Introduction
Ossification of the ligamentum flavum (OLF)
is the most common etiology of thoracic spinal stenosis (1). Although the causes and pathogeneses
are unclear, OLF is thought to occur due to genetic and
environmental factors, including diet, humidness, metabolic
disturbance and athletic injury (2). A number of surgical procedures are
used to treat OLF in the clinic, including lamina eroding
decompression, double-door lamina resection decompression, the
whole vertebral plate decompression peel method, and lamina
decompression and implant surgery (3). The lamina eroding decompression
method is a relatively simple operation; however, the double-door
and vertebral plate decompression methods require high-speed drills
and osteotomes (1). The loss of
the spinous process and vertebral plate weaken the stability of the
posterior column and, after a significant period of time, may
produce unstable regions of reduced pressure (4). To maintain the intact structure of
the canalis vertebralis, lamina decompression and implant
surgery are performed (5).
Implanted vertebral plates ensure that the structure of the
canalis spinalis is maintained and prevents scar tissue from
invading the canalis spinalis (6). In addition, vertebral plates provide
attachment points for paravertebral muscles and prevent amyotrophy.
The fixation of miniature titanium plates maintains the integrity
of the spinal cord. However, when compared with laminectomies,
vertebral plate implant surgery is more complex, with longer
operating times and increased levels of bleeding (7).
Mechanical stress stimulation serves an important
regulatory function in bone formation and remodeling (8). Mechanical stimuli target bone tissues
and are transmitted as one of three local signals, which include
fluid shear stress, cytomorphosis or stress-generated potentials
(9). Cells sensitive to
transformation stress transduce these local signals into primary
signals via the following three pathways: The integrin-cytoskeleton
system, the calcium channel pathway and the primary cilium pathway
(10). These primary signals are
subsequently transmitted as downstream signals via signal
transduction pathways (11). These
signals induce a number of alterations, including those associated
with genetic expression, energy metabolism and material
synthesis.
OLF is a common disease, which is characterized by
heterotopic osteogenesis of the posterior longitudinal ligament of
the cervical vertebra in China (12). Unregulated growth of an ossific
mass in the canalis spinalis may lead to severe spinal cord
and nerve root entrapment; however, the pathogenesis of OLF is
currently unclear (13). The aim
of the present study was to determine the effect of mechanical
stress on the rate of osteogenic differentiation of human
ligamentum flavum cells, and to elucidate the underlying
mechanisms involved.
Materials and methods
Cell culture and generation of the
mechanical stress model
Human OLF tissues (3–5 g) were obtained during
surgery from a patient admitted to the Shanghai Tenth People's
Hospital, Shanghai, China (aged 53 years; male; stage IV lumbar
degenerative disease, during March 2015) that was diagnosed with
lumbar degenerative disease. Written informed consent was provided
by the patient for the use of their tissue samples in the present
study. The tissues were cut into 1–2-mm3sections and
digested with 2% type-I collagenase (Sunshine Biotechnology Co.,
Ltd., Nanjing, China) at 37°C for 30 min. Nucleated cells (10,000
cells/cm2) were recovered and plated as mononuclear
cells to obtain a higher yield. Cells were cultured in α-minimum
essential medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 15% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C in 5%
CO2; the medium was refreshed every 2 days. Following 1
week of culture, the cells were transferred to culture flasks
coated with fibronectin (5 µg/cm2, Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). A specially-designed four-point bending
apparatus with flexible silicon-bottomed chambers was generated as
described previously (14), and
used to induce the mechanical stress model. The cells were then
subjected to uniaxial tensile strain (0.5 Hz, 2,000 min, three
times/day).
Cell viability
Cells were distributed into control, unstretched and
stretched groups.
In the control group, cells were treated with PBS;
in unstretched group, cells were subjected to uniaxial tensile
strain without voltage; in stretched group, cells were subjected to
uniaxial tensile strain (0.5 Hz, 2,000 min, three times/day).
Following 3 weeks of mechanical stress, cells
(1–2×103) were cultured in a 96-well plate for 24 h. A
total of 20 µl MTT assay reagent (5 mg/ml; Beyotime Institute of
Biotechnology, Haimen, China) was then added to each well, and the
plates were incubated at 37°C and 5% CO2 for 4 h.
Following incubation, the culture medium was removed and 150 µl
dimethyl sulfoxide (DMSO) was added to each well and allowed to
dissolve the formazan crystals for 20 min at 37°C. Cell viability
was measured using a microplate reader (Omega Bio-Tek, Inc.,
Norcross, GA, USA) at a wavelength of 490 nm.
Osteogenic differentiation
Cells (1×105) from the different groups
were fixed in 95% ethanol overnight at 4°C, and stained with Oil
Red O (0.25%) for 15 min at room temperature. The cells were then
washed three times with 70% ethanol for 5 min. Osteogenic
differentiation was observed using a microscope in three field of
view (Axio Imager 2; Zeiss GmbH, Jena, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After 3 weeks mechanical stress, cells
(1×106) from the different groups were cultured on a
6-well plate for 24 h. Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. A total of 2 ng RNA was
reverse-transcribed into cDNA using a High-Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. For qPCR
analysis, 1 ng cDNA template was used for each reaction and
sequences were amplified using a StepOne™ Real-Time PCR system and
Power SYBR® Green PCR Master Mix (both from Applied
Biosystems; Thermo Fisher Scientific, Inc.). Amplification
reactions were conducted with an initial step at 95°C for 1 min,
followed by 40 cycles of 55°C for 30 sec and 60°C for 30 sec. The
primers used for qPCR analysis are listed in Table I and gene expression was normalized
to the levels of β-actin and analyzed using the 2−ΔΔCq
method (15).
 | Table I.Primer sequences used to
analyzetarget gene expression levels by reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used to
analyzetarget gene expression levels by reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward sequence
(5′-3′) | Reverse
sequence(5′-3′) |
|---|
| OC |
ATGAGAGCCCTCACACTCCTC |
GCCGTAGAAGCGCCGATAGGC |
| ALP |
TGGAGCTTCAGAAGCTCAACACCA |
ATCTCGTTGTCTGAGTACCAGTCC |
| RUNX-2 |
ATCTCGTTGTCTGAGTACCAGTCC |
ATCTCGTTGTCTGAGTACCAGTCC |
| SOX-2 |
CCCCCCTGTGGTTACCTCTTC |
CCCCCCTGTGGTTACCTCTTC |
| Ets-1 |
GAGTTCAGCCTGAAGGGTGT |
CACATCCTCTTTCTGCAGGATCT |
| β-actin |
GTGGGGCGCCCCAGGCACCA |
CTTCCTTAATGTCACGCACGATTTC |
Western blot analysis
Cells (1×106) after 3 weeks mechanical
stress from the different groups were lysed with ice-cold lysis
buffer containing a 10 µg/ml of protein inhibitor mixture (1:100;
Roche Diagnostics, Indianapolis, IN, USA). Protein concentrations
were then determined using a DC Protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). A total of 50 µg protein
was separated by 8–12% SDS-PAGE and electrophoretically transferred
onto 0.22 µm polyvinylidenedifluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were then washed with5% non-fat
dried milk in Tris-buffered solution plus 0.05% Tween-20 (TBST) for
2 h, prior to incubation with anti-Src (catalog no. sc-8995,
dilution, 1:200), anti-bone morphogenetic protein (catalog no.
sc-9003, BMP; dilution, 1:200) (both from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-phosphorylated (p)-mothers against
decapentaplegic homolog-1 (catalog no. 13820, p-Smad-1; dilution,
1:400; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-p-p38-mitogen-activated protein kinases (catalog no.
sc-7975-R, p38MAPK; dilution, 1:300) and anti-β-actin (catalog no.
sc-7210, dilution, 1:400) (both from Santa Cruz Biotechnology,
Inc.) at 4°C overnight. The membranes were washed with TBST for 5
min three times and were subsequently incubated with anti-rabbit
horseradish peroxidase-conjugated secondary antibody (catalog no.
A0208, dilution, 1:4,000; Beyotime Institute of Biotechnology) at
room temperature for 1 h, and detected with
electrochemiluminescence western blot detection reagents (Beyotime
Institute of Biotechnology). Protein expression was analyzed using
Quantity One software version 3.0 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation, and represent the mean values of at least three
independent experiments using SPSS software version 17.0 (SPSS,
Inc., Chicago, IL, USA). Comparisons among groups were analyzed
using one-way analysis of variance, followed by the Tukey Kramer
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell morphology
OLF cells were extracted using a fibronectin
differential-adhesion assay, and OLF cell morphology was observed
following 1 week of culture. As shown in Fig. 1, OLF cells exhibited a uniform
morphology and were well-distributed in the culture flasks. In
addition, the cells exhibited a shuttle shape until the end of week
1 (Fig. 1).
Cell viability
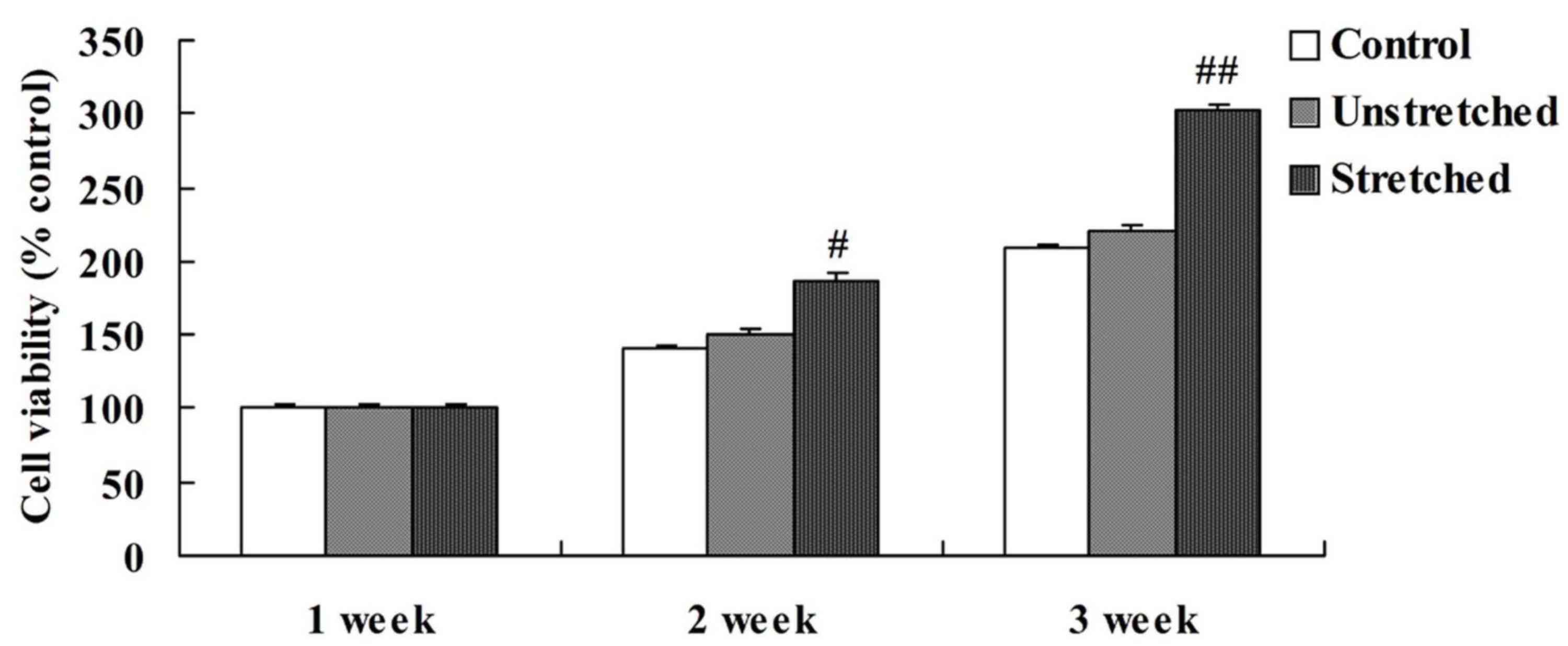
When comparing the viability of OLF cells among the
different experimental groups, the control and unstretched groups
demonstrated similar levels of cell viability at 1, 2 and 3 weeks
(Fig. 2). However, mechanical
stress significantly increased OLF cell viability following 2
(P<0.05) and 3 weeks (P<0.01) of mechanical stimulation when
compared with the controls (Fig.
2).
Osteogenic differentiation
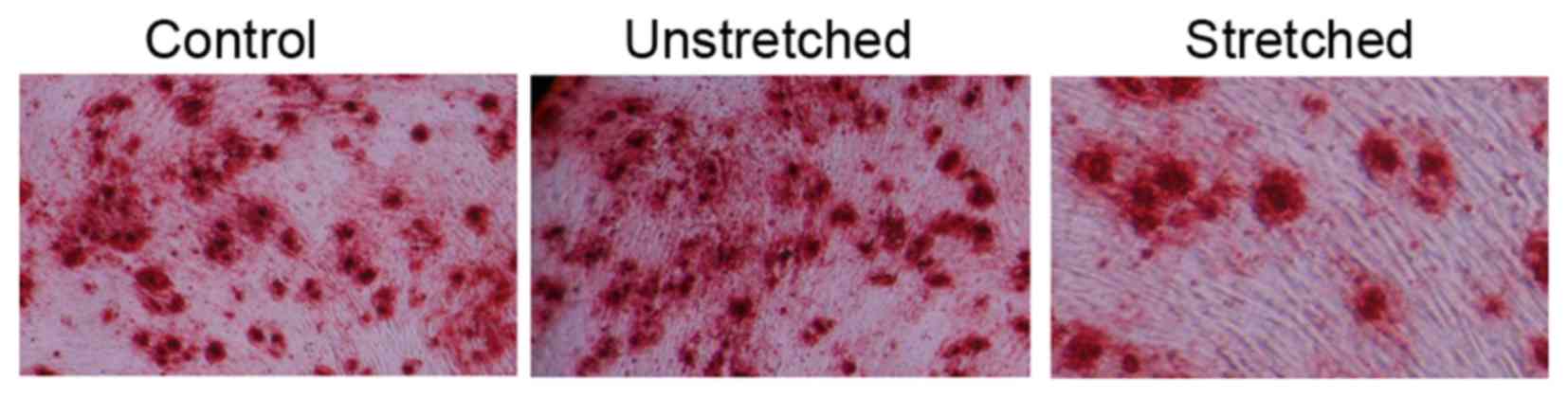
Oil Red O staining was performed to analyze the
effect of 3 weeks of mechanical stress on the osteogenic
differentiation of OLF cells. The osteogenic differentiation rate
of the control group appeared to be similar to that of the
unstretched group (Fig. 3). By
contrast, mechanical stress effectively promoted the osteogenic
differentiation rate and increased the size of OLF cells, when
compared with the control group (Fig.
3).
Expression of osteocalcin (OC),
alkaline phosphatase (ALP) and runt-related transcription factor 2
(RUNX-2) mRNA
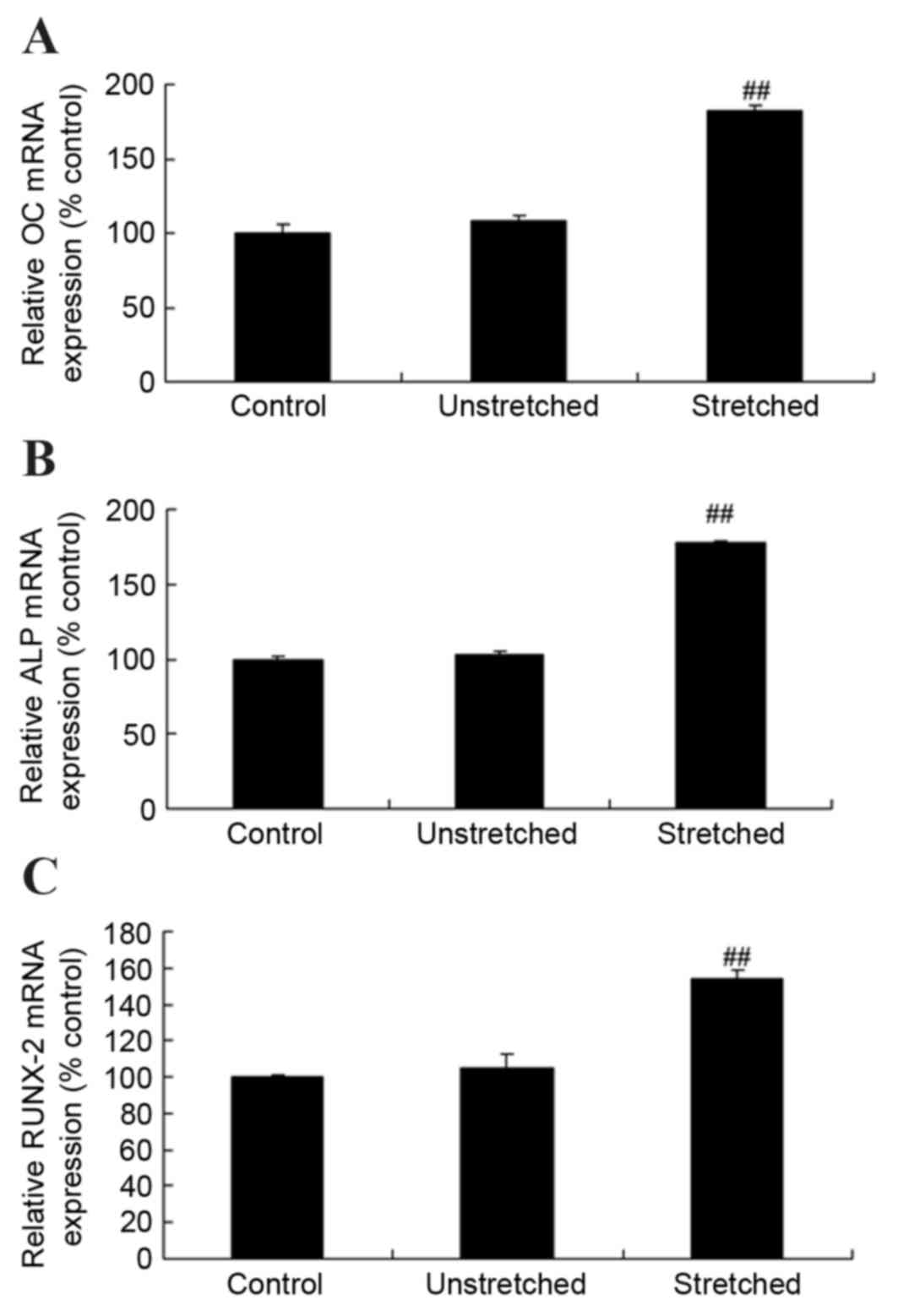
RT-qPCR analysis was performed to investigate OC,
ALP and RUNX-2 mRNA expression levels following mechanical stress
stimulation at 3 weeks. As shown in Fig. 4, the mRNA expression levels of OC,
ALP and RUNX-2 were comparable when comparing the control group and
unstretched groups. By contrast, mechanical stress significantly
increased the expression of OC, ALP and RUNX-2 mRNA in OLF cells
when compared with the control group (P<0.01; Fig. 4).
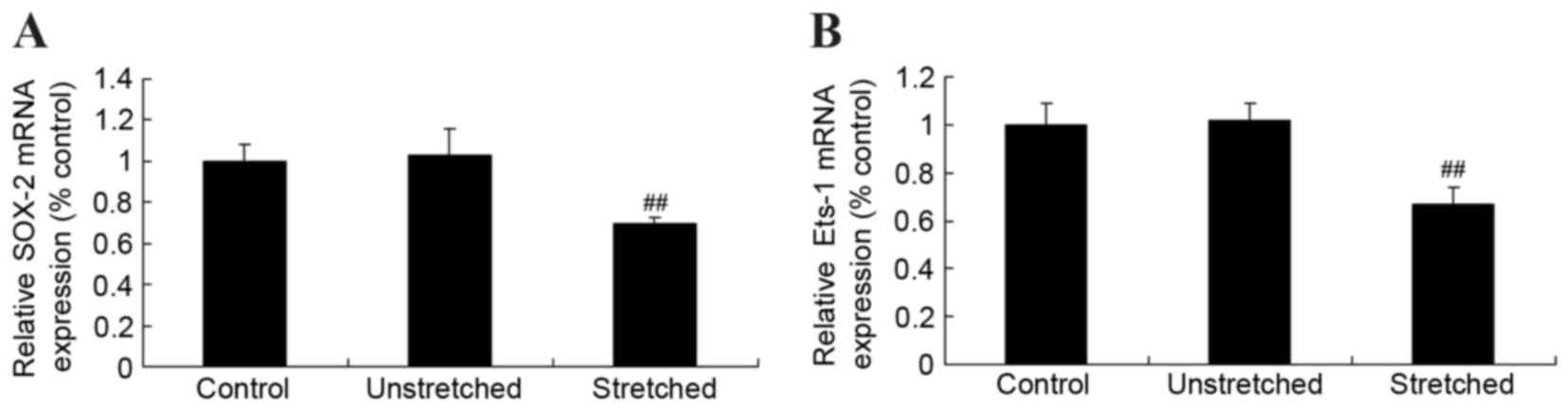
Expression of Ets proto-oncogene 1
(Ets-1) and sex determining region Y-box 2 (SOX-2) mRNA
In order to investigate the mechanisms underlying
the effects of mechanical stress on osteogenic OLF cell
differentiation, Ets-1 and SOX-2 mRNA expression was determined by
RT-qPCR analysis. Following quantitative analysis, no significant
difference in Ets-1 and SOX-2 miRNA expression levels were observed
between the control and unstretched groups (Fig. 5). However, mechanical stress
significantly inhibited Ets-1 and SOX-2 mRNA expression in OLF
cells when compared with the control cells (P<0.01; Fig. 5).
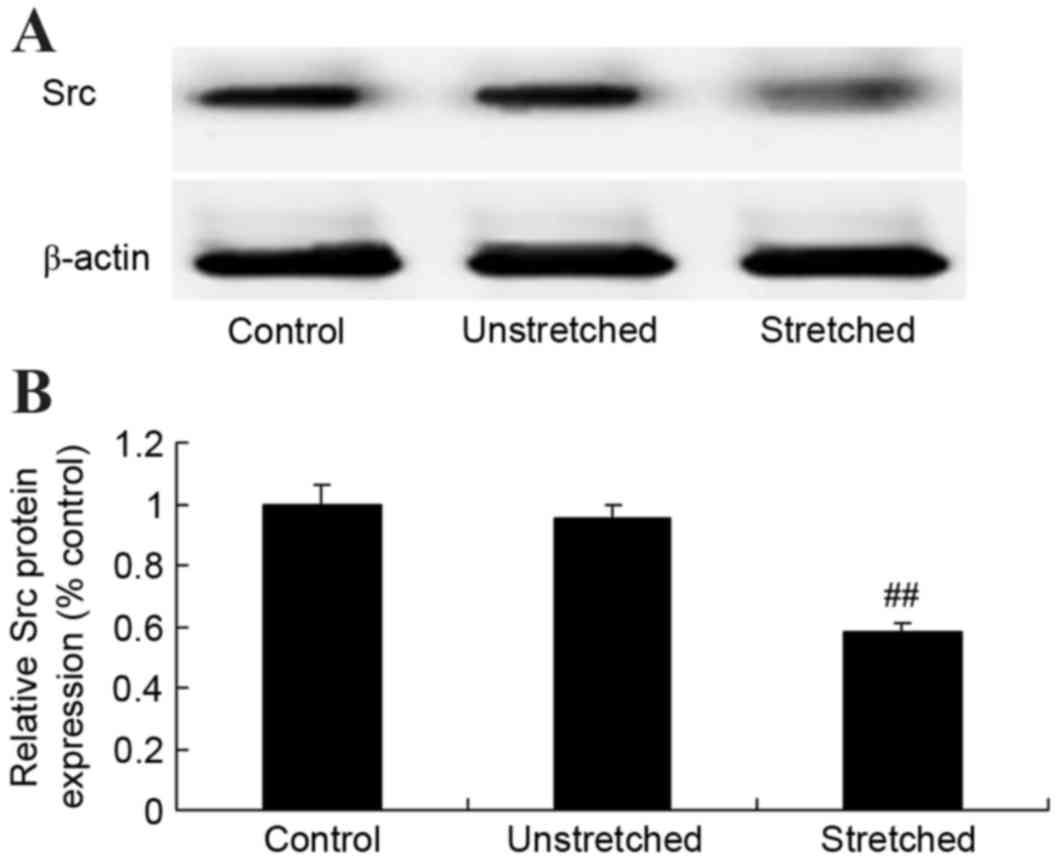
Expression of Src protein
Western blot analysis revealed that the control and
unstretched groups exhibited similar levels of Src protein
expression (Fig. 6). By contrast,
mechanical stress significantly inhibited Src protein expression in
OLF cells when compared with the control group (P<0.01; Fig. 6).
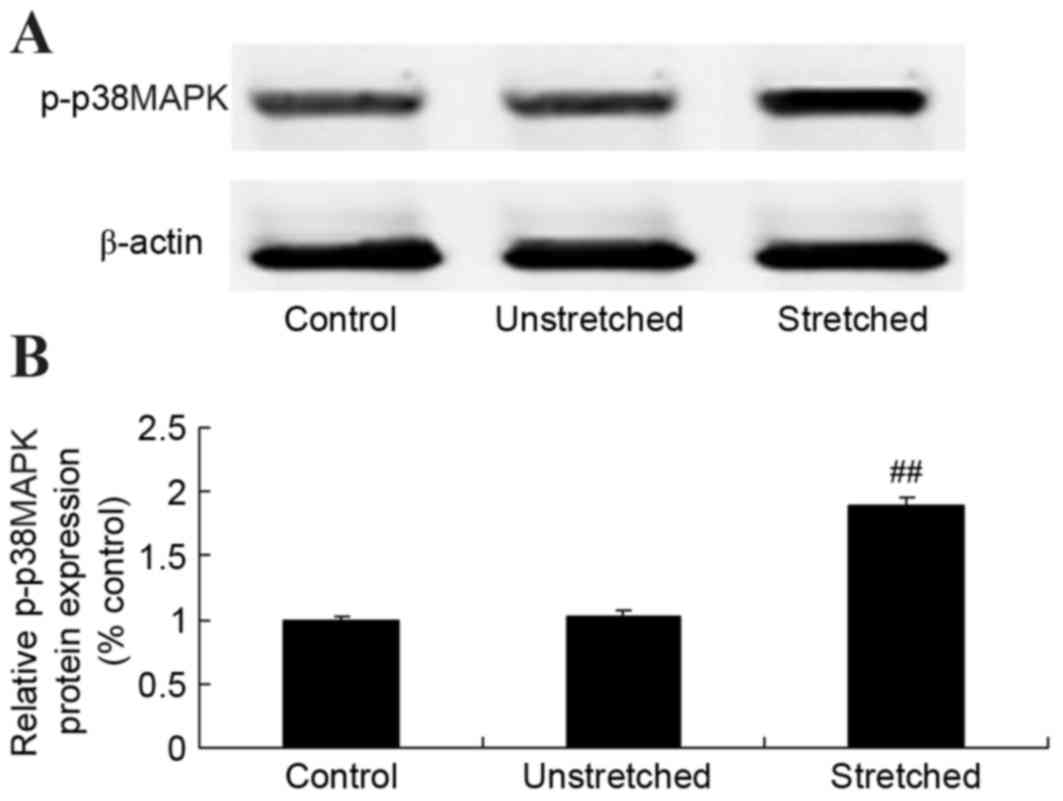
Expression of p-p38MAPK protein
To further elucidate the underlying mechanisms of
mechanical stress on osteogenic OLF cell differentiation, p-p38MAPK
protein was detected using western blot analysis. No significant
differences inp-p38MAPK protein expression were observed between
the control and unstretched groups (Fig. 7). However, mechanical stress
significantly increased p-p38MAPK protein expression, when compared
with the control group (P<0.01; Fig. 7).
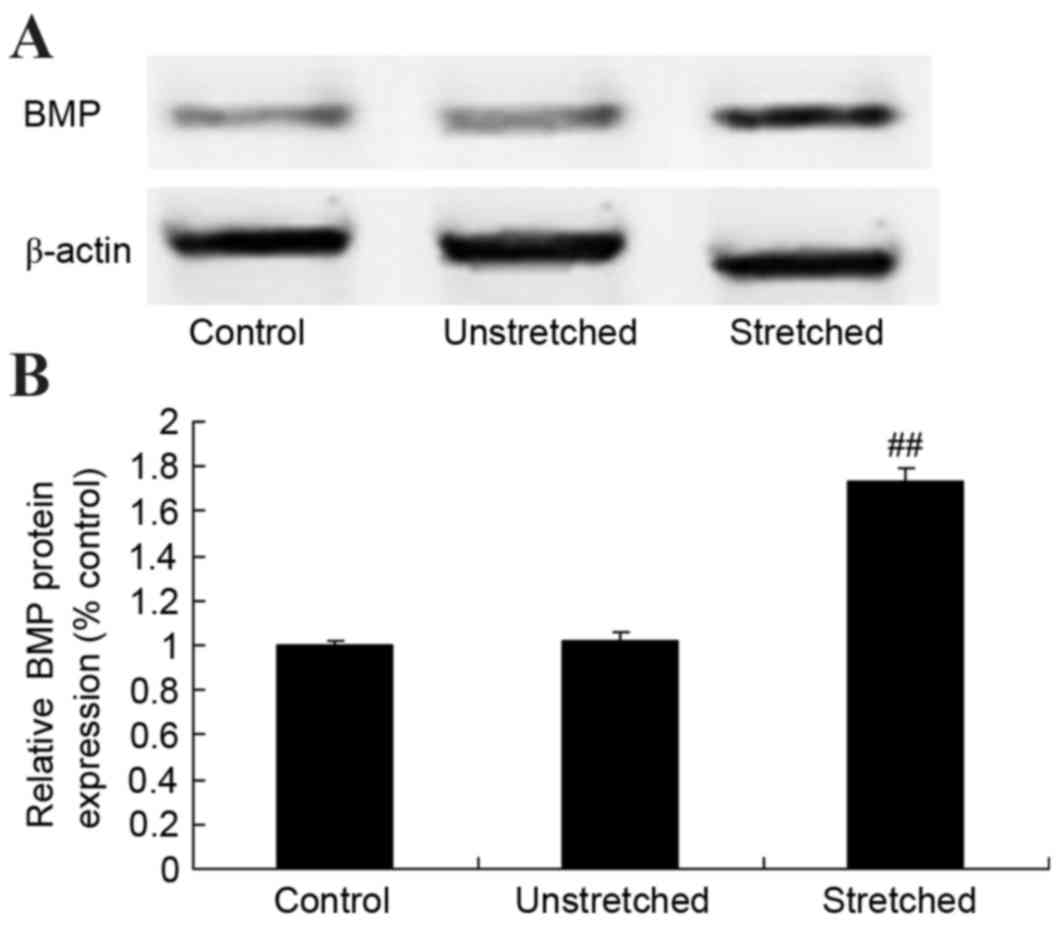
Expression of BMP protein
BMP protein expression was determined by western
blot analysis. As shown in Fig. 8,
no significant difference in BMP protein expression was observed
between the control and unstretched groups. However, mechanical
stress significantly increased BMP protein expression in OLF cells
when compared with the control group (P<0.01; Fig. 8).
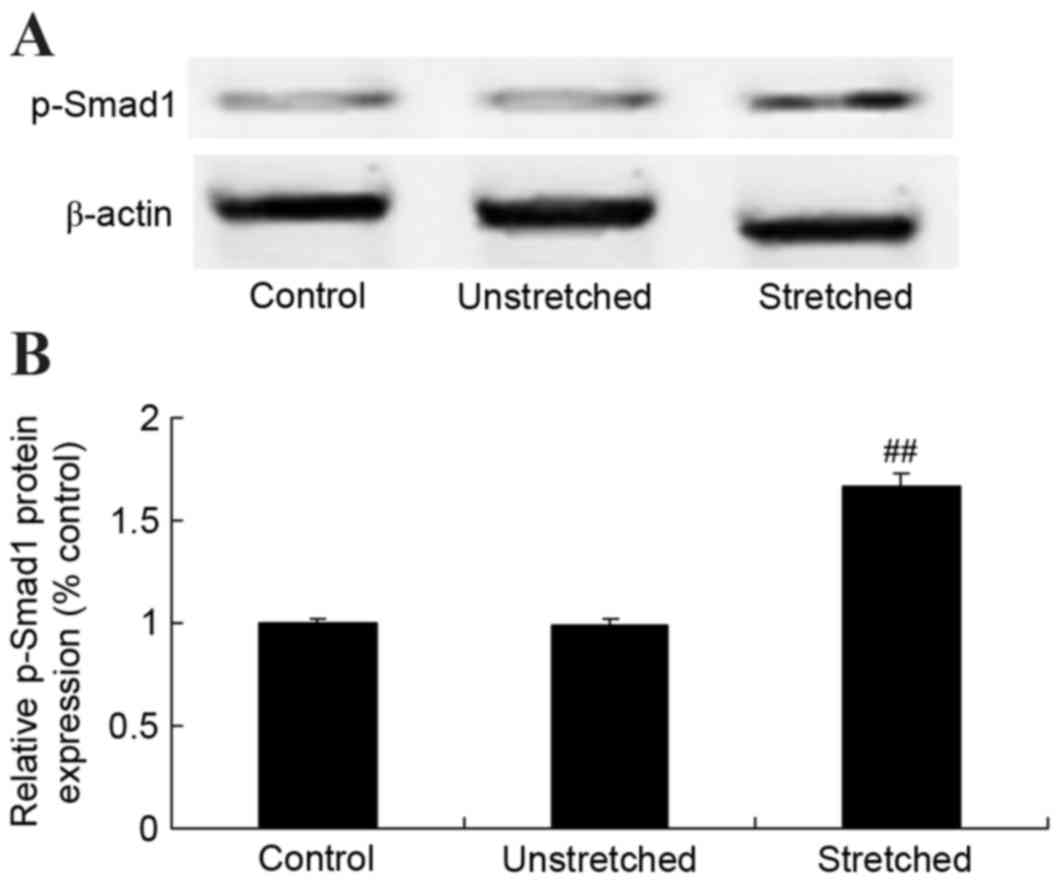
Expression of Smad-1 protein
p-Smad-1 protein expression in OLF cells was
analyzed using western blot analysis. As shown in Fig. 9, no significant difference in the
protein expression of p-Smad-1 was observed between the control and
unstretched groups. However, mechanical stress significantly
increased p-Smad-1 protein expression in OLF cells, when compared
with the control group (P<0.01; Fig. 9).
Discussion
Following the action of mechanical stress stimuli,
bone tissues transform mechanical stress signals into local
signals, which target stress-sensitive cells via the integrating
integrin-cytoskeleton system, the calcium channel pathway and the
primary cilium pathways (16).
Stress-sensitive cells transform these primary biochemical signals
into downstream signals through a signal transduction channel
(6). These signals subsequently
induce alterations in gene expression, energy metabolism and
material synthesis. In the present study, mechanical stress
effectively increased cell viability and promoted the osteogenic
differentiation rate of OLF cells. Mechanical stimuli induce
alterations in bone microstructure and produce a number of local
signals, which are recognized by mechanical stress-sensitive cells
(17). Li et al (18) demonstrated that mechanical strain
regulates osteogenic differentiation in bone marrow stromal cells
(BMSCs).
An ongoing study showed the complex underlying
mechanisms of signal transduction are currently unclear and are
therefore the focus of a study (19). The process by which cells produce
extracellular chemical energy from mechanical energy is of
particular interest (19,20). Previous studies have demonstrated
that cyclicalmechanical stress is regulated by kinases to promote
the proliferation of chondrocytes by functioning as extracellular
signaling molecules (19,21). In the present study, mechanical
stress significantly increased OC, ALP and RUNX-2 mRNA expression
levels, while significantly inhibiting Ets-1 and SOX-2 miRNA
expression in OLF cells. Li et al (18) demonstrated that mechanical strain
regulates osteogenic differentiation in BMSCs via Runx2, Osterix,
and collagen-I.
Src, a member of the Src family, possesses tyrosine
protein kinase activity. Phosphorylation of the Tyr residue on the
activation loop activates specific enzymes and the delivery of
extracellular signals to the cells, thereby facilitating cell
proliferation and migration (22).
A previous study demonstrated that the migration activity of murine
fibroblast cells in Src-knockout rats is reduced, which further
supports the role of Src in cell migration (22). The interactions between Src, the
extracellular matrix and integrin are essential for the induction
of ras homolog gene family, member A (RhoA), and in turn, the
activation of RhoA is closely associated with cell migration
(23). The present study revealed
that mechanical stress significantly suppressed Src protein
expression in OLF cells. Paravicini et al (24) demonstrated that mechanical stretch
influences vascular fibrosis during hypertension, which appeared to
be independent of c-Src and p38MAPK.
p38MAPK is a core component of the MAPK cascade.
Phosphorylation of p38MAPKinduces enzyme activation and serves a
fundamental role in cell proliferation, differentiation and
migration (25). p38MAPK at
different cellular locations possess different functions. p38MAPK
on the cytomembrane participates in cellular effects, and following
mechanical trauma, induces the migration and differentiation of
stomach smooth muscle cells (26).
This function does not depend on the intranuclear transcription.
The present study demonstrated that mechanical stress significantly
increased p-p38MAPK protein expression in OLF cells.
A study confirmed that the BMP-Smad-1 signaling
pathway regulates specific aspects of the osteoblast life cycle,
including differentiation from mesenchymal stem cells to
osteoblasts, osteogenic cell proliferation, the vitality of
osteoblast mineralization and osteoclast coupling (27). However, abnormal differentiation is
triggered by downregulated Runx2 and Osterix expression (28). Empirical data collected from a
recent study indicated that the loss of BMP-Smad signaling
significantly increased Osterix and Runx2 protein expression
(29,30). The results of the present study
demonstrated that mechanical stress significantly increased BMP and
p-Smad1 protein expression in OLF cells. Kido et al
(31) revealed that mechanical
stress activates the Smad pathway in osteoblasts. In addition,
Grottkau et al (32)
demonstrated that mechanical stretching induced osteogenesis
through BMP-2 mRNA expression in adipose-derived stem cells and
BMSCs.
In conclusion, the present study demonstrated that
mechanical stress effectively increased cell viability and promoted
the osteogenic differentiation rate of OLF cells potentially via
the activation of OC, ALP and RUNX-2, and the suppression of Ets-1
and SOX-2 signaling. These results suggest that the BMP-Smad-1
signaling pathway may serve an important role in mediating the
intracellular signaling effects of mechanical stress on osteogenic
differentiation via the Src and p38MAPK signaling pathways in OLF
cells.
References
|
1
|
Yamazaki M, Koda M, Okawa A and Aiba A:
Transient paraparesis after laminectomy for thoracic ossification
of the posterior longitudinal ligament and ossification of the
ligamentum flavum. Spinal Cord. 44:130–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dorenbeck U, Schreyer AG, Schlaier J, Held
P, Feuerbach S and Seitz J: Degenerative diseases of the cervical
spine: Comparison of a multiecho data image combination sequence
with a magnetisation transfer saturation pulse and cervical
myelography and CT. Neuroradiology. 46:306–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yabe Y, Hagiwara Y, Tsuchiya M, Honda M,
Hatori K, Sonofuchi K, Kanazawa K, Koide M, Sekiguchi T, Itaya N
and Itoi E: Decreased elastic fibers and increased proteoglycans in
the ligamentum flavum of patients with lumbar spinal canal
stenosis. J Orthop Res. 34:1241–1247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang T, Pan M, Yin CQ, Zheng XJ, Cong YN,
Wang DC and Li SZ: Spinal cord kinking in thoracic myelopathy
caused by ossification of the ligamentum flavum. Chin Med J (Engl).
128:2595–2598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li S, Xia H and Han C: Retrospective
analysis on correlation factors of preserving the ligamentum flavum
in microendoscopic discectomy. Clin Neurol Neurosurg. 139:46–50.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiang CW, Chen WC, Liu HW, Wang IC and
Chen CH: Evaluating osteogenic potential of ligamentum flavum cells
cultivated in photoresponsive hydrogel that incorporates bone
morphogenetic protein-2 for spinal fusion. Int J Mol Sci.
16:23318–23336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tani T, Kawasaki M, Taniguchi S and Ushida
T: Functional importance of degenerative spondylolisthesis in
cervical spondylotic myelopathy in the elderly. Spine (Phila Pa
1976). 28:1128–1134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oliva A, Llabrés M and Fariña JB: Fitting
bevacizumab aggregation kinetic data with the Finke-Watzky two-step
model: Effect of thermal and mechanical stress. Eur J Pharm Sci.
77:170–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kameo Y and Adachi T: Interstitial fluid
flow in canaliculi as a mechanical stimulus for cancellous bone
remodeling: In silico validation. Biomech Model Mechanobiol.
13:851–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mander L, Wesseln CJ, McElwain JC and
Punyasena SW: Tracking taphonomic regimes using chemical and
mechanical damage of pollen and spores: An example from the
Triassic-Jurassic mass extinction. PLoS One. 7:e491532012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cremers NA, Suttorp M, Gerritsen MM, Wong
RJ, van Run-van Breda C, van Dam GM, Brouwer KM, Kuijpers-Jagtman
AM, Carels CE, Lundvig DM and Wagener FA: Mechanical stress changes
the complex interplay between HO-1, inflammation and fibrosis,
during excisional wound repair. Front Med (Lausanne).
2:862015.PubMed/NCBI
|
|
12
|
Li WJ, Guo SG, Sun ZJ and Zhao Y:
Multilevel thoracic ossification of ligamentum flavum coexisted
with/without lumbar spinal stenosis: Staged surgical strategy and
clinical outcomes. BMC Musculoskelet Disord. 16:2062015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue H, Seichi A, Kimura A, Endo T and
Hoshino Y: Multiple-level ossification of the ligamentum flavum in
the cervical spine combined with calcification of the cervical
ligamentum flavum and posterior atlanto-axial membrane. Eur Spine
J. 22 Suppl 3:S416–S420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi MC, Hu J, Zou SJ, Chen HQ, Zhou HX and
Han LC: Mechanical strain induces osteogenic differentiation: Cbfa1
and Ets-1 expression in stretched rat mesenchymal stem cells. Int J
Oral Maxillofac Surg. 37:453–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang
G, Dong Y, Xu X, Liu Q, Huang D and Shi FD: Combination of the
immune modulator fingolimod with alteplase in acute ischemic
stroke: A pilot trial. Circulation. 132:1104–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fal K, Landrein B and Hamant O: Interplay
between miRNA regulation and mechanical stress for CUC gene
expression at the shoot apical meristem. Plant Signal Behav.
11:e11274972016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li R, Liang L, Dou Y, Huang Z, Mo H, Wang
Y and Yu B: Mechanical strain regulates osteogenic and adipogenic
differentiation of bone marrow mesenchymal stem cells. Biomed Res
Int. 2015:8732512015.PubMed/NCBI
|
|
19
|
Akimoto T, Okuhira K, Aizawa K, Wada S,
Honda H, Fukubayashi T and Ushida T: Skeletal muscle adaptation in
response to mechanical stress in p130cas−/− mice. Am J
Physiol Cell Physiol. 304:C541–C547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uyttewaal M, Burian A, Alim K, Landrein B,
Borowska-Wykręt D, Dedieu A, Peaucelle A, Ludynia M, Traas J,
Boudaoud A, et al: Mechanical stress acts via katanin to amplify
differences in growth rate between adjacent cells in Arabidopsis.
Cell. 149:439–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Zhang XY, Wu TJ, Cheng W, Liu X,
Jiang TT, Wen J, Li J, Ma QL and Hua ZC: Endoplasmic reticulum
stress regulates rat mandibular cartilage thinning under
compressive mechanical stress. J Biol Chem. 288:18172–18183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chong YP, Ia KK, Mulhern TD and Cheng HC:
Endogenous and synthetic inhibitors of the Src-family protein
tyrosine kinases. Biochim Biophys Acta. 1754:210–220. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marton N, Baricza E, Érsek B, Buzás EI and
Nagy G: The emerging and diverse roles of Src-like adaptor proteins
in health and disease. Mediators Inflamm. 2015:9525362015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paravicini TM, Montezano AC, Yusuf H and
Touyz RM: Activation of vascular p38MAPK by mechanical stretch is
independent of c-Src and NADPH oxidase: Influence of hypertension
and angiotensin II. J Am Soc Hypertens. 6:169–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Tao J, Zhang J, Tian X, Liu S, Sun
M, Zhang X, Yan C and Han Y: Cellular repressor E1A-stimulated
genes controls phenotypic switching of adventitial fibroblasts by
blocking p38MAPK activation. Atherosclerosis. 225:304–314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao WL, Zhang DZ, Fan CH and Yu BJ:
Intermittent stretching and osteogenic differentiation of bone
marrow derived mesenchymal stem cells via the p38MAPK-Osterix
signaling pathway. Cell Physiol Biochem. 36:1015–1025. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsumoto Y, Otsuka F, Takano M, Mukai T,
Yamanaka R, Takeda M, Miyoshi T, Inagaki K, Sada KE and Makino H:
Estrogen and glucocorticoid regulate osteoblast differentiation
through the interaction of bone morphogenetic protein-2 and tumor
necrosis factor-alpha in C2C12 cells. Mol Cell Endocrinol.
325:118–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Susperregui AR, Viñals F, Ho PW, Gillespie
MT, Martin TJ and Ventura F: BMP-2 regulation of PTHrP and
osteoclastogenic factors during osteoblast differentiation of C2C12
cells. J Cell Physiol. 216:144–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okamoto M, Murai J, Yoshikawa H and
Tsumaki N: Bone morphogenetic proteins in bone stimulate
osteoclasts and osteoblasts during bone development. J Bone Miner
Res. 21:1022–1033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghosh-Choudhury N, Singha PK, Woodruff K,
St Clair P, Bsoul S, Werner SL and Choudhury GG: Concerted action
of Smad and CREB-binding protein regulates bone morphogenetic
protein-2-stimulated osteoblastic colony-stimulating factor-1
expression. J Biol Chem. 281:20160–20170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kido S, Kuriwaka-Kido R, Umino-Miyatani Y,
Endo I, Inoue D, Taniguchi H, Inoue Y, Imamura T and Matsumoto T:
Mechanical stress activates Smad pathway through PKCδ to enhance
interleukin-11 gene transcription in osteoblasts. PLoS One. 5(pii):
e130902010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grottkau BE, Yang X, Zhang L, Ye L and Lin
Y: Comparison of effects of mechanical stretching on osteogenic
potential of ASCs and BMSCs. Bone Res. 1:282–290. 2013. View Article : Google Scholar : PubMed/NCBI
|