Introduction
Ovarian cancer (OC) is a fatal disease in women.
According to studies on the global patterns of cancer there are
~152,000 mortalities and 239,000 new OC cases annually (1). The 5-year survival rate of OC
following diagnosis is only 20–30% (2). A previous study indicated that the
high mortality rate and poor prognosis may be associated with OC
cell invasion and metastasis (3).
Epithelial-mesenchymal transition (EMT) is a process
by which an epithelial cell loses its cell-cell adhesion, gains the
capacity for invasion and migration, and turns into a mesenchymal
stem cell (4). EMT is recognized
as one of the crucial steps in OC pathogenesis (5), and serves an important role in OC
metastasis and invasion (6).
Therefore, an improved understanding of the EMT process and
cellular influences in OC would supply novel insights for the
diagnosis and treatment of OC.
MicroRNAs (miRs) are epigenetic factors that can
regulate a number of human biological processes, including cell
proliferation, tissue differentiation, cancer formation and
metastasis (7–10). Up- and downregulation of miR is
involved in the regulation of EMT in OC progression (11,12).
miR-141 has been reported to be associated with a number of types
of cancer, including colonic cancer (13), gastric cancer (14) and renal cell carcinoma (15). Tamagawa et al (16) reported that miR-141 serves a key
role in the regulation of migration and EMT in head and neck
squamous cell carcinoma. In addition, upregulation of miR-141 is
confirmed to inhibit cell proliferation and invasion by suppressing
the Wnt signaling pathway in renal cell carcinoma (17). However, the effects of miR-141 on
EMT and human OC migration and invasion remain to be
demonstrated.
In the present study, miR-141 expression in SKOV3
cells was measured, in addition to the mRNA and protein levels of
EMT markers: Vimentin; epithelial-cadherin (E-cadherin);
integrin-β; β-catenin and zinc finger E-box-binding homeobox (ZEB),
using the reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting, respectively. Then cell
proliferation, invasion and migration assays were performed. The
aims of the present study were to determine the effects of miR-141
on EMT, and on OC cell migration and invasion.
Materials and methods
Cell culture
The human OC cell line SKOV3 was obtained from the
Shanghai Institute of Biochemistry and Cell Biology, Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences
(Shanghai China) was cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin. The cell
line was cultured at 37°C with 5% CO2. The experiment
was conducted following the protocol approved by Tongji University
(Shanghai China).
Cell transfection
SKOV3 cells were seeded in 6-well plates at a
density of 4×105 cells/ml, 24 h prior to transfection.
When the cells reached 60% confluence (~24 h), the cells were
divided into four groups and transfected with 50 nM miR-141 mimic
(mimic group, 5′-UAACACUGUCUGGUAAAGAUGG-3′), miR-141 inhibitor
(inhibitor group, 5′-CCATCTTTACCAGACAGTGTTA-3′) or miR-141
nonspecific sequences (NC group, 5′-UUCUCCGAACGUGUCACGUTT-3′), or
left untransfected (blank group) using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
mimic, inhibitor and NC of miR-141 were purchased from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China).
RNA extraction and RT-qPCR
Cells were harvested 48 h following transfection.
RNA was extracted from the cells with TRIzol® reagent
and chloroform, according to the manufacturer's protocol (Guangzhou
RiboBio Co., Ltd.). The RNA was used as the template for the
synthesis of DNA using an RT-PCR kit (Guangzhou RiboBio Co.,
Ltd.).
Analysis of the miR-141 expression level in the
transfected OC cell line SKOV3 was performed using the Bulge-Loop™
miR RT-qPCR kits (miRQ0000432-1-1; Guangzhou RiboBio Co., Ltd.),
according to the manufacturer's protocol, and U6 (MQP-0201;
Guangzhou RiboBio Co., Ltd.) was measured as endogenous control to
perform relative quantification. qPCR was carried out at 95°C for
10 min, followed by 40 cycles at 95°C for 10 sec, 60°C for 30 sec
and 72°C for 1 min.
In order to evaluate the effects of miR-141 on EMT,
qPCR assays were performed using SYBR® Green
(Invitrogen; Thermo Fisher Scientific, Inc.) for the expression of
vimentin (forward primer, 5′-AAGGAGGAAATGGCTCGTCAC-3′; reverse
primer, 5′-CTCAGGTTCAGGGAGGAAAAGT-3′), E-cadherin (forward primer,
5′-GTCACTGACACCAACGATAATCCT-3′; reverse primer,
5′-TTTCAGTGTGGTGATTACGACGTTA-3′), integrin-β (forward primer,
5′-AATGTAACCAACCGTAGC-3′; reverse primer,
5′-GGTCAATGGGATAGTCTTC-3′), β-catenin (forward primer,
5′-GGGCGGCACCTTCCTACTTC-3′; reverse primer,
5′-AGCTCCCTCGCGGTTCAT-3′) and ZEB (forward primer,
5′-AAGTGGGCGGTAGATGGTA-3′; reverse primer,
5′-TTGTAGCGACTGGATTTT-3′). GAPDH was regarded as an internal
control gene. The method of quantification was 2−ΔΔCq
(18). The PCR primers (Table I) were designed by Primer Premier
version 5 (Premier Biosoft International, Palo Alto, CA, USA). The
reaction was performed at 95°C for 10 min, followed by 40 cycles at
95°C for 10 sec, 60°C for 30 sec, and 72°C for 1 min. Amplified
products were checked using an Applied Biosystems 7300 Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
 | Table I.Polymerase chain reaction primer
sequences. |
Table I.
Polymerase chain reaction primer
sequences.
|
| Primer sequence
(5′-3′) |
|
|---|
|
|
|
|
|---|
| Gene name | Forward | Reverse | Tm,°C |
|---|
| Vimentin |
AAGGAGGAAATGGCTCGTCAC |
CTCAGGTTCAGGGAGGAAAAGT | 60 |
| E-cadherin |
GTCACTGACACCAACGATAATCCT |
TTTCAGTGTGGTGATTACGACGTTA | 60 |
| Integrin-β |
AATGTAACCAACCGTAGC |
GGTCAATGGGATAGTCTTC | 52 |
| β-catenin |
GGGCGGCACCTTCCTACTTC |
AGCTCCCTCGCGGTTCAT | 61 |
| ZEB |
AAGTGGGCGGTAGATGGTA |
TTGTAGCGACTGGATTTT | 60 |
Protein extraction and western blot
analysis
The cellular proteins vimentin, β-catenin,
integrin-β, E-cadherin and ZEB were extracted for western blotting
as previously described (19). BCA
protein assay kit (Thermo Scientific, USA) was used to determine
the protein concentration, 100 µg proteins were separated on 10%
SDS-PAGE for every cell line, and the gel was subsequently
transferred onto a nitrocellulose (NC) filter. Then, the NC filter
was immersed in the blocking buffer (Sigma, USA) and agitated for
1–3 h at room temperature. Specific proteins were detected with
monoclonal mouse anti-vimentin (MAB2105), mouse anti-β-catenin
(MAB13291), mouse anti-integrin-β (MAB1778), mouse anti-E-cadherin
(MAB1838) and mouse anti-ZEB antibody (MAB6708; all from R&D
Systems, Inc., Minneapolis, MN, USA) for the primary antibodies
(1:100 dilution), agitated for 1–3 h at room temperature or
overnight at 4°C, and then were washed with TBST 3 times. The
secondary antibodies used were goat anti-mouse antibodies (sc-2005;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
anti-β actin (4967; 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA), shaking 1–3 h at room temperature. Then, they
were washed with TBST 3 times. Protein bands on the membranes were
visualized by chemiluminescence (ECL) reagent (WBKLS0500; Merck
KGaA, Darmstadt, Germany) and imaged with ImageQuant LAS4000 mini
analysis system (GE Healthcare Life Sciences, Little Chalfont,
UK).
MTT assay
SKOV3 cells were seeded into a 96-well plate at a
density of 5×104 cells/well and transfected as described
above. At 48 h following transfection, 10 µl MTT solution (5 mg/ml,
Sigma-Aldrich; Merck KGaA) was added to each well and incubated at
37°C for 4 h. Then, 100 µl solubilization solution [50%
dimethylsulfoxide and 20% SDS (pH 4.8)] was added to each well
followed by agitation for 10 min. The absorption values were
measured at 570 nm.
Invasion assay
Invasion assays were performed in triplicate using
polycarbonate membranes (8-µm pore size) in 6-well tissue culture
plates, which were coated with Matrigel® (BD
Biosciences, Franklin Lakes, NJ, USA) and were cultured for 48 h.
SKOV3 cells (3×104/chamber) of the different groups and
DMEM containing 10% fetal calf serum (Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd., Zhejiang, China) was added to the
upper and to the lower chambers, respectively. Following incubation
for 24 h, cells on the top filter were scrapped off, rinsed into
the lower chamber tissue culture, fixed in 4% paraformaldehyde for
15 min at room temperature, washed with PBS twice and stained with
0.1% crystal violet for 20 min at room temperature. A total of five
random fields was calculated.
Scratch migration assay
SKOV3 cells were transfected for 24 h then a scratch
(repeated six times) in the cell monolayer was made with a cell
scratch spatula. Cells were washed three times with PBS and
cultured under standard conditions for 72 h. Following discarding
of the supernatant, the cells were fixed in 4% paraformaldehyde for
30 min, and images were captured at 0, 24, 48, and 72 h, under an
optical microscope. The potential of migration was calculated by
counting the number of cells that migrated from the wound edge.
Statistical analysis
Data were presented as the mean ± standard
deviation. The statistical significance of differences between
groups was determined using one-way analysis of variance followed
by a Fisher's least significant difference post hoc test.
Statistical analysis was performed with SPSS software (version
20.0; IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-141 overexpression in SKOV3
cells
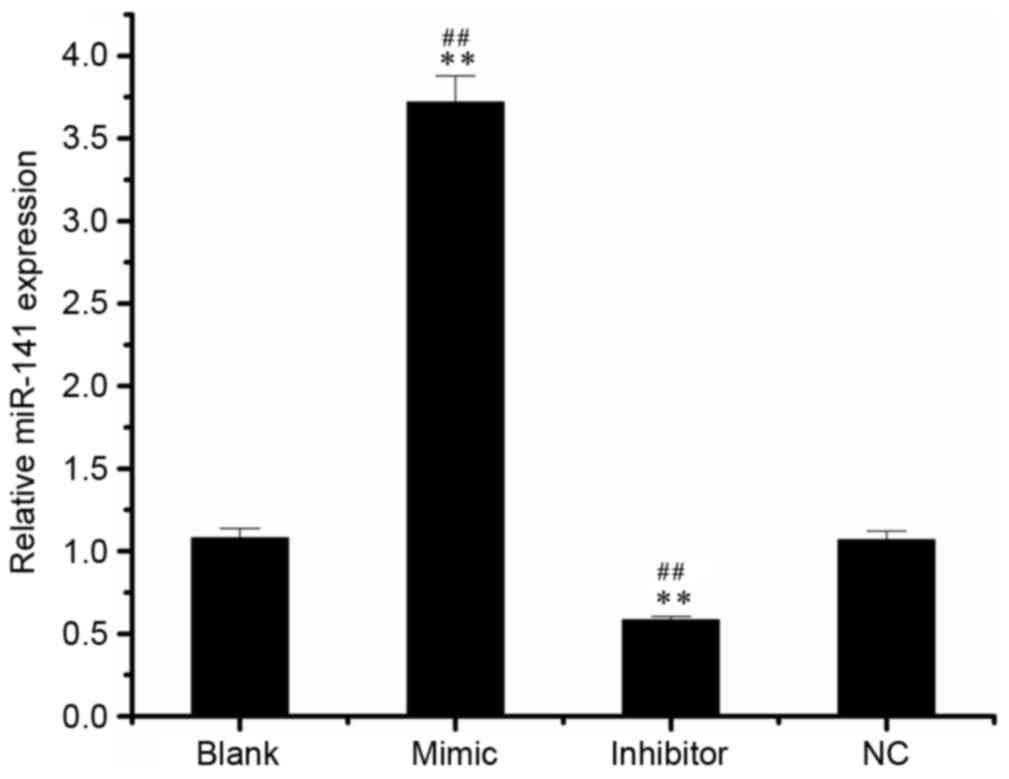
Following the detection of miR-141 expression in
SKOV3 cell by RT-qPCR, it was demonstrated that miR-141 levels in
the mimic group (3.72±0.16) were increased compared with the blank
(1.08±0.06) and NC groups (1.07±0.05; Fig. 1). In addition, the miR-141 level in
the inhibitor group (0.59±0.02) was significantly decreased
compared with the blank or NC groups.
Expression of EMT markers in different
groups
To investigate the effect of miR-141 on EMT, mRNA
and protein levels of EMT markers were detected in the mimic,
inhibitor, NC and blank groups. As exhibited in Fig. 2A, the mRNA expression levels of
E-cadherin and integrin-β were increased in the mimic group and
decreased in the inhibitor group compared with the NC or blank
groups. Compared with the NC or blank groups, the mRNA level of ZEB
was decreased in the mimic group and increased in the inhibitor
group. In addition, the mRNA level of vimentin was increased in the
mimic and inhibitor groups compared with the NC or blank groups.
However, no significant differences were identified in the
expression of β-catenin between mimic and blank or NC groups.
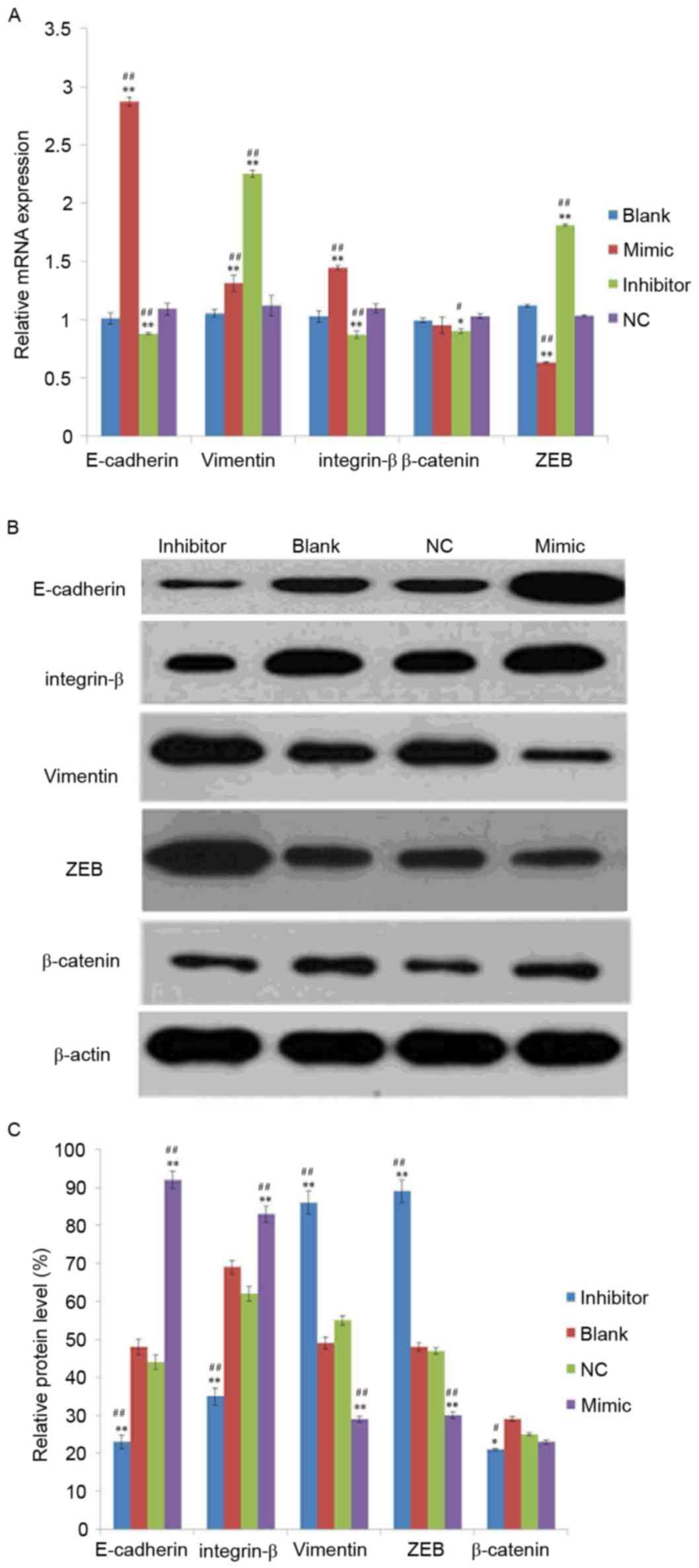 | Figure 2.Analysis of epithelial mesenchymal
transition-marker expression in SKOV3 cells. (A) mRNA and (B)
protein expression of vimentin, E-cadherin, integrin-β, β-catenin
and ZEB in transfected-SKOV3 cells. (C) The relative protein level
was normalized using β-actin. SKOV3 cells were transfected with 50
nM microRNA-141 mimic group, inhibitor group or NC, or left
untransfected. *P<0.05, **P<0.01 vs. NC group,
#P<0.05, ##P<0.01 vs. blank group.
E-cadherin, epithelial-cadherin; ZEB, zinc finger E-box-binding
homeobox; NC, nonspecific sequences. |
Following analysis of the protein expression of
vimentin, β-catenin, integrin-β, E-cadherin and ZEB using western
blotting, it was demonstrated that all were expressed in the SKOV3
transfected cells (Fig. 2B) and
their relative protein expression exhibited a similar tend to the
mRNA expression (Fig. 2C).
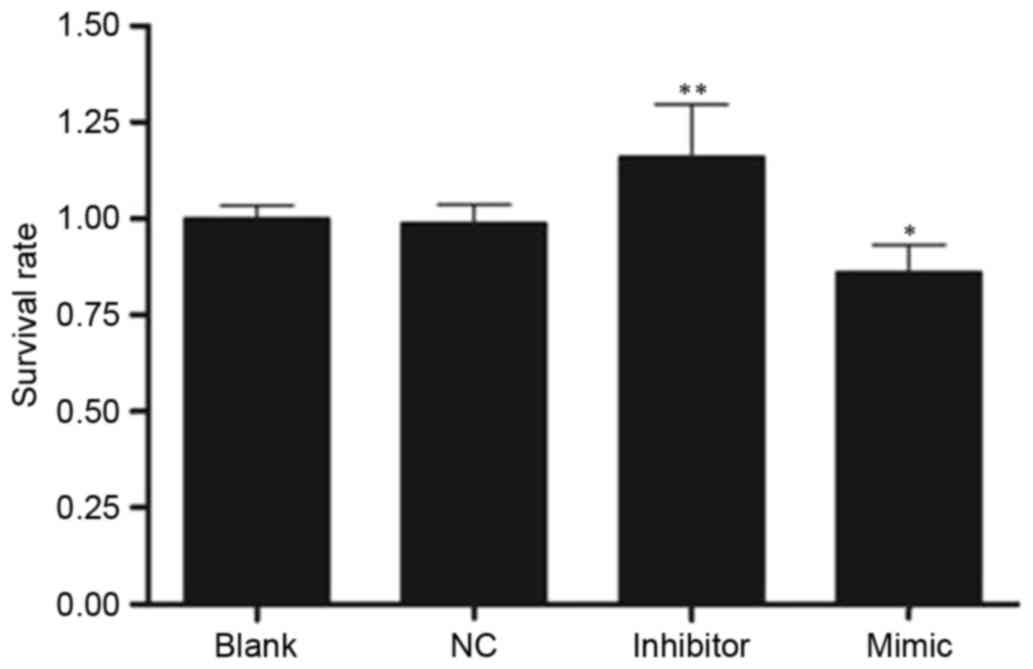
MTT assay
Cell proliferation was assessed using the MTT assay
and the value of optical density at 570 nm (OD570) in the four
groups is exhibited in Fig. 3. The
rate of cell proliferation was increased in the inhibitor group
(1.10±0.13) and decreased in the mimic group (0.80±0.07) compared
with the NC group (0.92±0.05; both P<0.05).
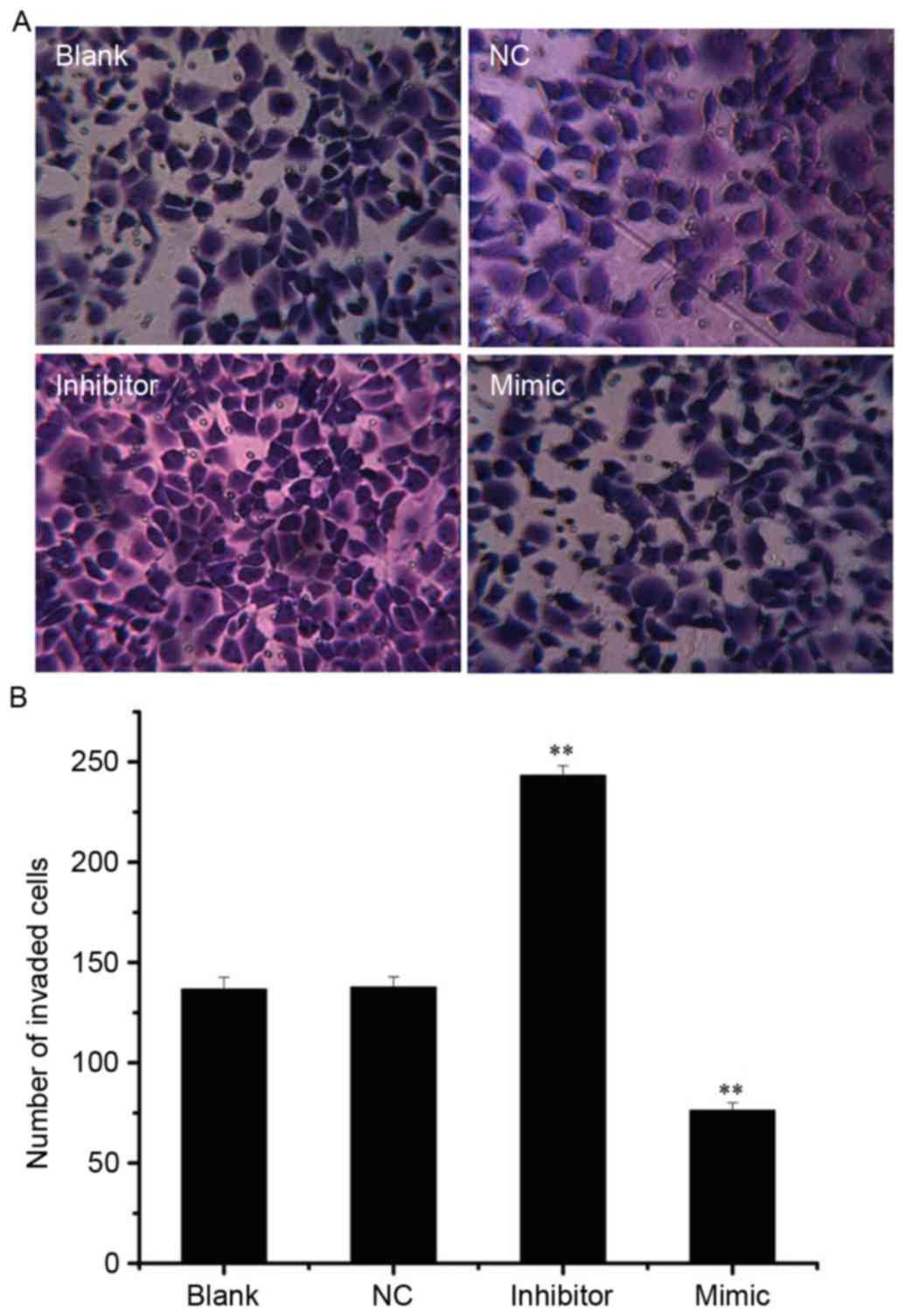
miR-141 inhibits the invasion and
migration of SKOV3 cells in vitro
Following analysis of the effect of miR-141 on SKOV3
cell invasion, the invasive cells were visualized in the mimic,
inhibitor, NC and blank groups (Fig.
4A). Compared with the NC group (137.67±4.04), the number of
invaded cells was significantly increased in the inhibitor group
(243.00±6.24) and decreased in the mimic group (76.33±2.08; both
P<0.01; Fig. 4B).
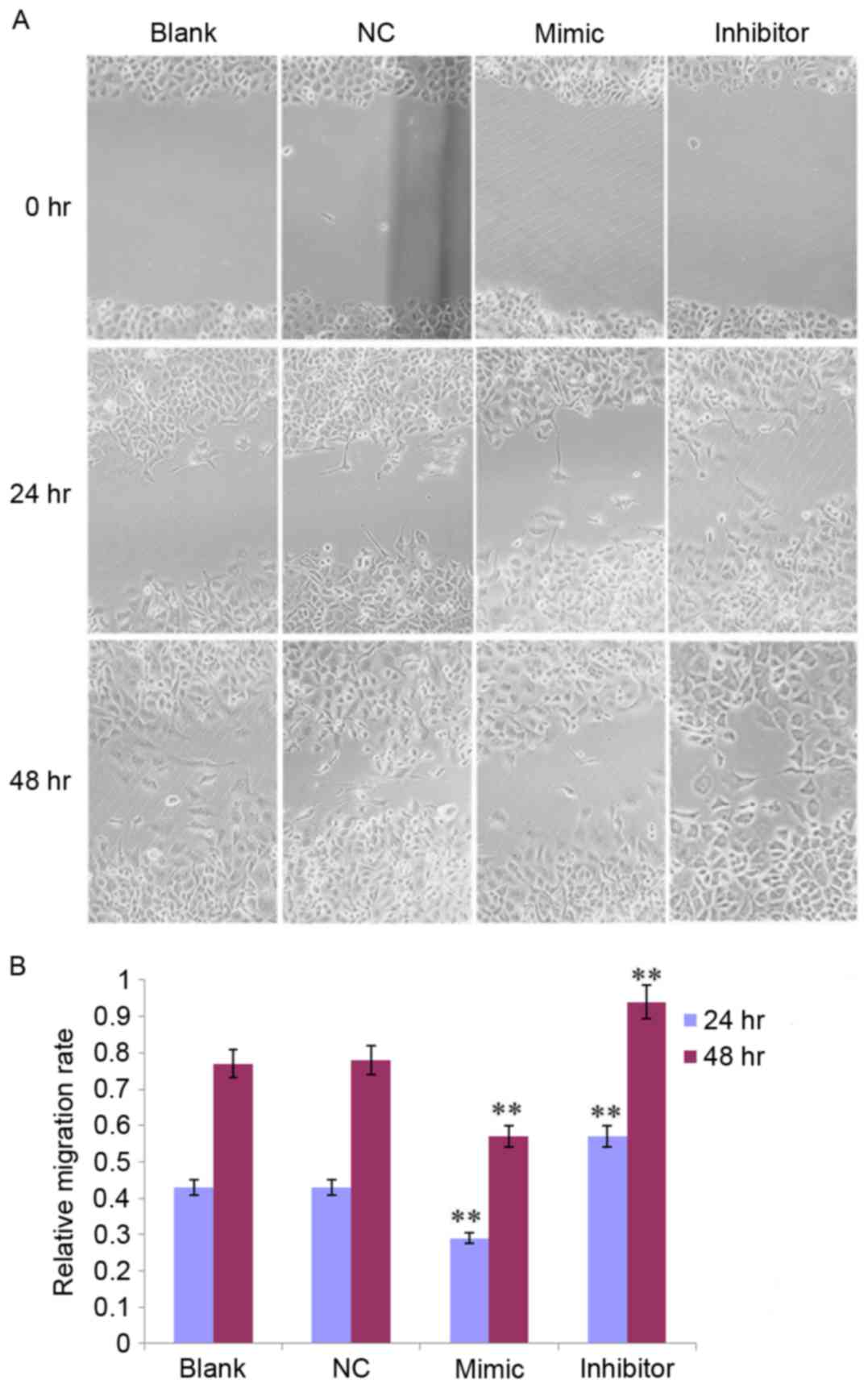
In addition, the effect of miR-141 on SKOV3 cell
migration was observed at 0, 24 and 48 h, respectively (Fig. 5A). The migratory rate was decreased
in the mimic group and increased in the inhibitor group at 24 and
48 h compared with the NC group (all P<0.01; Fig. 5B).
Discussion
In the present study, miR-141 expression was
investigated in the OC cell line SKOV3 following transfection with
an miR-141 mimic, inhibitor or NC, or no transfection.
Overexpression of miR-141 caused upregulation of E-cadherin,
inhibited cell proliferation and EMT in the SKOV3 cell line, and
decreased cell invasion and migration.
Cancer progression has similarities with the
developmental process of EMT (20). EMT results in the morphological
alteration of an epithelial cell to a mesenchymal cell, and
enhances the ability of cancer cells to metastasize and invade
(21). The molecular
characteristics of the alteration of epithelial cells to
mesenchymal cells are the downregulation of epithelial cell
markers, including E-cadherin, β-catenin, integrin-β, cytokeratin
and mucin expression, as well as upregulation of mesenchymal
markers, including vimentin and fibronectin, followed by
alterations of epithelial mesenchymal transition-associated
transcription factor expression (22–24).
Among them, E-cadherin is an important molecule in the maintenance
of the epithelial phenotype, and its decreased expression is an
important marker of EMT. ZEB1 and ZEB2, two members of the ZEB
family, are important regulators of EMTs, and can bind to the
enhancer box motif E2 [CACCT (G)] on the E-cadherin promoter,
inhibit transcription of E-cadherin, induce EMT, and enhance cell
invasion and metastasis (25,26).
As a subunit of integrin-β, integrin-β5 adhesion serves an
important role in transforming growth factor-β-induced EMT
(27). Vimentin is used as a
marker of EMT. A previous study reported that vimentin is essential
for alterations in cell shape, adhesion and motility during EMT
(28).
In the present study, E-cadherin and integrin-β mRNA
expression was increased in the mimic group and decreased in the
inhibitor group compared with the NC group. In addition, ZEB was
decreased in the mimic group and increased in the inhibitor group,
and vimentin was increased in the mimic and inhibitor group
compared with the NC or blank groups. A previous study demonstrated
that the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141 and
miR-29) is involved in the regulation of the EMT process, and
negatively correlated with the expression of ZEB1 and ZEB2
(29). Neves et al
(30) suggested that the EMT
process was accompanied by DNA hypermethylation and transcriptional
silencing of the miR-200c/141 promoter. Wellner et al
(31) indicated that miR-200
family members, including miR-141, induce epithelial
differentiation, thereby suppressing EMT by inhibiting translation
of mRNA for the EMT-activators ZEB1 and ZEB2. In addition, the
expression of endogenous miR-200 in normal and lung cancer cells
directly acts on ZEB1 and Mothers against decapentaplegic homolog 3
interacting protein 1 mRNA, promotes E-cadherin expression and
inhibition of EMT in cancer cells, and reduces the incidence of
invasion in breast cancer cells (32). In addition, increased miR-141
levels increased expression of E-cadherin and reduced EMT, which
agreed with a previous study reported by Tamagawa et al
(16) that suggested that
overexpression of miR-141 reduced the cell capacity of migration in
head and neck squamous cell carcinoma through regulation of EMT.
Therefore, these results suggested that miR-141 may inhibit EMT in
the SKOV3 OC cell line.
As a member of miR-200 family, miR-141
overexpression has been reported to inhibit invasion and migration
in a number of types of cancer, including colorectal cancer, breast
cancer and pancreatic cancer (30,33,34).
When miR-141 was downregulated, cancer cell migration was induced
via targeting E-cadherin transcriptional repressor genes (35), which improved tumor motility and
increased carcinogenicity. Consistent with previous studies, in the
present study, it was demonstrated that the number of invasive
cells was increased in the inhibitor group and decreased in the
mimic group compared with the NC group. In addition, the migratory
rate was decreased in the mimic group and increased in the
inhibitor group, at 24 and 48 h compared with the NC group. These
data suggest that miR-141 can inhibit invasion and migration in
SKOV3 cells in vitro.
In the present study, overexpression of miR-141 in
the OC cell line SKOV3 significantly inhibited cell proliferation.
As a member of miR-200, miR-141 has been demonstrated to be
decreased and served as a tumor suppressor in numerous cancer types
(36). miR-141 has been reported
to be downregulated in childhood renal neoplasms (37). Poell et al (38) demonstrated that miR-141 could
inhibit the proliferation of melanoma cells. In addition, miR-141
was decreased in gastric cancer and involved in gastric cancer cell
growth (14). Van Jaarsveld et
al (39) identified that
miR-141 could promote cisplatin sensitivity in OC cells by
targeting kelch-like ECH-associated protein 1. In the present
study, the MTT assay results demonstrated that the inhibitor group
exhibited an increased OD570 value compared with the blank or NC
groups, suggesting that overexpression of miR-141 could decrease
proliferation of SKOV3 cells.
Certain limitations remain in the present study. For
example, the effects of miR-141 on OC are long and complex, and
cell cycle progression and apoptosis were not included in the
present study. Therefore, additional studies are required to
confirm the results of the present study and the long-term impact
of miR-141 on OC development should be further investigated.
In conclusion, the present study revealed that
overexpression of miR-141 caused upregulation of E-cadherin,
inhibited cell proliferation and EMT in the SKOV3 cell line, and
decreased cell invasion and migration. The results of the present
study provide novel insight into the functional mechanism of OC
progression and suggest that miR-141 represents a novel molecular
target for OC therapy.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vergara D, Merlot B, Lucot JP, Collinet P,
Vinatier D, Fournier I and Salzet M: Epithelial-mesenchymal
transition in ovarian cancer. Cancer Lett. 291:59–66. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ju JA, Huang YC, Lan SH, Wang TH, Lin PC,
Lee JC, Niu KC, Tian YF and Liu HS: Identification of colorectal
cancer recurrence-related microRNAs. Gen Med Biomark Health Sci.
4:19–20. 2012.
|
|
8
|
Blenkiron C and Miska EA: miRNAs in
cancer: Approaches, aetiology, diagnostics and therapy. Hum Mol
Genet. 16:R106–R113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bommer GT, Gerin I, Feng Y, Kaczorowski
AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Wang L, Matyunina LV, Hill CG and
McDonald JF: Overexpression of miR-429 induces
mesenchymal-to-epithelial transition (MET) in metastatic ovarian
cancer cells. Gynecol Oncol. 121:200–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bendoraite A, Knouf EC, Garg KS, Parkin
RK, Kroh EM, O'Briant KC, Ventura AP, Godwin AK, Karlan BY,
Drescher CW, et al: Regulation of miR-200 family microRNAs and ZEB
transcription factors in ovarian cancer: Evidence supporting a
mesothelial-to-epithelial transition. Gynecol Oncol. 116:117–125.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma MiR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PLoS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamura M, Watanabe M, Nakajima A, Kurai D,
Ishii H, Takata S, Nakamoto K, Sohara E, Honda K, Nakamura M, et
al: Serial quantification of procalcitonin (PCT) predicts clinical
outcome and prognosis in patients with community-acquired pneumonia
(CAP). J Infect Chemother. 20:97–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakada C, Matsuura K, Tsukamoto Y,
Tanigawa M, Yoshimoto T, Narimatsu T, Nguyen LT, Hijiya N, Uchida
T, Sato F, et al: Genome-wide microRNA expression profiling in
renal cell carcinoma: Significant down-regulation of miR-141 and
miR-200c. J Pathol. 216:418–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tamagawa S, Beder LB, Hotomi M, Gunduz M,
Yata K, Grenman R and Yamanaka N: Role of miR-200c/miR-141 in the
regulation of epithelial-mesenchymal transition and migration in
head and neck squamous cell carcinoma. Int J Mol Med. 33:879–886.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamura S, Sharanjot S, Shahana M, Hirata
H, Ueno K, Chang I, Chiyomaru T, Tanaka Y and Dahiya R:
MicroRNA-141 inhibits proliferation and invasion by suppressing the
Wnt signaling pathway in renal cell carcinoma. Cancer Res.
72:31492012. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong Z, Shi Y, Zhu Z, Li X, Ye Y, Zhang J,
Li A, Li G and Zhou J: JWA deficiency suppresses
dimethylbenz[a]anthracene-phorbol ester induced skin papillomas via
inactivation of MAPK pathway in mice. PLoS One. 7:e341542012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Savagner P: Leaving the neighborhood:
Molecular mechanisms involved during epithelial-mesenchymal
transition. Bioessays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perez-Pinera P, Alcantara S, Dimitrov T,
Vega JA and Deuel TF: Pleiotrophin disrupts calcium-dependent
homophilic cell-cell adhesion and initiates an
epithelial-mesenchymal transition. Proc Natl Acad Sci USA.
103:17795–17800. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Herreros AG, Peiró S, Nassour M and
Savagner P: Snail family regulation and epithelial mesenchymal
transitions in breast cancer progression. J Mammary Gland Biol
Neoplasia. 15:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang SY, Miah A, Pabari A and Winslet M:
Growth Factors and their receptors in cancer metastases. Front
Biosci (Landmark Ed). 16:531–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dave N, Guaita-Esteruelas S, Gutarra S,
Frias À, Beltran M, Peiró S and de Herreros AG: Functional
cooperation between Snail1 and twist in the regulation of ZEB1
expression during epithelial to mesenchymal transition. J Biol
Chem. 286:12024–12032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S, et al:
Epithelial-mesenchymal transition and mesenchymal-epithelial
transition via regulation of ZEB-1 and ZEB-2 expression in
pancreatic cancer. J Surg Oncol. 105:655–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bianchi A, Gervasi ME and Bakin A: Role of
β5-integrin in epithelial-mesenchymal transition in response to
TGF-β. Cell Cycle. 9:1647–1659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes in cell shape, motility, and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neves R, Scheel C, Weinhold S, Honisch E,
Iwaniuk KM, Trompeter HI, Niederacher D, Wernet P, Santourlidis S
and Uhrberg M: Role of DNA methylation in miR-200c/141 cluster
silencing in invasive breast cancer cells. BMC research notes.
3:2192010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
Hausen AZ, et al: The EMT-activator ZEB1 promotes tumorigenicity by
repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu T, Zhao Y, Cao C, Hao R, Li C, Yi Y,
Gao S, Hui L and Liang A: Material and mechanisms induced pseudo
allergic reactions of Yuxingcao injection. Zhongguo Zhong Yao Za
Zhi. 35:1603–1606. 2010.(In Chinese). PubMed/NCBI
|
|
34
|
Burk U, Schubert J, Wellner U, Schmalhofer
O, Vincan E, Spaderna S and Brabletz T: A reciprocal repression
between ZEB1 and members of the miR-200 family promotes EMT and
invasion in cancer cells. EMBO Rep. 9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshino H, Enokida H, Itesako T, Tatarano
S, Kinoshita T, Fuse M, Kojima S, Nakagawa M and Seki N:
Epithelial-mesenchymal transition-related microRNA-200s regulate
molecular targets and pathways in renal cell carcinoma. J Hum
Genet. 58:508–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Senanayake U, Das S, Vesely P, Alzoughbi
W, Fröhlich LF, Chowdhury P, Leuschner I, Hoefler G and Guertl B:
miR-192, miR-194, miR-215, miR-200c and miR-141 are downregulated
and their common target ACVR2B is strongly expressed in renal
childhood neoplasms. Carcinogenesis. 33:1014–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Poell JB, van Haastert RJ, de Gunst T,
Schultz IJ, Gommans WM, Verheul M, Cerisoli F, van Noort PI,
Prevost GP, Schaapveld RQ and Cuppen E: A functional screen
identifies specific microRNAs capable of inhibiting human melanoma
cell viability. PLoS One. 7:e435692012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van Jaarsveld MT, Helleman J, Boersma AW,
van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH,
Berns EM, Verweij J, et al: miR-141 regulates KEAP1 and modulates
cisplatin sensitivity in ovarian cancer cells. Oncogene.
32:4284–4293. 2013. View Article : Google Scholar : PubMed/NCBI
|