Introduction
Hydrogen has potential protective effects on cardiac
remodeling. Hydrogen inhalation, commenced at the start of
hyperoxic cardiopulmonary resuscitation (CPR), significantly
improves brain and cardiac function in a rat model of cardiac
arrest (CA) (1). Inhalation of
hydrogen attenuates left ventricular remodeling induced by
intermittent hypoxia (2,3), ischemia-reperfusion injury (4), and germinal matrix hemorrhage in
neonatal rats (5). The
ischemia-reperfusion injury of the heart in rats can also be
attenuated by hydrogen-rich saline (6,7).
Chronic hydrogen-rich saline treatment attenuates left ventricular
hypertrophy in spontaneous hypertensive rats (8). Previous studies from our group also
revealed that intraperitoneal injection of hydrogen prevents
ISO-induced cardiac hypertrophy and dysfunction in mice (9), and suppresses abdominal aortic
coarctation-induced vascular hypertrophy in rats (10). However, the molecular mechanisms by
which hydrogen has a blocking effect on cardiac hypertrophy induced
by β-adrenoceptor stimuli remain poorly understood.
Abnormal autophagic responses have been revealed as
critical contributors of cardiac hypertrophy under various
cardiovascular stresses (11–14).
Autophagy is a mechanism whereby cytoplasmic components are
sequestered in a double-membraned vesicle (autophagosome) towards
delivery to the lysosome for breakdown in response to nutrient
limitation, cellular stress, reactive oxygen species (ROS), or
accumulation of protein aggregates or damaged organelles (15–18).
The cellular events during autophagy follow distinct stages:
Vesicle nucleation (formation of the isolation
membrane/phagophore), vesicle elongation and completion (growth and
closure), fusion of the double-membraned autophagosome with the
lysosome to form an autolysosome, and lysis of the autophagosome
inner membrane and breakdown of its contents inside the
autolysosome (19). These stages
can be identified by a set of autophagy markers, including
autophagy-related protein (Atg)1, Beclin1, Atg7 and
microtubule-associated protein 1 light chain 3β II (LC3B II). A
window of optimal autophagic activity appears to be critical to the
maintenance of cardiovascular homeostasis and function; excessive
or insufficient levels of autophagic flux can each contribute to
heart disease pathogenesis (20).
Whether hydrogen can influence cardiac autophagy during
β-adrenoceptor stimulation remains unclear. The aim of the present
study was, therefore, to determine the effects of hydrogen on
autophagy in isoproterenol (ISO)-mediated cardiomyocyte hypertrophy
in vivo and in vitro models.
Materials and methods
Drugs and antibodies
ISO (cat no. I5627; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was dissolved using high pressure deionized
water for cell culture study or dissolved in normal saline (5 mg/10
ml), under sterile conditions immediately prior subcutaneous
injection, for the animal model study. Hydrogen (99.999%; Guang
Zhou Guang Qi Gas Co., Ltd., Guangzhou, China) was stored in the
seamless steel gas cylinder, and it was injected into an aseptic
soft plastic infusion bag under sterile conditions immediately
prior to intraperitoneal injection. Primary antibodies against Atg7
(cat no. 2631) and LC3B (cat no. 3868) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The antibody against
β-actin (cat no. sc-81178) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The anti-Beclin1 antibody
(cat no. 3495; Cell Signaling Technology, Inc.) was kindly provided
by Dr Zhi Zhao (Department of Gastrointestinal Surgery, The First
Affiliated Hospital of Sun Yat-sen University). The goat
anti-rabbit immunoglobulin (Ig)G horseradish peroxidase
(HRP)-conjugated (cat no. 7074) and the horse anti-mouse IgG
HRP-conjugated (cat no. 7076) secondary antibodies were purchased
from Cell Signaling Technology, Inc.
Preparation of hydrogen-rich medium
and measurement of hydrogen concentration
Hydrogen-rich medium was prepared as previously
described (9,10). Briefly, 20 ml Dulbecco's Modified
Eagle Medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 1% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin was injected into a vacuumed aseptic soft
plastic infusion bag (100 ml; CR Double-Crane Pharmaceuticals Co.,
Ltd, Anhui, China). Then, 99.999% hydrogen from the seamless steel
gas cylinder was bubbled into the aseptic soft plastic infusion bag
until the bag was full of gas with no dead volume. The bag with
hydrogen and medium was maintained at 4°C for >6 h prior to use.
Hydrogen concentration was measured by the reaction of MB-Pt
reagent (generously provided by Ming Yan, Shanghai Nanobubble
Technology Co., Ltd., Shanghai, China.) with hydrogen-rich water,
as previously described (10).
Cell culture and treatment
H9c2 rat cardiac myoblasts (a cardiomyoblast cell
line derived from embryonic rat heart tissue, generously provided
by Dr Runmin Guo, Guangdong Medical University, Zhanjiang, China)
were grown in DMEM containing 5.5 mM glucose supplemented with 10%
FBS and 100 U/ml penicillin/streptomycin under a humidified
atmosphere of 95% air and 5% CO2 at 37°C. ISO powder was
dissolved as 10 mM stock solution using high pressure deionized
water 30 min prior to use. To investigate the effect of ISO on
autophagy, cells were starved for 18 h in DMEM/1% FBS and then
treated with 10 µM ISO in DMEM/1% FBS for the indicated time
(21). The 10 µM ISO concentration
was selected as it is routinely used for inducing cardiomyocyte
hypertrophy (21). Cell Counting
Kit-8 (cat no. CK04; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was used to analyze the possible cytotoxity of
hydrogen-rich medium in cardiomyocytes as the product description.
In order to investigate the effect of hydrogen on autophagy in
response to ISO, the medium was replaced with hydrogen-rich medium
for 30 min. Subsequently, the ISO (10 µM) treatment was continued
in hydrogen-rich medium for the indicated time (21).
Animal model of cardiac hypertrophy
and treatment protocol
C57BL/6J mice (aged 8–10 weeks; male; n=24; weight,
23.25±1.36 g) were obtained from the Laboratory Animal Center of
Sun Yat-sen University (Guangzhou, China). The animals were housed
with 12-h light/dark cycles and allowed access to food and water
ad libitum. All experimental procedures and protocols were
approved by the Institutional Animal Care and Use Committee
(Zhongshan School of Medicine, Sun Yat-sen University), and
conformed to the Guide for the Care and Use of Laboratory Animals
published by the National Institutes of Health (NIH publication no.
85-23, revised 1996).
Cardiac hypertrophy was induced by subcutaneous
injection of ISO (0.5 mg/100 g/day) for 7 days as previously
described (9,22). Mice were randomly assigned to four
groups: Control group (Con, n=6), ISO group (n=6), ISO plus
hydrogen group (ISO+H2, n=6), and hydrogen group
(H2, n=6). Mice in the ISO+H2 group received
hydrogen (1 ml/100 g/day, intraperitoneal injection) for 7 days
prior to ISO administration (0.5 mg/100 g/day, subcutaneous
injection), and then received ISO with hydrogen for another 7 days
(9,10,23).
Mice in the ISO or H2 groups received only ISO or
hydrogen administration alone, respectively. Control mice were
untreated. Mice were sacrificed on the day 8 of ISO administration.
Following sacrifice, hearts were excised, rinsed with ice-cold PBS,
and blotted dry. Hearts were weighed, and the heart weight/body
weight (HW/BW) ratios were calculated and expressed as mg HW per g
BW. Then hearts were snap frozen in liquid nitrogen within min and
stored at −80°C until further analysis.
Western blotting
Total protein was extracted from left ventricles and
H9c2 cardiomyocytes, as previously described (9). The protein concentration was
determined using a bicinchoninic acid protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Protein samples (30 µg) were
separated by SDS-PAGE (15% gel for LC3B and 10% gel for other
proteins), and transferred to polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). The membranes were incubated
with Beclin1, Atg7, LC3B and β-actin primary antibodies (all,
1:2,000 incubated at 4°C overnight) and their corresponding
secondary antibodies [goat anti-rabbit immunoglobulin G (IgG)
horseradish peroxidase (HRP)-conjugated for Beclin1, Atg7 and LC3B;
and horse anti-mouse IgG HRP-conjugated for β-actin; all, 1:2,000;
incubated at room temperature for 60 min] by standard techniques.
Signals were detected using enhanced chemiluminescence (ChemiDoc
XRS+ System; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein expression was quantified using the Image Lab 3.0.1 (Bate
1) (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as mean ± standard deviation.
Statistical comparisons were performed with SPSS 20.0 software (IBM
Corp., Armonk, NY, USA). Differences among groups were tested by
one-way analysis of variance followed by Bonferroni's method for
post hoc analysis. Comparisons between two groups were performed by
unpaired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
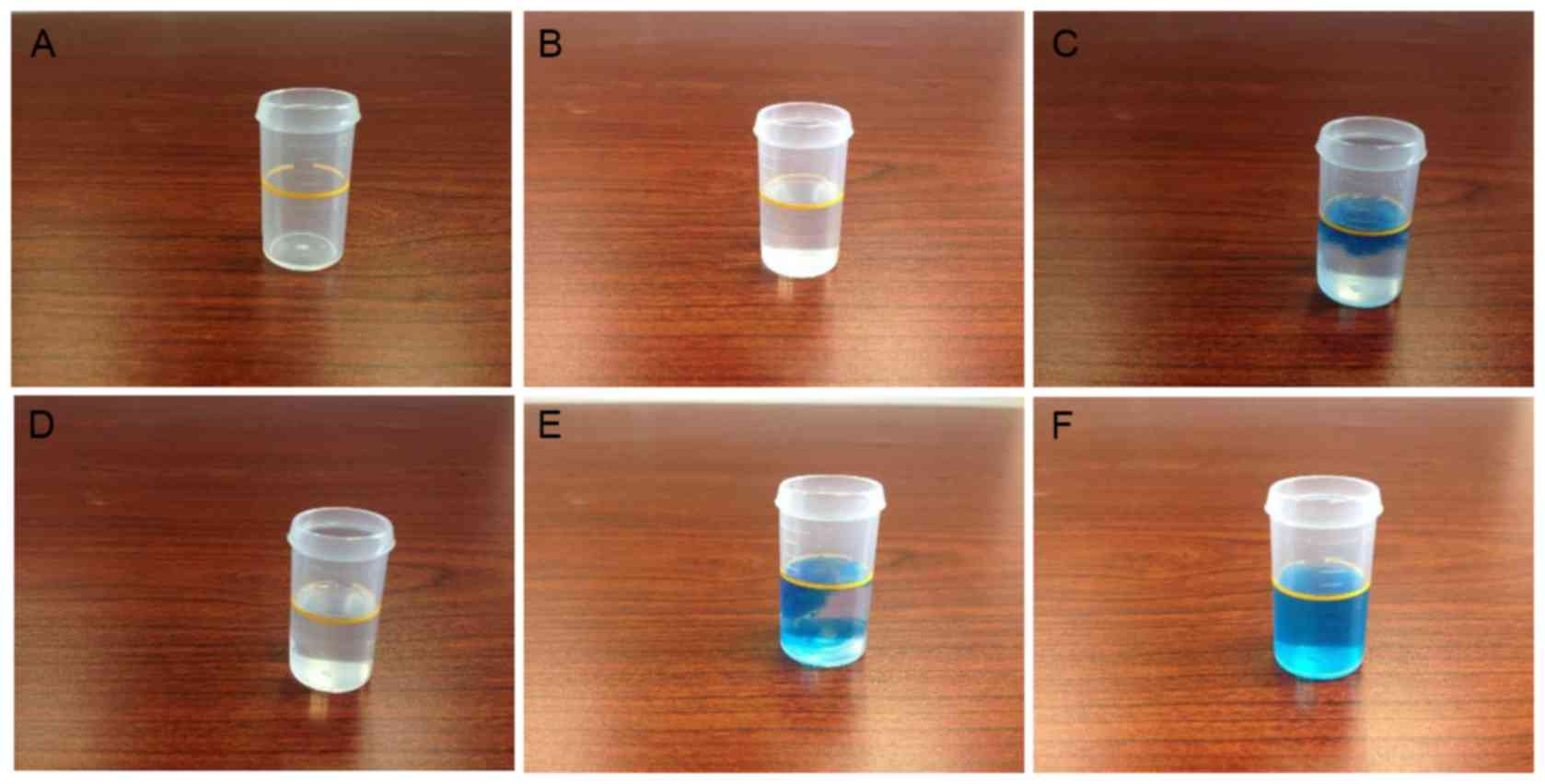
Hydrogen concentration analysis
DMEM is a complex mixture that contains reducing
reagents, thus, it is not suitable for determining the hydrogen
concentration by the MB-Pt reagent method directly. For this
reason, hydrogen-rich water was prepared with the identical
technique as the hydrogen-rich medium, and using the same volume of
purified water instead of DMEM. The hydrogen concentration in the
water was then determined in order to indirectly measure its
concentration in hydrogen-rich medium. MB-Pt reagent was added into
hydrogen-rich water until the solution was permanently stained blue
(Fig. 1) (24). Using this method, the hydrogen
concentration in normal purified water was determined to be <0.1
ppm, while in hydrogen-rich water it was ~0.6–0.9 ppm. These
findings suggest that the medium infused by hydrogen in the present
study was hydrogen-rich medium.
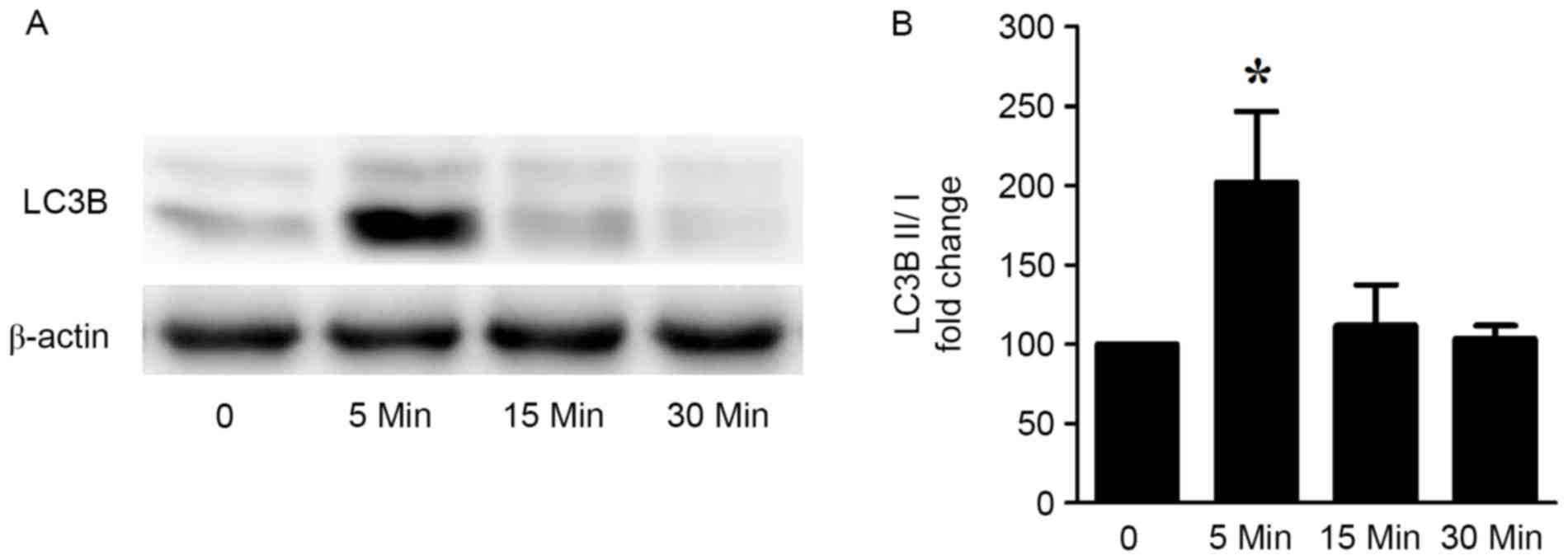
ISO stimulation induces cardiomyocyte
excessive autophagy in vitro
To investigate the effects of ISO on autophagy in
cardiomyocytes in vitro, 10 µM ISO was used to stimulate
H9c2 cardiomyocytes for different durations (5, 15 and 30 min). The
autophagic marker LC3B was used to evaluate cardiomyocyte
autophagy. The ratio of the protein expression levels of LC3B II/I
was significantly increased following 5 min of ISO stimulation, and
this increase was reduced back to basal levels at 30 min (Fig. 2). These results indicated that ISO
induced an acute increased cardiomyocyte autophagy response.
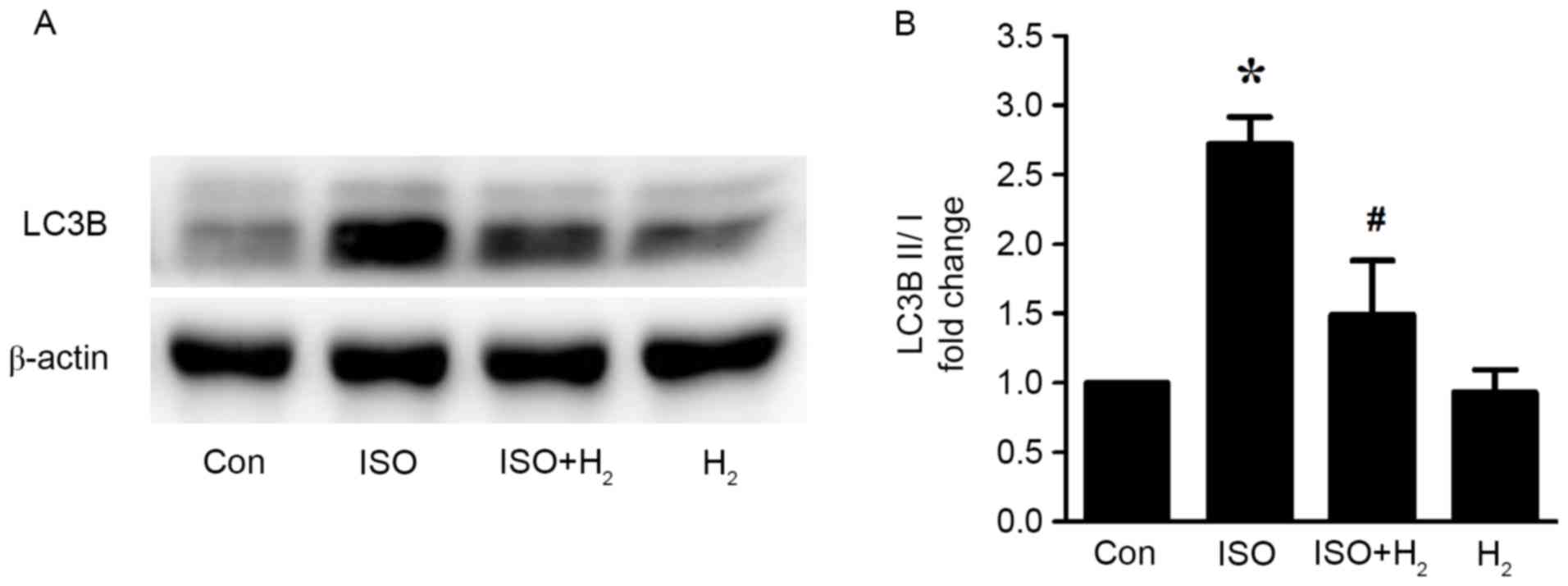
Hydrogen-rich medium pretreatment
inhibits the ISO-induced excessive autophagy in vitro
As discussed in our previous study, hydrogen-rich
medium incubation can effectively inhibit cardiomyocyte hypertrophy
in vitro (9). Therefore, in
the present study, we investigated whether hydrogen may have an
effect in regulating autophagy as a potential mechanism to inhibit
cardiomyocyte hypertrophy. Firstly, the possible cytotoxity of
hydrogen-rich medium in cardiomyocytes was assessed by Cell
Counting Kit-8 assay. Hydrogen-rich medium treatment for 48 h was
not cytotoxic in cardiomyocytes (data not shown). When examining
the protein expression levels of LC3B by western blotting, the
results revealed that the ratio of LC3B II/I was significantly
increased following 5 min of ISO stimulation in H9c2 cardiomyocytes
(Fig. 3). The ISO-mediated
increased autophagic activity, however, was significantly
attenuated by pretreatment with hydrogen-rich medium for 30 min
(Fig. 3). These data indicated
that pretreatment with hydrogen-rich medium blocked the ISO-induced
excessive autophagy in cardiomyocytes in vitro.
Hydrogen administration inhibits the
ISO-induced excessive autophagy in a cardiac hypertrophy model in
vivo
To further confirm the in vitro results, an
ISO-induced cardiac hypertrophy in vivo model was generated
in mice. Then, the effect of intraperitoneal administration of
hydrogen on cardiomyocyte autophagy activity was evaluated. The
increased heart weight and HW/BW ratio that were observed in the
ISO-treated mice indicated that the cardiac hypertrophy model was
successfully established in vivo (Table I). Similar to the results from our
previous study (9),
intraperitoneal administration of hydrogen effectively inhibited
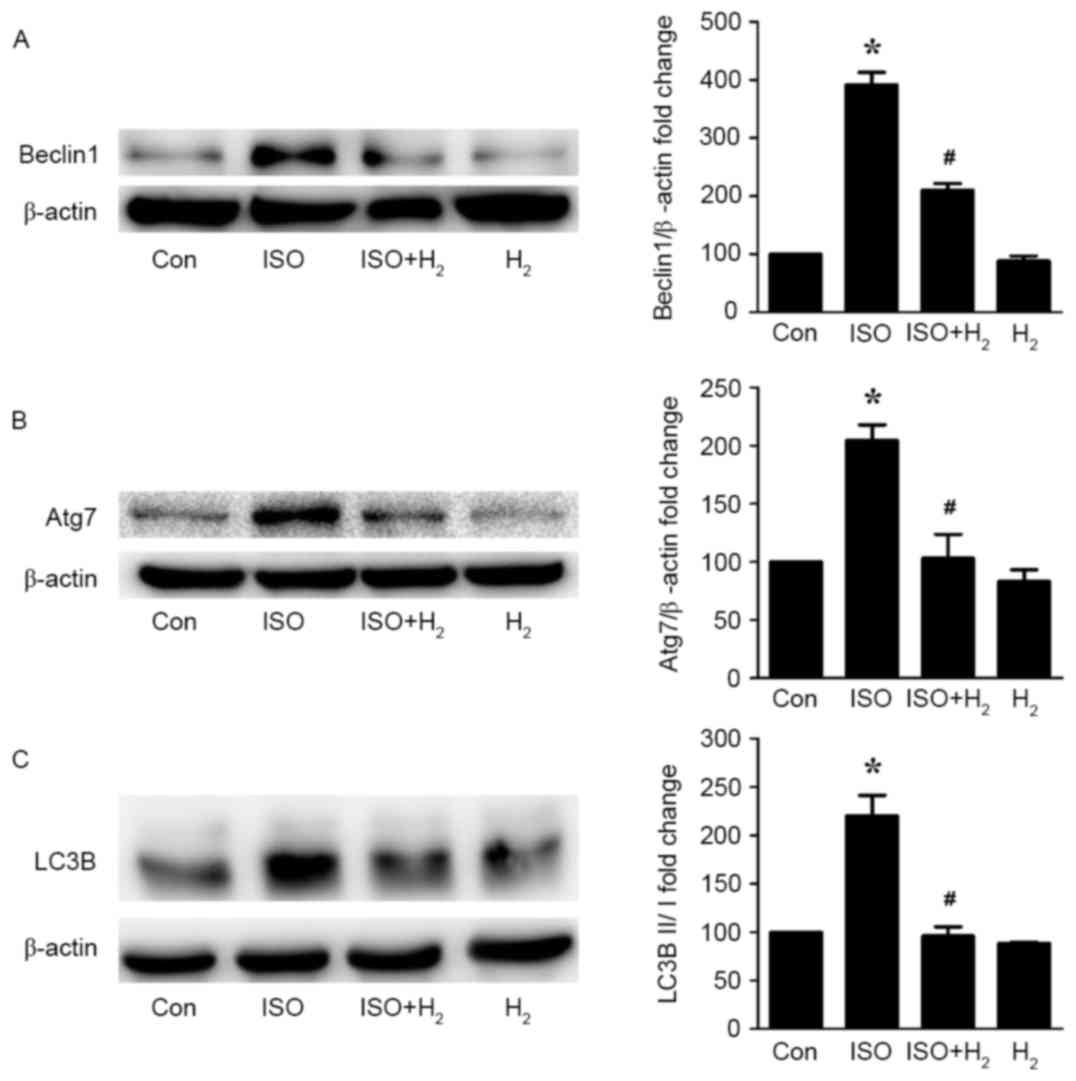
the ISO-mediated cardiac hypertrophic responses (Table I). Western blot analysis revealed
that ISO administration significantly induced the autophagy
response in the left ventricles of the mice, as indicated by
increased protein expression levels of the autophagic markers
Beclin1, Atg7 and LC3B II in the ISO group compared with the
control group (Fig. 4).
Intraperitoneal administration of hydrogen significantly reversed
the ISO-induced excessive autophagy in the heart, as measured by
reduced autophagy marker expression in the ISO+H2 group
compared with the ISO group (Fig.
4). Of note, hydrogen administration alone had no effect on
autophagy activity in the heart. The present findings revealed that
hydrogen administration inhibited the ISO-induced excessive
autophagy in a cardiac hypertrophy model in vivo.
 | Table I.Heart weight and body weight
measurements in the experimental mice. |
Table I.
Heart weight and body weight
measurements in the experimental mice.
| Parameter | Control | ISO |
ISO+H2 | H2 |
|---|
| Number | 6 | 6 | 6 | 6 |
| HW (mg) | 0.12±0.01 |
0.15±0.01a |
0.13±0.01b | 0.12±0.01 |
| BW (g) | 25.17±1.17 | 25.33±1.51 | 25.67±1.97 | 24.92±1.24 |
| HW/BW | 4.78±0.21 |
5.74±0.13a |
5.16±0.15b | 4.73±0.14 |
Discussion
The present study demonstrated that ISO induced an
excessive and acute autophagy response in H9c2 cardiomyocytes in
vitro and that hydrogen-rich medium pretreatment suppressed
this ISO-induced excessive autophagy. In addition, using a mouse
model of cardiac hypertrophy, the present study demonstrated that
intraperitoneal administration of hydrogen significantly blocked
the β-adrenoceptor agonist-mediated excessive autophagy in
vivo.
Autophagy is an intracellular process that mediates
protein degradation, organelle turnover, and recycling of
cytoplasmic components in response to cellular stress or nutrient
starvation (20,25). The process commences with formation
of the autophagosome, a double-membrane structure of reticular
origin that sequesters cytoplasmic components and ultimately fuses
with a lysosome, where engulfed cargo is degraded by
lysosome-derived acid hydrolases (20). Whereas basal levels of autophagy
are required for cell survival, excessive levels or perhaps
distinct forms of autophagic flux contribute to disease
pathogenesis. In an angiotensin II-induced cardiomyopathy model,
angiotensin II (1.1 mg/kg/d) infusion for 4 weeks induces cardiac
mitochondrial damage, autophagy and biogenesis through
mitochondrial ROS (11).
Angiotensin II type 2 receptor has been reported to antagonize
angiotensin II type 1 receptor-mediated cardiomyocyte autophagy
(12). Zhu et al (13) have also revealed that excess
autophagy contributes to pressure overload-induced cardiac
hypertrophy and heart failure (13). In the present study, an ISO-induced
cardiomyocyte hypertrophy model was established in vitro and
in vivo. The results demonstrated that the autophagic marker
LC3B was acutely activated in vitro following ISO
stimulation. Similarly, activated LC3B and autophagy-related
markers Beclin1 and Atg7 were significantly upregulated by ISO
administration in vivo. These findings indicated that an
excess autophagic response occurred during ISO-induced
cardiomyocyte hypertrophy both in vitro and in
vivo.
Hydrogen has been demonstrated to have
anti-autophagy effects on neuronal protection (26,27).
Treatment with hydrogen significantly attenuates neuronal injury
and autophagy in the hippocampal cornu ammonis 1 sector, as well as
reduces brain edema, following 24 h of reperfusion (27). Hydrogen-rich saline decreases the
degree of autophagy in the later stage of acute carbon monoxide
poisoning, thus maintaining homeostasis and enhancing neuronal
survival (26). Consistent with
these findings, the present results demonstrated that hydrogen-rich
medium blocked ISO-induced cardiomyocyte excessive autophagy in
vitro and in vivo, as measured by decreased expression
of Beclin1, Atg7 and LC3B II. It is well known that ROS contributes
to the increase in autophagy. We have recently revealed that
hydrogen can block ISO-induced accumulation of ROS in the heart
(9). Thus, the anti-autophagy
activity of hydrogen in the heart may be related to its antioxidant
effect. However, it should be noted that autophagy is regulated by
a complicated signaling network, and the molecular mechanism by
which hydrogen may block autophagy needs further investigation.
In summary, the present findings indicated that
hydrogen-rich medium and intraperitoneal administration of hydrogen
attenuated excessive autophagy in β-adrenoceptor agonist-induced
cardiomyocyte hypertrophy models in vitro and in
vivo, respectively. Therefore, hydrogen may be a useful natural
agent for inhibiting stress-induced autophagy under certain
conditions.
Acknowledgements
The present work was supported by the National
Natural Science Foundation of China (to Professor Tinghuai Wang,
grant nos. 81572585 and 81372818) and the Science and Technology
Department of Guangdong Province (to Professor Tinghuai Wang, grant
no. 2012A032500002).
References
|
1
|
Hayashida K, Sano M, Kamimura N, Yokota T,
Suzuki M, Maekawa Y, Kawamura A, Abe T, Ohta S, Fukuda K and Hori
S: H(2) gas improves functional outcome after cardiac arrest to an
extent comparable to therapeutic hypothermia in a rat model. J Am
Heart Assoc. 1:e0034592012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayashi T, Yoshioka T, Hasegawa K,
Miyamura M, Mori T, Ukimura A, Matsumura Y and Ishizaka N:
Inhalation of hydrogen gas attenuates left ventricular remodeling
induced by intermittent hypoxia in mice. Am J Physiol Heart Circ
Physiol. 301:H1062–H1069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato R, Nomura A, Sakamoto A, Yasuda Y,
Amatani K, Nagai S, Sen Y, Ijiri Y, Okada Y, Yamaguchi T, et al:
Hydrogen gas attenuates embryonic gene expression and prevents left
ventricular remodeling induced by intermittent hypoxia in
cardiomyopathic hamsters. Am J Physiol Heart Circ Physiol.
307:H1626–H1633. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayashida K, Sano M, Ohsawa I, Shinmura K,
Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et
al: Inhalation of hydrogen gas reduces infarct size in the rat
model of myocardial ischemia-reperfusion injury. Biochem Biophys
Res Commun. 373:30–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lekic T, Manaenko A, Rolland W, Fathali N,
Peterson M, Tang J and Zhang JH: Protective effect of hydrogen gas
therapy after germinal matrix hemorrhage in neonatal rats. Acta
Neurochir Suppl. 111:237–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang
JH, Denoble PJ, Tao H and Sun X: Hydrogen-rich saline protects
myocardium against ischemia/reperfusion injury in rats. Exp Biol
Med (Maywood). 234:1212–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Sun Q, He B, Xiao J, Wang Z and
Sun X: Anti-inflammatory effect of hydrogen-rich saline in a rat
model of regional myocardial ischemia and reperfusion. Int J
Cardiol. 148:91–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu YS and Zheng H: Chronic hydrogen-rich
saline treatment reduces oxidative stress and attenuates left
ventricular hypertrophy in spontaneous hypertensive rats. Mol Cell
Biochem. 365:233–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Xu J, Long Z, Wang C, Wang L, Sun
P, Li P and Wang T: Hydrogen (H2) Inhibits Isoproterenol-Induced
Cardiac Hypertrophy via Antioxidative pathways. Front Pharmacol.
7:3922016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang YX, Xu JT, You XC, Wang C, Zhou KW,
Li P, Sun P, Wang L and Wang TH: Inhibitory effects of hydrogen on
proliferation and migration of vascular smooth muscle cells via
Down-Regulation of mitogen/activated protein kinase and
Ezrin-Radixin-Moesin signaling pathways. Chin J Physiol. 59:46–55.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai DF, Johnson SC, Villarin JJ, Chin MT,
Nieves-Cintrón M, Chen T, Marcinek DJ, Dorn GW II, Kang YJ, Prolla
TA, et al: Mitochondrial oxidative stress mediates angiotensin
II-induced cardiac hypertrophy and Galphaq overexpression-induced
heart failure. Circ Res. 108:837–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porrello ER, D'Amore A, Curl CL, Allen AM,
Harrap SB, Thomas WG and Delbridge LM: Angiotensin II type 2
receptor antagonizes angiotensin II type 1 receptor-mediated
cardiomyocyte autophagy. Hypertension. 53:1032–1040. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu H, Tannous P, Johnstone JL, Kong Y,
Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA and Hill
JA: Cardiac autophagy is a maladaptive response to hemodynamic
stress. J Clin Invest. 117:1782–1793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakai A, Yamaguchi O, Takeda T, Higuchi Y,
Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et
al: The role of autophagy in cardiomyocytes in the basal state and
in response to hemodynamic stress. Nat Med. 13:619–624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohsumi Y: Historical landmarks of
autophagy research. Cell Res. 24:9–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizushima N, Yoshimori T and Ohsumi Y: The
role of Atg proteins in autophagosome formation. Annu Rev Cell Dev
Biol. 27:107–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levine B and Yuan J: Autophagy in cell
death: An innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lavandero S, Chiong M, Rothermel BA and
Hill JA: Autophagy in cardiovascular biology. J Clin Invest.
125:55–64. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong K, Kwon H, Min C and Pak Y:
Modulation of the caveolin-3 localization to caveolae and STAT3 to
mitochondria by catecholamine-induced cardiac hypertrophy in H9c2
cardiomyoblasts. Exp Mol Med. 41:226–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tshori S, Gilon D, Beeri R, Nechushtan H,
Kaluzhny D, Pikarsky E and Razin E: Transcription factor MITF
regulates cardiac growth and hypertrophy. J Clin Invest.
116:2673–2681. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang G, Zhou J, Zhan W, Xiong Y, Hu C, Li
X, Li X, Li Y and Liao X: The neuroprotective effects of
intraperitoneal injection of hydrogen in rabbits with cardiac
arrest. Resuscitation. 84:690–695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seo T, Kurokawa R and Sato B: A convenient
method for determining the concentration of hydrogen in water: Use
of methylene blue with colloidal platinum. Med Gas Res. 2:12012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakatogawa H, Suzuki K, Kamada Y and
Ohsumi Y: Dynamics and diversity in autophagy mechanisms: Lessons
from yeast. Nat Rev Mol Cell Biol. 10:458–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Tian L, Li Y, Wang X, Xia F, Li L,
Li J and Zhang Z: Effects of hydrogen-rich saline on rats with
acute carbon monoxide poisoning. J Emerg Med. 44:107–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagatani K, Wada K, Takeuchi S, Kobayashi
H, Uozumi Y, Otani N, Fujita M, Tachibana S and Nawashiro H: Effect
of hydrogen gas on the survival rate of mice following global
cerebral ischemia. Shock. 37:645–652. 2012. View Article : Google Scholar : PubMed/NCBI
|