Introduction
Diabetes mellitus (DM) is the most common metabolic
disease worldwide (1). DM may
become the seventh leading cause of human death by the year 2030
(2). The complications of DM
include microvascular (retinopathy, nephropathy and neuropathy) and
macrovascular complications (cardiovascular disease) (3). Diabetic retinopathy (DR) is
characterized by the increased vessel permeability and progressive
vascular occlusion (4). DR is a
main cause of visual impairment and blindness in adults (5). The pathogenesis of DR is
multifactorial, such as hyperglycemia, advanced glycation end
products, oxidative stress and inflammation (6,7).
Retinal ganglion cells (RGCs) are the final neurons
in the retina that output vision signals to brain visual centers
(8). The death of RGCs contributes
to irreversible visual loss (9).
Ciliary neurotrophic factor (CNTF) is a neurotrophic cytokine of
the IL-6 family and can induce neuron differentiation in the
central nervous system (10).
Mathews et al have reported that CNTF administration
promotes RGC survival and provides neuroprotection against
ischaemic optic nerve injury in animal models (11). Aizu et al have found that
topical instillation of CNTF via eye drops protects STZ-induced
diabetic rats from retinal degeneration (12).
RhoA is a GTPase protein that regulates cytoskeleton
reorganization in stress fiber formation (13). RhoA exerts its downstream effect
via effector proteins, Rho-associated protein kinase 1 (Rock1) and
Rock2 (14). Lu et al have
demonstrated that RhoA/Rock1 signaling is involved in the
protection of microvascular endothelial cell dysfunction induced by
hyperglycemia in an in vitro model of DR (15). Nogo receptor (NgR) is a neural
regeneration-associated membrane receptor for Nogo,
oligodendrocyte-myelin glycoprotein and myelin-associated
glycoprotein (16). Our previous
study has demonstrated that NgR and Rock1 expression levels are
elevated in the retina of diabetic rats. Downregulation of NgR
inhibits retinal ganglion cell apoptosis and decreases Rock1
expression (17).
Our study focused on the effect of NgR inhibition
and CNTF treatment on RGCs in DR in vivo. Furthermore, we
evaluated the synergistic effect of combination therapy and the
possible protective mechanisms. Our study provides a novel strategy
for DR treatment.
Materials and methods
Animals and treatment
Male Sprague Dawley (SD) rats (weighting 200–250 g,
purchased from Vital River, Beijing, China) were randomly assigned
to 6 groups (n=24 per group): i) Control group, ii) STZ group, iii)
STZ+control siRNA group, iv) STZ+NgR siRNA group, v) STZ+CNTF group
and vi) STZ+NgR siRNA+CNTF group. The rats in the STZ group were
treated intraperitoneally with a single injection of 65 mg/kg STZ
(Solarbio, Beijing, China) dissolved in 0.01 M cold sodium citrate
buffer and treated with saline. The control siRNA or NgR siRNA (5
µl and 3 µM) (GenePharma, Shanghai, China) were injected into the
vitreous of diabetic rats in the STZ+control siRNA group or STZ+NgR
siRNA group, respectively (This injection was repeated after 6
weeks). CNTF (1 µg) (Sino Biological, Beijing, China) were
administrated into the vitreous of diabetic rats in the STZ+CNTF
group once a week for 12 weeks. The rats in the STZ+NgR siRNA+CNTF
group received NgR siRNA and CNTF after induction of diabetes. The
rats in the control group received sodium citrate buffer and
saline. Blood glucose level (mM) was detected 3 days post-STZ
administration for diabetes induction confirmation (>16.7
mmol/l). All the rats were sacrificed after 12 weeks. The retinal
tissues were excised and fixed in 4% paraformaldehyde for further
analyses. The experiments were performed in accordance with the
Guide for the Care and Use of Laboratory Animals and approved by
Animal Care and Use Committee of Guangxi Medical University
(Nanning, China).
H&E staining
The paraffin-embedded retinal tissues were sectioned
into 5-µm slices. The sections were deparaffinized with xylene and
graded ethanol (100, 95, 85 and 75% ethanol). The sections were
stained with hematoxylin for 5 min and incubated with 1%
hydrochloric acid alcohol for 3 sec, followed by staining with
eosin for 3 min. Subsequently, the sections were dehydrated with
graded ethanol (75, 85, 95, and 100% ethanol) and xylene. The
sections were mounted with neutral gum and captured by OLYMPUS DP73
microscope (Olympus Corp., Tokyo, Japan).
TUNEL
The paraffin-embedded sections (5-µm thick) of
retinal tissues were deparaffinized, treated with 0.1% Triton X-100
and blocked with 3% H2O2. After washing with
PBS, the sections were incubated with the mixture of terminal
deoxynucleotidyl transferase (TdT) and fluorescein-labeled dUTP and
then treated with converter-POD according to the manufacturer's
protocol (Roche, Basel, Switzerland). DAB was added and the
sections were counterstained with hematoxylin. Finally, the
sections were dehydrated and photographed under OLYMPUS DP73
microscope.
Western blotting
The retinal tissues were lysed on the ice, followed
by protein extraction. The protein concentration was determined by
BCA assay kit (Wanleibio, Shenyang, China). The proteins were
subjected to SDS-PAGE (8–13%) and transferred to PVDF membranes.
Then, membranes were blocked with non-fat milk dissolved in
Tween-20/TBS buffer. Subsequently, the membranes were incubated
with primary antibodies against NgR (1:10,000; ab184556; Abcam,
Cambridge, UK), RhoA (1:200; sc-197; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), Rock1 (1:200; sc-374388; Santa Cruz
Biotechnology, Inc.), Bcl-2 (1:400; BA0412; Boster, Wuhan, China),
Bax (1:400; BA0315; Boster), Caspase-3 (1:1000; ab2302; Abcam),
F-actin (1:500; ab205; Abcam), growth-associated protein-43
(GAP-43) (1:200; sc-33705; Santa Cruz Biotechnology, Inc.) at 4°C
overnight and then with secondary antibody (1:5,000; horseradish
peroxidase-labeled IgG; WLA023 and WLA024; Wanleibio) for 45 min at
37°C. The protein bands were visualized via ECL reagent and
analyzed by Gel-Pro Analyzer (Media Cybernetics, Rockville, MD,
USA).
Immunohistochemistry
Retinal tissue sections (5-µm thick) were
deparaffinized with xylene and gradient ethanol (100, 95, 85 and
75% ethanol). After antigen retrieval, the sections were treated
with H2O2 and blocked with goat serum. Then,
sections were incubated with primary antibodies against F-actin
(1:200; bs-1571R; Bioss, Beijing, China) and GAP-43 (1:200;
D163002; Sangon Biotech, Shanghai, China) at 4°C overnight and
biotin-labeled secondary antibody (1:200; A0277; Beyotime Institute
of Biotechnology, Haimen, China) at 37°C for 30 min, followed by
incubation with HRP-labeled avidin (A0303; Beyotime) at 37°C for 30
min. The sections were visualized with DAB, counterstained with
hematoxylin and imaged under a microscope (Olympus Corp.).
Quantitative PCR (qPCR)
Total RNAs were extracted from the retinal tissues
using RNApure Total RNA Extraction kit (BioTeke, Beijing, China)
and reverse-transcribed to cDNAs using M-MLV Reverse Transcriptase
(BioTeke). The volume of obtained cDNAs was 20 µl. qPCR analysis
was carried out following the reaction conditions on Real-Time PCR
system (BIONEER, Daejeon, Korea): 95°C for 10 min, followed by 40
cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 30 sec. The
primers used were as follows: NgR forward,
5′-GTCCCTTCCAGACCAATCAGC-3′ and reverse,
5′-GCCATTGCCTGGTGGAGTGT-3′; RhoA forward,
5′-TCGGAATGATGAGCACACAA-3′ and reverse, 5′-GCTTCACAAGATGAGGCAC-3′;
Rock1 forward, 5′-GTGATGGCTATTATGGACG-3′ and reverse,
5′-AGGAAGGCACAAATGAGAT-3′; F-actin forward,
5′-GAAGAGAAAGCAGCAGTGTTA-3′ and reverse, 5′-GGAGCCAGAGGGTGGTTAT-3′;
GAP-43 forward, 5′-AGGGAGATGGCTCTGCTAC-3′ and reverse,
5′-CACATCGGCTTGTTTAGGC-3′; GAPDH forward,
5′-CGGCAAGTTCAACGGCACAG-3′ and reverse,
5′-CGCCAGTAGACTCCACGACAT-3′. The primers were synthesized by Sangon
Biotech. Gene expression was normalized to GAPDH and calculated
using the 2−ΔΔCq formula.
Statistical analysis
Data are expressed as the mean ± SD and analyzed by
Student's t-test for the comparison of every two groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of NgR siRNA and CNTF injection
on the number of cells in GCL in diabetic rats
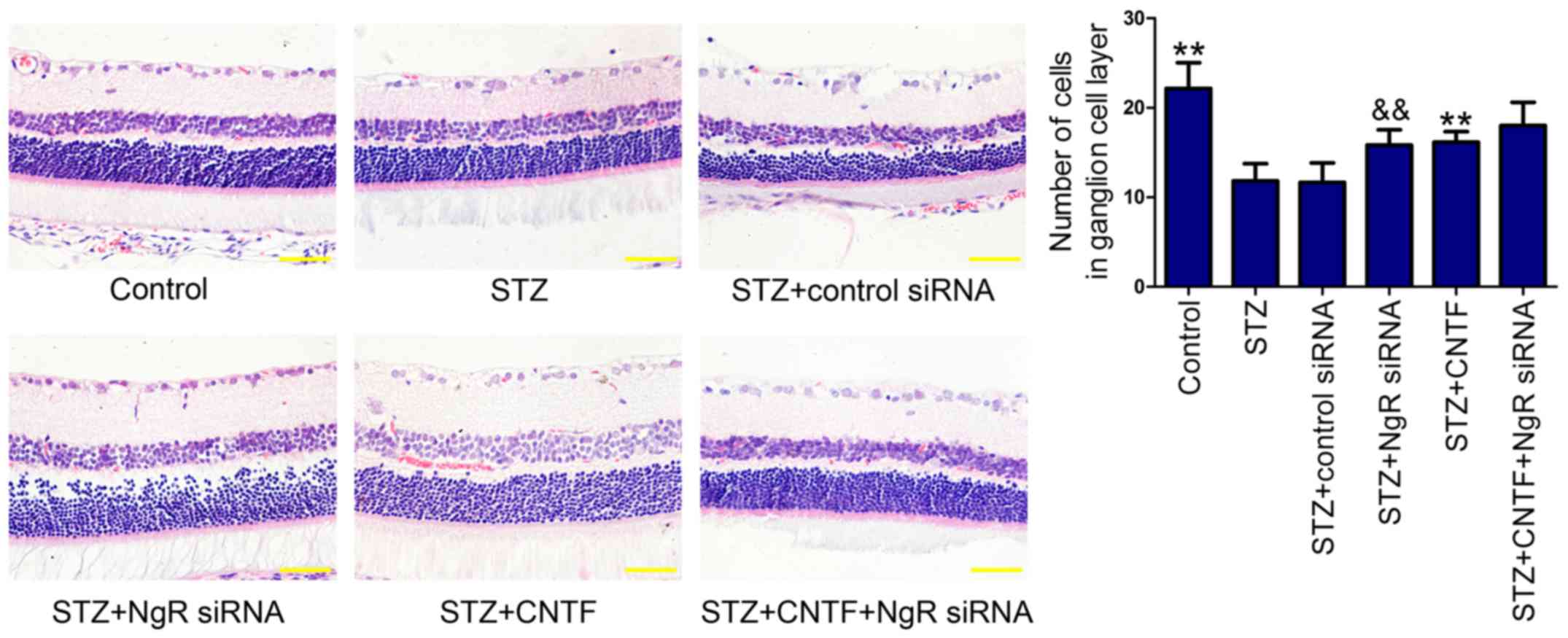
Histopathological examination showed that the cell
numbers in GCL (Fig. 1) were
decreased in diabetic rats compared with those in non-diabetic
control rats. We found that NgR siRNA or CNTF injection alone
increased the number of cells in GCL. Similarly, combination
treatment further enhanced the ability of single treatment,
although the difference was not statistically significant.
Effect of NgR siRNA and CNTF injection
on cell apoptosis in the retinal tissues of diabetic rats
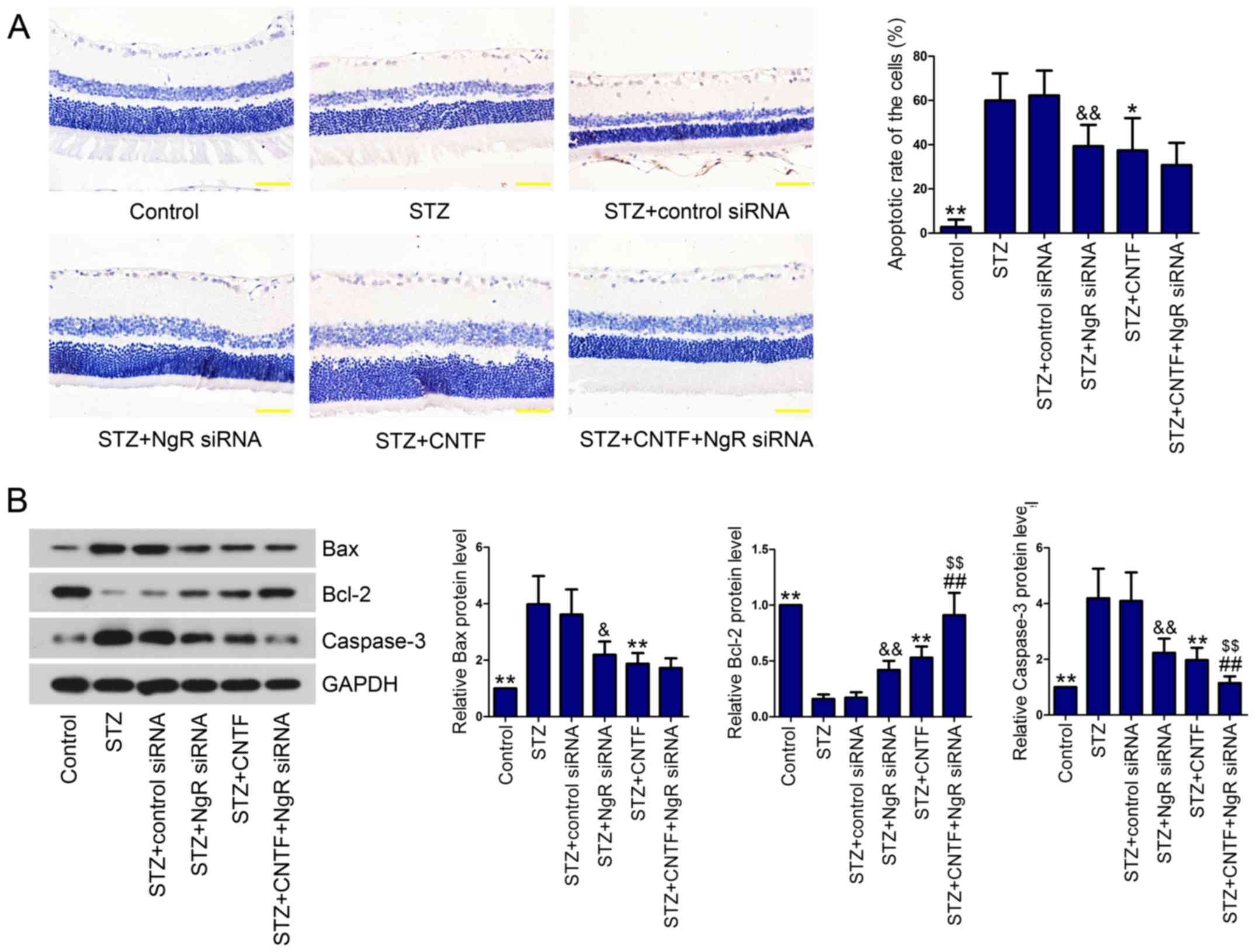
Death of RGCs is one of the earliest events in DR
occurrence and progression (18).
Therefore, we evaluated cell apoptosis using TUNEL assay. As
expected, diabetic rats presented higher apoptosis rate of the
cells in the retina than control rats (Fig. 2A). However, NgR knockdown or CNTF
incubation significantly protected RGCs against apoptosis in
diabetic rats. Combined injection of NgR siRNA and CNTF decreased
the apoptotic rate of RGCs in diabetic rats in comparison with the
STZ+NgR siRNA group or STZ+CNTF group, however, no significant
difference was observed between the single treatment group and the
combination group. Next, we measured the levels of
apoptosis-related proteins (Bax, Bcl-2 and Caspase-3) in the
retinal tissues using western blotting. We demonstrated that Bax
and Caspase-3 levels were significantly upregulated in the retinal
tissues of diabetic rats compared with control rats, whereas
anti-apoptotic protein Bcl-2 was downregulated (Fig. 2B). NgR siRNA alone and CNTF
injection alone notably reversed diabetes-induced cell apoptosis by
decreasing Bax and Caspase-3 levels and increasing Bcl-2 level.
Compared with the STZ+NgR siRNA group or STZ+CNTF group, NgR siRNA
injection combined with CNTF further enhanced the anti-apoptotic
effect of NgR knockdown and CNTF.
Effect of NgR siRNA and CNTF injection
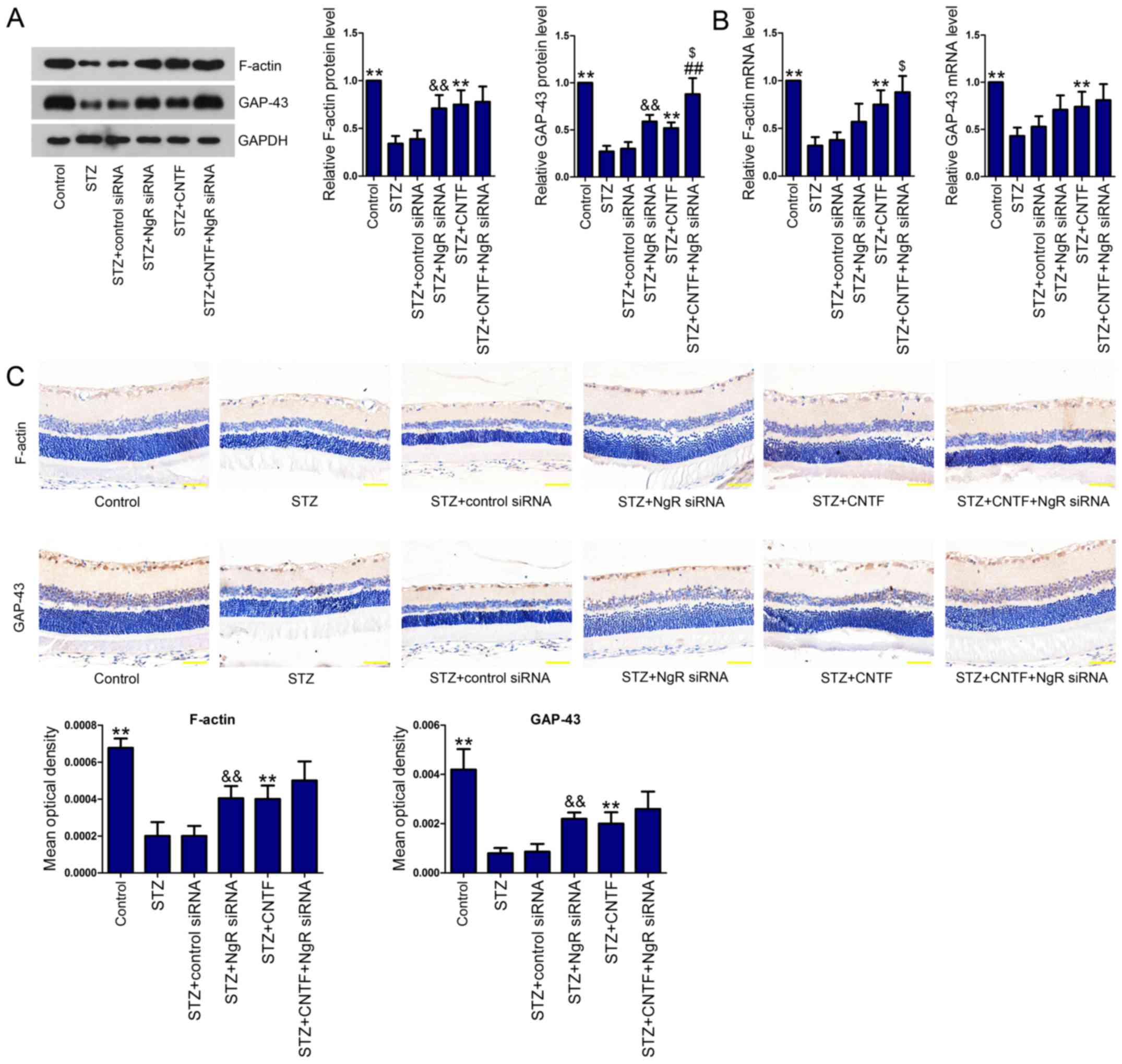
on growth cone cytoskeleton and axonal regeneration
To evaluate the effect of NgR knockdown and CNTF
treatment, either alone or in combination, on growth cone
cytoskeleton and axonal regeneration, we measured the expression
levels of F-actin and GAP-43 in the retinal tissues. We observed
the downregulated expression of F-actin and GAP-43 at both protein
(Fig. 3A) and mRNA levels
(Fig. 3B) in the retinal tissues
of diabetic rats. However, NgR siRNA or CNTF incubation
significantly elevated the levels of these two proteins. Moreover,
the combination of NgR siRNA and CNTF further increased F-actin and
GAP-43 levels. We then performed IHC analysis (Fig. 3C) and verified the results obtained
from western blotting and qPCR.
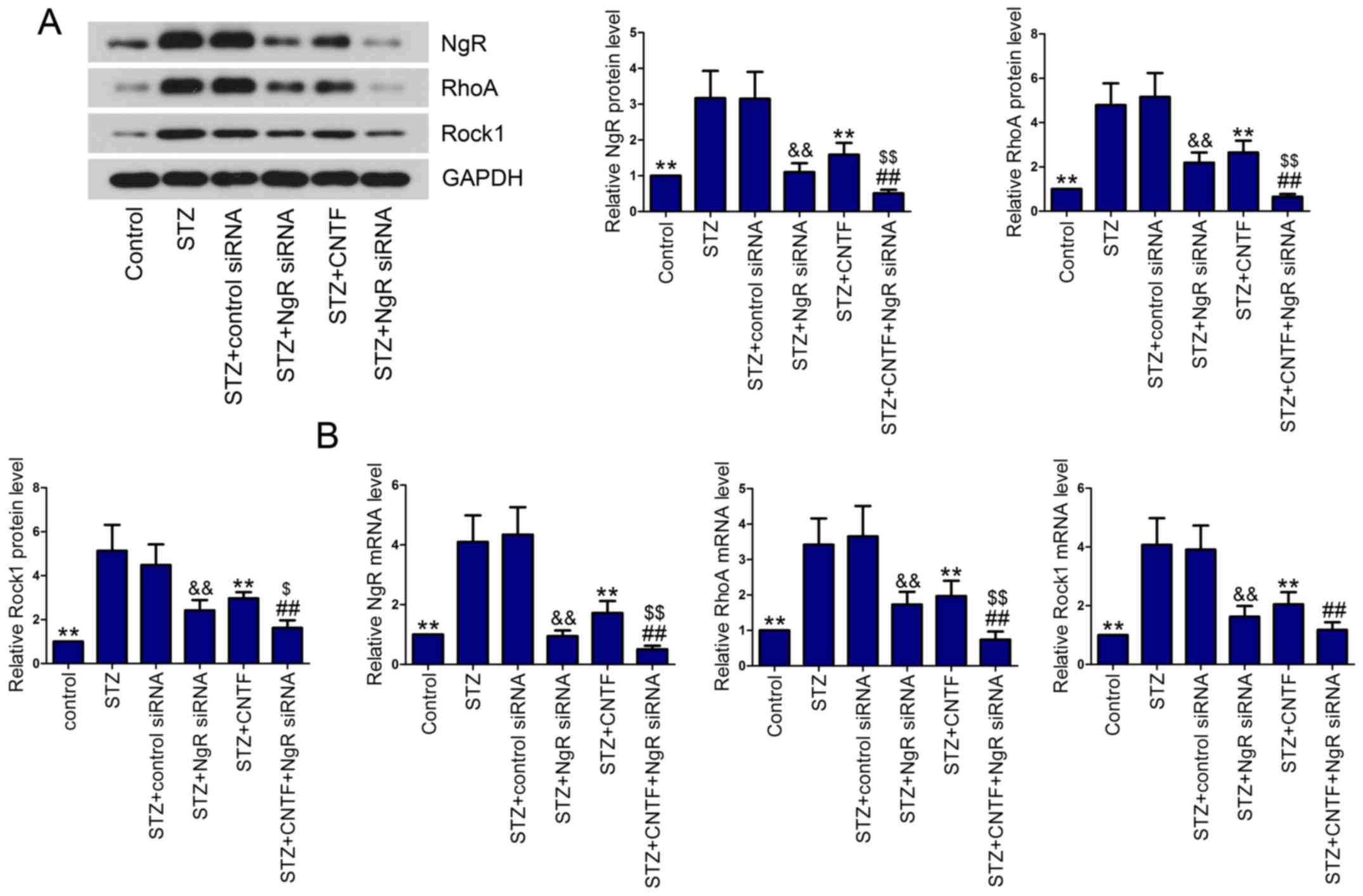
Effect of NgR siRNA and CNTF injection
on NgR/RhoA/Rock1 signaling pathway
We then examined the effect of NgR knockdown and
CNTF treatment, either alone or in combination, on the activation
of NgR/RhoA/Rock1 signaling pathway in diabetic rats. The results
showed that NgR, RhoA and Rock1 levels were elevated in diabetic
rats in comparison with control rats, as demonstrated by western
blotting (Fig. 4A) and qPCR
(Fig. 4B). After injection with
NgR siRNA or CNTF, the diabetic rats expressed lower levels of NgR,
RhoA and Rock1 in the retinal tissues than the corresponding model
rats. As expected, the expression of NgR, RhoA and Rock1 were
significantly inhibited by the combined administration of NgR siRNA
and CNTF compared with the single treatment group.
Discussion
Understanding the pathological mechanisms of DR and
targeting them are essential to DR prevention. In our study, we
aimed to investigate the effect of NgR/RhoA/Rock1 signaling pathway
inhibition and/or CNTF treatment on retinal ganglion cells in
diabetic rats.
RGCs are the main part of nervous tissues in the
retina and they relay visual signals to the brain (19). Apoptosis of RGCs plays an essential
role in onset and progression of DR (20). Tong et al have demonstrated
that NgR silencing inhibits C6 cell proliferation and promotes cell
apoptosis (21). Wang et al
have reported that CNTF protects neuroblastoma SH-SY5Y cells from
cytotoxicity and apoptosis induced by amyloid beta peptide
(Aβ1–42) (22).
However, the effect of NgR knockdown or CNTF treatment and their
synergistic effect on RGC-5 cells needed to be clarified. In our
study, a rat model of DM was induced by a single injection of STZ
(65 mg/kg). After administration of siRNAs or CNTF, the retinal
tissues were excised and subjected to H&E staining to measure
RGC count. The results showed that NgR siRNA or CNTF injection, as
well as combined therapy, prevented diabetes-induced the loss of
ganglion cells in GCL. B-cell-lymphoma 2 (Bcl-2) protein family
members regulate cell apoptosis and this family consists of
proapoptotic proteins (Bax and Bad) and antiapoptotic (Bcl-2 and
Bcl-xL) (23). Bax is a soluble
protein that usually exists in the cytosol. Bax translocates to
mitochondrial membranes, enhances membrane permeabilization,
results in cytochrome c release and thus induces apoptosis
(24). However, Bcl-2 can inhibit
the translocation of Bax in the cells undergo apoptosis (25). Apoptosis is the result of caspase
cascades. Caspase-3 is the downstream executor of these cascades
(26). The results showed that,
consistent with previous study (27), diabetes induced RGC apoptosis in
the retinal tissues, along with the increases of Bax and Caspase-3
and the decrease of Bcl-2. NgR siRNA or CNTF injection, as well as
combined therapy, protected cells from diabetes-induced apoptosis
by downregulating Bax and Caspase-3 and upregulating Bcl-2. Our
results suggested that both single agent treatment and combination
treatment alleviated diabetes-induced apoptosis by regulating the
expression of apoptosis-related proteins in diabetic rats.
F-actin is a main element of cytoskeleton and
functions in cell shape, motility and division (28). F-actin dynamics plays an important
role in regulating axon extension (29). GAP-43, which belongs to the
calmodulin-binding protein family, is a protein kinase C (PKC)
substrate and is highly expressed in adult RGCs (30,31).
Increased expression of GAP-43 correlates with cytoskeletal
organization in nerve ending, neurite outgrowth and axon
regeneration (32). Liu et
al have found that CNTF attenuates gp120-induced inhibition of
neurite outgrowth by elevating GAP-43 expression in dorsal root
ganglion (DRG) explants (33). Our
results showed that diabetes resulted in the loss of F-actin and
GAP-43 in the retina. NgR siRNA, CNTF or combination injection
prevented diabetes-induced loss of F-actin and GAP-43. Our results
suggested that NgR siRNA, CNTF or combination injection may promote
growth cone cytoskeleton and axonal regeneration by regulating
F-actin and GAP-43.
RhoA, a small GTPase protein, is associated with the
contractility of actin cytoskeleton (13). Moreover, RhoA has been demonstrated
to be involved in cell proliferation, apoptosis and metastasis
(34). Rock1 is a downstream
target of RhoA (35). Peng et
al have reported that RhoA/ROCK1 pathway is inhibited by
simvastatin in the treatment of early-stage of diabetic nephropathy
(DN) (36). Additionally,
RhoA/ROCK1 pathway has been shown to be involved in the pathology
of DR via triggering microvascular endothelial dysfunction
(15). In our study, we
demonstrated that NgR siRNA, CNTF or combination injection
inhibited the activation of NgR/RhoA/Rock1 signaling pathway
induced by diabetes.
In conclusion, the combination of NgR knockdown and
CNTF treatment exhibits obvious advantages over either therapy
alone. The combined therapy may be a potential therapeutic strategy
for the treatment of DR.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81571383).
References
|
1
|
Hossain M Kawser, Dayem A Abdal, Han J,
Saha S Kumar, Yang GM, Choi HY and Cho SG: Recent advances in
disease modeling and drug discovery for diabetes mellitus using
induced pluripotent stem cells. Int J Mol Sci. 17:2562016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roifman I, Ghugre N, Zia MI, Farkouh ME,
Zavodni A, Wright GA and Connelly KA: Diabetes is an independent
predictor of right ventricular dysfunction post ST-elevation
myocardial infarction. Cardiovasc Diabetol. 15:342016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amutha A and Mohan V: Diabetes
complications in childhood and adolescent onset type 2 diabetes-a
review. J Diabetes Complications. 30:951–957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van den Born JC, Hammes HP, Greffrath W,
van Goor H and Hillebrands JL: DFG GRK International Research
Training Group 1874 Diabetic Microvascular Complications
(DIAMICOM): Gasotransmitters in vascular complications of diabetes.
Diabetes. 65:331–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kur J, Burian MA and Newman EA: Light
adaptation does not prevent early retinal abnormalities in diabetic
rats. Sci Rep. 6:210752016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu S, Lin YU and Liu X: Protective
effects of SIRT1 in patients with proliferative diabetic
retinopathy via the inhibition of IL-17 expression. Exp Ther Med.
11:257–262. 2016.PubMed/NCBI
|
|
7
|
He K, Lv W, Zhang Q, Wang Y, Tao L and Liu
D: Gene set enrichment analysis of pathways and transcription
factors associated with diabetic retinopathy using a microarray
dataset. Int J Mol Med. 36:103–112. 2015.PubMed/NCBI
|
|
8
|
Nashine S, Liu Y, Kim BJ, Clark AF and
Pang IH: Role of C/EBP homologous protein in retinal ganglion cell
death after ischemia/reperfusion injury. Invest Ophthalmol Vis Sci.
56:221–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Chan A, Qin Y, Kwong JM, Caprioli
J, Levinson R, Chen L and Gordon LK: Programmed cell death-1 is
expressed in large retinal ganglion cells and is upregulated after
optic nerve crush. Exp Eye Res. 140:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seidel JL, Faideau M, Aiba I, Pannasch U,
Escartin C, Rouach N, Bonvento G and Shuttleworth CW: Ciliary
neurotrophic factor (CNTF) activation of astrocytes decreases
spreading depolarization susceptibility and increases potassium
clearance. Glia. 63:91–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mathews MK, Guo Y, Langenberg P and
Bernstein SL: Ciliary neurotrophic factor (CNTF)-mediated ganglion
cell survival in a rodent model of non-arteritic anterior ischaemic
optic neuropathy (NAION). Br J Ophthalmol. 99:133–137. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aizu Y, Katayama H, Takahama S, Hu J,
Nakagawa H and Oyanagi K: Topical instillation of ciliary
neurotrophic factor inhibits retinal degeneration in
streptozotocin-induced diabetic rats. Neuroreport. 14:2067–2071.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Wang D and Guo D: MiR-124 promote
neurogenic transdifferentiation of adipose derived mesenchymal
stromal cells partly through RhoA/ROCK1, but Not ROCK2 signaling
pathway. PLoS One. 11:e01466462016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rigassi L, Bozzolo F Barchiesi,
Lucchinetti E, Zaugg M, Fingerle J, Rosselli M, Imthurn B, Jackson
EK and Dubey RK: 2-Methoxyestradiol blocks the RhoA/ROCK1 pathway
in human aortic smooth muscle cells. Am J Physiol Endocrinol Metab.
309:E995–E1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu QY, Chen W, Lu L, Zheng Z and Xu X:
Involvement of RhoA/ROCK1 signaling pathway in
hyperglycemia-induced microvascular endothelial dysfunction in
diabetic retinopathy. Int J Clin Exp Pathol. 7:7268–7277.
2014.PubMed/NCBI
|
|
16
|
Cao Y, Dong YX, Xu J, Chu GL, Yang ZH and
Liu YM: Spatiotemporal expression of Nogo-66 receptor after focal
cerebral ischemia. Neural Regen Res. 11:132–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Zuo Z, Liu W, Wang Z, Hou Y, Fu Y
and Han Y: Upregulation of Nogo receptor expression induces
apoptosis of retinal ganglion cells in diabetic rats. Neural Regen
Res. 9:815–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin PM, Roon P, van Ells TK, Ganapathy
V and Smith SB: Death of retinal neurons in streptozotocin-induced
diabetic mice. Invest Ophthalmol Vis Sci. 45:3330–3336. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galan A, Dergham P, Escoll P, de-la-Hera
A, D'Onofrio PM, Magharious MM, Koeberle PD, Frade JM and Saragovi
HU: Neuronal injury external to the retina rapidly activates
retinal glia, followed by elevation of markers for cell cycle
re-entry and death in retinal ganglion cells. PLoS One.
9:e1013492014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang DD, Zhu HZ, Li SW, Yang JM, Xiao Y,
Kang QR, Li CY, Zhao Y, Zeng Y, Li Y, et al: Crude saponins of
Panax notoginseng have neuroprotective effects to inhibit
palmitate-triggered endoplasmic reticulum stress-associated
apoptosis and loss of postsynaptic proteins in staurosporine
differentiated RGC-5 retinal ganglion cells. J Agric Food Chem.
64:1528–1539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong S, Xiong N and Shen J: RNA
interference suppression of Nogo-66 receptor prevents
Nogo-66-mediated inhibition of invasion and adhesion and
simultaneously increases cell apoptosis in C6 cells. Oncol Rep.
30:2171–2178. 2013.PubMed/NCBI
|
|
22
|
Wang K, Xie M, Zhu L, Zhu X, Zhang K and
Zhou F: Ciliary neurotrophic factor protects SH-SY5Y neuroblastoma
cells against Aβ1-42-induced neurotoxicity via activating the
JAK2/STAT3 axis. Folia Neuropathol. 53:226–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng CH, Cheng YP, Chang IL, Chen HY, Wu
CC and Hsieh CP: Dodecyl gallate induces apoptosis by upregulating
the caspase-dependent apoptotic pathway and inhibiting the
expression of anti-apoptotic Bcl-2 family proteins in human
osteosarcoma cells. Mol Med Rep. 13:1495–1500. 2016.PubMed/NCBI
|
|
24
|
Jia HM, Li Q, Zhou C, Yu M, Yang Y, Zhang
HW, Ding G, Shang H and Zou ZM: Chronic unpredictive mild stress
leads to altered hepatic metabolic profile and gene expression. Sci
Rep. 6:234412016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Xiong Z, Wang J, Zhang S, Lei L,
Yang L and Zhang Z: Glucagonlike peptide1 protects cardiomyocytes
from advanced oxidation protein productinduced apoptosis via the
PI3K/Akt/Bad signaling pathway. Mol Med Rep. 13:1593–1601.
2016.PubMed/NCBI
|
|
26
|
Zhang X, Jiang D, Jiang W, Zhao M and Gan
J: Role of TLR4-Mediated PI3K/AKT/GSK-3β signaling pathway in
apoptosis of rat hepatocytes. Biomed Res Int. 2015:6313262015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Foureaux G, Nogueira BS, Coutinho DC,
Raizada MK, Nogueira JC and Ferreira AJ: Activation of endogenous
angiotensin converting enzyme 2 prevents early injuries induced by
hyperglycemia in rat retina. Braz J Med Biol Res. 48:1109–1114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamazaki S, Yamamoto K, de Lanerolle P and
Harata M: Nuclear F-actin enhances the transcriptional activity of
β-catenin by increasing its nuclear localization and binding to
chromatin. Histochem Cell Biol. 145:389–399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Atkinson-Leadbeater K, Hehr CL, Johnston
J, Bertolesi G and McFarlane S: EGCG stabilizes growth cone
filopodia and impairs retinal ganglion cell axon guidance. Dev Dyn.
245:667–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu Q, Liu Z, Wang C, Nie L, He Y, Zhang
Y, Liu X and Su G: Lentiviral-mediated growth-associated protein-43
modification of bone marrow mesenchymal stem cells improves
traumatic optic neuropathy in rats. Mol Med Rep. 12:5691–5700.
2015.PubMed/NCBI
|
|
31
|
Ivanov D, Dvoriantchikova G, Nathanson L,
McKinnon SJ and Shestopalov VI: Microarray analysis of gene
expression in adult retinal ganglion cells. FEBS Lett. 580:331–335.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benowitz LI and Routtenberg A: GAP-43: An
intrinsic determinant of neuronal development and plasticity.
Trends Neurosci. 20:84–91. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Liu G and Bi Y: CNTF regulates
neurite outgrowth and neuronal migration through JAK2/STAT3 and
PI3K/Akt signaling pathways of DRG explants with gp120-induced
neurotoxicity in vitro. Neurosci Lett. 569:110–115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Y, Chen JB, Yang B, Shen H, Liang JJ
and Luo Q: RhoA/ROCK pathway regulates hypoxia-induced myocardial
cell apoptosis. Asian Pac J Trop Med. 7:884–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang J, Xie M, Shah VR, Schneider MD,
Entman ML, Wei L and Schwartz RJ: Activation of Rho-associated
coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays
an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci
USA. 103:14495–14500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng H, Luo P, Li Y, Wang C, Liu X, Ye Z,
Li C and Lou T: Simvastatin alleviates hyperpermeability of
glomerular endothelial cells in early-stage diabetic nephropathy by
inhibition of RhoA/ROCK1. PLoS One. 8:e800092013. View Article : Google Scholar : PubMed/NCBI
|