Introduction
Worldwide, breast cancer is the leading type of
cancer in women, accounting for 25% of all cases (1). Genetic predisposition is the main
reason for breast cancer (2–4).
There are three types of calcium channel blockers
(CCBs), including dihydropyridine, phenylalkylamine and
benzothiazepine. Nifedipine is the most common kind of CCB and is
used to treat high blood pressure and angina. It has been reported
that CCBs may be associated with cancer; a meta-analysis of 17
observational studies identified that the long-term use of CCBs
appears to have a significant association with breast cancer
(5). The use of particular types
of antihypertensive medications, including immediate-release CCBs
and certain diuretics, may increase the risk of breast carcinoma
among women aged between 65 and 79 years (6). Use of antihypertensive medication for
≥5 years, compared with no use, was associated with a modest
increased risk of invasive breast cancer (RR=1.18, 95% CI,
1.02–1.36) (7). In addition, a
previous study of the authors (8)
demonstrated that nifedipine could promote the proliferation and
migration of breast cancer cells in vivo and in
vitro. Others have considered that CCBs have no association
with cancer (9). Patients with
coronary heart disease (CHD) and treated with CCBs exhibited a
similar risk of cancer incidence and total and cancer-related
mortality as those not treated with CCBs (10). No statistically significant
association was observed between the use of CCBs and breast cancer
in 49,950 women in North Jutland (11). A mixed treatment comparison
meta-analysis of randomized, controlled (placebo, active or
untreated control) trials of antihypertensive drugs was conducted
to determine the association between commonly used antihypertensive
agents and the incidence of cancer and the results demonstrated
that commonly used antihypertensive drugs were not associated with
an increased chance of developing cancer (12).
Nifedipine is widely used in clinics, so it is
important to determine its effects on breast cancer and the
mechanism involved. The present study identified and confirmed that
nifedipine can promote breast cancer in vitro. In MCF-7
cells, the effects of nifedipine are via the protein kinase B
(Akt)-endothelial constitutive nitric oxide synthase (eNOS)-nitric
oxide (NO) axis. However, nifedipine exercises its effects upon
MDA-MB-231 cells via activation of the extracellular
signal-regulated kinases (ERK) pathway.
Materials and methods
Cell culture and reagents
MCF-7 and MDA-MB-231 cells were purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
MCF-7 cells were cultured in Dulbecco's Modified Eagle's medium
(PromoCell GmbH, Heidelberg, Germany) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified atmosphere of 95% air and 5%
CO2. MDA-MB-231 cells were grown in L15 medium
(PromoCell GmbH) containing 10% FBS at 37°C in a humidified
atmosphere of 100% air.
Other reagents purchased were nifedipine
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), MTT (5 mg/ml;
Sigma-Aldrich; Merck KGaA), paraformaldehyde (4%; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China), Crystal violet
(0.1%, Beijing Solarbio Science & Technology Co., Ltd.),
radioimmunoprecipitation assay (RIPA) buffer (Beijing Solarbio
Science & Technology Co., Ltd.), phenylmethylsulfonyl fluoride
(Beijing Solarbio Science & Technology Co., Ltd.), protease
inhibitor (25x; Roche Applied Science, Penzberg, Germany) and
dimethylsulfoxide (DMSO; Sigma-Aldrich; Merck KGaA).
Proliferation assays
For the determination of cell proliferation, an MTT
assay was conducted, as reported previously (8). MCF-7 and MDA-MB-231 cells were seeded
at a density of 1,000 cells/well in 96-well plates. Following a 1
day incubation, the culture medium was replaced with new medium
containing nifedipine or the same concentration of DMSO as control.
The cells were incubated for 2 further days. Next, 10 µl MTT was
added for 3 h. Then the culture medium was removed and 150 µl of
DMSO was added per well. The absorbance was measured at 540 nm
using a Multiskan microplate reader (Thermo Fisher Scientific,
Inc.).
Cell migration assays
For Transwell migration assays, harvested cells
(1×105 cells) supplemented with 100 µl serum-free medium were
replated onto the upper chamber (a 6.5 mm polycarbonate membrane
with 8.0 µm pores; Corning Incorporated, Corning, NY, USA). DMEM
and L15 medium containing 10% FBS was used as the chemoattractant
and added to the lower well of the plate. Non-migrating cells were
removed from the upper chamber using a cotton applicator. Migrating
cells on the underside of the filter were stained with 0.1% crystal
violet for 20 min and then were eluted by 33% glacial acetic acid.
Optical density values were read by a Multiskan microplate reader
(Thermo Fisher Scientific, Inc.) at 595 nm.
Immunoblotting
Nifedipine was used to stimulate the breast cancer
cells (MCF-7 cells, 10 µM; MDA-MB-231 cells 1 µM). Cells were lysed
in RIPA buffer containing protease and phosphatase cocktails (Roche
Applied Science). Equal amounts of protein (50 µg) were separated
by 10% SDS-PAGE and electro-transferred onto a PVDF membrane (Merck
KGaA). Membranes were blocked with 5% non-fat milk powder in TBST
(0.1% Tween-20 in 1X TBS) for 1 h at room temperature and then
incubated with appropriate primary antibodies at 4°C overnight,
followed by incubation with horseradish peroxidase-conjugated
secondary antibodies for 1 h at room temperature. The following
antibodies were used: Rabbit anti-P (Ser473)-Akt (1:1,000, no.
9271), rabbit anti-Akt (1:1,000, no. 9272), rabbit anti-P
(Thr202/Tyr204)-ERK (1:1,000, no. 9101), mouse anti-ERK (1:1,000,
no. 4696), all purchased from Cell Signalling Technology Inc.
(Danvers, MA, USA), and mouse anti-β-actin (1:5,000, no. M009;
Beijing TDY Biotech Co., Ltd., Beijing, China).
Statistical analysis
All data were derived from ≥3 independent
experiments. Statistical analyses were conducted using SPSS version
19.0 (IBM SPSS, Armonk, NY, USA). Values were calculated as mean ±
standard error of the mean. Significant differences between the
groups were determined using the unpaired one-way analysis of
variance test. P<0.05 was considered to indicate a statistically
significant difference.
Results
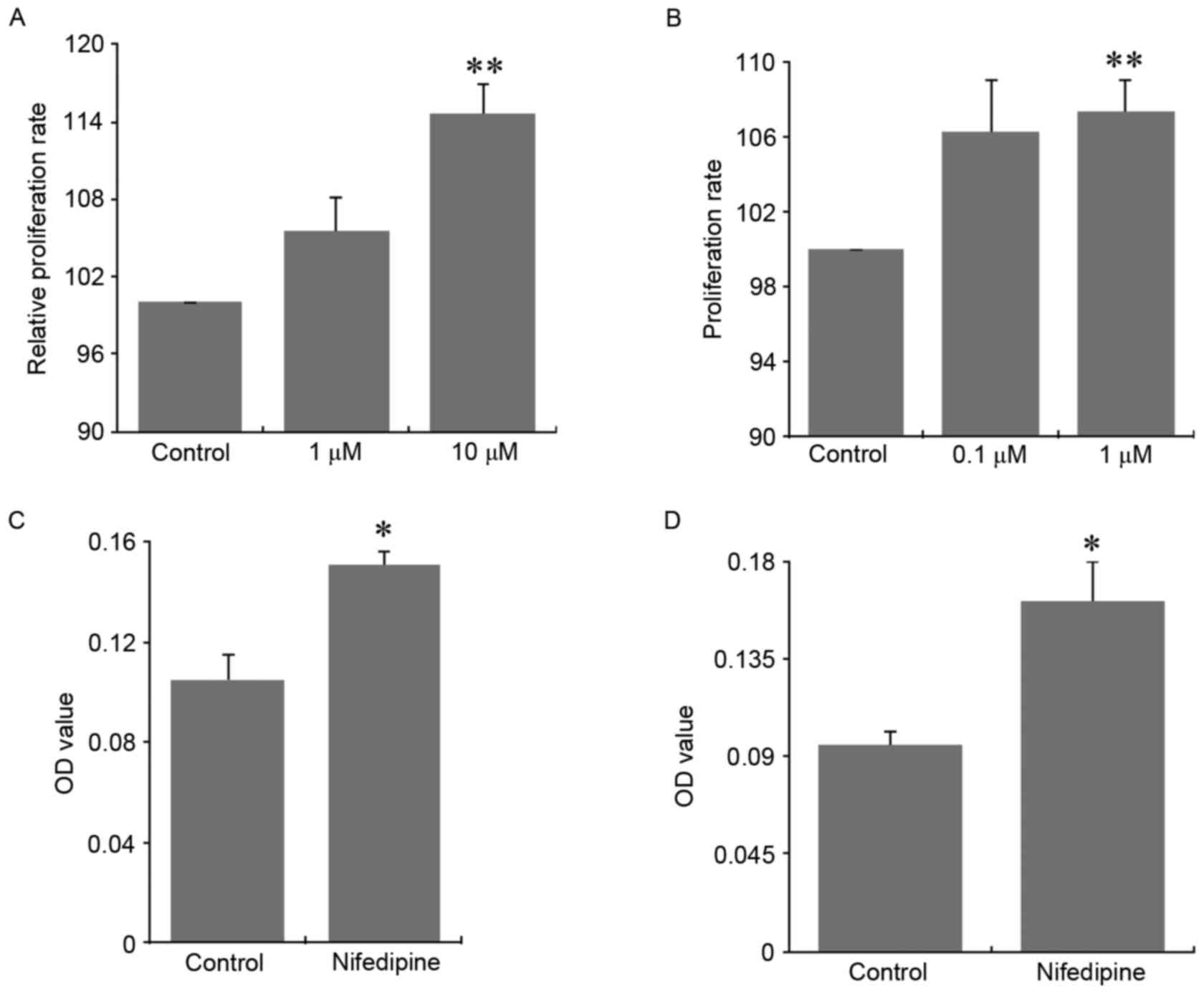
Nifedipine promoted the proliferation
and migration of breast cancer cells in vitro
Nifedipine as a CCB is commonly used in clinic to
treat angina and hypertension. MCF-7 breast cancer cells were
treated with nifedipine at the dosages of 1 µM and 10 µM.
Nifedipine at these two dosages significantly stimulated the
proliferation of MCF-7 cells, when compared with control cells
(Fig. 1A; P<0.01).
Consistently, nifedipine at a dosage of 1 µM also significantly
exhibited a similar stimulation on the proliferation of MDA-MB-231
breast cancer cells, compared with the control group (Fig. 1B; P<0.01). Nifedipine at a
dosage of 10 µM significantly promoted the migration of MCF-7 cells
compared with control cells (Fig.
1C; P<0.05). In addition, nifedipine (1 µM) exhibited a
similar stimulation on the migration of MDA-MB-231 cells (Fig. 1D; P<0.05).
Nifedipine stimulation effect on
breast cancer cells not via its blockage on calcium channels
To test whether the stimulatory effect of nifedipine
on breast cancer cells is due to alternation of the concentration
of the intracellular free Ca2+, the MCF-7 and MDA-MB-231
cells were preincubated in the presence of 1 µM nifedipine in DMEM
or L15 medium for 1 h at 37°C and then loaded with Fura-8 (AAT
Bioquest, Inc., Sunnyvale, CA, USA). No significant increase in
[Ca2+]i was observed with either nifedipine
alone or by increasing K+ concentration from 2.5 mM to
90 mM, indicating that MCF-7 and MDA-MB-231 cells did not express a
functional L-type calcium channel. It suggested that the effect of
nifedipine on the breast cancer cells was not due to calcium
channels and cellular Ca2+ levels (data not shown).
Verapamil had no effect on the tumor
growth in vitro
To test whether the stimulation of breast tumor is
nifedipine-specific, another CCB, verapamil, was used to treat
MCF-7 and MDA-MB-231 breast cancer cells. No significant change in
cell proliferation and migration was observed, indicating that the
specific effects of nifedipine on MDA-MB-231 cells were not a
common characteristic of L-type CCBs (data not shown).
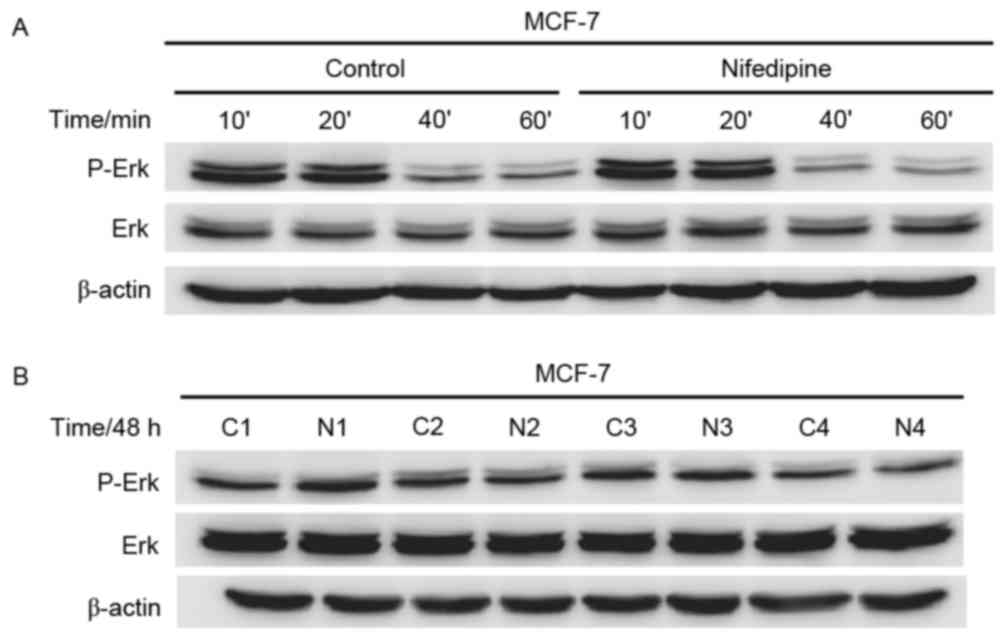
Nifedipine effect upon MCF-7 cells not
via ERK pathway
In our previous study (8), nifedipine could stimulate the ERK
pathway of MDA-MB-231 cells. Notably, no differences in
phosphorylation of ERK and total ERK were observed in MCF-7 cells
with or without nifedipine at 10, 20, 40 and 60 min. Membranes were
reprobed for b-actin for loading control (Fig. 2A). No differences were observed in
phosphorylation of ERK and total ERK in MCF-7 cells with or without
nifedipine at 48 h. Membranes were reprobed with β-actin for
loading control (Fig. 2B).
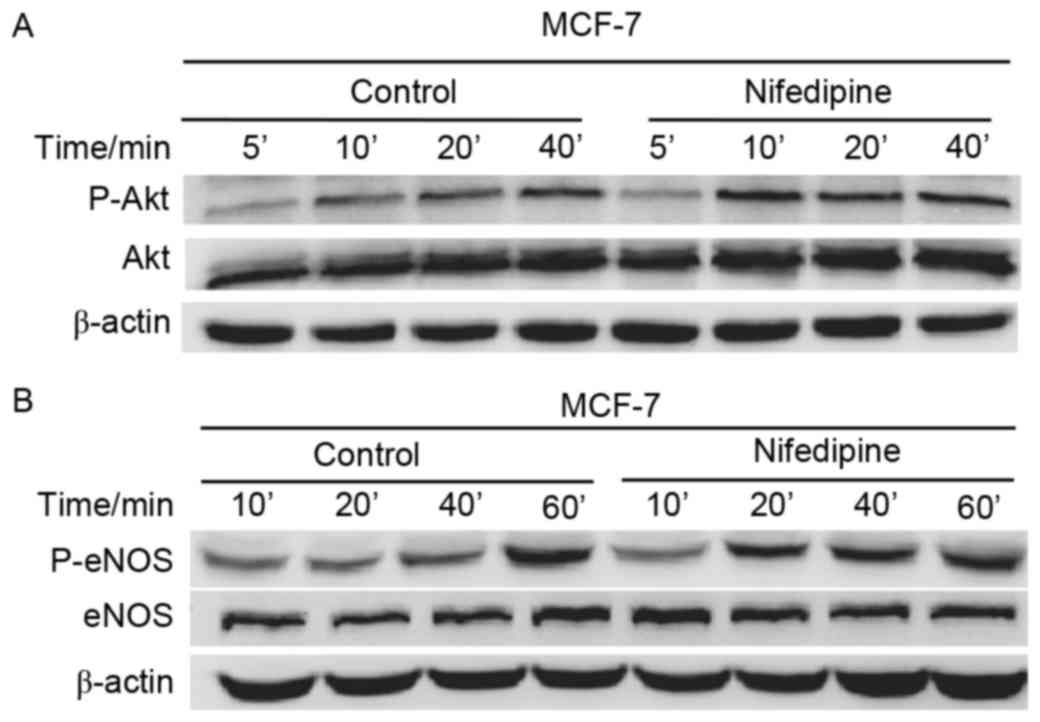
Akt activation in nifedipine treated
MCF-7 cells
Notably, Nifedipine increased phosphorylation of Akt
and total Akt following treatment at 5, 10, 20 and 40 min.
Membranes were reprobed for β-actin for loading control (Fig. 3A). Nifedipine also increased
phosphorylated eNOS (P-eNOS) and total eNOS in MCF-7 cells at 10,
20, 40 and 60 min. Membranes were reprobed for β-actin for loading
control (Fig. 3B). These results
suggested that nifedipine stimulated MCF-7 cells via the
Akt-eNOS-NO axis.
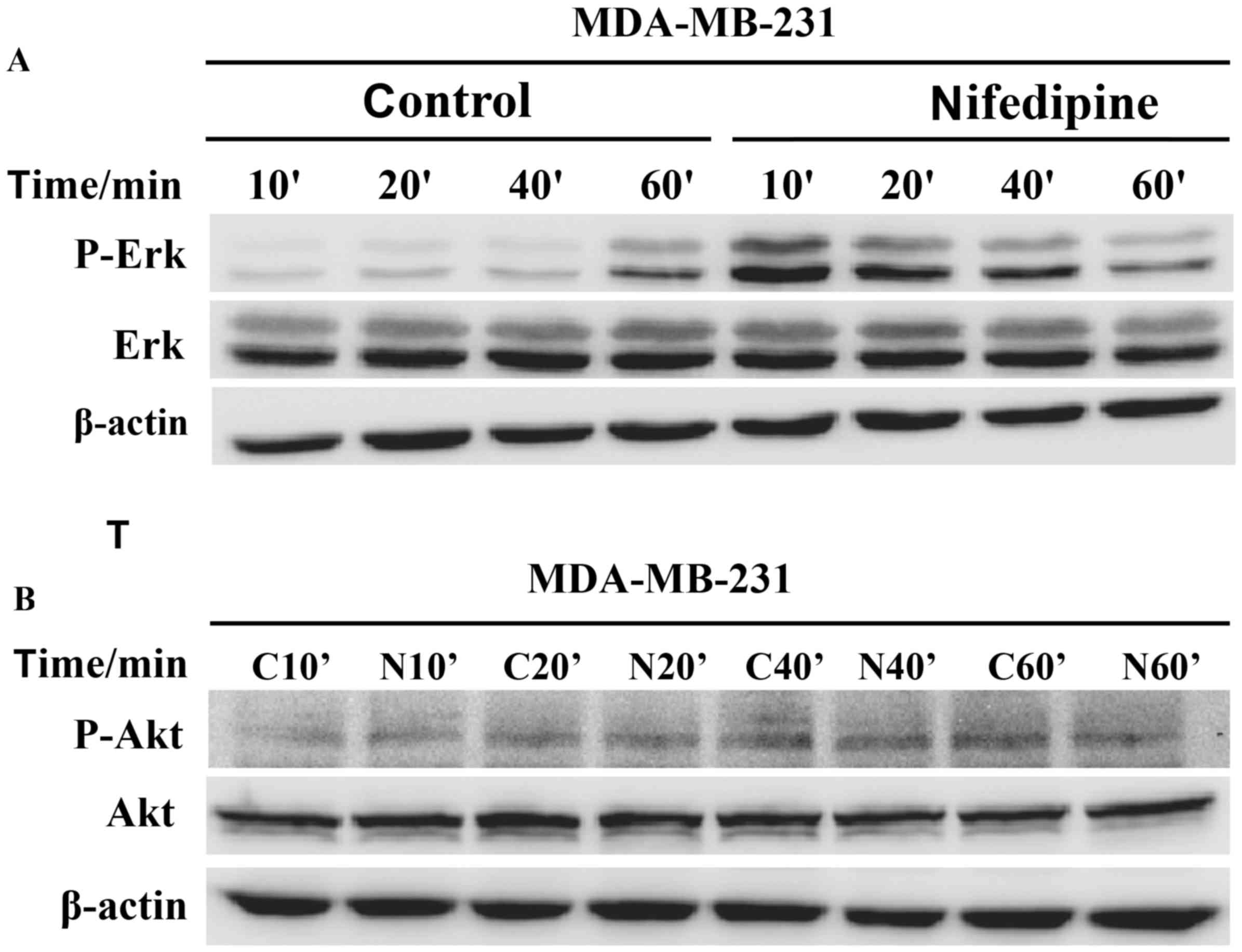
Nifedipine effect on MDA-MB-231 cells
via ERK activation not Akt pathway
Nifedipine increased the phosphorylation of ERK in
MDA-MB-231 cells following treatment at 10, 20, 40 and 60 min
compared with the control groups, respectively. The p-ERK level at
10 min demonstrated the strongest stimulatory effects of
nifedipine, and these decreased over time. Membranes were reprobed
for β-actin for loading control (Fig.
4A). However, nifedipine had no effect on the phosphorylation
of Akt in MDA-MB-231 cells. Membranes were reprobed for β-actin for
loading control (Fig. 4B).
Discussion
In the present study, nifedipine was identified to
significantly stimulate the proliferation and migration of MCF-7
and MDA-MB-231 breast cancer cells. This stimulatory effect was
nifedipine specific and verapamil, another calcium channel blocker,
demonstrated no observable effect on the breast cancer cells.
Notably, nifedipine effects upon MCF-7 cells were via the axis
Akt-eNOS-NO while its effects upon MDA-MB-231 cells were via
activation of ERK. The p-ERK level at 10 min exhibited the
strongest stimulatory effects of nifedipine and decreased over
time, demonstrating a transient activation that has been reported
elsewhere (13). These results
suggested distinct pathways in activation of cell proliferation and
migration presented in different cell lines by the same stimulator.
Additionally, the present study advises that nifedipine promotes
breast cancers and that nifedipine ought to be avoided in clinical
practice, particularly for women who suffer from breast cancer and
hypertension.
MCF-7 (14,15) and MDA-MB-231 (16) cells are derived from invasive
ductal breast carcinoma and represent metastasis (pleural effusion)
tumor type without ERBB2 amplification. However, there are distinct
differences in the expression of estrogen receptor (ER),
progesterone receptor and TP53 mutation between them. ER expression
is one of most important criteria to distinguish the breast cancer
type. Therefore, MCF-7 and MDA-AB-231 cells were utilized in the
present study to understand the collective effects of nifedipine on
breast cancer.
Previous studies on whether CCBs promote cancer
cells are controversial (5,17).
The present study confirmed nifedipine as one of the CCBs that can
potentiate breast cancer, although it is inconsistent with previous
studies that this effect is nifedipine specific (18). With respect to the possible
mechanism, [Ca2+]i modulation was excluded by
the evidence that MCF-7 and MDA-MB-231 cells do not express the
CACNA1C and CACNA1D subtypes and 1 µM nifedipine failed to alter
[Ca2+]i, although previous studies (19–22)
have described the connection of CCBs and cellular calcium
alternation in cancer cells.
In addition to their function as the channel
blockers, CCBs are also suggested to affect growth
hormone-releasing hormone receptors in cancers (21), the expression of P-glycoprotein
(23), the function of breast
cancer resistant protein (17,24),
Na channel activity (25) and
microRNA, resulting in alterations to cell proliferation and
migration (8). In contrast to the
activation of the miRNA-524-5P-BRI3-ERK pathway in MDA-MB-231 by
nifedipine, there is a distinct pathway (AKt-eNOS-NO) present in
MCF-7 following treatment with nifedipine. A previous study
(26) revealed that eNOS and weak
iNOS were expressed in MCF-7 cells and served an important role in
cell proliferation. In general, NO can stimulate the proliferation
and migration of epithelial cells in addition to gene profile
expression (27).
Women comprise ~1/3 of all hypertension patients. A
number of them may be suffering from, or are genetically
predisposed to develop, breast cancer. Nifedipine may be dangerous
for patients with breast cancers and indeed promotes breast cancer.
Clinics should avoid administering nifedipine to women who suffer
from breast cancer and hypertension.
Acknowledgements
This work was supported by research grants held by
Y.G. from the 973 Project (grant nos. 2013CB531206 and
2012CB517803) and the National Natural Science Foundation of China
(grant nos. 81170236, 31127001, 81570245 and 31221002).
References
|
1
|
Needleman H and Huff J: The international
agency for research on cancer and obligate transparency. Lancet
Oncol. 6:920–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ellis MJ, Ding L, Shen D, Luo J, Suman VJ,
Wallis JW, van Tine BA, Hoog J, Goiffon RJ, Goldstein TC, et al:
Whole-genome analysis informs breast cancer response to aromatase
inhibition. Nature. 486:353–360. 2012.PubMed/NCBI
|
|
3
|
Ruark E, Snape K, Humburg P, Loveday C,
Bajrami I, Brough R, Rodrigues DN, Renwick A, Seal S, Ramsay E, et
al: Mosaic PPM1D mutations are associated with predisposition to
breast and ovarian cancer. Nature. 493:406–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stephens PJ, Tarpey PS, Davies H, van Loo
P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell
GR, et al: The landscape of cancer genes and mutational processes
in breast cancer. Nature. 486:400–404. 2012.PubMed/NCBI
|
|
5
|
Li W, Shi Q, Wang W, Liu J, Li Q and Hou
F: Calcium channel blockers and risk of breast cancer: A
meta-analysis of 17 observational studies. PLoS One. 9:e1058012014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li CI, Malone KE, Weiss NS, Boudreau DM,
Cushing-Haugen KL and Daling JR: Relation between use of
antihypertensive medications and risk of breast carcinoma among
women ages 65–79 years. Cancer. 98:1504–1513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Largent JA, Bernstein L, Horn-Ross PL,
Marshall SF, Neuhausen S, Reynolds P, Ursin G, Zell JA, Ziogas A
and Anton-Culver H: Hypertension, antihypertensive medication use,
and breast cancer risk in the California teachers study cohort.
Cancer Causes Control. 21:1615–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo DQ, Zhang H, Tan SJ and Gu YC:
Nifedipine promotes the proliferation and migration of breast
cancer cells. PLoS One. 9:e1136492014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahr HJ, Bomhard E, Enzmann H, Karbe E,
Mager H, Sander E and Schlüter G: Calcium channel blockers and the
risk of cancer: A preclinical assessment. Cardiovasc Drugs Ther.
12:157–169. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Braun S, Boyko V, Behar S, Reicher-Reiss
H, Laniado S, Kaplinsky E and Goldbourt U: Calcium channel blocking
agents and risk of cancer in patients with coronary heart disease.
Benzafibrate infarction prevention (BIP) study research group. J Am
Coll Cardiol. 31:804–808. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fryzek JP, Poulsen AH, Lipworth L,
Pedersen L, Nørgaard M, McLaughlin JK and Friis S: A cohort study
of antihypertensive medication use and breast cancer among Danish
women. Breast Cancer Res Treat. 97:231–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coleman CI, Baker WL, Kluger J and White
CM: Antihypertensive medication and their impact on cancer
incidence: A mixed treatment comparison meta-analysis of randomized
controlled trials. J Hypertens. 26:622–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alfonzo-Méndez MA, Castillo-Badillo JA,
Romero-Ávila MT, Rivera R, Chun J and García-Sáinz JA: Carboxyl
terminus-truncated alpha1D-adrenoceptors inhibit the ERK pathway.
Naunyn Schmiedebergs Arch Pharmacol. 389:911–920. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soule HD, Vazguez J, Long A, Albert S and
Brennan M: A human cell line from a pleural effusion derived from a
breast carcinoma. J Natl Cancer Inst. 51:1409–1416. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabei I, Nishiyama S, Yamashita S,
Hashimoto H, Tachibana T, Uchida K and Ishikawa H: Establishment
and characterization of HER2-positive cell line derived from
pleural effusion of human breast scirrhous carcinoma Hum. Cell.
21:105–112. 2008.
|
|
16
|
Cailleau R, Young R, Olivé M and Reeves WJ
Jr: Breast tumor cell lines from pleural effusions. J Natl Cancer
Inst. 53:661–674. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Gupta A, Wang H, Zhou L,
Vethanayagam RR, Unadkat JD and Mao Q: BCRP transports dipyridamole
and is inhibited by calcium channel blockers. Pharm Res.
22:2023–2034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Timcheva CV and Todorov DK: Does verapamil
help overcome multidrug resistance in tumor cell lines and cancer
patients? J Chemother. 8:295–299. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Correale P, Tagliaferri P, Celio L, Genua
G, Montagnani S and Bianco AR: Verapamil upregulates sensitivity of
human colon and breast cancer cells to LAK-cytotoxicity in vitro.
Eur J Cancer. 27:1393–1395. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fine RL, Patel J and Chabner BA: Phorbol
esters induce multidrug resistance in human breast cancer cells.
Proc Natl Acad Sci USA. 85:582–586. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garcia-Fernandez MO, Schally AV, Varga JL,
Groot K and Busto R: The expression of growth hormone-releasing
hormone (GHRH) and its receptor splice variants in human breast
cancer lines; the evaluation of signaling mechanisms in the
stimulation of cell proliferation. Breast Cancer Res Treat.
77:15–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nie L, Oishi Y, Doi I, Shibata H and
Kojima I: Inhibition of proliferation of MCF-7 breast cancer cells
by a blocker of Ca(2+)-permeable channel. Cell Calcium. 22:75–82.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung SY, Sung MK, Kim NH, Jang JO, Go EJ
and Lee HJ: Inhibition of P-glycoprotein by natural products in
human breast cancer cells. Arch Pharm Res. 28:823–828. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou XF, Yang X, Wang Q, Coburn RA and
Morris ME: Effects of dihydropyridines and pyridines on multidrug
resistance mediated by breast cancer resistance protein: In vitro
and in vivo studies. Drug Metab Dispos. 33:1220–1228. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roger S, Le Guennec JY and Besson P:
Particular sensitivity to calcium channel blockers of the fast
inward voltage-dependent sodium current involved in the invasive
properties of a metastastic breast cancer cell line. Br J
Pharmacol. 141:610–615. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loibl S, Bratengeier J, Farines V, von
Minckwitz G, Spänkuch B, Schini-Kerth V, Nepveu F, Strebhardt K and
Kaufmann M: Investigations on the inducible and endothelial nitric
oxide synthases in human breast cancer cell line MCF-7-estrogen has
an influence on e-NOS, but not on i-NOS. Pathol Res Pract. 202:1–7.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng RY, Basudhar D, Ridnour LA, Heinecke
JL, Kesarwala AH, Glynn S, Switzer CH, Ambs S, Miranda KM and Wink
DA: Gene expression profiles of NO- and HNO-donor treated breast
cancer cells: Insights into tumor response and resistance pathways.
Nitric Oxide. 43:17–28. 2014. View Article : Google Scholar : PubMed/NCBI
|