Introduction
Preeclampsia (PE) is as a major factor in maternal
and fetal morbidity and mortality. It is one of the most common
complications during pregnancy, occurring in 3–4% of pregnancies,
and up to 10% in developing countries (1). The main clinical presentations are
proteinuria, hypertension and edema. Early research focused on
understanding hypertension and renal dysfunction; however,
additional studies on the syndrome are lacking. Over the past 20
years, increasing evidence has indicated that PE is a multisystemic
syndrome that is associated with endothelial dysfunction (2), inflammatory activation (3), an imbalance of angiogenic factors and
metabolic changes (4,5). Studies are currently focusing on the
process of trophoblast invasion, which is an important feature of
PE (6).
Wnt signaling is an essential pathway in the
regulation of cell proliferation, migration and death, and is
conserved from hydras to humans. Over 30 years ago, Nusse et
al (7) identified Wnt genes in
mice that lead to tumorigenesis. Since then, an implicit connection
has been made between the physiological role of Wnt genes in
development and a potential pathophysiological role in
carcinogenesis (8). Numerous
studies have demonstrated that the Wnt-signaling pathway may lead
to a variety of human diseases, ranging from birth defects to
cancers, and our previous studies have focused on PE (9–13).
Results from one of our previous studies confirmed that the levels
of Wnt2 were decreased in the placenta of patients with PE
(14). Additional experiments are
required to investigate the abnormal activation of Wnt/β-catenin
signaling pathway in PE.
The present review summarizes recent reports on the
pathophysiology of PE, particularly trophoblast invasion, and
explores the involvement of Wnt/β-catenin signaling pathway in the
trophoblast and PE pathophysiology, to further understand the
pathogenesis of PE and to discover better treatments.
Role of trophoblast in PE
The clinical symptoms of PE quickly subside after
childbirth. During pregnancy, the placenta acts as an interface
between the mother and the fetus, suggesting that the placenta has
an important role in PE (15). One
highly recognized hypothesis suggests that PE may result from
placental dysfunction (16).
Development of the human placenta can directly affect the pregnancy
outcomes, failure development in placenta can lead pregnant
diseases. According to previous clinical, pathological and
experimental findings, reduced placental perfusion is the most
significant feature of the placenta in PE (17). Placental abnormalities that may be
involved in the pathogenic process of PE include abnormal
implantation and trophoblast invasion of spiral arterioles, and
improper vascular development in the placenta (18,19).
A better understanding of abnormal trophoblasts and placentas may
contribute to the elucidation of PE pathogenesis.
A previous study revealed that cytotrophoblastic
invasion occurs in two stages during pregnancy: Initially after 2
weeks of gestation, and then at 12 and 20 weeks gestation (20). During this time, extravillous
cytotrophoblast (CTB) cells invade the maternal spiral uterine
arteries. Trophectodermal cells that make up the outer epithelial
layer of the blastocyst begin to differentiate into various types
of trophoblastic cells after implantation (21). The primitive syncytium, possibly
the earliest type of invasive trophoblastic cells are formed by
cellular fusions and migrates into the maternal endometrium; CTBs
originating from the trophectodermal layer through the primitive
syncytium to invade and proliferate which can produce primary villi
(22). Subsequent formation of
secondary and tertiary villi takes place throughout pregnancy;
these villi characteristically invade ectomesenchymal cells,
forming villous branches and blood vessels. Two types of mature
villi are formed during the first trimester: i) Floating villi,
which are the transport units of the human placenta and are
directly connected to the intervillous space where nutrients and
oxygen are exchanged with maternal blood; and ii) anchoring villi,
which can invade the decidua, the muscular layer and blood vessels
(23). Interactions between these
villi ensure proper fetal development and growth. Villi that are
connected to the basal plate of the placenta produce proliferative
cell columns, which in turn give rise to differentiated
extravillous trophoblast (EVT) cells (24). During the early stages of
pregnancy, successful invasion of the endovascular CTB (eCTB) cells
and the maternal arterioles may prevent the premature onset of
blood flow into the intervillous space (25). Complications during pregnancy may
lead to failures in this process, possibly due to premature rises
in oxygen levels, which may induce oxidative stress and cause harm
to the placental villi (26).
Proliferative CTBs differentiate into EVTs and then invade decidual
tissue and blood vessels which is thought to encompass a series of
precise biological process.
In addition to endovascular invasion, migrating
interstitial CTB (iCTB) cells enter the maternal decidua where they
are likely to interact with different uterine cell types, such as
uterine natural killer cells, macrophages and decidual stromal
cells (27,28). These mutual effects have an
important role in the immunological acceptance of the
placental/fetal allograft and the depth of trophoblast invasion
(29). For example, interactions
between paternal human leukocyte antigen C and maternal killer-cell
immunoglobulin-like receptors are considered to be important for
placentation and reproductive success (30). When blood flow is absent, invasion
of the trophoblast is highly dependent on epidermal growth factor
and vascular endothelial growth factor (VEGF), which contribute to
establishing maternal-placental circulation (31). Once the maternal-placental
circulation has formed, the trophoblastic plugs are dissolved and
extensive remodeling occurs, including the transformation of
maternal spiral arteries into large diameter vessels that ensure an
adapted nutrient supply, reduced vessel contractility and constant
oxygen delivery to the developing fetus at low blood pressure
(32). Natural killer cells and
differentiated EVTs may serve a key role in vascular remodeling
(33). Maternal endothelial cells
are displaced by eCTBs, which then remodel the decidua and
myometrium on the surface of the spiral arteries, whereas iCTBs are
involved in elastolysis and disruption of the vascular wall, which
involves a series of trophoblast-induced events, such as apoptosis
of the vascular smooth muscle cells (34). Abnormal vascular pressure may cause
hypoxia/reoxygenation injury to floating villi, leading to the
secretion of various inflammatory factors and anti-angiogenic
molecules, such as interleukin 6, soluble fms-like tyrosine kinase
1 and syncytiotrophoblast (ST) microparticles (33,35).
Failures in EVT invasion have been noted in a number of
pregnancy-associated diseases, including PE (36,37).
Increased ST microparticle shedding is hypothesized to be involved
in the dysfunction of maternal endothelial cells, leading to the
systemic inflammatory response that may be involved in PE.
Wnt/β-catenin signaling pathway
How Wnt works: Components and
mechanism
The first Wnt gene was isolated in 1982 as a common
site of integration by the mouse mammary tumor virus and designated
Int (38). The gene was later
identified to be homologous to the Drosophila
segment-polarity gene, wingless (39). The Wnt gene family encode the Wnt
proteins that are able to activate intracellular signaling pathway
and participate in the development of different mechanisms. The
Wnt-signaling pathway is an important regulator of cell
proliferation, migration and death, and is conserved from hydras to
humans (40). There are three
Wnt-signaling pathways in humans: The canonical Wnt/β-catenin
pathway, the non-canonical Wnt/Ca2+ pathway and the
non-canonical planar cell polarity pathway (41). Wnt/β-catenin is a conserved
cell-signaling system that is involved numerous biological
processes such as organogenesis, axis differentiation in
multicellular organisms cancer pathogenesis and the
epithelial-mesenchymal transition (42).
Of the three Wnt-signaling pathways, the canonical
Wnt/β-catenin signaling pathway will be the focus of this review.
To date, 19 mammalian Wnt ligands have been identified that can
directly banding bind to eight out of the 10 frizzled (Fzd)
transmembrane G protein-coupled receptors; binding to the Fzd
receptor relays the Wnt-signal to the nucleus where it serves its
biological role (43). Currently,
the most widely studied ligands that can activate the classical
Wnt-signaling pathway include Wnt1, Wnt2, Wnt3a and Wnt8.
Activation of canonical Wnt signaling is highly dependent on the
interactions between Fzd and endogenous co-receptors such as
low-density lipoprotein related proteins 5 and 6 (44). β-catenin also serves an essential
role in the function of the Wnt/β-catenin signaling pathway. The
majority of β-catenin proteins and epithelial mucins (E-cadherin)
are located in the cell membrane, whereas fewer proteins are
located in the cytoplasm. When the Wnt ligands are absent
(off-state), there are low levels of free β-catenin in cytoplasm.
If not bound to E-cadherin in the cytomembrane, cytoplasmic
β-catenin is phosphorylated by a multiprotein destruction complex
[comprising the scaffold proteins axin and adenomatous polyposis
coli (APC), and the kinases that phosphorylate β-catenin, glycogen
synthase kinase 3β (GSK3β), casein kinase 1 (CK1) and protein
phosphatase 2A], which contributes to the degradation of β-catenin
in the cytoplasm through the addition of phosphate groups (45,46).
Through this mechanism, the level of β-catenin in the cytoplasm
remains low, and is inhibited from entering the nucleus and thus
cannot activate nuclear transcription. Extracellular Wnt proteins
bind to the Fzd receptor, which then recruits cytoplasmic proteins
and directly binds to Dishevelled (Dvl); Dvl subsequently
multimerizes and induces the formation of Wnt signalosomes
(Fig. 1) (47). Dvl then recruits axin, which is the
rate-limiting component of Wnt/β-catenin signaling, and other
associated kinases, such as GSK3β and CK1, thus destabilizing the
β-catenin destruction complex. This process leads to the
accumulation of β-catenin in the cytoplasm, which is able to enter
the nucleus. In the nucleus, β-catenin interacts with members of
the T cell factor/lymphocyte enhancer binding factor family of
transcription factors, which can activate the transcription of
downstream target genes, such as c-myc, cyclin D1 and matrix
metalloproteinase 7, as transcriptional activator, resulting in the
abnormal cellular proliferation and/or apoptosis, along with a
series of other biological effects (48).
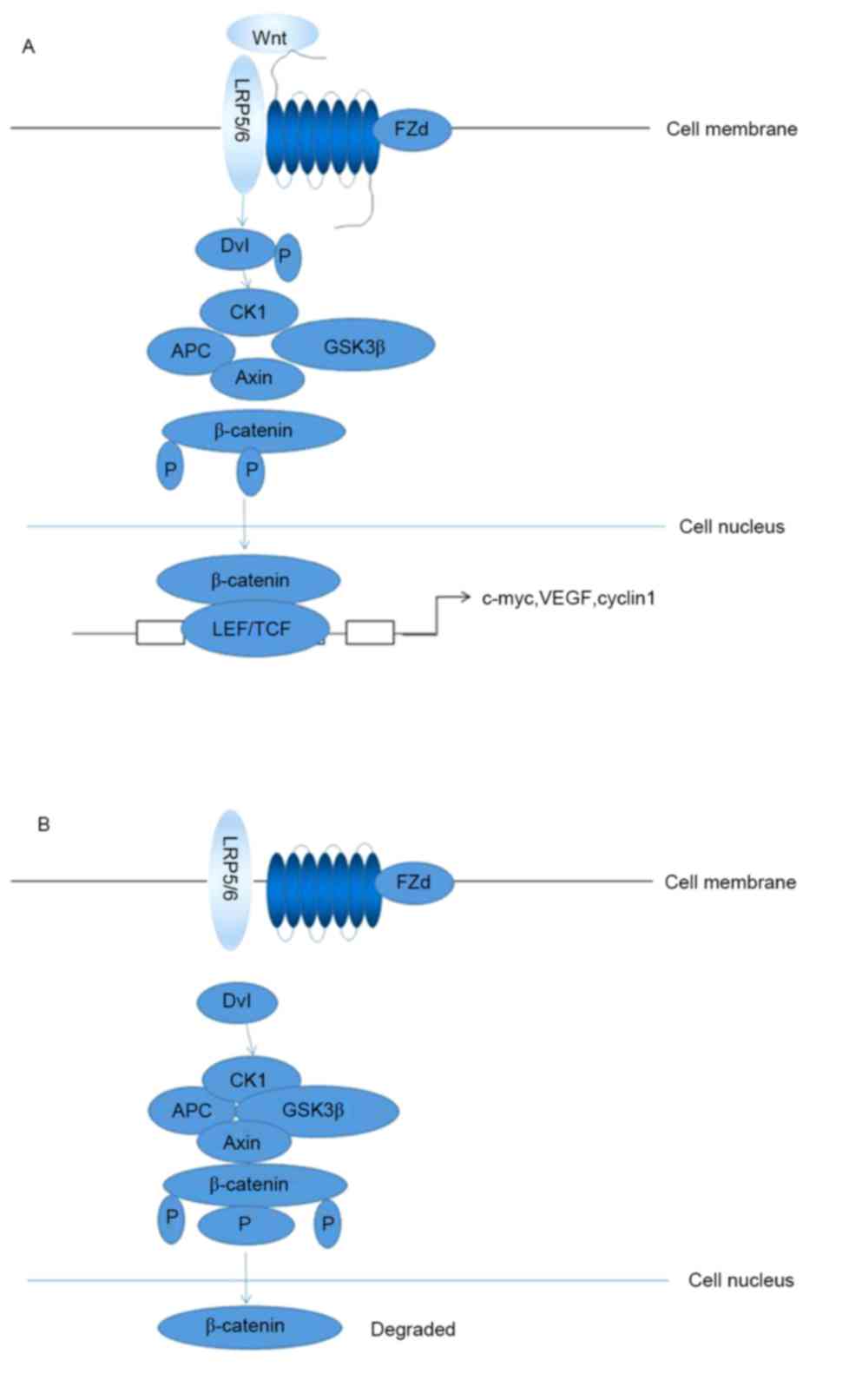 | Figure 1.The transmission mechanism of
Wnt/β-catenin signaling pathway. (A) In the presence of Wnt, Wnt
binding to cell-membrane receptor frees β-catenin from the complex
and prevents its degradation. β-catenin enter the nucleus to
activate target genes. (B) In the absence of Wnt or when Wnt is
prevented by an inhibitor, β-catenin is phosphorylated by a
complex. This phosphorylated β-catenin is rapidly degraded. LRP5/6,
low-density lipoprotein receptor-related protein 5/6; Fzd,
frizzled; Dvl, disheveled; P, phosphate group; CK1, casein kinase
1; APC, adenomatous polyposis coli; GSK3β, GSK3β, glycogen synthase
kinase 3β; LEF, lymphocyte enhancer binding factor; TCF, T cell
factor; VEGF, vascular endothelial growth factor. |
Functions of Wnt/β-catenin
signaling
Wnt/β-catenin signaling has been demonstrated to
contribute to the development of organ systems, including the
respiratory, digestive system, skeletal, nervous, cardiovascular,
hematopoietic and reproductive systems; in particular,
Wnt-signaling is important for the development of the cerebral
cortex, heart, skin, teeth, gut, lungs, eyes and lenses, somites,
neural crest, limbs, bones, pancreas, liver, kidneys and mammary
glands (49–52). Abnormal activation of Wnt/β-catenin
signaling is implicated in different types diseases, including
obstetrical and gynecological disease, metabolic diseases and
cancers (53–55).
The Wnt/β-catenin signaling pathway is involved in
multiple physiological processes, although numerous studies have
focused on its role in the pathogenesis of various types of tumor.
Aberrant activation of the Wnt/β-catenin signaling pathway may
result in tumor formation, suggesting that dysfunctional
Wnt/β-catenin signaling is a significant event that can contribute
to the development of cancer (56–59).
Abnormal activation of the Wnt/β-catenin signaling pathway has been
linked to primary hepatocellular carcinomas, renal cancer and
colorectal cancer, among others (10–13).
Wnt/β-catenin signaling pathway in
trophoblasts
The rapid generation of several subtypes of
trophoblast cells is well known to contribute to the development of
the placenta in mice and humans (60). The maternal uterus is then
remodeled, including the stromal cell differentiation, angiogenesis
and immunological alterations. These key processes are initiated
during the secretory phase of the menstrual cycle, and upon
implantation and during the early stages of placental development
(24). Since Wnt signaling serves
a crucial role in organ development and tissue homeostasis, it is
likely that the pathway also has important roles in the development
and differentiation of trophoblasts (61).
A recent study demonstrated that 14 Wnt ligands and
eight Fzd receptors are expressed in the human placenta, further
indicating a function for the Wnt-signaling pathway in placental
development (62). A number of
studies have identified Wnt ligands and other Wnt-signaling
components in the endometrium, suggesting that the Wnt pathway
could be associated with the diverse biological functions of
uterine cell types. The expression of Wnt ligand mRNA transcripts
has been investigated by microarray analyses of global gene
expressions: High levels of Wnt3 mRNA were detected in the
endometrium during the menstrual cycle, whereas the mRNA levels of
Dickkopf 1 (Dkk1) increased in in vitro decidualization of
endometrial stromal cells in the mid-secretory phase, suggesting a
possible role of the Wnt pathway in the differentiation and
implantation of the endometrium (63,64).
Similarly, a previous study using different trophoblast models have
revealed that the Wnt pathway may be closely associated with
implantation and the differentiation of trophoblasts (65). Treatment of JAr choriocarcinoma
cell spheroids with Dkk1 increased their attachment to Ishikawa
endometrial-like adenocarcinoma cells (66). In addition, Wnt4 and Fzd2
expression was shown to be downregulated in primary decidualized
endometrial stromal cells, suggesting that trophoblast-dependent
Wnt signaling modulates the decidualization process (67).
Wnt signaling serves an essential role in the
development of early trophoblasts. Treatment of embryonic stem
cells with Wnt3a induced the formation of trophectodermal stem
cells that have the ability to differentiate into
spongiotrophoblasts (68–70). Several studies have also
demonstrated the role of Wnt signaling in the development of
extraembryonic tissues; in particular, the vascularization of the
placenta (71,72). Krivega et al (73) detected Wnt3 ligands and β-catenin
in human blastocysts, and demonstrated that they could promote
progenitor trophoblast development during embryogenesis. Wnt
signaling has been indicated to function during trophoblast
differentiation. For example, Meinhardt et al (74) suggested that the Wnt-signaling
pathway may play a role in EVT differentiation by downregulating of
TCF4. A role for Wnt signaling during invasion was demonstrated
in vivo as well as in vitro, depending on the level
of nuclear β-catenin expression by explanting cultures the
chorionic villous (75).
Furthermore, stimulation with Wnt ligand was revealed to increase
the invasion of primary CTBs (24). However, the ability of cells to
migrate and invade decreased in the different trophoblast models
treated with recombinant Dkk1, suggesting that the canonical Wnt
proteins that are expressed in EVTs exert autocrine effects
(76). Wnt-Fzd5 signaling may lead
to the upregulation of VEGF expression in the chorion and the
subsequent vascularization of primary villi, suggesting that Fzd5
is involved in human trophoblast differentiation (77).
Wnt signaling also contributes to trophoblast
invasion. One study demonstrated high levels of β-catenin-positive
EVT nuclei in the placenta of complete hydatidiform moles (CHMs),
compared to normal cells, indicating that dysregulated Wnt
signaling could contribute to abnormal trophoblast development
(78). As Dkk1 inhibits canonical
Wnt signaling, the pathway may decrease the invasion of trophoblast
cells. Ectoplacental cones co-cultured with decidual cells were
demonstrated to promoted trophoblast invasion when treated with
recombinant Dkk1, whereas treatment with Dkk1 antibodies and
antisense oligonucleotides reduced invasiveness (79). β-catenin activation is a strong
promoter of HTR8/SVneo cell (normal trophoblast cell line)
invasion, leading to the outgrowth and migration in villous
explants (80). The levels of
Wnt1, Wnt7A, Wnt10A and Wnt10B expression were revealed to be
higher in first trimester trophoblasts compared with term
trophoblasts, whereas Wnt1 and Wnt2B were more strongly expressed
in EVTs, suggesting that Wnt may regulate trophoblast invasiveness
(62). Hyperactivation of
Wnt/β-catenin signaling may lead to trophoblast disorders such as
choriocarcinoma, whereas the downregulation of Wnt/β-catenin
signaling may lead to PE.
Recent epigenetic studies have demonstrated a
general level of activation of Wnt signaling in isolated
trophoblasts (81,82). As important components of the Wnt
signaling pathway, APC and secreted Fzd-related protein 2 (sFRP2)
were revealed to be hypermethylated in trophoblasts compared with
the placental fibroblasts or leukocytes (83). This finding suggested that
activation of the Wnt-signaling pathway in trophoblasts may
contribute to placentation. Effectors other than Wnt ligands are
likely to serve a role in the stabilization of β-catenin, as well
as the proliferation and invasion of trophoblasts. A study
demonstrated that Dkk1 is able to induce apoptosis and inhibit
proliferation in JEG3 and BeWo trophoblast cell lines (84). The expression levels of Dkk1 and
sFRP4 were demonstrated to be higher in PE compared with normal
placental tissues, whereas the levels of Wnt2 and β-catenin
expression were decreased (14,16),
indicating that the Wnt-signaling pathway may serve a role in the
development of placental tissues.
Wnt/β-catenin signaling pathway in the
process of PE
PE is a major cause of maternal and perinatal
morbidity and mortality in developing countries (14). To reduce the danger of this
disease, the most important task is to determine the pathogenesis
of PE, of which there are various theories. Although the precise
underlying molecular mechanisms for PE remain unknown, endothelial
cell dysfunction, maternal-fetal immune balance disorders,
inflammation and abnormal recasting of blood vessels are considered
to contribute to PE (85–87). Numerous placenta-induced factors
appear in PE, such as shallow placenta implantation, an imbalance
between trophoblast proliferation and apoptosis (88,89).
An improved understanding of the nature of the placenta can help us
to identify which factor lead to the PE (90). It is speculated that humans have
the tendency to develop PE for a number of reasons, but the
following factors are essential for PE development: Trophoblast
differentiation disorder, hypoxia-ischemia of the placenta and the
extent of trophoblast-induced uterine artery transformation.
Trophoblasts are a highly specialized cell type.
They grow faster than normal cells and they have the ability to
migrate and invade maternal myometrium, which is similar to the
process in which tumor cells invade the surrounding tissue.
However, trophoblast migration is tightly controlled by the body,
both temporally and spatially, which is an essential difference
compared with tumor cell migration. There are two types of
trophoblastic cells: CTBs and STs. With further advances,
lymphocyte proliferation could be inhibited by artificially
generated CTB membrane fragments, and leading to T lymphocyte
apoptosis, which may damage the formation of endothelial cell
monolayers and then contribute the pathogenesis of PE (91). It is increasingly accepted that
CTBs contribute to PE.
In a normal pregnancy, during the process of the
placental formation, the Sertoli cell matrix of spiral arteries is
transformed into large-capacity, low-resistance blood vessels
during a normal pregnancy (25).
This ensures the nutrition of the fetus and the demand for oxygen
are adequately met. Abnormal Sertoli cell differentiation can
interfere with their function, damage trophoblast migration
ability, cause disorder to the invasion of the myometrium, cause
shallow placenta implantation, lead to placental ischemia-hypoxia
and induce PE.
To date, the factors governing blastocyst activation
remain poorly understood; however, recent studies have shown that
multiple signaling pathways are involved in regulating the
differentiation, apoptosis and invasion of trophoblasts (24,92,93).
Advances in our understanding of normal nourishing cells revealed
some unique biological characteristics that are more similar to
malignant tumors. Activation of the Wnt/β-catenin signaling pathway
promotes tumor cell apoptosis (53). It has been hypothesized that the
Wnt/β-catenin signaling pathway may also affect blastocysts and may
be the main cause for shallow trophoblast invasion and disruption
to the remodeling of the spiral artery, which is one of the most
essential and crucial pathological changes that occurs during PE
and is followed by a series of complications.
Wnt-signaling components were revealed to be
involved in the pathogenesis of various diseases, including
gestational diseases. β-catenin-positive EVT nuclei were detected
at higher levels in the placenta of a CHM compared with normal
tissues, indicating that improper Wnt signaling could lead to
abnormal invasion and differentiation in CHM. APC and sFRP2 genes
were revealed to be hypermethylated in choriocarcinoma cells,
suggesting that the inactivation of Wnt signaling may serve a major
role in the pathogenesis of trophoblastic cancer cells (82).
The expression levels of Dkk1 and sFRP4 were
increased in placental tissues from patients with PE, whereas the
levels of Wnt2 and β-catenin expression were reduced (14,16).
Results from our previous study revealed a stronger expression of
E-cadherin in the cytomembrane of villous ST and EVT in PE tissue,
compared with normal tissue (94).
These results provide direct evidence that the Wnt-signaling
pathway is closely associated with PE.
Conclusion
Canonical Wnt/β-catenin signaling is an essential
pathway that promotes implantation, blastocyst activation and
implantation. It serves crucial roles in the differentiation,
differentiation and invasion of trophoblasts. Abnormal
Wnt/β-catenin signaling was observed in numerous diseases including
PE, which is one of the major causes of the perinatal morbidity and
mortality. A better understanding of PE pathogenesis is essential
and may reduce the mortality of the fetus and the mother. In this
review, recent studies that have investigated the pathophysiology
of PE were examined; in particular, those concerning the possible
role of Wnt/β-catenin signaling pathway were reviewed in detail. A
number of studies suggest that the Wnt/β-catenin signaling pathway
may have an essential role in the trophoblast and the development
of PE. However, direct evidence of a role for Wnt/β-catenin
signaling pathway in the development of PE is lacking. Future
studies will help verify whether Wnt/β-catenin signaling within
trophoblasts participates in the development of PE.
Acknowledgements
The present study was supported by the National
Natural Science Foundation (grant no. 81501285).
Glossary
Abbreviations
Abbreviations:
|
APC
|
adenomatous polyposis coli
|
|
CK1
|
casein kinase 1
|
|
CTB
|
cytotrophoblast
|
|
Dkk1
|
Dickkopf-1
|
|
Dvl
|
disheveled
|
|
eCTB
|
endovascular CTB
|
|
EVT
|
extravillous trophoblast
|
|
Fzd
|
frizzled
|
|
GSK3β
|
glycogen synthase kinase 3β
|
|
iCTB
|
interstitial CTB
|
|
LEF
|
lymphocyte enhancer binding factor
|
|
PE
|
preeclampsia
|
|
sFRP
|
secreted frizzled-related protein
|
|
ST
|
syncytiotrophoblast
|
|
TCF
|
T cell factor
|
References
|
1
|
Wang A, Rana S and Karumanchi SA:
Preeclampsia: The role of angiogenic factors in its pathogenesis.
Physiology (Bethesda). 24:147–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts JM, Taylor RN, Musci TJ, Rodgers
GM, Hubel CA and McLaughlin MK: Preeclampsia: An endothelial cell
disorder. Am J Obstet Gynecol. 161:1200–1204. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Redman CW, Sacks GP and Sargent IL:
Preeclampsia: An excessive maternal inflammatory response to
pregnancy. Am J Obstet Gynecol. 180:499–506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mongraw-Chaffin ML, Cirllo PM and Cohn BA:
Preeclampsia and cardiovascular disease death: Prospective evidence
from the child health and development studies cohort. Hypertension.
56:166–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hubel CA, McLaughlin MK, Evans RW, Hauth
BA, Sims CJ and Roberts JM: Fasting serum triglycerides, free fatty
acids, and malondialdehyde are increased in preeclampsia, are
positively correlated, and decrease within 48 hours post partum. Am
J Obstet Gynecol. 174:975–782. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaufmann P, Black S and Huooertz B:
Endovascular trophoblast invasion: Implications for the
pathogenesis of intrauterine growth retardation and preeclampsia.
Biol Reprod. 69:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nusse R, van Ooyen A, Cox D, Fung YK and
Varmus H: Mode of proviral activation of a putative mammary
oncogene (int-1) on mouse chromosome 15. Nature. 307:131–136. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito-Diaz K, Chen TW, Wang X, Thorne CA,
Wallace HA, Page-McCaw A and Lee E: The way Wnt works: Components
and mechanism. Growth Factors. 31:1–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonderegger S, Pollheimer J and Knöfler M:
Wnt signalling in implantation, decidualisation and placental
differentiation-review. Placenta. 31:839–847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma XR, Sim UH Edmund, Pauline B, Patricia
L and Rahman J: Overexpression of WNT2 and TSG101 genes in
colorectal carcinoma. Trop Biomed. 25:46–57. 2008.PubMed/NCBI
|
|
11
|
Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ
and Liu XH: Loss of Wnt5a and Ror2 protein in hepatocellular
carcinoma associated with poor prognosis. World J Gastroenterol.
18:1328–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bui TD, Zhang L, Rees MC, Bicknell R and
Harris AL: Expression and hormone regulation of Wnt2, 3, 4, 5a, 7a,
7b and 10b in normal human endometrium and endometrial carcinoma.
Br J Cancer. 75:1131–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirata H, Hinoda Y, Nakajima K, Kawamoto
K, Kikuno N, Ueno K, Yamamura S, Zaman MS, Khatri G, Chen Y, et al:
Wnt antagonist DKK1 acts as a tumor suppressor gene that induces
apoptosis and inhibits proliferation in human renal cell carcinoma.
Int J Cancer. 128:1793–1803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Zhang L, Zhang L, Jia L, Wang P
and Gao Y: Association of Wnt2 and sFRP4 Expression in the Third
trimester placenta in women with severe preeclampsia. Reprod Sci.
20:981–989. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roberts JM and Escudero C: The placenta in
preeclampsia. Pregnancy Hypertens. 2:72–83. 2012.PubMed/NCBI
|
|
16
|
Zhang Z, Li H, Zhang L, Jia L and Wang P:
Differential expression of beta-catenin and dickkopf-1 in the third
trimester placentas from normal and preeclamptic pregnancies: A
comparative study. Reprod Biol Endocrinol. 11:172013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roberts JM and Gammill HS: Preeclampsia:
Recent insights. Hypertension. 46:1243–1249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
LaMarca BD, Gilbert J and Granger JP:
Recent progress toward the understanding of the pathophysiology of
hypertension during preeclampsia. Hypertension. 51:982–988. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Red-Horse K, Zhou Y, Genbacev O,
Prakobphol A, Foulk R, McMaster M and Fisher SJ: Trophoblast
differentiation during embryo implantation and formation of the
maternal-fetal interface. J Clin Invest. 114:744–754. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh HJ: Pre-eclampsia: Is it all in the
placenta? Malays J Med Sci. 16:7–15. 2009.PubMed/NCBI
|
|
21
|
Cross JC, Werb Z and Fisher SJ:
Implantation and the placenta: Key pieces of the development
puzzle. Science. 266:1508–1518. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carter AM, Enders AC and Pijnenborg R: The
role of invasive trophoblast in implantation and placentation of
primates. Philos Trans R Soc Lond B Biol Sci. 370:201400702015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caniggia I, Winter J, Lye SJ and Post M:
Oxygen and placental development during the first trimester:
Implications for the pathophysiology of pre-eclampsia. Placenta. 21
Suppl A:S25–S30. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Knöfler M and Pollheimer J: Human
placental trophoblast invasion and differentiation: A particular
focus on Wnt signaling. Front Genet. 4:1902013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pijnenborg R, Vercruysse L and Hanssens M:
The uterine spiral arteries in human pregnancy: Facts and
controversies. Placenta. 27:939–958. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jauniaux E, Hempstock J, Greenwold N and
Burton GJ: Trophoblastic oxidative stress in relation to temporal
and regional differences in maternal placental blood flow in normal
and abnormal early pregnancies. Am J Pathol. 162:115–125. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bulmer JN, Williams PJ and Lash GE: Immune
cells in the placental bed. Int J Dev Biol. 54:281–294. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oreshkova T, Dimitrov R and Mourdjeva M: A
cross-talk of decidual stromal cells, trophoblast, and immune
cells: A prerequisite for the success of pregnancy. Am J Reprod
Immunol. 68:366–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rauwel B, Mariamè B, Martin H, Nielsen R,
Allart S, Pipy B, Mandrup S, Devignes MD, Evain-Brion D, Fournier T
and Davrinche C: Activation of peroxisome proliferator-activated
receptor gamma by human cytomegalovirus for de novo replication
impairs migration and invasiveness of cytotrophoblasts from early
placentas. J Virol. 84:2946–2954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiby SE, Walker JJ, O'shaughnessy KM,
Redman CW, Carrington M, Trowsdale J and Moffett A: Combinations of
maternal KIR and fetal HLA-C genes influence the risk of
preeclampsia and reproductive success. J Exp Med. 200:957–965.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burton GJ, Jauniaux E and Charnock-Jones
DS: Human early placental development: Potential roles of the
endometrial glands. Placenta. 28 Suppl A:S64–S69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burton GJ, Jauniaux E and Charnock-Jones
DS: The influence of the intrauterine environment on human
placental development. Int. Int J Dev Biol. 54:303–312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robson A, Harris LK, Innes BA, Lash GE,
Aljunaidy MM, Aplin JD, Baker PN, Robson SC and Bulmer JN: Uterine
natural killer cells initiate spiral artery remodeling in human
pregnancy. FASEB J. 26:4876–4885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harris LK: IFPA Gabor Than Award lecture:
Transformation of the spiral arteries in human pregnancy: Key
events in the remodelling timeline. Placenta. 32 Suppl 2:S154–S158.
2012. View Article : Google Scholar
|
|
35
|
Tal R: The role of hypoxia and
hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol
Reprod. 87:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pijnenborg R, Anthony J, Davey DA, Rees A,
Tiltman A, Vercruysse L and van Assche A: Placental bed spiral
arteries in the hypertensive disorders of pregnancy. Br J Obstet
Gynaecol. 98:648–655. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Y, Gormley MJ, Hunkapiller NM,
Kapidzic M, Stolyarov Y, Feng V, Nishida M, Drake PM, Bianco K,
Wang F, et al: Reversal of gene dysregulation in cultured
cytotrophoblasts reveals possible causes of preeclampsia. J Clin
Invest. 123:2862–2872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miller JR: The Wnts. Genome Biol.
3:REVIEWS30012002.PubMed/NCBI
|
|
41
|
Kestler HA and Kühl M: From individual Wnt
pathways towards a Wnt signalling network. Philos Trans R Soc Lond
B Biol Sci. 363:1333–1347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bender W and Reifer M: Oncogenes take
wing. Cell. 50:519–520. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wodarz A and Nusse R: Mechanisms of Wnt
signaling in development. Annu Rev Cell Dev Biol. 14:59–88. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ng SS, Mahmoudi T, Danenberg E, Bejaoui I,
de Lau W, Korswagen HC, Schutte M and Clevers H:
Phosphatidylinositol 3-kinase signaling does not activate the Wnt
cascade. J Biol Chem. 284:35308–35313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kimelman D and Xu W: beta-catenin
destruction complex: Insights and questions from a structural
perspective. Oncogene. 25:7482–7491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wong HC, Bourdelas A, Krauss A, Lee HJ,
Shao Y, Wu D, Mlodzik M, Shi DL and Zheng J: Direct binding of the
PDZ domain of Dishevelled to a conserved internal sequence in the
C-terminal region of Frizzled. Mol Cell. 12:1251–1260. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jho EH, Zhang T, Domon C, Joo CK, Freund
JN and Costantini F: Wnt/beta-catenin/Tcf signaling induces the
transcription of Axin2, a negative regulator of the signaling
pathway. Mol Cell Biol. 22:1172–1183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cadigan KM and Peifer M: Wnt signaling
from development to disease: Insights from model systems. Cold
Spring Harb Perspect Biol. 1:a0028812009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
van Amerongen R and Nusse R: Towards an
integrated view of Wnt signaling in development. Development.
136:3205–3214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Polakis P: Drugging Wnt signalling in
cancer. EMBO J. 31:2737–2746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chien AJ, Conrad WH and Moon RT: A Wnt
survival guide: From flies to human disease. J Invest Dermatol.
129:1614–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
58
|
Phelps RA, Broadbent TJ, Stafforini DM and
Jones DA: New perspectives on APC control of cell fate and
proliferation in colorectal cancer. Cell Cycle. 8:2549–2556. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tarapore RS, Siddiqui IA and Mukhtar H:
Modulation of Wnt/β-catenin signaling pathway by bioactive food
components. Carcinogenesis. 33:483–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Georgiades P, Ferguson-Smith AC and Burton
GJ: Comparative developmental anatomy of the murine and human
definitive placentae. Placenta. 23:3–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gough NR: Focus issue: Wnt and β-catenin
signaling in development and disease. Sci Signal.
5:eg22012.PubMed/NCBI
|
|
62
|
Sonderegger S, Husslein H, Leisser C and
Knöfler M: Complex expression pattern of Wnt ligands and frizzled
receptors in human placenta and its trophoblast subtypes. Placenta.
28 Suppl A:S97–S102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tulac S, Nayak NR, Kao LC, van Waes M,
Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E and
Giudice LC: Identification, characterization, and regulation of the
canonical Wnt signaling pathway in human endometrium. J Clin
Endocrinol Metab. 88:3860–3866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Carson DD, Lagow E, Thathiah A, Al-Shami
R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA and Lessey B:
Changes in gene expression during the early to mid-luteal
(receptive phase) transition in human endometrium detected by
high-density microarray screening. Mol Hum Reprod. 8:871–879. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen Y, Zhang Y, Deng Q, Shan N, Peng W,
Luo X, Zhang H, Baker PN, Tong C and Qi H: Wnt5a inhibited human
trophoblast cell line HTR8/SVneo invasion: Implications for early
placentation and preeclampsia. J Matern Fetal Neonatal Med.
29:3532–3538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng
EH, Yeung WS, Ho PC and Lee KF: Excessive ovarian stimulation
up-regulates the Wnt-signaling molecule DKK1 in human endometrium
and may affect implantation: An in vitro co-culture study. Hum
Reprod. 25:479–490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hess AP, Hamilton AE, Talbi S, Dosiou C,
Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K,
Kruessel J, et al: Decidual stromal cell response to paracrine
signals from the trophoblast: Amplification of immune and
angiogenic modulators. Biol Reprod. 76:102–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chawengsaksophak K, de Graaff W, Rossant
J, Deschamps J and Beck F: Cdx2 is essential for axial elongation
in mouse development. Pro Natl Acad Sci USA. 101:7641–7645. 2004.
View Article : Google Scholar
|
|
69
|
Miller C and Sassoon DA: Wnt-7a maintains
appropriate uterine patterning during the development of the mouse
female reproductive tract. Development. 125:3201–3211.
1998.PubMed/NCBI
|
|
70
|
Vainio S, Heikkilä M, Kispert A, Chin N
and McMahon AP: Female development in mammals is regulated by Wnt-4
signalling. Nature. 397:405–409. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
71
|
Newman AC and Hughes CC: Macrophages and
angiogenesis: A role for Wnt signaling. Vasc Cell. 4:132012.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Herr F, Horndasch M, Howe D, Baal N, Goyal
P, Fischer S, Zygmunt M and Preissner KT: Human placenta-derived
Wnt-5a induces the expression of ICAM-1 and VCAM-1 in
CD133(+)CD34(+)-hematopoietic progenitor cells. Reprod Biol.
14:262–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Krivega M, Essahib W and Van de Velde H:
WNT3 and membrane-associated β-catenin regulate trophectoderm
lineage differentiation in human blastocysts. Mol Hum Repro.
21:711–722. 2015. View Article : Google Scholar
|
|
74
|
Meinhardt G, Haider S, Haslinger P,
Proestling K, Fiala C, Pollheimer J and Knöfler M: Wnt-dependent
T-cell factor-4 controls human extravillous trophoblast motility.
Endocrinology. 155:1908–1920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bilic J, Huang YL, Davidson G, Zimmermann
T, Cruciat CM, Bienz M and Niehrs C: Wnt induces LRP6 signalosomes
and promotes dishevelled-dependent LRP6 phosphorylation. Science.
316:1619–1622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sonderegger S, Haslinger P, Sabri A,
Leisser C, Otten JV, Fiala C and Knöfler M: Wingless (Wnt)-3A,
induces trophoblast migration and matrix metalloproteinase-2
secretion through canonical Wnt signaling and protein kinase B/AKT
activation. Endocrinology. 151:211–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lu J, Zhang S, Nakano H, Simmons DG, Wang
S, Kong S, Wang Q, Shen L, Tu Z, Wang W, et al: A positive feedback
loop involving Gcm1 and Fzd5 directs chorionic branching
morphogenesis in the placenta. PLoS Biol. 11:e10015362013.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pollheimer J, Loregger T, Sonderegger S,
Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P and Knöfler M:
Activation of the canonical wingless/T-cell factor signaling
pathway promotes invasive differentiation of human trophoblast. Am
J Pathol. 168:1134–1147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Peng S, Li J, Miao C, Jia L, Hu Z, Zhao P,
Li J, Zhang Y, Chen Q and Duan E: Dickkopf-1 secreted by decidual
cells promotes trophoblast cell invasion during murine
placentation. Reproduction. 135:367–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhuang B, Luo X, Rao H, Li Q, Shan N, Liu
X and Qi H: Oxidative stress-induced C/EBPβ inhibits β-catenin
signaling molecule involving in the pathology of preeclampsia.
Placenta. 36:839–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lavergne E, Hendaoui I, Coulouarn C,
Ribault C, Leseur J, Eliat PA, Mebarki S, Corlu A, Clément B and
Musso O: Blocking Wnt signaling by SFRP-like molecules inhibits in
vivo cell proliferation and tumor growth in cells carrying active
β-catenin. Oncogene. 30:423–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wong NC, Novakovic B, Weinrich B, Dewi C,
Andronikos R, Sibson M, Macrae F, Morley R, Pertile MD, Craig JM
and Saffery R: Methylation of the adenomatous polyposis coli (APC)
gene in human placenta and hypermethylation in choriocarcinoma
cells. Cancer Lett. 268:56–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Novakovic B, Rakyan V, Ng HK, Manuelpillai
U, Dewi C, Wong NC, Morley R, Down T, Beck S, Craig JM and Saffery
R: Specific tumour-associated methylation in normal human term
placenta and first-trimester cytotrophoblasts. Mol Hum Reprod.
14:547–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cui H, Li H, Li QL, Chen J, Na Q and Liu
CX: Dickkopf-1 induces apoptosis in the JEG3 and BeWo trophoblast
tumor cell lines through the mitochondrial apoptosis pathway. Int J
Oncol. 46:2555–2561. 2015.PubMed/NCBI
|
|
85
|
Ducat A, Doridot L, Calicchio R, Méhats C,
Vilotte JL, Castille J, Barbaux S, Couderc B, Jacques S, Letourneur
F, et al: Endothelial cell dysfunction and cardiac hypertrophy in
the STOX1 model of preeclampsia. Sci Rep. 6:191962016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Szarka A, Rigó J Jr, Lázár L, Beko G and
Molvarec A: Circulating cytokines, chemokines and adhesion
molecules in normal pregnancy and preeclampsia determined by
multiplex suspension array. BMC Immunol. 11:592010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Crocker I: Gabor Than Award Lecture 2006:
pre-eclampsia and villous trophoblast turnover: perspectives and
possibilities. Placenta. 28 Suppl A:S4–S13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Goldman-Wohl D and Yagel S: Regulation of
trophoblast invasion: From normal implantation to pre-eclampsia.
Mol Cell Endocrinol. 187:233–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Can M, Guven B, Bektas S and Arikan I:
Oxidative stress and apoptosis in preeclampsia. Tissue Cell.
46:477–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Schrocksnadel H, Daxenbichler G, Artner E,
Steckel-Berger G and Dapunt O: Tumor markers in hypertensive
disorders of pregnancy. Gynecol Obstet Invest. 35:204–208. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dey SK, Lim H, Das SK, Reese J, Paria BC,
Daikoku T and Wang H: Molecular cues to implantation. Endocr Rev.
25:341–373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang J, Dunk CE and Lye SJ: Sphingosine
signaling regulates decidual NK cell angiogenic phenotype and
trophoblast migration. Hum Reprod. 28:3026–3037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fan M, Xu Y, Hong F, Gao X, Xin G, Hong H,
Dong L and Zhao X: Rac1/β-catenin signalling pathway contributes to
trophoblast cell invasion by targeting snail and MMP9. Cell Physiol
Biochem. 38:1319–1332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang Zhan, LI Wei, Zhang Lin-Lin, Jia
Li-Ting, Yu Hai-Yang and Liu Li-Sha: Detection of E-cadherin
expression in preeclampsia by placenta tissue microarray. Chinese
Journal of Health Laboratory Technology. 9:1232–1235. 2014.
|















