Introduction
The mechanism maintaining homeostasis in the immune
system is very complex and remains to be fully elucidated.
Homeostasis in the immune system may be disturbed by increased cell
apoptosis or proliferation, which may lead to the development of
fatal inflammatory disease. A previous study determined that a
group of superfamily proteins are essential for the maintenance of
the homeostasis in the immune system (1), particularly the protein with
hexameric helix bundle structure termed death-effector domain
(DED), similar to death domain and caspase activation and
recruitment domain (CARD), which participate in apoptosis and other
signaling pathways (2). Tumor
necrosis factor α-induced protein 8-like 2 (TIPE2) has been
previously identified to contribute to immune homeostasis (3). The primary mechanism is associated
with the regulation of T cell receptors and toll-like receptor
(TLR) signaling pathways (3,4). In
addition to high expression in inflamed tissues, TIPE was also
expressed in medullary tissue and a variety of tumor cells,
indicating that it may have different functions (5). It has been previously reported that
TIPE2 may regulate the expression of TLR (3). However, it remains to be determined
whether the activation of upstream of TLR affects the expression of
TIPE2 or the activity and proliferation of human monocytes. The
present study was performed from January 2014 to December 2015 and
used the THP-1 human monocyte cell line as the study subject, to
investigate the effect of different TLR agonists on TIPE2
expression and the associated molecular mechanisms.
Materials and methods
Cell culture and TLR agonist
treatments
THP-1 cells were purchased from Shanghai Cell Bank,
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China); newborn calf serum (NCS), Dulbecco's modified
Eagle's medium (DMEM) and 0.25% trypsin were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA); human TLR1-9 agonists
were purchased from InvivoGen, Inc. (San Diego, CA, USA).
Antibodies of human TIPE2 (cat. no. ab110389) and caspase-8 (cat.
no. ab25901) were purchased from Abcam (Cambridge, MA, USA). Other
reagents were domestic analytical grade. THP-1 cells were cultured
in DMEM medium containing 10% NCS and 100 U/ml ampicillin, which
was placed in an incubator at a temperature of 37°C and 5%
CO2, and the medium was changed every 2–3 days. Cells in
the logarithmic growth phase were selected for subsequent
experiments. The cells were counted (1×104 cells per
well), the TLR1-9 specific agonists were diluted with DMEM medium,
resulting in the final concentration of 0.1 and 1 µg/ml. After 24 h
incubation, cells were lysed using TRIzol (Thermo Fisher
Scientific, Inc.). The RNA was extracted and the expression level
of TIPE2 mRNA was detected as subsequently described.
Cytoinhibition experiments
The growth inhibition rate was analyzed using an MTT
assay. THP-1 cells at a density of 1×104/ml were seeded
in 6-well or 12-well plates, placed in the incubator at 37°C for 24
h. 10 mg/ml stock solution of polyinosinic:polycytidylic acid (Poly
I:C) was diluted with culture medium and respectively added to at a
final concentration of 1 or 0.1 µg/ml, and PBS was set as the
control group. Cells were collected after 24 h treatment at 37°C
and 5 mg/ml MTT solution (Sangon Biotech Co., Ltd., Shanghai,
China) was added and the mixture was incubated in 37°C for 4 h. The
supernatant was discarded and 150 µl DMSO was added to each well.
Then the absorbance (OD) at 570 nm was detected using a microplate
reader. The experiment was repeated for three times. The inhibition
rate was calculated as follows: Inhibition rate = (1 -
ODployI:C /ODblank) × 100. The detection of
the growth inhibition rate following silencing TIPE2 expression of
was similar to the aforementioned protocol. Briefly, 200 µl cells
(1×104/ml) were seeded in 96-well plates, set as shTIPE2
silencing group (THP-1 cells after silencing the expression of
TIPE2 screened using puromycin) and shScramble group (the empty
vector screened using puromycin). After 24 h incubation with Poly
I:C (1 µg/ml) at 37°C, MTT was added to detect the absorbance (OD)
at 570 nm.
Construction of lentiviral vector and
the establishment of TIPE2-slienced cell lines
The synthetic oligonucleotides of shRNA (3 pairs of
TIPE2 shRNA were synthesized by Sangon Biotech, Co., Ltd.,
(Shanghai, China) were designed and the specific sequences were as
follows: shTIPE2-1 forward (F)
5′-CCGGGTGGCTCATCTCTTCATAGATCTCGAGATCTATGAAGAGATGAGCCACTTTTTG-3′;
shTIPE2-1 reverse (R)
5′-AATTCAAAAAGTGGCTCATCTCTTCATAGATCTCGAGATCTATGAAGAGATGAGCCAC-3′;
shTIPE2-2
F5′-CCGGTTCAATCTTCAGGCTTCATTCCTCGAGGAATGAAGCCTGAAGATTGAATTTTTG-3′;
shTIPE2-2
5′-AATTCAAAAATTCAATCTTCAGGCTTCATTCCTCGAGGAATGAAGCCTGAAGATTGAA-3′;
shTIPE2-3F
5′-CCGGGCCACGTGTTTGATCACTTCTCTCGAGAGAAGTGATCAAACACGTGGCTTTTTG-3′;
shTIPE2-3
R'-AATTCAAAAAGCCACGTGTTTGATCACTTCTCTCGAGAGAAGTGATCAAACACGTGGC-3′.
Double stranded DNA fragments were formed after annealing, and then
linked to pLKO.1-TRC vector (includes GFP encoding sequence,
BioVector NTCC Inc., Beijing, China) double digested by restriction
endonucleases AgeI and EcoRI. The 4 µl products was used to
transform DH5α competent cells (BioVector NTCC Inc.) and incubated
overnight at 37°C. The 3 separated monoclonal colonies were
inoculated into LB broth with ampicillin resistant, shaking at 37°C
with 8 × g overnight. Following the small extraction of plasmids by
the SanPrep Column Plasmid Mini-Preps kit (Sangon Biotech Co.,
Ltd.), the combined plasmids were identified using a 1% agarose gel
electrophoresis following digestion with EcoRI and NcoI enzymes.
The identified plasmid was termed pLKO.1-TRC-TIPE2-shRNA1. The
bacteria, which contained the confirmed plasmids were sent to
Shanghai Shenggong Co., Ltd. (Shanghai, China) for sequencing.
For the packaged lentivirus, the 293T cells were
divided the day prior to transfection. After combination of shTIPE2
or shScramble plasmid with the packaging plasmid pΔ8.91 and pMD2.G
(BioVector NTCC Inc., the weight ratio of 10:10:1), Lipofectamine
2000 (Thermo Fisher Scientific, Inc.) was mixed and incubated at
room temperature for 20 min and then the total mixture was added to
the 293T cells. Following incubation at 37°C for 12 h, the medium
was replaced with complete DMEM medium. After 48 h, the expression
of green fluorescence was observed under fluorescence microscope.
The supernatant was collected during 48–72 h and filtrated through
a 0.45 µm filter. For the infection, THP-1 cells at logarithmic
growth phase were selected and seeded into 6-wells
(1.5×105 cells per well) for 12 h incubation at 37°C.
The supernatant of each well was discarded and 200 µl
virus-containing liquid was added instead. Subsequently, the
culture medium with polybrene (concentration of 4 µg/ml) was added
for infection for 8 h at 37°C, and then transferred the cells to
the DMEM medium. After 48 h the culture was added to a medium with
puromycin (concentration of 0.5 µg/ml) and the medium was replaced
every 2–3 days. After screening for 2 months, normal medium was
added and the successfully transfected cells were used for the
subsequent experiments.
Detection of cell apoptosis
In order to determine the apoptotic rate, a Cell
Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA)
was used for Annexin V-fluorescein isothiocyanate (FITC)/propidium
iodide (PI) double staining. Following treatment with different
concentrations of Poly I:C for 24 h, THP-1 cells were collected,
washed with PBS and re-suspended with 300 µl binding buffer. Then 5
µl FITC-labeled Annexin V and 5 µl PI were added, and followed with
addition of 200 µl binding buffer to the suspension. After
incubation in dark at room temperature for 15 min, the mixture was
filtered by 200-mesh nylon mesh and analyzed on BD Accuri C6 flow
cytometer (BD Biosciences). The ratio of apoptotic cells and
survival cells was calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells from each treatment group were collected. The
total RNA was extracted using TRIzol (Thermo Fisher Scientific,
Inc.) and then the cDNA was synthesized using MMLV reverse
transcriptase (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. cDNA from each group was used as the
template for the subsequence PCR reaction. Primer sequences used
were as follows: TIPE2, upstream 5′-GGAACATCCAAGGCAAGACTG-3′,
downstream 5′-AGCACCTCACTGCTTGTCTCATC-3′; GAPDH (internal control)
upstream 5′-GTCGTATTGGGCGCCTGGTCACC-3′, downstream
5′-CACACCCATGACGAACATGGGGGC-3′. The total reaction volume of 20 µl
contained 2×SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.,
Dalian, China) 10 µl, upstream primer (10 µM) 0.5 µl, downstream
primer (10 µM) 0.5 µl, cDNA 1 µl and ddH2O 8 µl. The PCR
thermocycling protocol was as follows: 95°C pre-denaturation for 3
min; followed by 30 cycles of 95°C for 10 sec, 55°C for 30 sec,
72°C for 30 sec. Each cDNA sample was measured in triplicate in the
qPCR reaction, the mean of the quantification cycle (Cq) was used
to calculate the ∆Cq in each group (Cq target gene-Cq reference
gene), and used the normal group as 1 for homogenization and the
formula (2−∆∆Cq) represented the relative expression
level of the gene (6).
Immunoblotting
THP-1 cells were treated with Poly I:C for 24 h.
Cells were washed once with cold PBS and lysed by RIPA buffer
(Sangon Biotech Co., Ltd.). Following protein collection, the
protein concentration was determined using a bicinchonic acid assay
kit (Sangon Biotech Co., Ltd.). SDS-PAGE electrophoresis was
performed with 20 µg protein/lane and then transferred onto PVDF
membranes which were blocked with 5% non-fat milk. Primary
antibodies, including TIPE2 (1:2,000, cat. no. ab110389), caspase-8
(1:2,000, cat. no. ab25901), GAPDH (1:4,000, cat. no. ab9485),
β-actin (1:4,000, cat. no. ab8227) purchased from Abcam (Cambridge,
UK) and p41/42 (1:2,000, cat. no. 9748) purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA), were added.
Following overnight incubation at 4°C, the membrane was taken out
and washed with TBST for three times, then incubated with
HRP-labeled secondary antibody (1:10,000, cat. no. L3042-2)
obtained from SAB Biotherapeutics, Inc. (Sioux Falls, SD, USA) at
room temperature for 1 h. The membranes were washed with TBST three
times and visualized using SuperSignal West Femto Trial kit (Thermo
Fisher Scientific, Inc.). Relative expression of protein was
scanned and calculated using LI-COR western blot imaging system
(LI-COR Biosciences, Lincoln, NE, USA) and β-actin was used as
internal control.
Statistical analysis
Significant difference analysis was performed by
GraphPad Prism software version 6.01 (GraphPad Software, Inc., La
Jolla, CA, USA). The data was the mean of three independent
experiments, and is presented as the mean ± standard deviation.
When the data had a normal distribution, data from two groups was
compared using independent samples Student's t-test and multiple
groups comparison were performed by an analysis of variance
(one-way ANOVA followed by Dunnett's post hoc test). If the data
did not have a normal distribution, the data should be analyzed
using the nonparametric Mann-Whitney test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of different TLR agonists on
TIPE2 expression
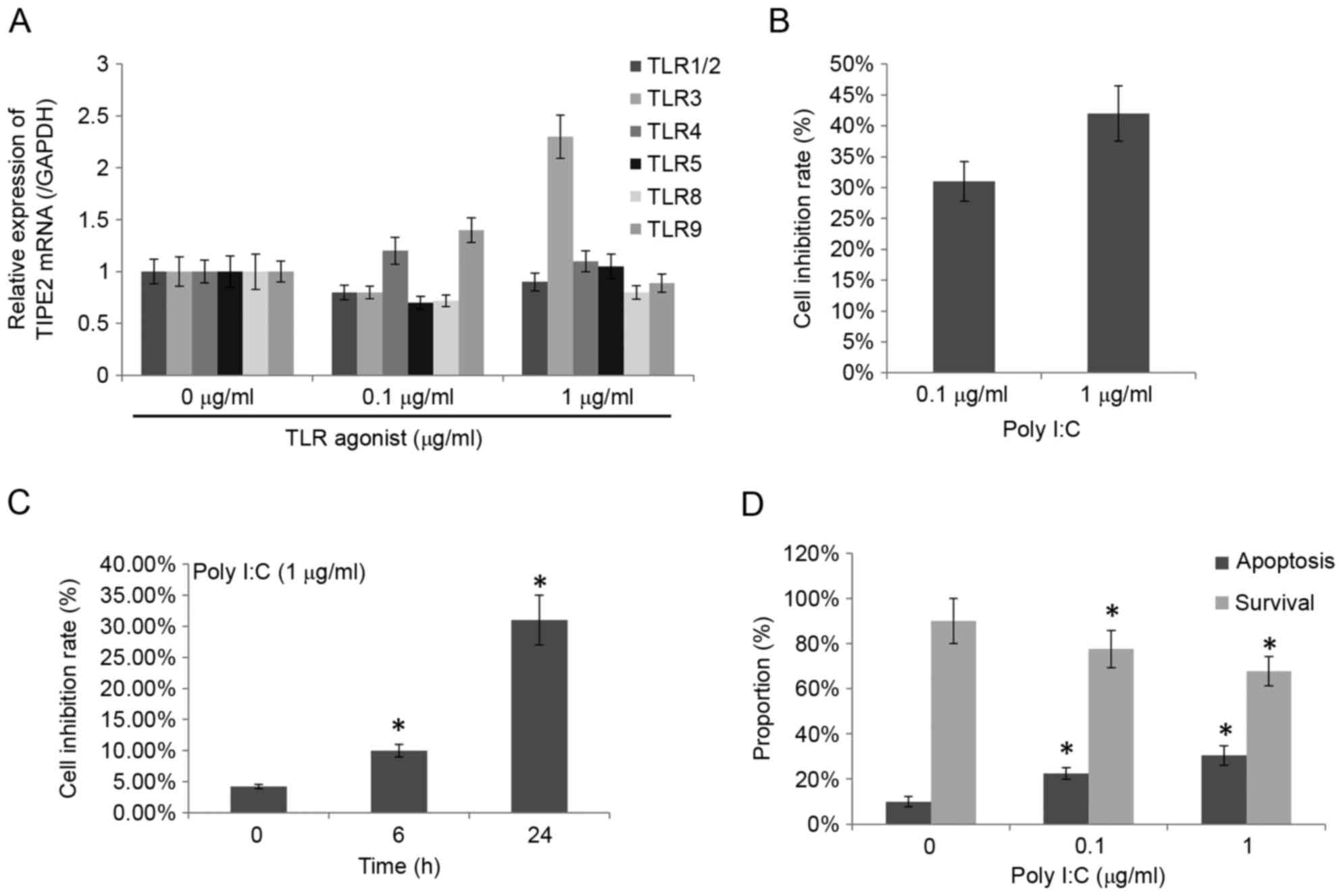
TIPE2 was affected by Poly I:C activation and the
other specific activators revealed specific changes (Fig. 1A). However, the group treated with
Poly I:C had the highest significant upregulation of TIPE2
expression (0.8±0.06 and 2.3±0.21 with the relative expression
intensity of 0.1 and 1 µg/ml, respectively) and it was the main
agonist for TLR3 specific agonist Poly I:C. A previous study
revealed that Poly I:C may inhibit the proliferation of a variety
of cells and induce apoptosis (7).
The effect of different Poly I:C concentrations and different
incubation times on the growth inhibition rate of THP-1 cells was
detected using an MTT assay and the results are presented in
Fig. 1B and C. Poly I:C
significantly inhibited the THP-1 cells, with the growth inhibition
rate reaching 42% at a concentration of 1 µg/ml Poly I:C and the
rate increased with greater incubation time. Subsequently, using
Annexin V/PI double staining to detect the effect of Poly I:C on
THP-1 apoptosis, it was determined that the apoptotic cells
gradually increased and the survival cells reduced significantly
with greater Poly I:C concentration (P<0.05; Fig. 1D).
Construction of cell culture for
silencing TIPE2 expression
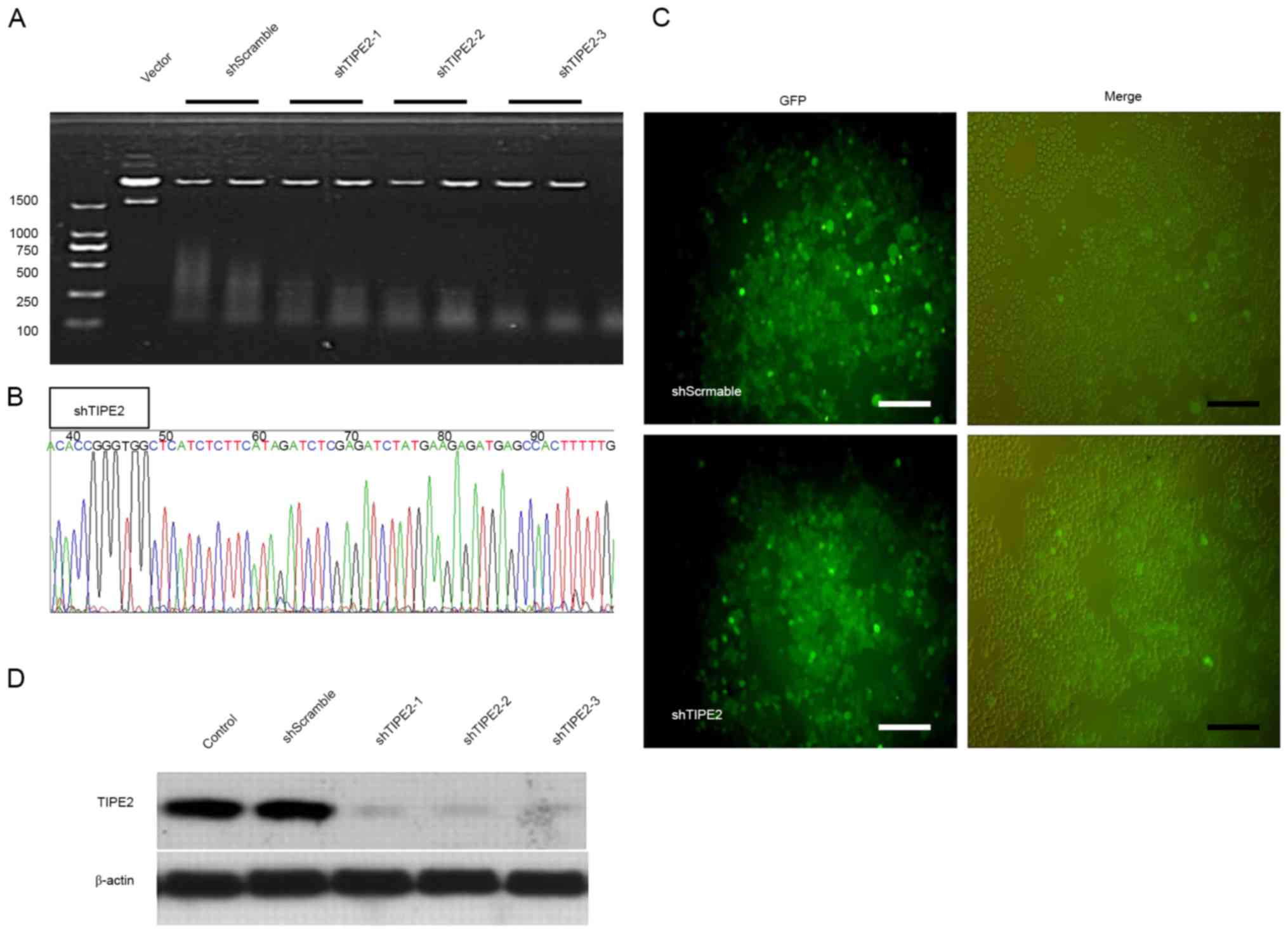
Previous studies have revealed that TIPE2 may be
involved in the activation of caspase-8 (3). In order to confirm that TLR3-specific
inhibition of the THP-1 cells and the induced apoptosis by Poly I:C
treatment may be associated with TIPE2 upregulation, the expression
of TIPE2 in THP-1 cells was silenced using lentivirus technology
(Fig. 2A). The constructed vector
was transformed into competent cells DH5α. Following the
extraction, the plasmids were identified by enzyme digestion, with
the fragment sizes from the original plasmid digested to be 7,872,
2,155 and 190 bp, whereas the sizes from plasmids inserted with
shRNA should be 7,872, 190, 303 and 42 bp. The plasmids with the
appropriate digestion fragment sizes were sequenced for
verification (Fig. 2B). The
verified plasmids were selected for extraction and the endotoxin
was removed for transfecting the packaging virus. As presented in
Fig. 2C, the higher of the viral
titer was, the better effect of infection was on THP-1 cells. The
successful transfection of shTIPE2 was verified using western
blotting (Fig. 2D), and it was
revealed that the three types of shRNA designed successfully
silenced TIPE2 expression.
Influence of silencing the expression
of TIPE2 on Poly I:C-induced apoptosis
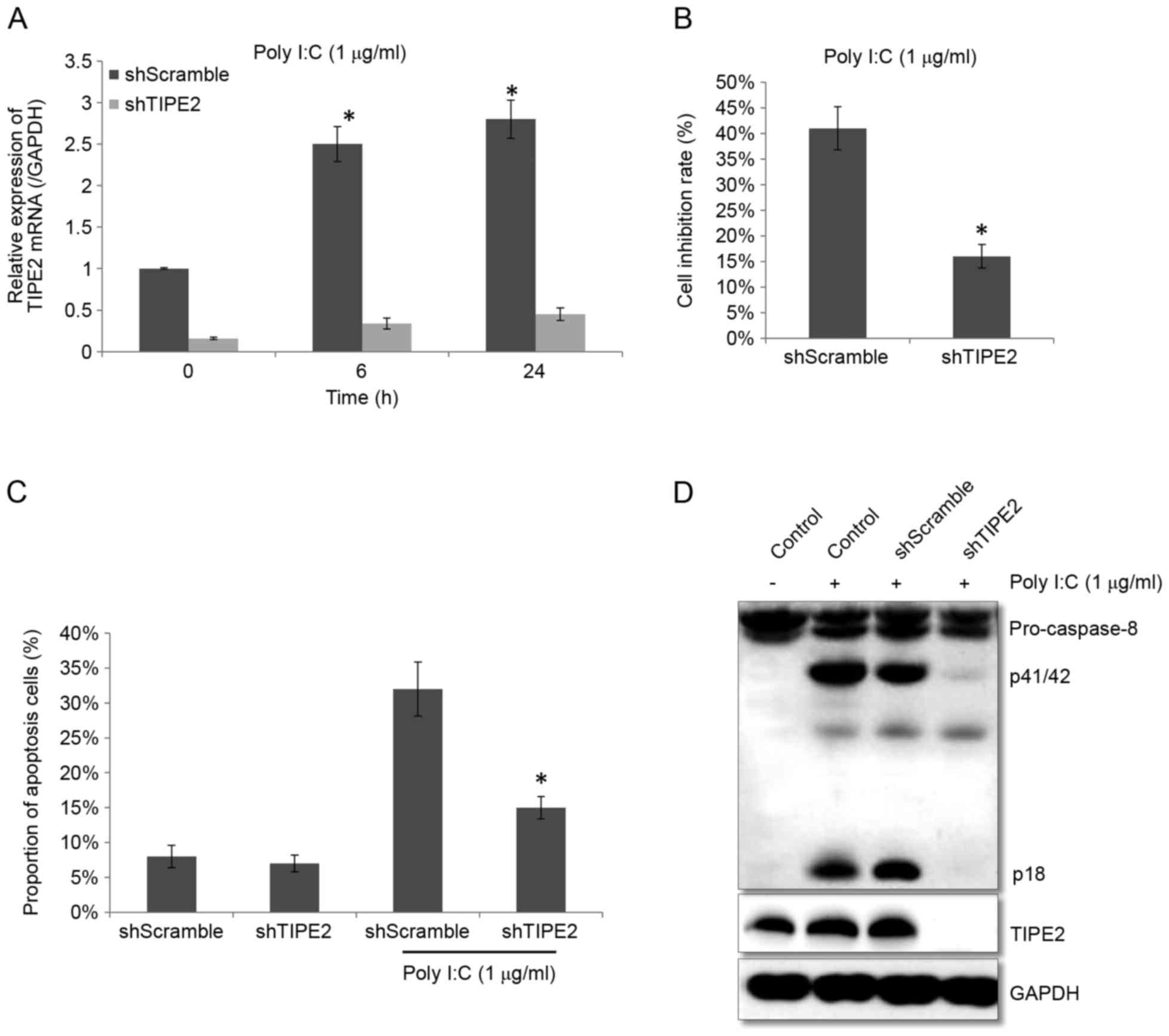
As presented in Fig.
3A, after using shRNA to silence the expression of TIPE2 in
THP-1 cells, the TIPE2 expression in the shScramble group was
higher when treated with Poly I:C, which may significantly increase
the expression of TIPE2, whereas in shTIPE2 group, the upregulation
of TIPE2 expression that was stimulated by Poly I:C treatment was
significantly inhibited (P<0.05; Fig. 3A). Comparing the effect of Poly I:C
treatment on the cell growth inhibition rate in the shScramble
group with the shTIPE2 group revealed that the growth inhibition
rate in the shScramble group was 41.2±4.3%, whereas the rate in the
shTIPE2 group was significantly reduced at 16±2.3% (P<0.05;
Fig. 3B). Apoptosis was
subsequently detected by flow cytometry, which revealed that the
percentage of apoptotic cells in the shTIPE2 group was 15.2±1.6%
after Poly I:C treatment for 24 h, whereas in the shScramble group
apoptotic cells were significantly greater at 32.2±3.9% (P<0.05;
Fig 3C). Subsequently, expression
of caspase-8 was determined to be reduced as the expression of
p41/42 was decreased in shTIPE2 group. Additionally, western blot
analysis revealed that the protein expression level of TIPE2 was
significantly reduced in the shTIPE2 group, whereas that had no
influence in shScramble group, suggesting that the silencing of the
TIPE2 gene may reduce the apoptosis of THP-1 cells induced by Poly
I:C.
Discussion
TLR is part of the innate immune-mediated
transmembrane signaling receptor family, which has an important
role in cell activation signal transduction, and links innate
immunity with adaptive immunity. Previous studies revealed that
TLR-conducted innate immunity had the same significance in the
injury process of inflammatory cells induced by virus (8,9).
TLR3 is an important member from TLR family, which is composed of
extracellular leucine-rich repeats, transmembrane domain and
cytoplasmic kinase domains (10).
It can recognize specific double-stranded RNA (dsRNA) of pathogenic
viruses. dsRNA viruses have pathogen associated molecular patterns,
which may be identified by TLR3. During the replication period of a
variety of viruses, large quantities of dsRNA were produced.
Therefore, TLR3 may be used as an important anti-viral defense.
TLR3 contributes to the anti-viral process in the host and also has
a close association with some autoimmune diseases. Previous studies
determined that the RNA released by necrotic cells or mRNA produced
from transcription in vitro may activate TLR3, indicating
that the RNA released by necrotic cells may be used as the
endogenous ligand to activate or regulate immune response (11). As most autoimmune diseases have not
been identified to have a direct contact with the virus infection,
endogenous ligands may have greater significance on the
pathogenesis mechanism of TLR3 activation in autoimmune diseases
(12).
TIPE2, as a new protein molecule, has partial
sequences which are the similar to those of tumor necrosis
factor-induced protein 8. TIPE2 is selectively expressed in
lymphoid and myeloid-derived immune cells and it has a negative
regulation on the innate immunity and cellular immunity. It may
inhibit the activation of transcription factor activator protein-1
and nuclear factor-kB (3). Unlike
the TIPE2 expressed primarily in mice lymphocytes, TIPE2 is
expressed in various human tissues and cell types, including stem
cells, neurons in the brain and brainstem, esophagus, cervical
squamous cells, the junction of the bladder and urethra, colon,
gastric epithelial cells and the appendix (13). It was identified to be expressed at
high levels particularly terminal differentiated cells and its
expression was downregulated in precursor cells, indicating that
TIPE2 may be associated with the cell proliferation and
differentiation (13). TIPE2 has
been previously revealed to exhibit abnormal expression in various
diseases, including hepatitis (14), liver cancer, stroke (15), kidney rejection (16), hepatitis C virus, asthma (17), myasthenia gravis (18), lupus erythematosus (19). The expression of TIPE2 in
peripheral blood of the patients was reduced in a variety of
autoimmune diseases, suggesting that immune cells were in a
highly-activated state. It may aid in the reduction of the
inhibition of autoimmune cell activity and the damage to normal
tissues by specifically inducing the expression of TIPE2 in immune
cells (20).
As an agonist of endogenous TLR3, Poly I:C may
induce apoptosis in a variety of cell types, such as the epithelial
cells of the bile duct and it may contribute to the inflammatory
lesions of liver cirrhosis (21).
Exogenous or endogenous dsRNA may induce various types of cell
death via the TLR3 or IRF-3 pathway, including pancreatic β cell
death (22), prostate cancer
(23) and salivary gland
epithelial cells (24). TLR3 is a
pattern recognition receptor, and the apoptosis caused by TLR3 is
primarily associated with caspase-8 (25). As an important chaperonin of
caspase-8, TIPE2 has an essential role in its activity; therefore,
it is considered that activation of caspase-8 and TIPE2 may be the
underlying mechanism which triggers the apoptosis following Poly
I:C-induced TLR3 activation. In addition, previous studies about
tumors (26) have revealed that
the expression of TIPE2 was reduced in some tumors and TIPE2 was
the primary inhibitor of Ras, which inhibited the formation of Ras
complex by inhibiting ral guanine nucleotide dissociation
stimulator, ultimately leading to the inhibition of Ral and Akt. If
the expression was upregulated, it may reduce the activation of Ras
and in turn decrease cell proliferation and migration. Therefore,
it is possible that that Poly I:C activates TLR3 and upregulates
the expression of TIPE2, which may lead to further inhibition of
Ras, thereby inhibiting cell proliferation. Poly I:C may influence
the migration of cells and their phagocytic and antibiotic
abilities. The cell proliferation and migration speed increased
after following silencing of TIPE2 and it may inhibit the
exocytosis, conversely after overexpression of TIPE2, it would
suppress the hepatoma cell proliferation and the invasion in
vivo and in vitro (27,28).
Previous studies revealed that TIPE2 is associated with reducing
Rac1 and F-actin levels and the activation of urokinase (29). The present study observed that
THP-1 cell proliferation increased following silencing of TIPE2,
which was consistent with previous studies (30).
Therefore, TIPE2 has an important role in autoimmune
diseases and may also contribute to the development of tumors.
Artificial upregulation of TIPE2 expression in tumor cells may
inhibit the cancer cell proliferation and migration; however, it
may also affect the activation of immune cells. Therefore, the
potential negative impact on the normal immune system should be
considered if the TIPE2 is used as a drug target in future cancer
therapy.
Acknowledgements
The present study was funded by the Social
Development Project of Ningbo (grant no. 2013C50043).
References
|
1
|
Freundt EC, Bidere N and Lenardo MJ: A
different TIPE of immune homeostasis. Cell. 133:401–402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tibbetts MD, Zheng L and Lenardo MJ: The
death effector domain protein family: Regulators of cellular
homeostasis. Nat Immunol. 4:404–409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng Y, Zhao Q, Zhang H, Han B and Liu S,
Han M and Liu S: TIPE2, a negative regulator of TLR signaling,
regulates p27 through IRF4-induced signaling. Oncol Rep.
35:2480–2486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lou Y and Liu S: The TIPE (TNFAIP8) family
in inflammation, immunity, and cancer. Mol Immunol. 49:4–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takemura R, Takaki H, Okada S, Shime H,
Akazawa T, Oshiumi H, Matsumoto M, Teshima T and Seya T:
PolyI:C-Induced, TLR3/RIP3-dependent necroptosis backs up immune
effector-mediated tumor elimination in vivo. Cancer Immunol Res.
3:902–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colonna M, Trinchieri G and Liu YJ:
Plasmacytoid dendritic cells in immunity. Nature Immunol.
5:1219–1226. 2004. View
Article : Google Scholar
|
|
10
|
Boehme KW and Compton T: Innate sensing of
viruses by toll-like receptors. J Virol. 78:7867–7873. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mori K, Yanagita M, Hasegawa S, Kubota M,
Yamashita M, Yamada S, Kitamura M and Murakami S: Necrosis-induced
TLR3 activation promotes TLR2 expression in gingival cells. J Dent
Res. 94:1149–1157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rifkin IR, Leadbetter EA, Busconi L,
Viglianti G and Marshak-Rothstein A: Toll-like receptors,
endogenous ligands, and systemic autoimmune disease. Immunol Rev.
204:27–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang
Y, Qu Z, Guo C, Chen Y, Zhang Y and Liu S: Tissue-specific
expression of TIPE2 provides insights into its function. Mol
Immunol. 47:2435–2442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y,
Qu Z, Cui J, Zhang G, Liang X, et al: Roles of TIPE2 in hepatitis B
virus-induced hepatic inflammation in humans and mice. Mol Immunol.
48:1203–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Wei X, Liu L, Liu S, Wang Z,
Zhang B, Fan B, Yang F, Huang S, Jiang F, et al: TIPE2, a novel
regulator of immunity, protects against experimental stroke. J Biol
Chem. 287:32546–32555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia L, Gui B, Tian P, Yao G, Fu R, Wang L,
Ge H and Ou Y: TIPE2, a novel biomarker for clinical chronic kidney
allograft rejection. Artif Organs. 37:221–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma Y, Liu X, Wei Z and Wang X, Wang Z,
Zhong W, Li Y, Zhu F, Guo C, Zhang L and Wang X: The expression and
significance of TIPE2 in peripheral blood mononuclear cells from
asthmatic children. Scand J Immunol. 78:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Shao Z, Zhang X, Jia X, Xia Y,
Zhang Y, Xin N, Guo M, Chen J, Zheng S, et al: TIPE2 play a
negative role in TLR4-mediated autoimmune T helper 17 cell
responses in patients with myasthenia gravis. J Neuroimmune
Pharmacol. 10:635–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li F, Zhu X, Yang Y, Huang L and Xu J:
TIPE2 alleviates systemic lupus erythematosus through regulating
macrophage polarization. Cell Physiol Biochem. 38:330–339. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qian J, Meng Z, Guan J, Zhang Z and Wang
Y: Expression and roles of TIPE2 in autoimmune hepatitis. Exp Ther
Med. 13:942–946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rong GH, Yang GX, Ando Y, Zhang W, He XS,
Leung PS, Coppel RL, Ansari AA, Zhong R and Gershwin ME: Human
intrahepatic biliary epithelial cells engulf blebs from their
apoptotic peers. Clin Exp Immunol. 172:95–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dogusan Z, García M, Flamez D, Alexopoulou
L, Goldman M, Gysemans C, Mathieu C, Libert C, Eizirik DL and
Rasschaert J: Double-stranded RNA induces pancreatic beta-cell
apoptosis by activation of the toll-like receptor 3 and interferon
regulatory factor 3 pathways. Diabetes. 57:1236–1245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gambara G, Desideri M, Stoppacciaro A,
Padula F, De Cesaris P, Starace D, Tubaro A, Del Bufalo D,
Filippini A, Ziparo E and Riccioli A: TLR3 engagement induces
IRF-3-dependent apoptosis in androgen-sensitive prostate cancer
cells and inhibits tumour growth in vivo. J Cell Mol Med.
19:327–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura H, Horai Y, Suzuki T, Okada A,
Ichinose K, Yamasaki S, Koji T and Kawakami A: TLR3-mediated
apoptosis and activation of phosphorylated Akt in the salivary
gland epithelial cells of primary Sjögren's syndrome patients.
Rheumatol Int. 33:441–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Estornes Y, Toscano F, Virard F, Jacquemin
G, Pierrot A, Vanbervliet B, Bonnin M, Lalaoui N, Mercier-Gouy P,
Pachéco Y, et al: dsRNA induces apoptosis through an atypical death
complex associating TLR3 to caspase-8. Cell Death Differ.
19:1482–1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YH, Yan HQ, Wang F, Wang YY, Jiang
YN, Wang YN and Gao FG: TIPE2 inhibits TNF-α-induced hepatocellular
carcinoma cell metastasis via Erk1/2 downregulation and NF-κB
activation. Int J Oncol. 46:254–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo
C, Zhu F, Wang Q, Wang J, Wang X, et al: Human tumor necrosis
factor (TNF)-alpha-induced protein 8-like 2 suppresses
hepatocellular carcinoma metastasis through inhibiting Rac1. Mol
Cancer. 12:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Shi YY, Wang Y, Zhu F, Wang Q, Ma
C, Chen YH and Zhang L: The unique expression profile of human
TIPE2 suggests new functions beyond its role in immune regulation.
Mol Immunol. 48:1209–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Li X, Liu G, Sun R, Wang L, Wang J
and Wang H: Downregulated TIPE2 is associated with poor prognosis
and promotes cell proliferation in non-small cell lung cancer.
Biochem Biophys Res Commun. 457:43–49. 2015. View Article : Google Scholar : PubMed/NCBI
|