Introduction
Diabetes is a global health challenge. According to
the recent report of Diabetes Atlas, ~380 million people have
diabetes, and the number is still increasing (1). Diabetic vascular complications are
considered as the leading cause of morbidity and mortality in
diabetic patients (2). In the
course of treatment for diabetes, the delay of diabetic
complications, such as macroangiopathy, is even more important than
the control of serum glucose (3,4).
Glucagon-like peptide-1 (GLP-1) and its analogues,
including liraglutide, serve a key role in stimulation of insulin
release and inhibition of glucagon release in a glucose-dependent
manner, and maintain the glycemic homeostasis. Previous studies
have revealed that GLP-1 can protect arteriosclerotic lesions by
ameliorating hyperglycemia, decreasing blood pressure, reducing
macrophage infiltration (5), and
improving vascular inflammation (6) and endothelial dysfunction (7).
Advanced glycation end products (AGEs) are a diverse
group of complex compounds which are formed via a chain of
non-enzymatic chemical reactions (8). The formation and accumulation of AGEs
progress under diabetic conditions. Accumulating evidence has
suggested that receptor for (R) AGE serves a pivotal role in
promoting inflammatory processes and endothelial activation, which
accelerates atherosclerosis in patients with diabetes (9,10).
Binding of AGEs to RAGE activates multiple intracellular signaling
pathways including p21ras, which recruits downstream targets such
as mitogen-activated protein kinase, and activates nuclear factor
κB (NF-κB). The AGE-RAGE interaction augments inflammatory
responses, and leads to vascular dysfunction and monocyte
activation (9).
Diabetes-associated atherosclerotic lesions exhibit increased
accumulation of RAGE ligands and enhanced expression of RAGE
(11,12). Therefore, inhibition of AGE
formation and blockage of RAGE may be a novel molecular target for
protecting diabetic vascular complications. In our previous
studies, it was demonstrated that atorvastatin can significantly
downregulate the expression of RAGE in the aortas of diabetic Goto
Kakiski rats, without an alteration in glucose levels (13). GLP-1 analogues can influence AGE
levels via lowering blood glucose and subsequently altering the
expression of RAGE. However, no research has been conducted on the
direct effects of GLP-1 analogue on the formation and deposition of
AGEs and the expression of RAGE in healthy animal models. The
present study aimed to investigate whether GLP-1 analogue could
decrease the AGE-induced increase in serum level of AGEs, and
suppress the expression of RAGE in the aorta in an
ApoE−/− mouse model in euglycemia conditions.
These results will further confirm the protective effects of GLP-1
analogue on arteriosclerotic lesions by targeting RAGE.
Materials and methods
Animals and treatment
Male C57BL/6 J ApoE−/− mice (age,
10 weeks; n=40) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China) and were acclimated in
their new environment for 2 weeks. Mice were housed in the animal
facility of Shanghai University of Traditional Chinese Medicine
(Shanghai, China) with free access to food and water and in a
pathogen-free environment with a 12-h light/dark cycle. After the
adjustment time, all the animals were fed a high-fat diet. Animals
were randomly assigned to the control group (n=10), GLP-1 group
(n=10), AGEs group (n=10) and AGEs+GLP-1 group (n=10). The GLP-1
and AGEs+GLP-1 groups received liraglutide (Novo Nordisk, Bagsværd,
Denmark; 0.4 mg/kg/day) for 9 weeks by subcutaneous injection. The
AGEs and AGEs+GLP-1 group received AGEs-modified bovine serum
albumin (BSA; 30 mg/kg/day) for 9 weeks by intraperitoneal
injection. Body weight, random blood glucose and individual food
intake were monitored weekly. After 9 weeks, the mice were killed
after 15 h of fasting, ~1 ml blood was drawn and the aorta from the
aortic root to the iliac bifurcation was rapidly procured. Sudan IV
staining requires a whole aorta, therefore, in each group 5 of the
aortas were assessed by Sudan IV staining, and the other 5 were
used for western blotting and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). The present study was approved
by the ethics committee of Tongji University School of Medicine
(Shanghai, China).
Preparation of AGEs-BSA
AGEs-BSA was prepared in vitro as previously
described (14). BSA (50 g/l;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 500 mmol/l
D-glucose (Sigma-Aldrich; Merck KGaA), 100 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 0.1 mg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) were dissolved
in phosphate buffer (0.2 M, pH 7.4), mixed and incubated overnight
at room temperature. The mixture was sterilized by 0.22 µm
bacterial filter and then incubated at 37°C in the dark for 90
days, followed by extensive dialysis using 0.1 M phosphate buffer
for 24 h to remove unincorporated glucose. Fluorescence
spectrophotometry (slit, 2.5 nm; voltage 700 mv) with excitation
and emission wavelengths of 370 nm and 440 nm, respectively,
AGEs-BSA was confirmed to be successfully prepared, which was
subsequently made into lyophilized powder using a lyophilized
powder machine.
Serum biochemical index
measurement
The serum levels of AGEs (cat. no. MU30166), soluble
(s)RAGE (cat. no. MU10877), stromal cell-derived factor (SDF)-1α
(cat. no. MU30235), total cholesterol (CHO; cat. no. MU30383) and
triacylglycerol (TG; cat. no. MU30320) were measured using
commercially available ELISA kits (Bio-Swamp Life Science Lab,
Hubei, China), according to the manufacturer's protocols.
RT-qPCR
Total RNA in aorta tissue was obtained from frozen
tissue (half of the aorta tissue) using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Purified RNA was used as template
for first-strand cDNA synthesis using a PrimeScript RT reagent kit
(Takara Bio, Inc., Otsu, Japan). qPCR was performed with a SYBR
Green PCR kit (Takara Bio, Inc.), using an ABI 7500 real-time PCR
system, according to the manufacturer's instructions, with the
following thermocycling conditions: 1 cycle at 95°C for 30 sec,
then 40 cycles of 95°C for 5 sec and 60°C for 34 sec. Gene
expressions were analyzed using the comparative Cq method (15) and normalized to GADPH. Primers
(Sangon Biotech, Co., Ltd., Shanghai, China) were as follows:
Forward, 5′-GAA-GGC-TCT-GTG-GGT-GAG-TC-3′ and reverse,
5′-ATT-CAG-CTC-TGC-ACG-TTC-CT-3′ for RAGE; and forward,
5′-CCT-GA-CCA-CCA-ACT-GCT-TAG-C-3′ and reverse,
5′-CCA-GTG-AGC-TTC-CCG-TCT-AGC-3′ for GAPDH.
Western blot analysis
Total proteins of the other half of the aorta tissue
were initially extracted by centrifugation (16,000 × g for 5 min at
4°C) in radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China). Protein concentrations
were determined using an Enhanced Bicinchoninic Acid Protein Assay
kit according to the manufacturer's instructions (Beyotime
Institute of Biotechnology). Equal amounts (20 µg) of protein
samples separated by 10 or 12% SDS-PAGE and then transferred onto a
polyvinylidene difluoride membrane. The non-specific proteins were
blocked with 5% non-fat dried milk for 1 h. The membranes were
incubated with anti-RAGE (cat. no. ab37647; Abcam, Cambridge, MA,
USA; 1:500) and anti-GAPDH (cat. no. BM3876; Boster Biological
Technology, Pleasanton, CA, USA; 1:2,000) primary antibodies
overnight at 4°C, followed by incubation with horseradish
peroxidase (HRP)-conjugated IgG secondary antibodies (cat. nos.
111-055-003 and 115-035-003; Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA; 1:2,000) for 1 h at room temperature.
HRP-conjugated secondary antibodies were used in conjunction with
an enhanced chemiluminescence detection system. Protein expression
was analyzed using Gel-Pro analyzer 4 software (Media Cybernetics,
Inc., Rockville, MD, USA) and normalized to that of GAPDH.
Quantification of atherosclerotic
lesions
The atherosclerotic lesions were assessed by Sudan
IV staining as previously described (16). The entire aorta was dissected from
the proximal ascending aorta to the bifurcation of the iliac
artery, fixed with 10% formalin for 36 h, and then stained with
Sudan IV for 10 min, differentiated in 70% alcohol for 15 min, and
washed in water for 30 min. The adventitial fat was removed and the
aorta was opened longitudinally and pinned flat onto a black
paraffin board using a dissecting microscope. The aorta was imaged
using a charged couple device camera. The images were merged into
one image using Adobe Photoshop Version 7.0 (Adobe Systems, Inc.,
San Jose, CA, USA). Total aortic and lesion areas were calculated
using Image-Pro Plus 6.0 (National Institutes of Health, Bethesda,
MA, USA). The results were reported as a percentage of the total
aortic area that contained lesions.
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed by one-way analysis of variance followed by a
Fisher's least significant difference post hoc test using SPSS
version 19.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Animal data and metabolic profile
Male mice (10 weeks old) matched for baseline body
weight were fed with a high-fat diet. After a 9-week intervention,
the body weight of ApoE−/− mice in the GLP-1
group reduced compared with the control group (P<0.01), the food
intake and random plasma glucose were reduced in GLP-1 treated
groups compared with non-treated groups (all P<0.01). Serum AGEs
and sRAGE increased in the AGEs group compared with the control
group (all P<0.01), and serum AGEs and sRAGE were reduced in
GLP-1 treated groups compared with non-treated groups, (all
P<0.01). SDF-1α levels were increased in both AGEs and GLP-1
group compared with the control group (all P<0.05), and SDF-1α
levels were increased in the AGEs+GLP-1 group compared with the
AGEs group (P<0.05). There were no significant differences in
serum TG and CHO levels between the four groups (Table I).
 | Table I.Characteristics and laboratory data of
apolipoprotein-E deficient mice treated for 9 weeks with a high-fat
diet. |
Table I.
Characteristics and laboratory data of
apolipoprotein-E deficient mice treated for 9 weeks with a high-fat
diet.
|
| Control group | GLP-1 group | AGEs group | AGEs+GLP-1 group |
|---|
| Body weight (g) |
31.2±1.6 |
29.0±0.8a |
29.8±1.0 |
29.4±1.9 |
| Food intake
(g/day) |
4.06±0.21 |
2.96±0.36a |
3.72±0.41 |
2.63±0.4b |
| Glucose (mmol/l) |
7.39±0.48 |
6.66±0.37a |
7.39±0.45 |
6.34±0.40b |
| AGEs (pg/ml) |
915.3±173.1 |
589.4±66.4a |
2198.25±478.7a |
1217.1±199.0b |
| sRAGE (pg/ml) |
428.3±41.9 |
340.5±65.3a |
617.0±123.0a |
389.6±83.3b |
| SDF-1α (ng/ml) |
6.14±1.01 |
7.87±0.74a |
7.95±1.01a |
9.95±2.07b |
| TG (mmol/l) |
2.57±0.35 |
2.99±0.41 |
2.85±0.46 |
2.96±0.32 |
| CHO (mmol/l) |
13.3±2.2 |
13.5±2.4 |
13.6±1.9 |
14.1±2.7 |
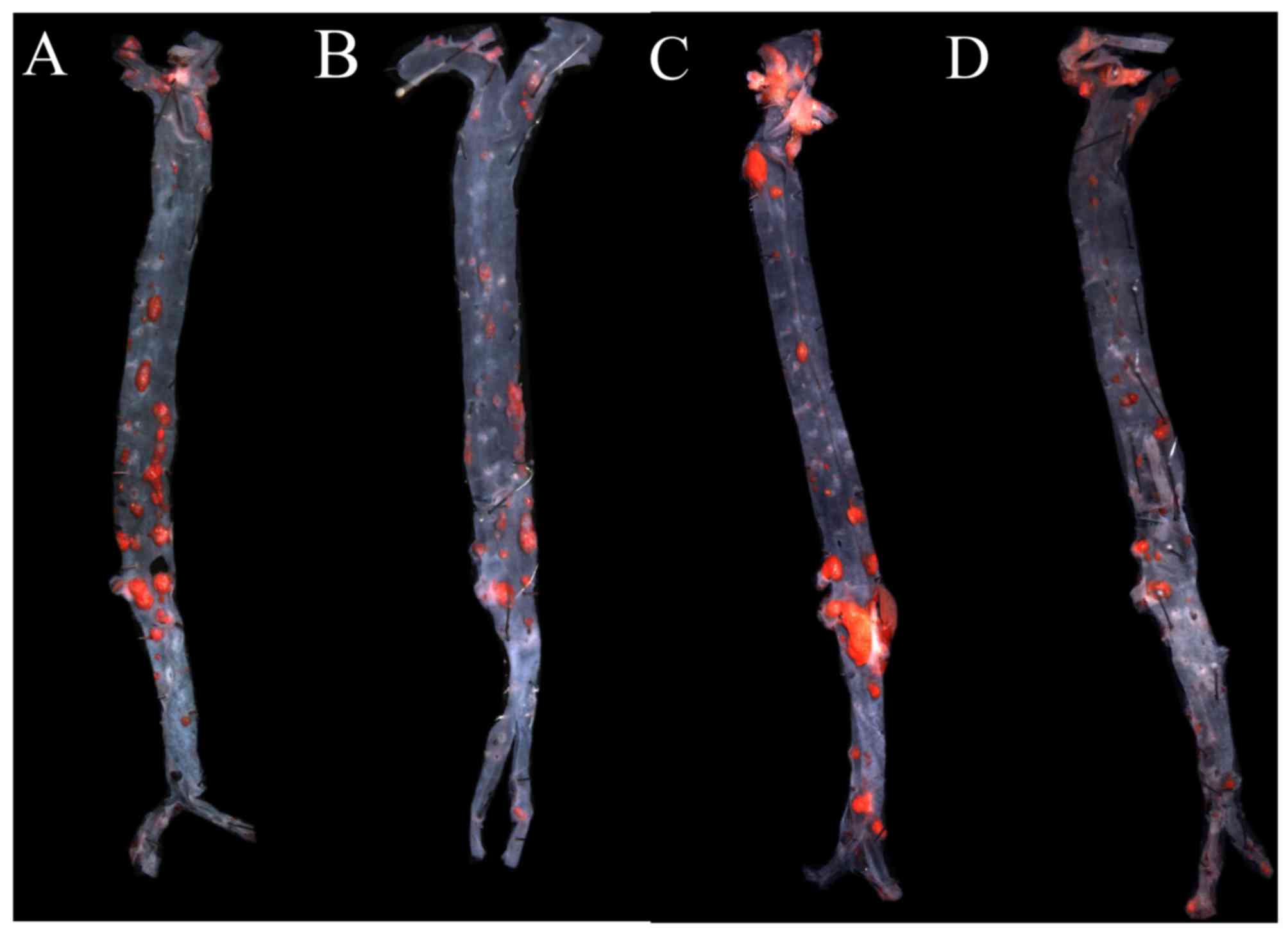
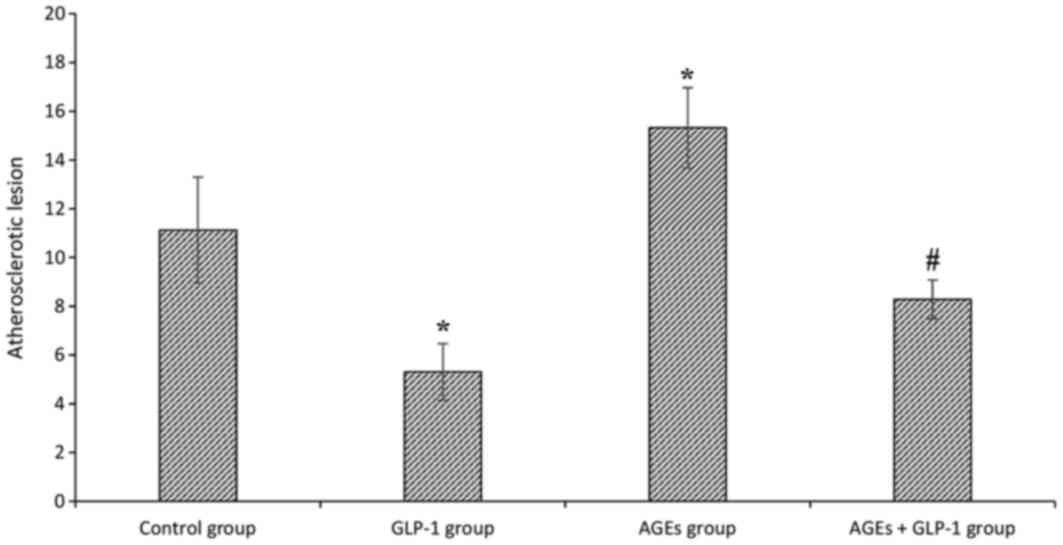
AGEs aggravate atherosclerotic
lesions, and GLP-1 treatment relieves it
To investigate the effect of AGEs on atherogenesis,
ApoE−/− mice were treated with intraperitoneal
injections of AGEs for 9 weeks, and then total plaque area in the
entire aorta were assessed by Sudan IV staining. The aortic lesion
size increased in AGEs groups compared with the control group
(P<0.01). Additionally, aortic lesion size decreased in GLP-1
treated groups compared with non-treated groups (all P<0.01;
Figs. 1 and 2).
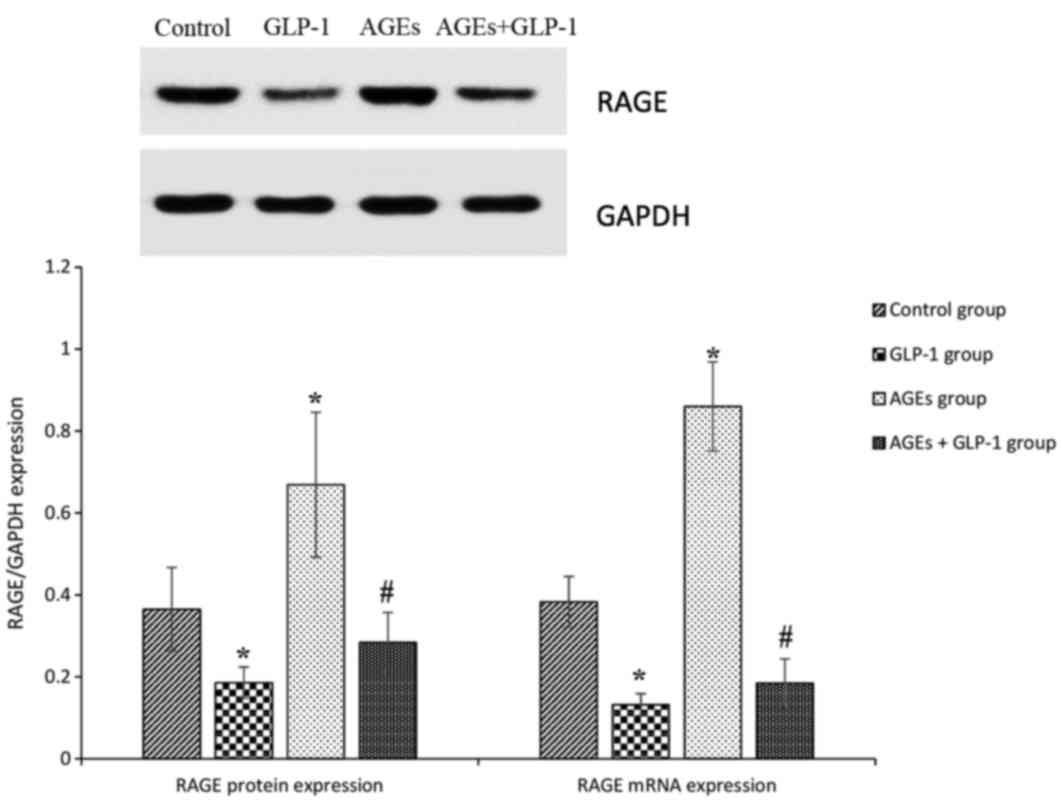
AGEs increase the expression of RAGE
in aorta tissues, and GLP-1 treatment reduces it
To further analyze the mechanisms mediating
aggravation in the atherosclerotic lesions in
ApoE−/− mice, RAGE expression was measured in
aorta tissues. In the AGEs groups, RAGEs protein and mRNA
expression levels were increased compared with the control group
(all P<0.01). GLP-1 treatment reduced RAGEs protein and mRNA
expression levels compared with the control and AGEs groups (all
P<0.01; Fig. 3).
Discussion
Previous studies have reported that GLP-1 reduces
the development of atherosclerosis in ApoE−/−
mice fed a high-fat diet (17).
However, the mechanism by which GLP-1 suppresses the development of
atherosclerosis remains to be fully elucidated. The majority of
studies have demonstrated that GLP-1 reduces vascular inflammation
via suppression of pro-inflammatory activation of
monocytes/macrophages (18). AGEs
serve a pivotal role for the initiation and development of
atherogenesis in type II diabetes mellitus (2-DM) via activing
RAGE. Therefore, the present study examined whether GLP-1 can
protect arteriosclerotic lesions via inhibiting AGEs-induced RAGE
expression. The present results demonstrated that AGEs aggravate
atherosclerotic lesions via increasing the expression of RAGE in
aorta tissues, and liraglutide relieved it via downregulating the
expression of RAGE in aorta tissues.
The present study subjected
ApoE−/− mice to a high-fat diet for 9 weeks to
facilitate the development of atherosclerotic lesions at 12 weeks.
Liraglutide reduced food intake and slowed the growth of body
weight. Liraglutide has previously demonstrated pleiotropic effects
on food intake, body weight, fat mass loss and energy expenditure
(19), consistent with the results
of the present study.
The present study revealed that intraperitoneal
injection of AGEs significantly increased serum AGEs, which
upregulated aortic RAGE expression and aggravated atherosclerotic
lesions in ApoE−/− mice. AGEs are formed by the
maillard process, a non-enzymatic reaction between reducing sugars
and the amino groups of proteins, lipids and nucleic acids that
contributes to the aging of macromolecules (20). In hyperglycemic and/or oxidative
stress conditions, this process begins with the conversion of
reversible Schiff base adducts to more stable, covalently-bound
Amadori rearrangement products (21). Over the course of days to weeks,
these Amadori products undergo further rearrangement reactions to
form irreversibly-crosslinked moieties, termed AGEs (22). RAGE, one of the most important
binding proteins of AGEs, is a signal-transducing receptor on the
cell surface, and is upregulated by AGEs (23). Increasing evidence has demonstrated
that activation of RAGE induced by AGEs elicits oxidative stress
generation (24) and activation of
NF-κB (21). The AGEs-RAGE-induced
oxidative stress generation further potentiates the formation and
accumulation of AGEs and subsequent RAGE overexpression. Therefore,
these positive feedback mechanisms may form a vicious cycle, and
cause atherosclerosis in diabetes.
The present study demonstrated that liraglutide
treatment decreased serum AGEs and the RAGE expression in the
aorta, relieving atherosclerotic lesions in
ApoE−/− mice. GLP-1 is one of the incretins, a
gut hormone secreted from L cells in the intestine in response to
food intake (25). GLP-1 suppress
oxidative stress generation induced by AGEs-RAGE (26), then blocks the positive feedback
loops between the AGEs-RAGE axis. GLP-1 receptor (GLP-1R) is
expressed in vascular endothelial cells, and GLP-1R small
interfering RNAs decrease RAGE mRNA expression levels. It has been
demonstrated that GLP-1R activation may attenuate the abnormal
expression of RAGE via the suppression of NF-κB (27).
Liraglutide decreased plasma glucose levels in the
GLP-1 group compared with the control group, causing a decrease of
serum AGEs. In the AGEs+GLP-1 group, serum AGEs were significantly
decreased compared with AGEs group, without significant decrease of
plasma glucose. Therefore, it was hypothesized that liraglutide has
direct effects on metabolizing serum AGEs, and subsequently
decreases RAGE expression in the aorta, relieving atherosclerotic
lesions. However, the underlying mechanisms remain unclear. Perhaps
these effects of liraglutide are associated with the activation of
GLP-1R.
RAGE has a C-truncated secreted isoform, termed
sRAGE. In contrast to cell surface RAGE, sRAGE blocks cell surface
RAGE-ligand blinding and subsequent signaling by acting as a decoy
(28). Previous studies have
reported serum sRAGE levels as an important novel biomarker in
patients with 2-DM (29) and in
nondiabetic subjects with coronary artery disease (30). In the present study, liraglutide
decreased serum sRAGE levels, which may be another reason for the
decreased expression of RAGE in the aorta.
In the present study, serum SDF-1α levels increased
in ApoE−/− mice treated with AGEs and/or
liraglutide. SDF-1 is a small peptide chemokine that regulates many
essential biological processes, including stem cell motility,
cardiac and neuronal development, neovascularization, and tissue
repair and regeneration (31).
SDF-1 is produced in reactive stromal tissue in response to
injuries, where it is believed to recruit bone marrow-drived
somatic stem cells involved in tissue repair (32). A previous study suggested that
GLP-1 could enhance the restorability of SDF-1 to beta cells
(33). The results of the present
study indicated that the increase of serum SDF-1 is a protective
factor for arteriosclerotic lesion.
In conclusion, the results of the present study
demonstrated that GLP-1 can protect arteriosclerotic lesions in
ApoE−/− mice. Additionally, the beneficial
effects of GLP-1 involved reducing diet and losing weight. These
observations indicated that GLP-1 has protective actions against
atherosclerosis via inhibiting AGEs-induced RAGE expression. As the
prevention and treatment of diabetic vascular complications remains
an important and challenging issue, liraglutide, an effective
medicine for diabetes, may provide attractive therapeutic options
for atherosclerosis and associated diseases.
Acknowledgements
The present study was supported by Shanghai Pudong
New District [grant no. 2011CB504006 (1)]. The authors would like to thank
Professor Yuzhen Zhang (Shanghai East Hospital) for providing the
method for Sudan IV staining of the entire aorta, and Professor
Zhanyu Guo (School of Life Sciences and Technology, Tongji
University) for the preparation of AGEs-BSA.
Glossary
Abbreviations
Abbreviations:
|
AGEs-BSA
|
advanced glycation end
products-modified bovine serum albumin
|
|
RAGE
|
receptor of advanced glycation end
products
|
|
GLP-1
|
glucagon-like peptide-1
|
References
|
1
|
Whiting DR, Guariguata L, Weil C and Shaw
J: IDF diabetes atlas: Global estimates of the prevalence of
diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94:311–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K
and Laakso M: Mortality from coronary heart disease in subjects
with type 2 diabetes and in nondiabetic subjects with and without
prior myocardial infarction. N Engl J Med. 339:229–234. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fowler MJ: Microvascular and Macrovascular
Complications of Diabetes. Clinical Diabetes. 26:77–82. 2008.
View Article : Google Scholar
|
|
4
|
Scheen AJ and Paquot N: Blood glucose
control and prevention of microangiopathy and macroangiopathy in
type 2 diabetes. Rev Med Liege. 58:265–270. 2003.PubMed/NCBI
|
|
5
|
Wang Y, Parlevliet ET, Geerling JJ, van
der Tuin SJ, Zhang H, Bieghs V, Jawad AH, Shiri-Sverdlov R, Bot I,
de Jager SC, et al: Exendin-4 decreases liver inflammation and
atherosclerosis development simultaneously by reducing macrophage
infiltration. Br J Pharmacol. 171:723–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dozier KC, Cureton EL, Kwan RO, Curran B,
Sadjadi J and Victorino GP: Glucagon-like peptide-1 protects
mesenteric endothelium from injury during inflammation. Peptides.
30:1735–1741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
le Kim Chung T, Hosaka T, Yoshida M,
Harada N, Sakaue H, Sakai T and Nakaya Y: Exendin-4, a GLP-1
receptor agonist, directly induces adiponectin expression through
protein kinase A pathway and prevents inflammatory adipokine
expression. Biochem Biophys Res Commun. 390:613–618. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamagishi S: Role of advanced glycation
end products (AGEs) and receptor for AGEs (RAGE) in vascular damage
in diabetes. Exp Gerontol. 46:217–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huttunen HJ, Fages C and Rauvala H:
Receptor for advanced glycation end products (RAGE)-mediated
neurite outgrowth and activation of NF-kappaB require the
cytoplasmic domain of the receptor but different downstream
signaling pathways. J Biol Chem. 274:19919–19924. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bierhaus A, Schiekofer S, Schwaninger M,
Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting
I, et al: Diabetes-associated sustained activation of the
transcription factor nuclear factor-kappaB. Diabetes. 50:2792–2808.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cipollone F, Iezzi A, Fazia M, Zucchelli
M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R,
et al: The receptor RAGE as a progression factor amplifying
arachidonate-dependent inflammatory and proteolytic response in
human atherosclerotic plaques: Role of glycemic control.
Circulation. 108:1070–1077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ
Jr, Chow WS, Stern D and Schmidt AM: Suppression of accelerated
diabetic atherosclerosis by the soluble receptor for advanced
glycation endproducts. Nat Med. 4:1025–1031. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng B, Xu L, Wang H, Yan X, Xue J, Liu F
and Hu JF: Atorvastatin exerts its anti-atherosclerotic effects by
targeting the receptor for advanced glycation end products. Biochim
Biophys Acta. 1812:1130–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vlassara H, Striker LJ, Teichberg S, Fuh
H, Li YM and Steffes M: Advanced glycation end products induce
glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad
Sci USA. 91:11704–11708. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yagyu H, Ishibashi S, Chen Z, Osuga J,
Okazaki M, Perrey S, Kitamine T, Shimada M, Ohashi K, Harada K, et
al: Overexpressed lipoprotein lipase protects against
atherosclerosis in apolipoprotein E knockout mice. J Lipid Res.
40:1677–1685. 1999.PubMed/NCBI
|
|
17
|
Chrysant SG and Chrysant GS: Clinical
implications of cardiovascular preventing pleiotropic effects of
dipeptidyl peptidase-4 inhibitors. Am J Cardiol. 109:1681–1685.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arakawa M, Mita T, Azuma K, Ebato C, Goto
H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R and Watada H:
Inhibition of monocyte adhesion to endothelial cells and
attenuation of atherosclerotic lesion by a glucagon-like peptide-1
receptor agonist, exendin-4. Diabetes. 59:1030–1037. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knudsen LB: Liraglutide: The therapeutic
promise from animal models. Int J Clin Pract Suppl. 4–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vlassara H and Palace MR: Diabetes and
advanced glycation endproducts. J Intern Med. 251:87–101. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukami K, Yamagishi S and Okuda S: Role of
AGEs-RAGE system in cardiovascular disease. Curr Pharm Des.
20:2395–2402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamagishi S, Fukami K and Matsui T:
Crosstalk between advanced glycation end products (AGEs)-receptor
RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic
vascular complications. Cardiovasc Diabetol. 14:22015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sourris KC and Forbes JM: Interactions
between advanced glycation end-products (AGE) and their receptors
in the development and progression of diabetic nephropathy-are
these receptors valid therapeutic targets. Curr Drug Targets.
10:42–50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daffu G, del Pozo CH, O'Shea KM,
Ananthakrishnan R, Ramasamy R and Schmidt AM: Radical roles for
RAGE in the pathogenesis of oxidative stress in cardiovascular
diseases and beyond. Int J Mol Sci. 14:19891–19910. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim W and Egan JM: The role of incretins
in glucose homeostasis and diabetes treatment. Pharmacol Rev.
60:470–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishibashi Y, Matsui T, Takeuchi M and
Yamagishi S: Glucagon-like peptide-1 (GLP-1) inhibits advanced
glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA
levels in endothelial cells by suppressing AGE receptor (RAGE)
expression. Biochem Biophys Res Commun. 391:1405–1408. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S, Yin L, Xu Z, An FM, Liu AR, Wang
Y, Yao WB and Gao XD: Inhibiting receptor for advanced glycation
end product (AGE) and oxidative stress involved in the protective
effect mediated by glucagon-like peptide-1 receptor on AGE induced
neuronal apoptosis. Neurosci Lett. 612:193–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hudson BI, Harja E, Moser B and Schmidt
AM: Soluble levels of receptor for advanced glycation endproducts
(sRAGE) and coronary artery disease: The next C-reactive protein?
Arterioscler Thromb Vasc Biol. 25:879–882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basta G, Sironi AM, Lazzerini G, Del Turco
S, Buzzigoli E, Casolaro A, Natali A, Ferrannini E and Gastaldelli
A: Circulating soluble receptor for advanced glycation end products
is inversely associated with glycemic control and S100A12 protein.
J Clin Endocrinol Metab. 91:4628–4634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Falcone C, Emanuele E, D'Angelo A, Buzzi
MP, Belvito C, Cuccia M and Geroldi D: Plasma levels of soluble
receptor for advanced glycation end products and coronary artery
disease in nondiabetic men. Arterioscler Thromb Vasc Biol.
25:1032–1037. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca
R, Wojakowski W and Ratajczak J: The pleiotropic effects of the
SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis.
Leukemia. 20:1915–1924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kucia M, Reca R, Jala VR, Dawn B,
Ratajczak J and Ratajczak MZ: Bone marrow as a home of heterogenous
populations of nonhematopoietic stem cells. Leukemia. 19:1118–1127.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Stanojevic V, Avadhani S, Yano T
and Habener JF: Stromal cell-derived factor-1 (SDF-1)/chemokine
(C-X-C motif) receptor 4 (CXCR4) axis activation induces
intra-islet glucagon-like peptide-1 (GLP-1) production and enhances
beta cell survival. Diabetologia. 54:2067–2076. 2011. View Article : Google Scholar : PubMed/NCBI
|