Introduction
Gastric cancer (GC) is a highly lethal malignancy
that ranks amongst the top ten most frequent cancers, and is a
leading cause of cancer-related mortality. In Asian countries,
including China, Japan and Korea, gastric cancer accounts for
approximately 60% of new cancer cases (1). GC is a complex and molecularly
heterogeneous disease, which is associated with lifestyle,
age-related, environmental and genetic factors. Smoking, alcohol
use and improper food intake are leading risk factors for GC
morbidity and mortality worldwide (2). Obesity and being overweight and are
also important causes of GC, especially in high-income countries
(3) Furthermore, H. pylori
infection is an established environmental risk factor for GC
(4). Several canonical oncogenic
signalling pathways, including the tumor protein p53,
wnt/β-catenin, nuclear factor (NF)-κB and
phosphatidylinositol-3-kinase (PI3K)/Akt pathways, are associated
with gastric carcinogenesis (5).
Caspases are cysteine-dependent, aspartate-specific proteases
associated with apoptosis, a process that is regulated by several
oncogenic pathways. Caspase-9 is a key effector caspase in the
intrinsic apoptosis pathway. Release of cytochrome c
(Cyt-c), a protein found in the mitochondrial inner
membrane, activates procaspase-9 to caspase-9, which in turn
converts procaspase-3 to cleaved caspase-3. These closely linked
events lead to cell apoptosis; thus, caspase-9 is considered a
therapeutic target in cancer treatment (6).
Caffeine is a methylxanthine derivative found in
coffee, cacao and tea, and is the most widely consumed psychoactive
substance in the world. Caffeine intake induces several normal
physiologic effects, including nervous and musculoskeletal system
stimulation, bronchial and vascular smooth muscle relaxation,
diuresis and intestinal motility stimulation (7–12).
Caffeine also exerts various pharmacological effects. Several
epidemiological and experimental studies have reported that
caffeine may have potential as an anticancer agent due to its
ability to suppress cell proliferation and induce apoptosis in
multiple organs, including the oesophagus, breast, liver and brain,
via several oncogenic pathways, including phosphatase and tensin
homolog (PTEN), PI3K/Akt, p53 and mammalian target of rapamycin
(mTOR) pathways (13–21). However, the caspases have received
limited attention. A few studies have demonstrated that caspase-9
and −3 may serve important roles in caffeine-induced cancer cell
apoptosis (22,23); however, the molecular signalling
pathways associated with these processes remain to be elucidated.
Furthermore, previous research has indicated that caffeine may have
sustained effects following withdrawal (20), the underlying mechanism of which
remains unknown.
In the present study, two gastric cell lines were
selected for examining the anticancer effect of caffeine on gastric
cancer; MGC-803 is classified as a poorly differentiated gastric
cancer cell line and SGC-7901 is classified as a well
differentiated gastric cancer cell line. Poorer cellular
differentiation is associated with a higher degree of malignancy,
therefore the present study aimed to investigate the anticancer
effects of caffeine on two types of malignant cancer cells.
Furthermore, the effects of treatment with graded concentrations of
caffeine were investigated on cell growth, apoptosis and the
apoptosis-related proteins B cell lymphoma 2 (Bcl-2), Cyt-c,
caspase-9 and caspase-3. Finally, the post-withdrawal sustained
effects of caffeine on cell viability, apoptosis and caspase-9/−3
signalling pathway activation were examined.
Materials and methods
Cell culture
The human gastric cancer cell lines MGC-803 (poorly
differentiated gastric cancer cell line), SGC-7901
(well-differentiated gastric cancer cell line) and GES-1 (human
gastric mucosa epithelial cells) were obtained from the Cell
Resource Center of Shanghai Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences (Shanghai, China). Cells were
cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(both from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). MGC-803 and SGC-7901 cells seeded at a density of
1×105 cells in a 100-mm plate were used in the cell
viability and colony formation assays, and for RNA and protein
extraction. MGC-803 and SGC-7901 cells at a density of
3×104 cells/well in a 6-well plate were used for flow
cytometry. MGC-803 and SGC-7901 cells at a density of
5×103 cells/well in a 96-well plate were used in the
Cell Counting Kit-8 (CCK-8) assay. GES-1 cells were seeded at a
density of 2×105 cells/well in a 100-mm plate and were
used as untreated normal control cells. All cells were cultured at
37°C in a 5% CO2 atmosphere.
Chemicals and experimental set-up
Caffeine (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was dissolved in RPMI-1640 and then added to the culture
medium. The cell growth inhibitor 5-fluorouracil (5-FU;
Sigma-Aldrich; Merck KGaA), which was used to evaluate the
inhibitory effects of caffeine on cell proliferation, was dissolved
in the culture medium. Cells treated with caffeine were considered
the experimental group, cells treated with 5-FU were considered the
control evaluation group and cells treated with equal amounts of
RPMI-1640 plus 5% fetal bovine serum were considered the normal
control group. Two inhibitors, Z-LEHD-FMK (a specific inhibitor of
caspase-9) and Z-DEVD-FMK (a specific inhibitor of caspase-3), were
obtained from BioVision, Inc. (Milpitas, CA, USA).
Cell viability and colony-forming unit
assays
Cell viability was analysed with CCK-8 reagent
(CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
MGC-803 and SGC-7901 cells were cultured at a density of
3×104 cells/well in 96-well plates, and incubated at
37°C overnight. GC cells were subsequently treated with 4 different
caffeine concentrations (0, 0.5, 1 or 2 mM) or 0.5 mg/ml 5-FU for
24 h. CCK-8 reagent (10 µl) was added to each well, and the plates
were incubated at 37°C for a further 3 h. In the
caffeine-withdrawal groups, GC cells were washed twice in PBS and
cultured in caffeine-free serum for 24 h following caffeine
treatment, and cell viability was evaluated via CCK-8 assay. The
absorbance of the coloured formazan product, produced by
mitochondrial dehydrogenases in metabolically active cells, was
measured at 450 nm and the optical density of each well was
recorded. A colony-forming unit assay was conducted to analyse cell
clusters. Cells were cultured at a density of 100 cells/plate with
caffeine (1 and 2 mM) for 2 weeks, and cells incubated in
caffeine-free medium were established as the control group. Viable
cells were subsequently stained with crystal violet and the results
were photographed. Each experiment was performed ≥3 times.
Flow cytometry analysis
MGC-803 and SGC-7901 cells were harvested following
caffeine treatment. Double staining with fluorescein isothiocyanate
(FITC)-Annexin V and propidium iodide (PI) was performed using an
FITC-Annexin V Apoptosis Detection kit (BD Biosciences, Franklin
Lakes, NJ, USA), according to the manufacturer's protocol. GC cells
were analysed using a FACScan flow cytometer equipped with
CellQuest Pro software version 5.1 (both from BD Biosciences). The
cells were assessed as viable, dead, early apoptotic and late
apoptotic, and the relative amounts of early apoptotic cells were
compared with those of the control groups. Cells used for cell
cycle analysis were stained with PI using a Cycletest®
Plus DNA reagent kit (BD Biosciences), according to the
manufacturer's protocol, and analysed using a FACScan flow
cytometer. The percentages of cells in G0-G1, S and G2-M phase were
counted and compared with those of the control groups.
Western blot analysis
Following treatment with various concentrations of
caffeine, GC cells were lysed using radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
on ice for 40 min to extract the proteins. The protein
concentration of the supernatants was determined via Bradford
assay. Lysates (20 µg/lane) were separated via 10% SDS-PAGE,
transferred to 0.22 µm polyvinylidene fluoride membranes
(Sigma-Aldrich; Merck KGaA), and incubated at 4°C overnight with
the following primary antibodies at a working dilution of 1:1,000,
all purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA): Anti-tubulin (cat no. 2148), anti-cyclin D1 (cat no. 2978),
anti-cyclin dependent kinase (CDK)4 (cat no. 12790), anti-p21 (cat
no. 2947), anti-Bcl-2 (cat no. 4223), anti-Bcl-2-associated death
promoter (Bad; cat no. 9239), anti-Bcl-2-associated X protein (Bax;
cat no. 5023), anti-Cyt-c (cat no. 11940), anti-caspase-9
(cat no. 9502), anti-cleaved caspase-9 (cat no. 20750),
anti-caspase-3 (cat no. 9665), and anti-cleaved caspase-3 (cat no.
9664). Membranes were then incubated with mouse anti-rabbit
secondary antibody (cat no. 3678; 1:1,000; Cell Signaling
Technology, Inc.) at 4°C for 2.5 h. Autoradiograms were
semi-quantified via densitometry using ImageJ software version
1.46r (National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the GC cell lines using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. cDNA was synthesized with
a PrimeScript™ RT reagent kit, and qPCR was performed with a
SYBR® Premix Ex Taq™ kit (both from Takara Biotechnology
Co., Ltd., Dalian, China) on a 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling
conditions were as follows: Initial 1 step at 95°C for 10 min,
followed by 40 cycles at 95°C for 15 sec and at 60°C for 1 min. PCR
primers (Sangon Biotech, Shanghai, China) for β-catenin, PTEN, AKT,
mTOR, P53 and vascular endothelial growth factor A (VEGF-A) are
listed in Table I. GAPDH served as
an internal control, and fold changes were calculated using the
2−ΔΔCq method (24).
 | Table I.Primers used for quantitative
polymerase chain reaction. |
Table I.
Primers used for quantitative
polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| β-catenin |
ATTGAAGCTGAGGGAGCCAC | TCCTGGCCATATCC
ACCAGA |
| PTEN |
ATGACAGCCATCATCAAAGAG |
GACTTTTGTAATTTGTGTATGCTGA |
| AKT |
GTATGCTGGCAGAGTAGGAGAAC |
CAGGTAACATCAGAGACAGACACA |
| mTOR |
AGGCCGCATTGTCTCTATCAA |
GCAGTAAATGCAGGTAGTCATCCA |
| P53 |
ACGACGGTGACACGCTTCCCTG |
CGCTAGGATCTGACTGCGGCTC |
| VEGF-A |
CCCACTGAGGAGTCCAACAT |
GATGATTCTGCCCTCCTCCTT |
| GAPDH |
ATCATCCCTGCCTCTACTGG |
GTCAGGTCCACCACTGACAC |
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The statistical significance of the differences between
groups was assessed using one-way analysis of variance followed by
a post hoc Student-Newman-Keuls test for multiple comparisons.
Statistical analysis was performed using SPSS software version 16.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference. GraphPad Prism
software version 6 (GraphPad Software, Inc., La Jolla, CA, USA) was
used to calculate sensitivity and specificity.
Results
Caffeine inhibits GC cell growth and
reduces viability in a concentration-dependent manner
MGC-803 and SGC-7901 cells were treated with
caffeine at concentrations of 0.5, 1, 2, 4 and 8 mM. Untreated GC
cells served as controls, and the 5-FU-treated group was used to
evaluate the effects of caffeine. Cell viability was measured by
CCK-8 assay. Cell viability was reduced by caffeine in both MGC-803
and SGC-7901 cells (Fig. 1A). The
survival ratio significantly decreased to <50% following 24 h
caffeine treatment at concentrations of 4 and 8 mM. Caffeine
treatment at high concentrations (>2 mM) resulted in marked
toxicity in normal gastric mucosa cells (data not shown),
therefore, a concentration range of 0–2 mM was selected for
subsequent experiments. To investigate the effects of caffeine on
cell viability, GC cells were treated with 2 mM caffeine and
harvested at 12, 24, 36, 48, 60 and 72 h, and cell viability was
assessed via CCK-8 assay. The results indicated that the numbers of
viable cells decreased as the concentration of caffeine increased
(Fig. 1B and C). Furthermore,
colony-forming unit assay indicated that colony forming efficiency
was lower following caffeine treatment at concentrations of 1 and 2
mM (Fig. 1D). These results
indicate that caffeine treatment significantly inhibits MGC-803 and
SGC-7901 cell viability and growth. Furthermore, this inhibitory
effect may be concentration-dependent.
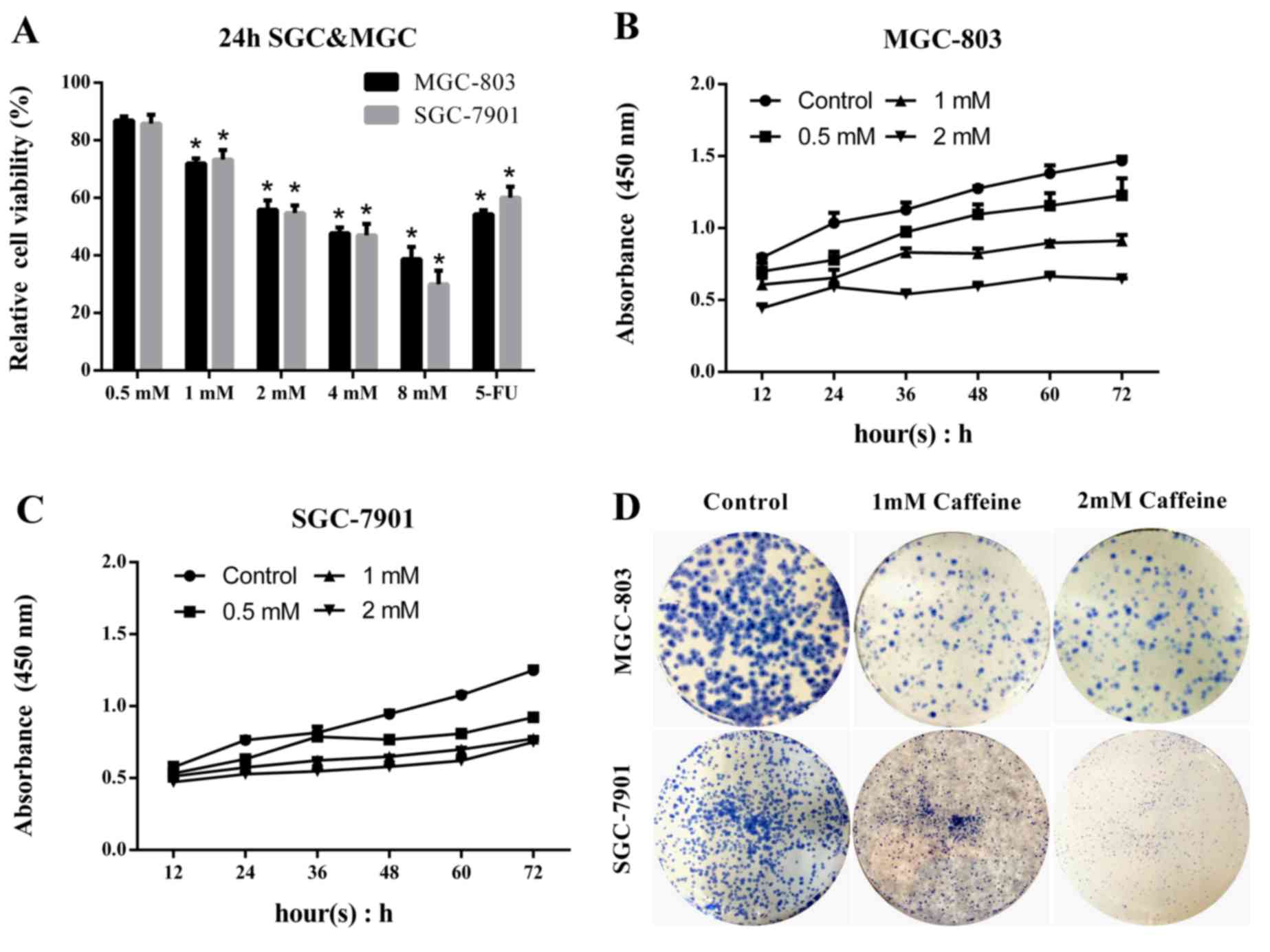 | Figure 1.Caffeine inhibits GC cell growth in a
concentration-dependent manner. GC cells were treated with the
indicated caffeine concentrations and harvested at the indicated
times. In addition, GC cells were treated with 5 mg/ml 5-FU in the
evaluation groups. Total cell survival was estimated by cell
counts. (A) Relative cell viability was compared with that of the
untreated control group. (B) MGC and (C) SGC cells were incubated
with caffeine in 96-well plates and harvested at 12, 24, 36, 48, 60
and 72 h. Cell Counting Kit-8 reagent was added to each well at a
dilution of 10 µl/100 µl, and the optical density at 450 nm was
detected and recorded. (D) Cells were cultured with caffeine at a
density of 100 cells/plate and were stained with crystal violet.
Data are expressed as the mean ± standard error of the mean of at
least three independent experiments. *P<0.01 vs. control. GC,
gastric cancer; SGC, SGC-7901 cells; MGC, MGC-803 cells; OD,
optical density; 5-FU, 5-fluorouracil. |
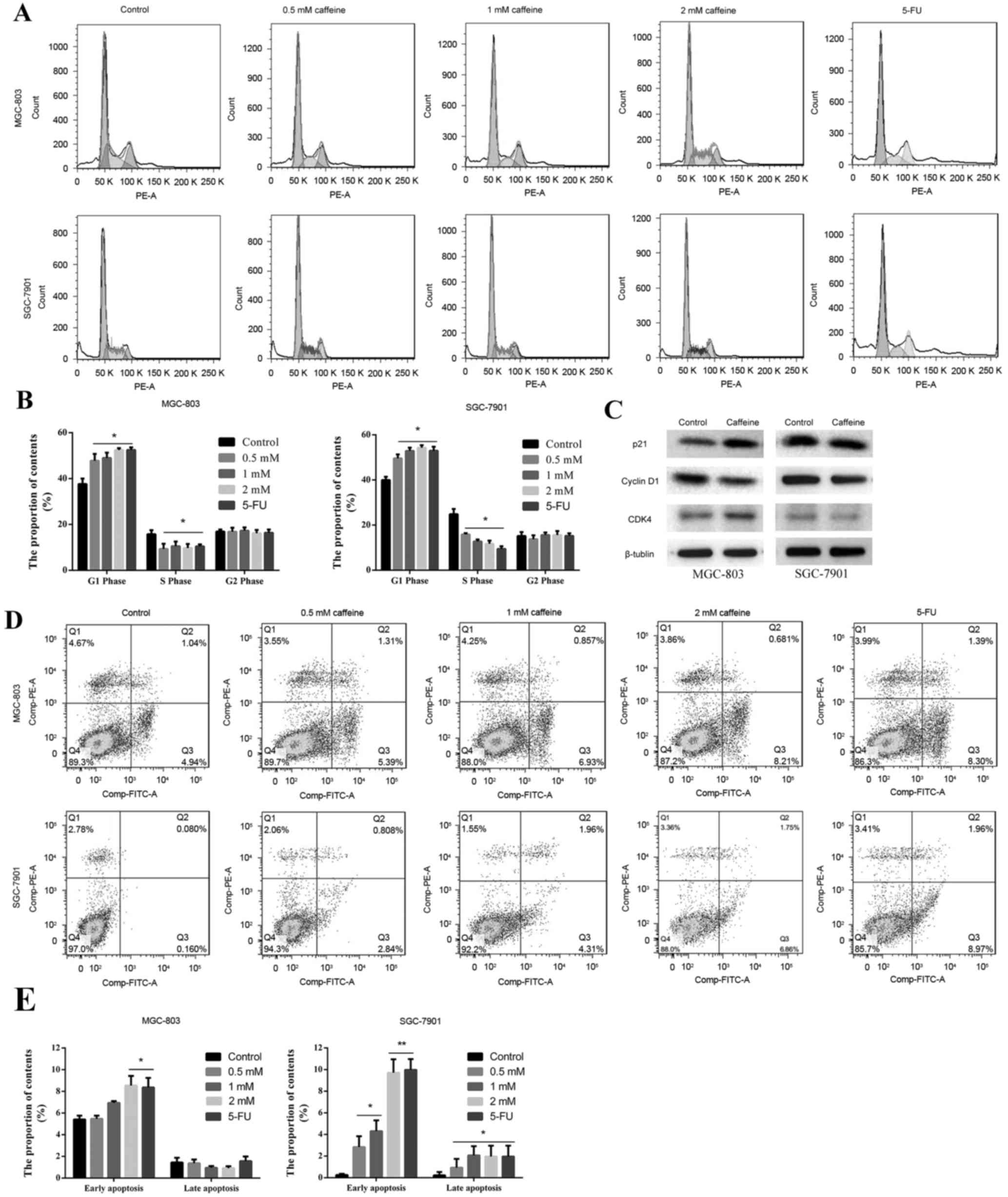
Caffeine inhibits cell cycle
progression and promotes GC cell apoptosis
To investigate whether caffeine is able to inhibit
cell cycle progression and promote GC cell apoptosis, flow
cytometry analysis was conducted. Caffeine treatment significantly
inhibited cell cycle progression beyond the G0/G1 phase,
furthermore, the % of cells in the S phase was lower compared with
the control group (Fig. 2A and B).
There were no differences in the % of cells in each cell cycle
phase amongst the caffeine-treated groups (Fig. 2A and B). Cell cycle-related protein
expression was determined by western blot analysis. The results
indicated that p21 levels were upregulated in MGC-803 cells and
cyclin D1 levels were downregulated in both GC cell lines,
following 2 mM caffeine treatment (Fig. 2C). However, no differences in CDK4
protein expression were noted (Fig.
2C). Furthermore, apoptosis was promoted by caffeine treatment
for 24 h, which was particularly marked in SGC-7901 cells. Early
apoptosis demonstrated the greatest increase, and this increase
appeared to be concentration-dependent (Fig. 2D and E). 5-FU served as a positive
control; flow cytometry indicated that the results for the 5-FU
groups were similar to the results for the 2 mM caffeine group.
These results indicated that caffeine treatment results in cell
cycle arrest and promotes MGC-803 and SGC-7901 cell apoptosis,
providing supporting evidence for the potential cytotoxic effects
of caffeine.
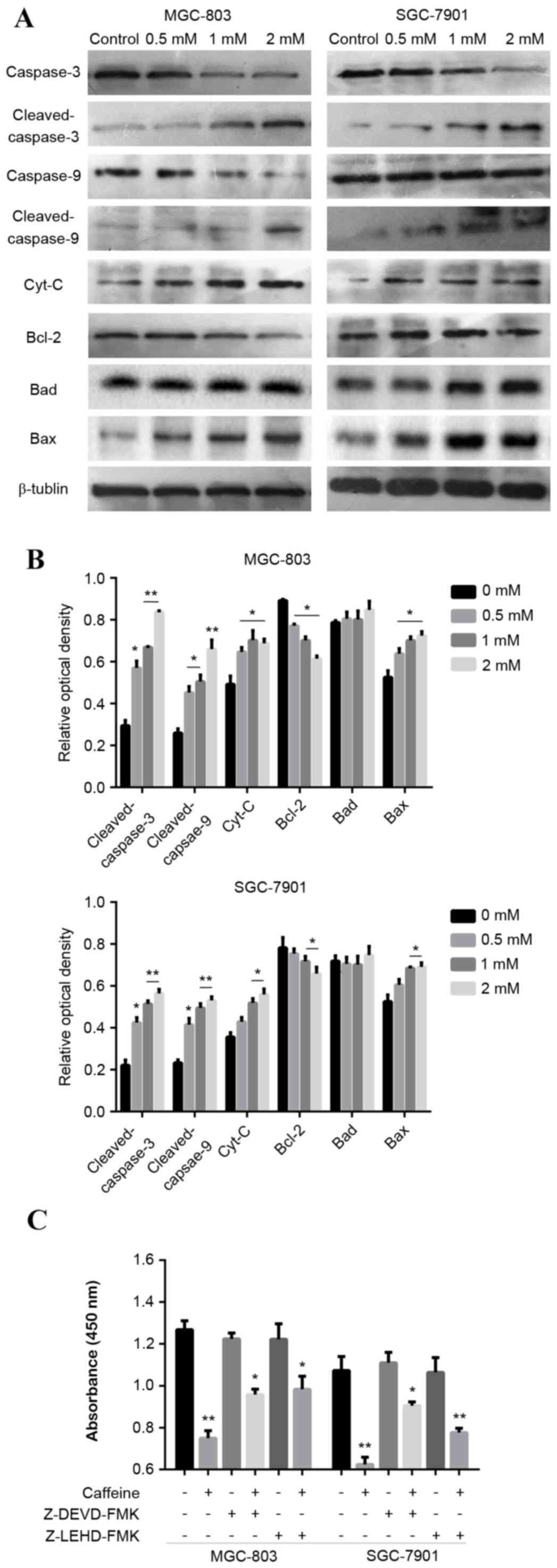
Caffeine induces GC cell apoptosis via
the caspase-9/−3 pathway
The mechanism underlying the ability of caffeine to
inhibit GC cell growth and induce apoptosis was investigated. It
was hypothesised that apoptosis was driven by the caspase pathway
in vitro. MGC-803 and SGC-7901 cells were treated with
graded concentrations of caffeine for 24 h in the presence of
serum. The expression of key apoptosis-related proteins (Bcl-2,
Bad, Bax, Cyt-c, caspase-9 and caspase-3) was assessed by
western blot analysis. Caffeine treatment increased the activation
of caspase-9 and −3 (cleaved caspase-9 and −3), and increased the
expression levels of Cyt-c in MGC-803 and SGC-7901 cells,
compared with the control group (Fig.
3A and B). Furthermore, Bcl-2 expression was reduced and Bax
expression was increased with the indicated concentrations of
caffeine, however, there were no significant differences in Bad
expression (Fig. 3A and B). GC
cells treated with caffeine at a concentration of 2 mM exhibited
the greatest differences in the expression of these proteins,
compared with control cells and lower caffeine concentrations
(Fig. 3A and B). These results
indicate that caffeine treatment markedly influenced the expression
of key proteins associated with apoptosis. Specific inhibitors of
caspase-9 (5 µM Z-LEHD-FMK) and caspase-3 (5 µM Z-DEVD-FMK) were
used to investigate the association between the caspase-9/−3
pathway activation and the caffeine effect. The pro-apoptotic
effects of caffeine were reversed by caspase-9 and −3 inhibition
(Fig. 3C). These data indicate
that caffeine induces cell apoptosis via activation of the
caspase-9/−3 pathway.
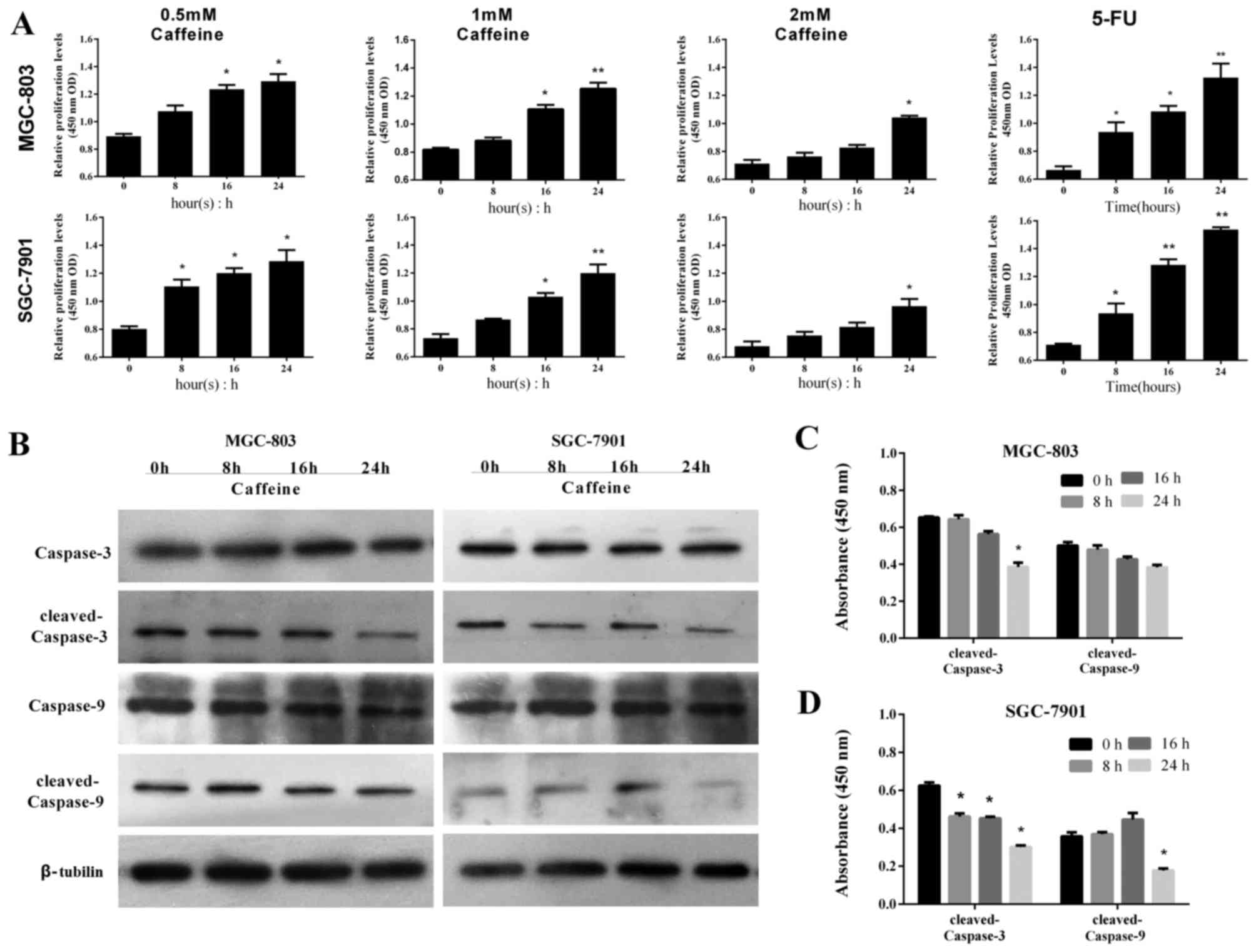
Caffeine exerts sustained effects on
cell apoptosis after withdrawal
The effects of caffeine withdrawal were investigated
to determine if the antiproliferative effects were sustained
following drug withdrawal. GC-803 and SGC-7901 cells were incubated
in caffeine-free medium for 24 h following caffeine treatment, and
the post-withdrawal effects of caffeine on apoptosis-related
pathways were investigated. GC cells were harvested at 0, 8, 16 and
24 h following caffeine withdrawal, and their viability was
assessed by CCK-8 assay. The numbers of viable cells increased over
time in all groups, however this was overall lower in the
caffeine-treated groups compared with the 5-FU treated group
(Fig. 4A). Notably, the number of
viable cells was markedly reduced in the 2 mM groups compared with
the other groups (Fig. 4A). Based
on these results, MGC-803 and SGC-7901 cells were harvested from
the 2 mM groups at 0, 8, 16 and 24 h following caffeine withdrawal,
and the levels of caspase-9 and −3 were determined by western blot
analysis. There were no significant differences in the levels of
cleaved caspase-9 and −3 in MGC-803 cells amongst these four time
points, with the exception of the level of cleaved caspase-3 at 24
h (Fig. 4B and C). Similarly,
there were no significant differences in the levels of cleaved
caspase-9 in SGC-7901 cells between the four time points, with the
exception of the levels of cleaved caspase-9 at 24 h. The levels of
cleaved caspase-3 were decreased following caffeine withdrawal,
with the greatest decrease in protein expression occurring at 24 h
(Fig. 4B and D). These results
suggest that the effects of caffeine on apoptosis and the
corresponding increases in caspase-9/−3 levels are sustained
following caffeine withdrawal.
The mRNA expression levels of several genes upstream
of the caspases were measured via RT-qPCR, including β-catenin,
PTEN, AKT, mTOR, p53 and VEGF-A. The results indicated that the
relative mRNA expression levels of these genes were influenced by
caffeine treatment. In MGC-803 and SGC-7901 cells, PTEN and p53
expression was upregulated and VEGF-A expression was downregulated
following caffeine treatment for 24 h, compared with control
(Fig. 5A). Furthermore, mTOR
expression was downregulated in SGC-7901 cells (Fig. 5A). Relative mRNA expression
quantification was also conducted in the caffeine-withdrawal
groups. The relative expression levels of β-catenin, PTEN, AKT,
mTOR, p53 and VEGF-A varied (against value ‘1’ represening the
endogenous control-normalized expression) over time. The results
were compared across the treatment conditions; expression levels
were observed to change with time following caffeine withdrawal.
Only a few groups demonstrated stable expression levels (against
value ‘1’) with time. PTEN and p53 (in both cell lines) remained
above value ‘1’ prior to 16 h, and mTOR (in SGC-7901) remained
below value ‘1’ prior to 8 h (Fig.
5B). These results suggest that caffeine treatment may
influence the mRNA expression levels of several signalling
molecules, which are vital components of cancer signalling
pathways.
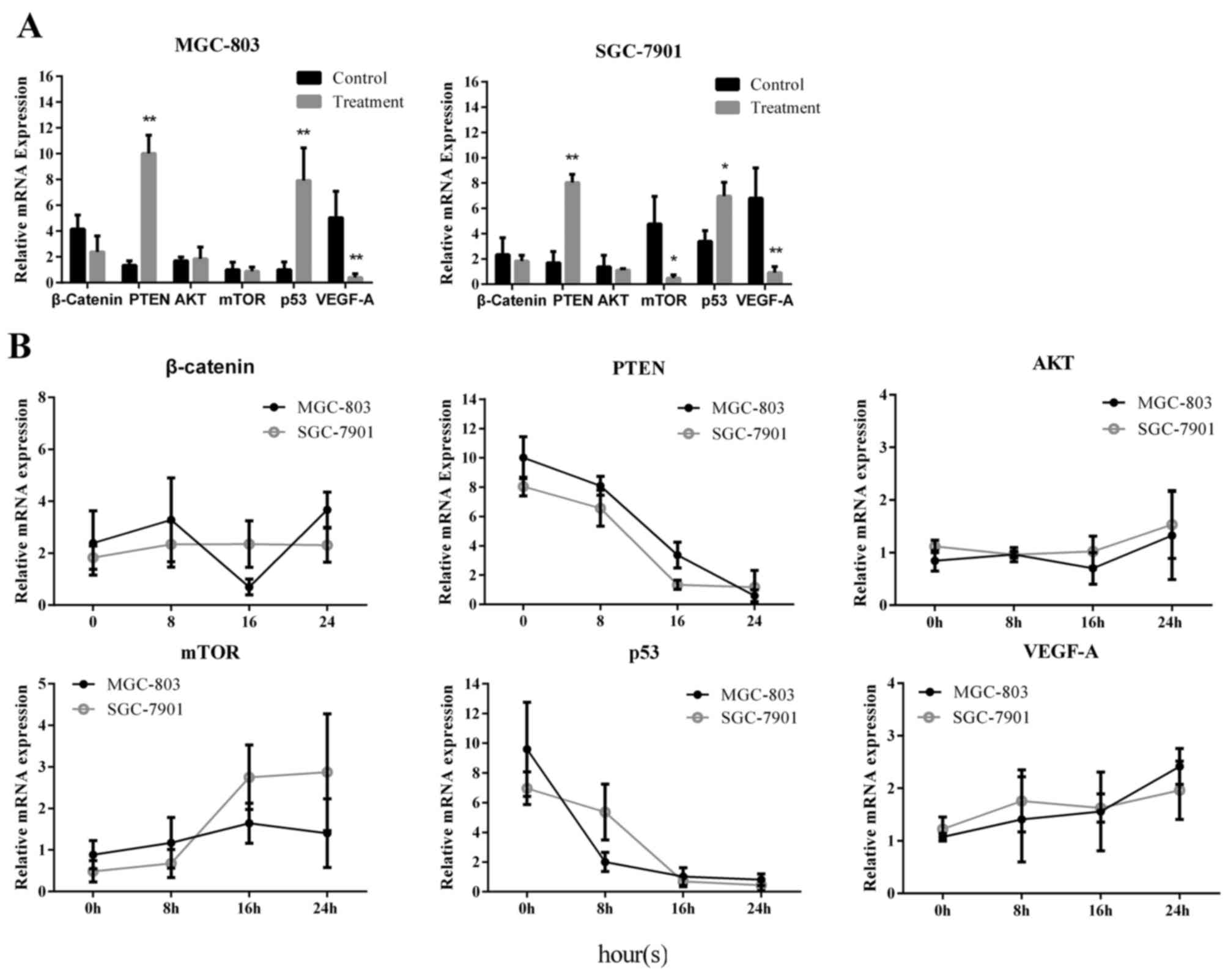 | Figure 5.Expression of cancer-related factors
was altered by caffeine treatment. MGC-803 and SGC-7901 cells were
obtained via enzymatic digestion following caffeine treatment for
24 h. Human gastric mucosa epithelial cells (GES-1) were used as an
internal control. Relative mRNA expression levels were detected by
reverse transcription-quantitative polymerase chain reaction, and
the data were analysed using the 2−ΔΔCq method. (A)
Relative mRNA expression levels of β-catenin, PTEN, AKT, mTOR, p53
and VEGF-A in MGC-803 and SGC-7901 cells were measured after 24 h
of caffeine treatment. GES-1 cells were used the untreated normal
control. (B) Relative mRNA expression levels of β-catenin, PTEN,
AKT, mTOR, p53 and VEGF-A in MGC-803 and SGC-7901 cells were
measured and recorded at the indicated time points after caffeine
withdrawal. Data are representative of at least three independent
experiments. *P<0.01 and **P<0.01 vs. control. PTEN,
phosphatase and tensin homolog; mTOR, mammalian target of
rapamycin; VEGF-A, vascular endothelial growth factor-A; AKT,
protein kinase B. |
Discussion
The worldwide popularity of caffeine is attributable
to its stimulatory effects on the nervous system, as it causes
enhanced alertness, mental hyperactivity, arousal, wakefulness,
enhanced cognitive abilities, enhanced pain tolerance and
improvements in workplace efficiency. As the structure of caffeine
is similar to that of adenosine, competitive binding of caffeine to
adenosine receptors impacts upon the nervous system, which
facilitates the release of various neurotransmitters at the
synaptic cleft, including acetylcholine, norepinephrine, dopamine
and others (25–28). In addition to these transient
effects, several reports have emphasized that caffeine exerts many
long-term beneficial effects, as it may protect against the
development of age-related dementia and cognitive decline (29,30),
as well as development of Alzheimer's disease and Parkinson's
disease (31–34), suggesting that caffeine has
neuro-protective effects. In addition, caffeine increases cAMP
levels by inhibiting phosphodiesterase activity (35). Higher amounts of caffeine than are
ordinarily possible are required by athletes to improve their
physical performance and induce weight loss (36). Notably, an inverse association
between coffee consumption and type 2 diabetes (T2DM) risk among
Dutch individuals was first reported in 2002 (37). To date, numerous meta-analyses and
prospective studies have demonstrated that regular coffee intake
may reduce the risk of T2DM (38–42).
These results demonstrate that coffee intake reduces T2DM risk via
regulation of plasma glucose levels, insulin-glucose homeostasis,
islet cell inflammation and pro-inflammatory mediators (43). It has also been reported that
caffeine exerts anticancer effects resulting in suppression of
carcinogenesis, proliferation, invasion and metastasis (44). Furthermore, several retrospective
and prospective studies indicate that long-term caffeinated coffee
intake reduces the morbidity and mortality associated with cancer
(45,46).
The present study demonstrated that caffeine
treatment reduced cell growth and induced GC cell apoptosis
(MGC-803 and SGC-7901) in vitro. MGC-803 cells are poorly
differentiated GC cells, whereas SGC-7901 cells are
well-differentiated GC cells. These cell lines were used to assess
the putative anticancer effects of caffeine in cancer cells of
various differentiation stages. The present findings indicated that
caffeine effectively inhibited the proliferation and induced
apoptosis in both cell lines. Caffeine has previously demonstrated
antiproliferative effects in a concentration-dependent
(particularly at high concentrations ≥1 mM) and time-dependent
manner (47–50). In the present study, cell cycle
arrest was induced by caffeine in a concentration- and
time-independent manner, demonstrating that the effects of caffeine
on MGC-803 and SGC-7901 cells are consistent with previous studies.
The protein expression levels of cell cycle-associated proteins
were detected. The present results revealed that p21 levels were
upregulated in MGC-803 cells, whereas cyclin D1 levels were
downregulated in both GC cell lines, thus supporting the cell
cycle-arresting effects of caffeine. The effects of caffeine on
caspase-9 and −3 were investigated, as these are key
apoptosis-related factors in cancer cells. The results indicated
that caffeine induces apoptosis via activation of the caspase-9/−3
pathway.
Notably, the effects of caffeine on cell growth
inhibition and apoptosis in MGC-803 and SGC-7901 cells appeared to
be sustained following caffeine withdrawal for a short period of
time (0–16 h). A previous study indicated that caffeine mediates
sustained growth inhibition of breast cancer-related myofibroblasts
(20), and the present study
provides additional evidence of the sustained effects of caffeine
in cancer cells. Cell cycle progression is controlled by cyclin-CDK
complexes, which include p21 CDK-interacting protein, kinase
inhibitory proteins (KIP: p27KIP1 and
p57KIP2), and CDK4 inhibitors (INK4:
p16INK4a, p15INK4b, p18INK4c,
p19INK4d). The G1 phase of the cell cycle is
the only period during which cells may respond to extracellular
cues, and progression depends on the balance between proliferative
and antiproliferative signals. A few studies have demonstrated that
caffeine affects cyclin D1 and INK4 expression and causes cell
cycle arrest in cancer cells. In the present study, caffeine
induced G0/G1 phase arrest in MGC-803 and
SGC-7901 cells, which supported the apoptotic effects of this drug.
Therefore, it was surmised that caffeine may induce sustained cell
apoptosis in GC cells by mediating cyclin-CDK complexes. However,
the mechanisms underlying the sustained effects of caffeine remain
unknown. Further studies should investigate cell cycle arrest
mechanisms in the sustained anticancer effects of caffeine and
other drugs. Furthermore, key genes and pathways must be confirmed,
and these may serve as novel targets in cancer prevention and
chemotherapy.
In addition to its effects on the cell cycle,
caffeine may exert sustained effects on gene and protein
expression. The present study measured Bcl-2 and Cyt-c
expression to determine the relationship between the caspase-9/−3
pathway and the antiproliferative effects of caffeine. Caspase-9/−3
are downstream proteins of numerous molecular pathways. It was
speculated that caffeine may induce sustained GC cell apoptosis via
various upstream mediators, the results supported this hypothesis;
caffeine treatment appeared to exert sustained effects on several
cancer-related signalling pathways. Furthermore, it was revealed
that the mRNA expression levels of PTEN and p53 were sensitive to
caffeine treatment. During the early period (8 h) following
caffeine withdrawal, the mRNA levels of these proteins remained
relatively high, compared with those of the internal controls.
Notably, psychotropic substances, including caffeine, may cause
withdrawal symptoms, and these are considered a type of
psychological syndrome (51).
Similar effects were noted in the present study, which were
attributed to changes in mRNA expression, as although the mRNA
levels of PTEN were downregulated following caffeine withdrawal,
these remained higher than value ‘1’, thus suggesting that mRNA
expression and translation was sustained (Fig. 5B). However, further studies are
required to fully elucidate the effects of caffeine and explore the
molecular mechanisms that are involved.
MicroRNAs (miRNAs), which are members of the
non-coding RNA family, are widely regarded as key modulators of
anticancer processes (52,53). miRNAs also serve as downstream
transcriptional targets of several genes in response to internal or
external stimuli. Numerous studies have established that
chemotherapeutic agents, such as 5-FU (54) and decitabine (55), can profoundly alter miRNA gene
expression patterns. Notably, some non-chemotherapeutic drugs, such
as caffeine and non-steroidal anti-inflammatory drugs, have been
reported to alter miRNA gene expression (56,57).
miRNAs have been associated with sustained effects on cancer cells;
mutant p53-273H promotes sustained epidermal growth factor
(EGF)-induced extracellular signal-regulated kinase 1/2 activation
via the miR-27a/EGF receptor axis, thereby facilitating cell
proliferation and tumourigenesis (58). As caffeine appears to alter miRNA
expression, miRNAs may serve as effectors of the sustained
anticancer effects of caffeine. Further research is required on
this hypothesis; the potential association between caffeine and
miRNA expression may be of therapeutic interest in the clinical
management of cancer.
The anticancer effects of caffeine have been
reported in various human cells and tissues, and similar results
were noted in MGC-803 and SGC-7901 GC cells in the present study.
Furthermore, caffeine was observed to induce GC cell apoptosis. The
present study provided further experimental evidence for the
effects of caffeine observed in several previous studies, however,
the mechanisms underlying the sustained effects of caffeine on
cancer cells require further investigation. Optimization of drug
doses is a critical factor in balancing drug safety and efficacy.
In the present study, 1–2 mM caffeine was effective in suppressing
caspase-9 expression and inducing GC cell apoptosis in
vitro. Although the efficacy of caffeine increased with
increasing concentrations of the drug, doses >2 mM were toxic to
normal gastric cells (data not shown). Previously, dose-escalation
studies have been performed in order to establish potentially
suitable oral administration doses for caffeine in clinical
treatments or animal experiments (59,60).
The relationship between everyday caffeine intake and the risk of
cancer is currently undefined (61,62).
Notably, there is potential for caffeine to be used in the adjuvant
setting during chemotherapy application (63,64).
The differences in efficacy between oral or local delivery also
need to be investigated. Furthermore, the mild analgesic effect of
caffeine may be useful for post-operative recovery, however,
further investigations into the potential clinical benefits of
caffeine are required.
In conclusion, the present study investigated the
role of caffeine in GC in targeting the apoptosis-related
caspase-9/−3 pathway. These results may serve as supporting
evidence for further studies regarding the anticancer effects of
caffeine on gastrointestinal cancers.
Acknowledgements
The present study was supported by a research grant
from the Jiangsu Province Department of Health (grant no.
Z2010023).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danaei G, Vander Hoorn S, Lopez AD, Murray
CJ, Ezzati M, et al: Comparative Risk Assessment collaborating
group (Cancers): Causes of cancer in the world: Comparative risk
assessment of nine behavioural and environmental risk factors.
Lancet. 366:1784–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olefson S and Moss SF: Obesity and related
risk factors in gastric cardia adenocarcinoma. Gastric Cancer.
18:23–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi J, Qu YP and Hou P: Pathogenetic
mechanisms in gastric cancer. World J Gastroenterol. 20:13804–1319.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim B, Srivastava SK and Kim SH: Caspase-9
as a therapeutic target for treating cancer. Expert Opin Ther
Targets. 19:113–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldstein A, Kaizer S and Warren R:
Psychotropic effects of caffeine in man. II. Alertness, psychomotor
coordination, and mood. J Pharmacol Exp Ther. 150:146–151.
1965.PubMed/NCBI
|
|
8
|
Arnaud MJ: The pharmacology of caffeine.
Prog Drug Res. 31:273–313. 1987.PubMed/NCBI
|
|
9
|
Dunwiddie TV: Interactions between the
effects of adenosine and calcium on synaptic responses in rat
hippocampus in vitro. J Physiol. 350:545–559. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Keefe JH, Bhatti SK, Patil HR,
DiNicolantonio JJ, Lucan SC and Lavie CJ: Effects of habitual
coffee consumption on cardiometabolic disease, cardiovascular
health, and all-cause mortality. J Am Coll Cardiol. 62:1043–1051.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilson DF: Effects of caffeine on
neuromuscular transmission in the rat. Am J Physiol. 225:862–865.
1973.PubMed/NCBI
|
|
12
|
Rousseau E, Ladine J, Liu QY and Meissner
G: Activation of the Ca2+ release channel of skeletal
muscle sarcoplasmic reticulum by caffeine and related compounds.
Arch Biochem Biophys. 267:75–86. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bessler H, Salman H, Bergman M and
Djaldetti M: Caffeine alters cytokine secretion by PBMC induced by
colon cancer cells. Cancer Invest. 30:87–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bode AM and Dong Z: The enigmatic effects
of caffeine in cell cycle and cancer. Cancer Lett. 247:26–39. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conney AH, Zhou S, Lee MJ, Xie JG, Yang
CS, Lou YR and Lu Y: Stimulatory effect of oral administration of
tea, coffee or caffeine on UVB-induced apoptosis in the epidermis
of SKH-1 mice. Toxicol Appl Pharmacol. 224:209–213. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ku BM, Lee YK, Jeong JY, Ryu J, Choi J,
Kim JS, Cho YW, Roh GS, Kim HJ, Cho GJ, et al: Caffeine inhibits
cell proliferation and regulates PKA/GSK3beta pathways in U87MG
human glioma cells. Mol Cells. 31:275–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miwa S, Sugimoto N, Shirai T, Hayashi K,
Nishida H, Ohnari I, Takeuchi A, Yachie A and Tsuchiya H: Caffeine
activates tumor suppressor PTEN in sarcoma cells. Int J Oncol.
39:465–472. 2011.PubMed/NCBI
|
|
18
|
Hashimoto T, He Z, Ma WY, Schmid PC, Bode
AM, Yang CS and Dong Z: Caffeine inhibits cell proliferation by
G0/G1 phase arrest in JB6 cells. Cancer Res. 64:3344–3349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okano J, Nagahara T, Matsumoto K and
Murawaki Y: Caffeine inhibits the proliferation of liver cancer
cells and activates the MEK/ERK/EGFR signalling pathway. Basic Clin
Pharmacol Toxicol. 102:543–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Ansari MM and Aboussekhra A: Caffeine
mediates sustained inactivation of breast cancer-associated
myofibroblasts via up-regulation of tumor suppressor genes. PLoS
One. 9:e909072014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosendahl AH, Perks CM, Zeng L, Markkula
A, Simonsson M, Rose C, Ingvar C, Holly JM and Jernström H:
Caffeine and caffeic acid inhibit growth and modify estrogen
receptor and insulin-like growth factor I receptor levels in human
breast cancer. Clin Cancer Res. 21:1877–1887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu JD, Song LJ, Yan DJ, Feng YY, Zang YG
and Yang Y: Caffeine inhibits the growth of glioblastomas through
activating the caspase-3 signaling pathway in vitro. Eur Rev Med
Pharmacol Sci. 19:3080–3038. 2015.PubMed/NCBI
|
|
23
|
Matsuoka S, Moriyama T, Ohara N, Tanimura
K and Maruo T: Caffeine induces apoptosis of human umbilical vein
endothelial cells through the caspase-9 pathway. Gynecol
Endocrinol. 22:48–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgan ME and Vestal RE: Methylxanthine
effects on caudate dopamine release as measured by in vivo
electrochemistry. Life Sci. 45:2025–39. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Degubareff T and Sleator W Jr: Effects of
caffeine on mammalian atrial muscle, and its interaction with
adenosine and calcium. J Pharmacol Exp Ther. 148:202–214.
1965.PubMed/NCBI
|
|
27
|
Solinas M, Ferré S, You ZB, Karcz-Kubicha
M, Popoli P and Goldberg SR: Caffeine induces dopamine and
glutamate release in the shell of the nucleus accumbens. J
Neurosci. 22:6321–6324. 2002.PubMed/NCBI
|
|
28
|
Daly JW, Bruns RF and Snyder SH: Adenosine
receptors in the central nervous system: Relationship to the
central actions of methylxanthines. Life Sci. 28:2083–2097. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jarvis MJ: Does caffeine intake enhance
absolute levels of cognitive performance? Psychopharmacology
(Berl). 110:45–52. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corley J, Jia X, Kyle JA, Gow AJ, Brett
CE, Starr JM, McNeill G and Deary IJ: Caffeine consumption and
cognitive function at age 70: The Lothian Birth Cohort 1936 study.
Psychosom Med. 72:206–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lindsay J, Laurin D, Verreault R, Hébert
R, Helliwell B, Hill GB and McDowell I: Risk factors for
Alzheimer's disease: A prospective analysis from the Canadian Study
of Health and Aging. Am J Epidemiol. 156:445–453. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen ZX: Brain cholinesterases: II. The
molecular and cellular basis of Alzheimer's disease. Med
Hypotheses. 63:308–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ross GW, Abbott RD, Petrovitch H, Morens
DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL,
Curb JD, et al: Association of coffee and caffeine intake with the
risk of Parkinson disease. JAMA. 283:2674–2679. 2000. View Article : Google Scholar
|
|
34
|
Johnson-Kozlow M, Kritz-Silverstein D,
Barrett-Connor E and Morton D: Coffee consumption and cognitive
function among older adults. Am J Epidemiol. 156:842–850. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferreira DD, Stutz B, de Mello FG, Reis RA
and Kubrusly RC: Caffeine potentiates the release of GABA mediated
by NMDA receptor activation: Involvement of A1 adenosine receptors.
Neuroscience. 281:208–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hursel R and Westerterp-Plantenga MS:
Catechin- and caffeine-rich teas for control of body weight in
humans. Am J Clin Nutr. 98:(6 Suppl). S1682–S1693. 2013. View Article : Google Scholar
|
|
37
|
van Dam RM and Feskens EJ: Coffee
consumption and risk of type 2 diabetes mellitus. Lancet.
360:1477–1478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tuomilehto J, Hu G, Bidel S, Lindström J
and Jousilahti P: Coffee consumption and risk of type 2 diabetes
mellitus among middle-aged Finnish men and women. JAMA.
291:1213–1219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salazar-Martinez E, Willett WC, Ascherio
A, Manson JE, Leitzmann MF, Stampfer MJ and Hu FB: Coffee
consumption and risk for type 2 diabetes mellitus. Ann Intern Med.
140:1–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sartorelli DS, Fagherazzi G, Balkau B,
Touillaud MS, Boutron-Ruault MC, de Lauzon-Guillain B and
Clavel-Chapelon F: Differential effects of coffee on the risk of
type 2 diabetes according to meal consumption in a French cohort of
women: The E3N/EPIC cohort study. Am J Clin Nutr. 91:1002–1012.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carlsson S, Hammar N, Grill V and Kaprio
J: Coffee consumption and risk of type 2 diabetes in Finnish twins.
Int J Epidemiol. 33:616–617. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hamer M, Witte DR, Mosdøl A, Marmot MG and
Brunner EJ: Prospective study of coffee and tea consumption in
relation to risk of type 2 diabetes mellitus among men and women:
The Whitehall II study. Br J Nutr. 100:1046–1053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akash MS, Rehman K and Chen S: Effects of
coffee on type 2 diabetes mellitus. Nutrition. 30:755–763. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng YC, Ding YM, Hueng DY, Chen JY and
Chen Y: Caffeine suppresses the progression of human glioblastoma
via cathepsin B and MAPK signaling pathway. J Nutr Biochem.
33:63–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hashibe M, Galeone C, Buys SS, Gren L,
Boffetta P, Zhang ZF and La Vecchia C: Coffee, tea, caffeine
intake, and the risk of cancer in the PLCO cohort. Br J Cancer.
113:809–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu S, Han J, Song F, Cho E, Gao X, Hunter
DJ and Qureshi AA: Caffeine intake, coffee consumption and risk of
cutaneous malignant melanoma. Epidemiology. 26:898–908. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dubrez L, Coll JL, Hurbin A, Solary E and
Favrot MC: Caffeine sensitizes human H358 cell line to p53-mediated
apoptosis by inducing mitochondrial translocation and
conformational change of BAX protein. J Biol Chem. 276:38980–38987.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jafari M and Rabbani A: Dose and time
dependent effects of caffeine on superoxide release, cell survival
and DNA fragmentation of alveolar macrophages from rat lung.
Toxicology. 149:101–108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fernandez MJ, López A and Santa-Maria A:
Apoptosis induced by different doses of caffeine on Chinese hamster
ovary cells. J Appl Toxicol. 23:221–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ito K, Nakazato T, Miyakawa Y, Yamato K,
Ikeda Y and Kizaki M: Caffeine induces G2/M arrest and apoptosis
via a novel p53-dependent pathway in NB4 promyelocytic leukemia
cells. J Cell Physiol. 196:276–283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Juliano LM, Huntley ED, Harrell PT and
Westerman AT: Development of the caffeine withdrawal symptom
questionnaire: Caffeine withdrawal symptoms cluster into 7 factors.
Drug Alcohol Depend. 124:229–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gong Y, Ren J, Liu K and Tang LM: Tumor
suppressor role of miR-133a in gastric cancer by repressing IGF1R.
World J Gastroenterol. 21:2949–2958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ren J, Huang HJ, Gong Y, Yue S, Tang LM
and Cheng SY: MicroRNA-206 suppresses gastric cancer cell growth
and metastasis. Cell Biosci. 4:262014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rossi L, Bonmassar E and Faraoni I:
Modification of miR gene expression pattern in human colon cancer
cells following exposure to 5-fluorouracil in vitro. Pharmacol Res.
56:248–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Garzon R, Pichiorri F, Palumbo T,
Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder
H, et al: MicroRNA gene expression during retinoic acid-induced
differentiation of human acute promyelocytic leukemia. Oncogene.
26:4148–4157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yiannakopoulou E: Targeting epigenetic
mechanisms and microRNAs by aspirin and other non steroidal
anti-inflammatory agents-implications for cancer treatment and
chemoprevention. Cell Oncol (Dordr). 37:167–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Varma SD and Kovtun S: Protective effect
of caffeine against high sugar-induced transcription of microRNAs
and consequent gene silencing: A study using lenses of galactosemic
mice. Mol Vis. 19:493–500. 2013.PubMed/NCBI
|
|
58
|
Wang W, Cheng B, Miao L, Mei Y and Wu M:
Mutant p53-R273H gains new function in sustained activation of EGFR
signaling via suppressing miR-27a expression. Cell Death Dis.
4:e5742013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Altman RD, Lang AE and Postuma RB:
Caffeine in Parkinson's disease: A pilot open-label,
dose-escalation study. Mov Disord. 26:2427–2431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee IA, Low D, Kamba A, Llado V and
Mizoguchi E: Oral caffeine administration ameliorates acute colitis
by suppressing chitinase 3-like 1 expression in intestinal
epithelial cells. J Gastroenterol. 49:1206–1216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Guercio BJ, Sato K, Niedzwiecki D, Ye X,
Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, et al:
Coffee intake, recurrence and mortality in stage III colon cancer:
Results from CALGB 89803 (Alliance). J Clin Oncol. 33:3598–3607.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sanikini H, Dik VK, Siersema PD,
Bhoo-Pathy N, Uiterwaal CS, Peeters PH, González CA, Zamora-Ros R,
Overvad K, Tjønneland A, et al: Total, caffeinated and
decaffeinated coffee and tea intake and gastric cancer risk:
Results from the EPIC cohort study. Int J Cancer. 136:E720–E730.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hayashi M, Tsuchiya H, Yamamoto N, Karita
M, Shirai T, Nishida H, Takeuchi A and Tomita K:
Caffeine-potentiated chemotherapy for metastatic carcinoma and
lymphoma of bone and soft tissue. Anticancer Res. 25:2399–2405.
2005.PubMed/NCBI
|
|
64
|
Miwa S, Kitamura S, Shirai T, Hayashi K,
Nishida H, Takeuchi A, Nojima T and Tsuchiya H: Desmoplastic small
round cell tumour successfully treated with caffeine-assisted
chemotherapy: A case report and review of the literature.
Anticancer Res. 30:3769–3774. 2010.PubMed/NCBI
|