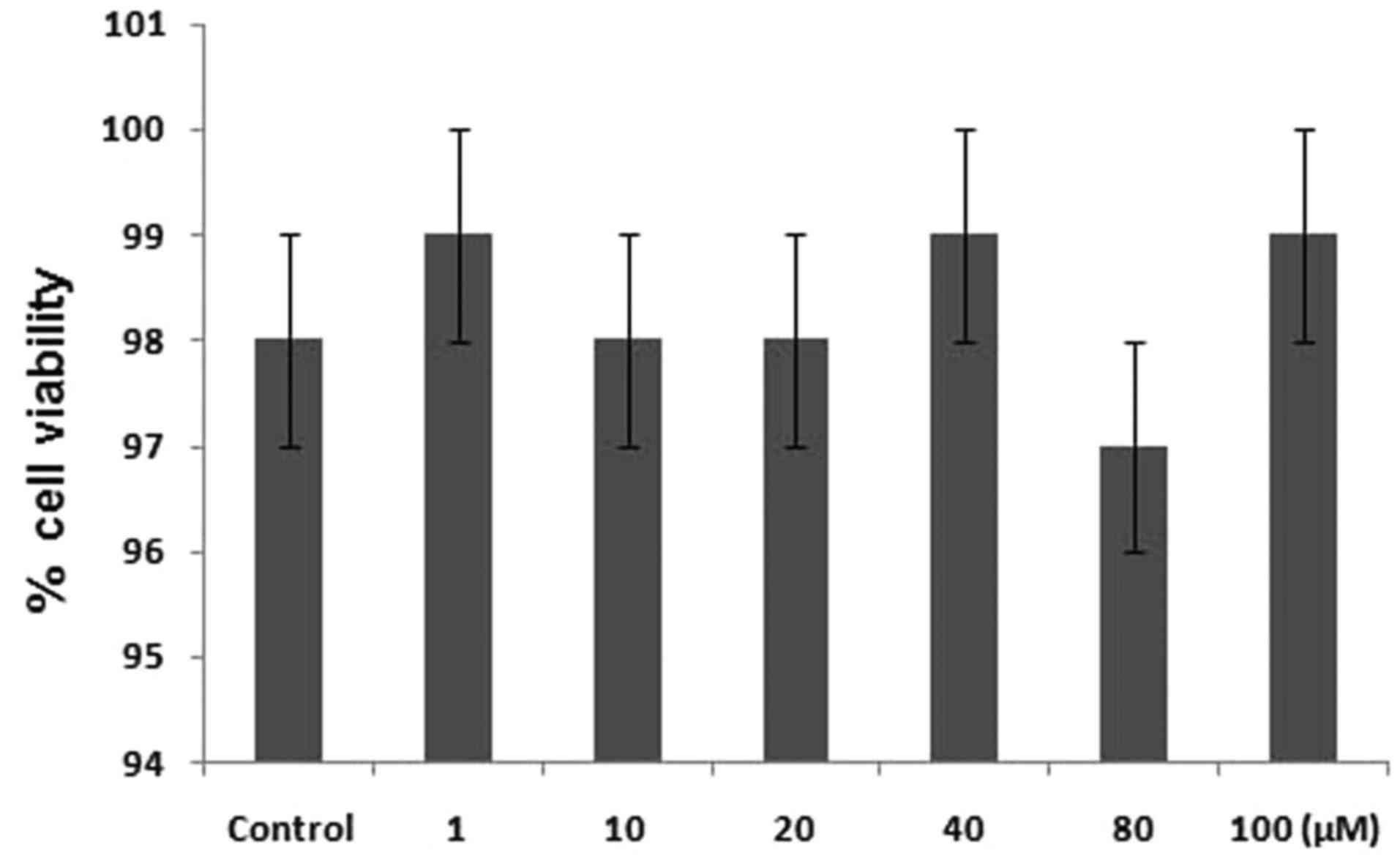
treatment of the PC12 cells with different concentrations of
quercetin for 24 h induce no toxicity (Fig. 2).
Protective effect of quercetin against
Aβ-induced cell cytotoxicity in PC12 cells
For investigating the effect of quercetin on the
reduced viability of PC12 cells induced by Aβtreatment, an MTT
assay was used. The PC12 cells were exposed to a range of quercetin
concentrations (10–100 µM) prior to incubation with Aβ. The results
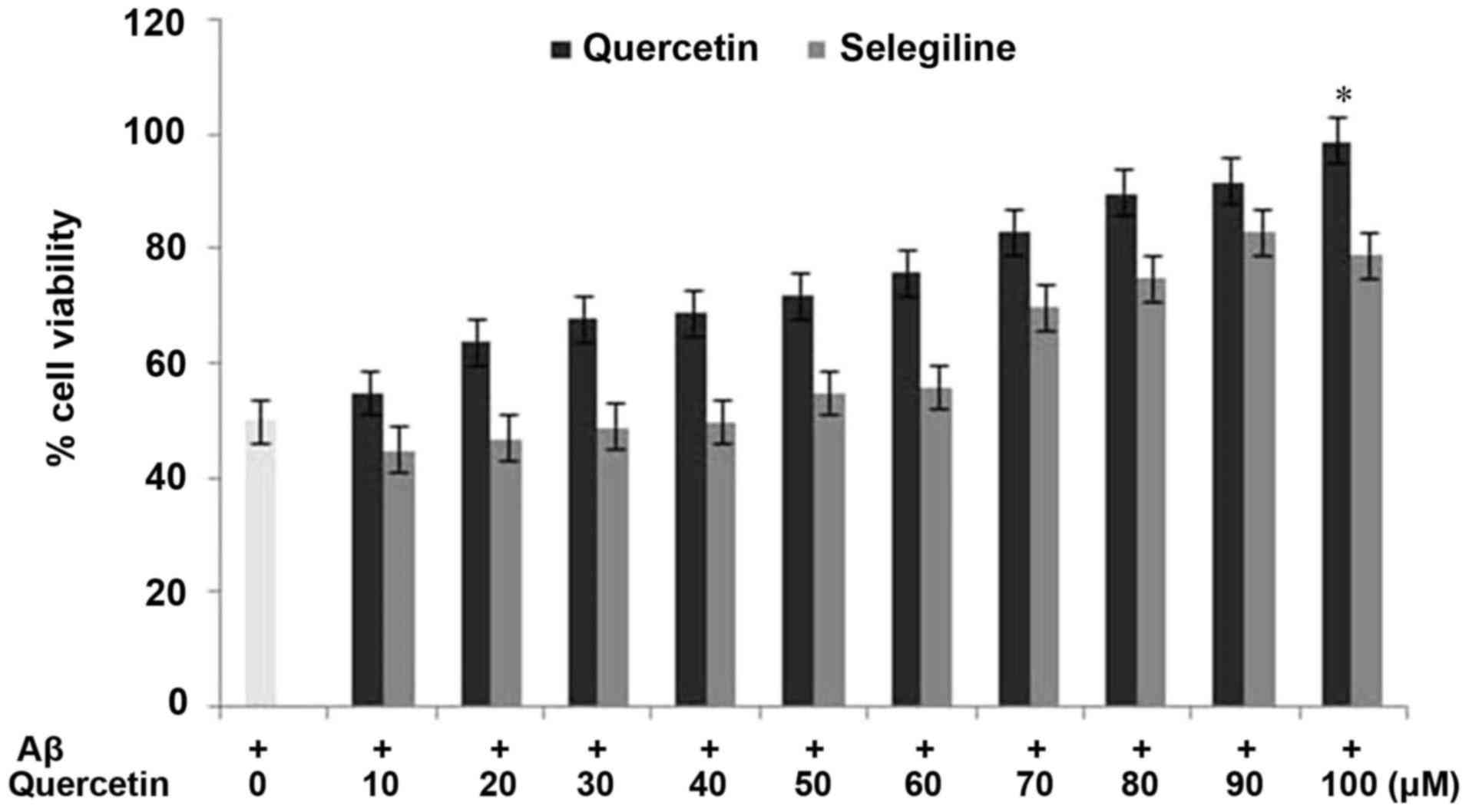
revealed that pretreatment of the PC12 cells with quercetin
inhibited the decrease in cell viability induced by Aβ (Fig. 3). The rates of cell viability in
the cells exposed to quercetin prior to Aβ treatment and in the
Aβ-only treated cells were 97 and 28%, respectively, compared with
98% in the control cells (Fig. 3).
Pretreatment of the cells with quercetin exhibited a
concentration-dependent effect on the inhibition of cell viability.
The inhibition of the Aβ-induced reduction in PC12 cell viability
by quercetin was significant (P<0.002) at a concentration of 100
µM after 24 h. These findings indicated that quercetin exhibited a
protective effect against Aβ-induced cytotoxicity in the PC12
cells.
Quercetin inhibits Aβ-induced memory
degeneration in mice
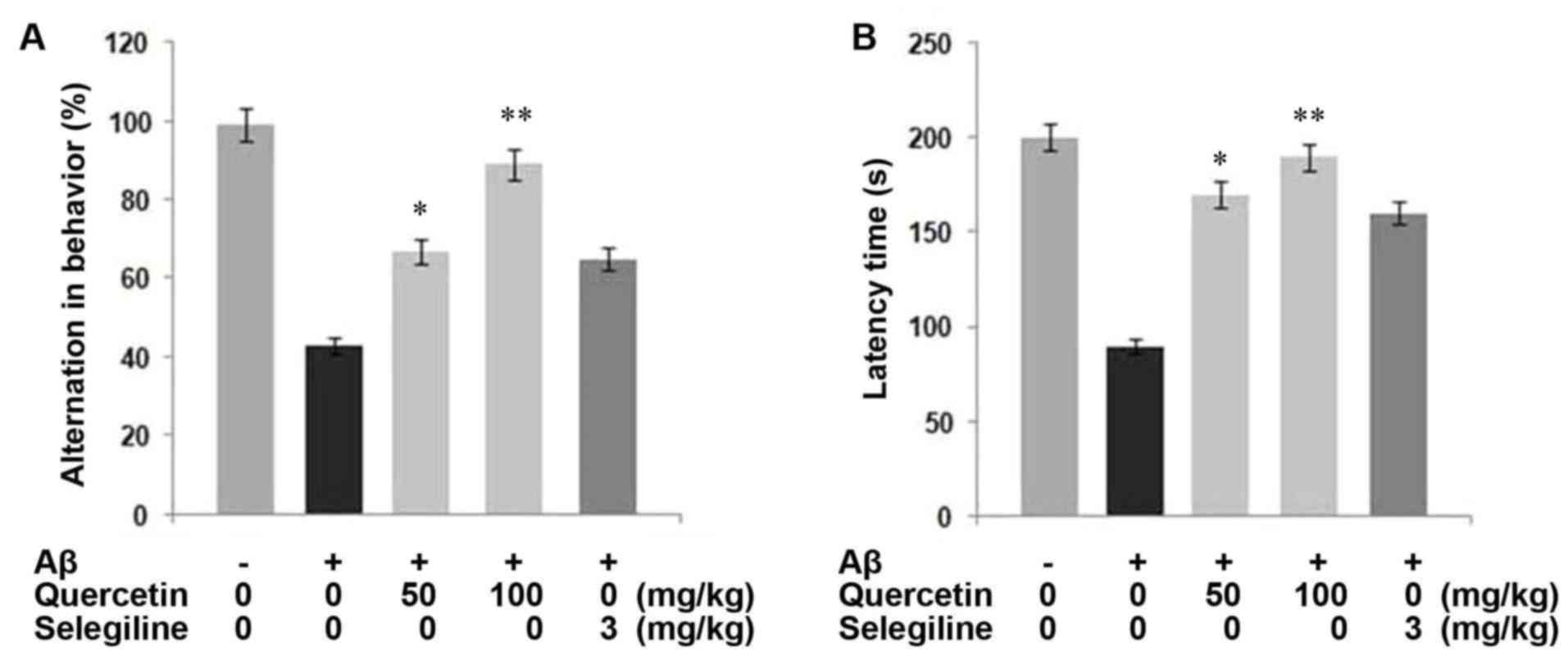
Examination of the Aβ1-42-injected group of mice
showed a marked decrease in behavioral alternations, compared with
the untreated group (P<0.05; Fig.
4A). By contrast, treatment of the mice with quercetin prior to
Aβ1-42-injection prevented the behavioral alternations induced by
Aβ1-42. Treatment of the mice with 50 and 100 mg/kg body weight of
quercetin led to spatial working memory of 14.4 and 19.5%,
respectively.
The learning ability of the mice was examined by
measuring response latency, which revealed that treatment of the
mice with Aβ1-42 led to the degradation of learning ability,
compared with the untreated rats. Compared with the control group
with a latency of 199.3 sec, the latency in the Aβ1-42-treated
group was 85.0 sec. However, treatment of the mice with quercetin
significantly inhibited Aβ-induced memory degeneration, compared
with the control (Fig. 4B). The
inhibition of Aβ-induced memory degeneration by quercetin was
significantly higher, compared with that by selegiline, which was
used as the positive control. In terms of the selegiline-treated
group of mice, the latency was 155 sec (P<0.05). Therefore,
quercetin treatment exhibited a potential inhibitory effect on the
degeneration of learning memory in mice.
LD50 of quercetin in mice
The mice were administered with a single dose of
quercetin followed by examination of their alterations in behavior
and number of deaths over a period of 100 h. The results showed a
concentration-dependent enhancement in behavioral alterations and
the number of deaths. The administration of 100 mg/kg of quercetin
resulted in a mortality rate of 72% within the 100 h treatment
period, with altered behavior observed in the remaining mice
(Table I). The behavioral
alterations induced by quercetin treatment included reduced walking
speed, hair loss and unconsciousness. The LD50 value of
orally administered quercetin was estimated to be 575 mg/kg body
weight in the mice.
 | Table I.Quercetin-induced effects in mice
following oral administration. |
Table I.
Quercetin-induced effects in mice
following oral administration.
| Quercetin
(mg/kg) | Total mice (n) | Deaths (n) | Latency (h) | Symptoms |
|---|
|
0 | 5 | 0 | – | – |
| 100 | 5 | 0 | – | – |
| 400 | 5 | 1 | >28 | Reduced walking
speed; hair loss |
| 800 | 5 | 4 | >30 | Reduced walking
speed; hair loss; unconsciousness. |
Discussion
The present study aimed to investigate the effect of
quercetin on protection against neuronal cell death, Aβ-induced
oxidative stress and memory degradation. To investigate the
anti-oxidant nature of a compound, a scavenging activity assay
against oxygen species is used (19). In neurological disorders, including
AD, the dysfunction of neurons and their death has been found to be
associated with oxidative stress (20,21).
Previous in vitro studies have demonstrated that quercetin
has a promising ability at quenching oxygen free radicals,
suppressing the peroxidation of membrane lipids and altering the
redox status of glutathione (15,16).
The results of the present study revealed that quercetin had a
potential role in the inhibition of DPPH radical activity, and
reduced radical activity by 76.5%. Treatment of the PC12 cells with
different concentrations of quercetin for 24 h induced no toxic
effects.
It has been reported that the presence of Aβ peptide
is responsible for the production of reactive oxygen species, which
in turn induces the peroxidation of membrane lipids and cell
apoptosis (22,23). There are reports that antioxidants
prevent neurons from apoptosis, and can inhibit the degradation of
cognition and prevent memory loss induced by the Aβ peptide
(24). Extracted brain tissue
specimens from patients with AD have also shown the presence of
peroxidized lipids, modified proteins and oxidized DNA in cells
(25). The results of the present
study demonstrated that quercetin treatment prevented the neuronal
cells from Aβ-induced cytotoxicity. Quercetin treatment also
protected the cells from Aβ-induced degradation of learning and
loss of memory. It appeared that the protective effect of quercetin
was the result of its ability to inhibit oxidative stress by
quenching reactive oxygen species.
For any biologically active molecule, the
determination of acute oral toxicity is an important parameter. The
results of the present study revealed that quercetin had a
significantly lower toxicity, compared with selegiline. In
conclusion, quercetin treatment prevented neuronal cells from
Aβ-induced oxidative stress, and prevented the degradation of
learning and loss of memory induced by Aβ. Therefore, quercetin may
be a potential candidate for the prevention of AD.
References
|
1
|
Zhu Y, Li C, Sun A, Wang Y and Zhou S:
Quantification of microRNA-210 in the cerebrospinal fluid and
serum: Implications for Alzheimer's disease. Exp Ther Med.
9:1013–1017. 2015.PubMed/NCBI
|
|
2
|
Chambers JK, Uchida K, Harada T, Tsuboi M,
Sato M, Kubo M, Kawaguchi H, Miyoshi N, Tsujimoto H and Nakayama H:
Neurofibrillary tangles and the deposition of a beta amyloid
peptide with a novel-N-terminal epitope in the brains of wild
Tsushima leopard cats. PLoS One. 7:e464522012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo J, Chang L, Zhang X, Pei S, Yu M and
Gao J: Ginsenoside compound K promotes β-amyloid peptide clearance
in primary astrocytes via autophagy enhancement. Exp Ther Med.
8:1271–1274. 2014.PubMed/NCBI
|
|
4
|
Floyd RA and Hensley K: Oxidative stress
in brain aging. Implications for therapeutics of neurodegenerative
diseases. Neurobiol Aging. 23:795–807. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mattson MP, Chan SL and Duan W:
Modification of brain aging and neurodegenerative disorders by
genes, diet, and behavior. Physiol Rev. 82:637–672. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X, Atwood CS, Hartshorn MA, Multhaup
G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD,
et al: The A beta peptide of Alzheimer's disease directly produces
hydrogen peroxide through metal ion reduction. Biochemistry.
38:7609–7616. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christen Y: Oxidative stress and Alzheimer
disease. Am J Clin Nutr. 71:621s–629s. 2000.PubMed/NCBI
|
|
8
|
Sultana R, Perluigi M and Butterfield DA:
Oxidatively modified proteins in Alzheimer's disease (AD), mild
cognitive impairment and animal models of AD: Role of Abeta in
pathogenesis. Acta Neuropathol. 118:131–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu X, Su B, Wang X, Smith MA and Perry G:
Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci.
64:2202–2210. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mark RJ, Lovell MA, Markesbery WR, Uchida
K and Mattson MP: A role for 4-hydroxynonenal, an aldehydic product
of lipid peroxidation, in disruption of ion homeostasis and
neuronal death induced by amyloid beta-peptide. J Neurochem.
68:255–264. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Butterfield DA, Hensley K, Harris M,
Mattson M and Carney J: beta-amyloid peptide free radical fragments
initiates synaptosomal lipoperoxidation in a sequence-specific
fashion: Implications to Alzheimer's disease. Biochem Biophys Res
Commun. 200:710–715. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly GS: Quercetin. Monograph. Altern Med
Rev. 16:172–194. 2011.PubMed/NCBI
|
|
13
|
Heim KE, Tagliaferro AR and Bobilya DJ:
Flavonoid antioxidants: Chemistry, metabolism and
structure-activity relationships. J Nutr Biochem. 13:572–584. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heijnen CG, Haenen GR, Oostveen RM,
Stalpers EM and Bast A: Protection of flavonoids against lipid
peroxidation: The structure activity relationship revisited. Free
Radic Res. 36:575–581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Q, Rahn RO and Zhang R: Dietary
flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA
damage and lipid peroxidation and quench free radicals. Cancer
Lett. 119:99–107. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meyers KJ, Rudolf JL and Mitchell AE:
Influence of dietary quercetin on glutathione redox status in mice.
J Agric Food Chem. 56:830–836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rivera L, Morón R, Sánchez M, Zarzuelo A
and Galisteo M: Quercetin ameliorates metabolic syndrome and
improves the inflammatory status in obese Zucker rats. Obesity
(Silver Spring). 16:2081–2087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phan TT, Lim IJ, Chan SY, Tan EK, Lee ST
and Longaker MT: Suppression of transforming growth factor
beta/smad signaling in keloid-derived fibroblasts by quercetin:
Implications for the treatment of excessive scars. J Trauma.
57:1032–1037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung JC, Jang S, Lee Y, Min D, Lim E, Jung
H, Oh M, Oh S and Jung M: Efficient synthesis and neuroprotective
effect of substituted 1,3-diphenyl-2-propen-1-ones. J Med Chem.
51:4054–4058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heneka MT, O'Banion MK, Terwel D and
Kummer MP: Neuroinflammatory processes in Alzheimer's disease. J
Neural Transm (Vienna). 117:919–947. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MJ, Seung AR, Yoo JY, Jin CH, Lee YH,
Kim YJ, Lee J, Jun WJ and Yoon HG: Gallic acid, a histone
acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by
inhibiting microglial-mediated neuroinflammation. Mol Nutr Food
Res. 55:1798–1808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varadarajan S, Yatin S, Aksenova M and
Butterfield DA: Review: Alzheimer's amyloid beta-peptide-associated
free radical oxidative stress and neurotoxicity. J Struct Biol.
130:184–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kadowaki H, Nishitoh H, Urano F, Sadamitsu
C, Matsuzawa A, Takeda K, Masutani H, Yodoi J, Urano Y, Nagano T
and Ichijo H: Amyloid beta induces neuronal cell death through
ROS-mediated ASK1 activation. Cell Death Differ. 12:19–24. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park SY, Kim HS, Cho EK, Kwon BY, Phark S,
Hwang KW and Sul D: Curcumin protected PC12 cells against
beta-amyloid-induced toxicity through the inhibition of oxidative
damage and tau hyperphosphorylation. Food Chem Toxicol.
46:2881–2887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonda DJ, Wang X, Perry G, Numonura A,
Tabaton M, Zhu X and Smith MA: Oxidative stress in Alzheimer
disease: A possibility for prevention. Neuropharmacololgy.
59:290–294. 2010. View Article : Google Scholar
|