Introduction
Osteoarthritis (OA) represents the most prevalent
type of joint disease especially in the elderly and it is estimated
that about 3 million newly diagnosed OA could be presented each
year (1,2). In current clinical practice, joint
replacement remains the preferred treatment method for patients
with advanced OA (3). However, due
to the lacking of knowledge about the disease pathophysiology, only
a few effective disease-modifying therapies has been proposed for
OA treatment. Therefore, investigate the disease mechanism will
give insights for the treatment.
OA is characterized by extracellular matrix
destruction and chondrocyte function loss (apoptosis) and multiple
risk factors could result in this phenomenon, including mechanical
injury, aging and inflammation (4). Enhanced chondrocyte apoptosis is
considered as the sign of cartilage joint degeneration in OA
(5). Studies have shown that a
variety of stimuli including tumor necrosis factor-α (TNF-α)
(6), TNF-related
apoptosis-inducing ligand (TRAIL) (7) and nitric oxide (8) were involved in the chondrocytes
apoptosis process. Recent epidemiology and experimental studies
have shown that lipid peroxidation was involved in the pathogenesis
of OA (9,10). Associations were found between
hypercholesterolemia or hypertension and OA (9,10).
Joint manifestation are frequently presented in patients with
familial hypercholesterolemia, which is characterized by a
decreased removal of low-density lipoprotein (LDL), and treatment
with a lipid-lowering diet attenuates the incidence of joint
involvement (11). However, the
few studies has explored the detailed events involved in these
events.
Here, we aimed to investigate the role of oxidized
low density lipoprotein (ox-LDL) in TNF-α mediated chondrocyte
death and explore the mechanisms. Based on our patient data and
cell model experiment data, we demonstrated that ox-LDL could
enhance TNF-α mediated chondrocyte death via autophagy related
pathway.
Materials and methods
OA cartilage and SF samples
OA (n=40) cartilage and synovial fluid (SF) samples
were collected at the time of total knee arthroplasty at Shanghai
Sixth People's Hospital, Shanghai, China. Normal cartilage and SF
from patients (n=40) with no history of OA was obtained from
Shanghai Sixth People's Hospital. Cartilage and SF samples were
stored in −80°C before further processing. The demographic data of
these patients, including age, gender, body mass index (BMI),
disease duration, the total Western Ontario and McMasters
University Osteoarthritis Index (WOMAC) score, ox-LDL level and
Lox-1 level, were also collected and listed in Table I. Among these indexed, the total
WOMAC score includes WOMAC pain score and WOMAC function score, and
functional disability and pain were assessed by self-reported
questionnaires according to previous publications (12,13).
All research involving human participants was approved by the
Institutional Review Board of Shanghai Jiaotong University School
of Medicine, Shanghai, China. Written informed consent was
collected from all the participants.
 | Table I.Demographic data of the
osteoarthritis patients. |
Table I.
Demographic data of the
osteoarthritis patients.
|
| Healthy controls
(n=40) | Patient (n=40) |
|---|
| Age | 47.7±10.4 | 57.4±10.2 |
| Sex (female,
%) | 22 (55%) | 21(52.5%) |
| BMI
(kg/m2) | 27.5±4.5 | 26.2±3.9 |
| Disease
duration | – | 52.2±38.1 |
| Total WOMAC
score | – | 73.0±19.9 |
| LOX-1 level | 0.33±0.04 | 0.49±0.11 |
| Ox-LDL level
(mU/ml) | 34.5±15.7 | 64.8±18.3 |
Enzyme-linked immunosorbent assay
SF samples were examined with enzyme-linked
immunosorbent assay (ELISA) kit from Cell Biolabs Inc., (San Diego,
CA, USA) and the absorbance value was measured at 450 nm to
concentration measurement according to the manufacturer's
instructions.
Quantitative real-time reverse
transcription-polymerase chain reaction (qRT-PCR) assay
Total cellular RNA was extracted from of cartilage
tissue with TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA). RT-PCR was carried out using a One Step SYBR®
PrimeScript™ RT-PCR kit (Takara Bio, Dalian, China) and
an iQ5 Real-time PCR Detection system (Bio-Rad, Hercules, CA, USA).
Expression of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
gene was assayed simultaneously with samples as an internal
control. Relative gene expression was determined by the
2−ΔΔCT method (14).
Oligonucleotide primers specific for LOX-1 and GAPDH are listed in
Table II.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| LOX-1 | TTACTCTCCATG | AGCTTCTTCTGC |
|
| GTGGTGCC | TTGTTGCC |
| GAPDH | CAAAGCCAGAG | GATGGTCTTGGT |
|
| TCCTTCAGA | CCTTAGCC |
Cell culture and treatment
Human articular chondrocyte culture was purchased
from cell culture collection of Fudan University, Shanghai, China,
and cultured according to the manufacturer's instruction. Cell were
plated at a density of 1×104/cm2 in a human
chondrocyte medium containing chondrocyte growth supplement and
penicillin/streptomycin (ScienCell, Carlsbad, CA, USA) and
incubated at 37°C in a humidified 5% CO2 incubator. In
the setting of LOX-1 neutralization, chondrocytes at 70–80%
confluency were pre-incubated with or without anti-LOX-1 antibody
(10 µg/ml) before processing for vehicle control or TNF-α (50
ng/ml) or ox-LDL (20 µg/ml, Yiyuan biotech, Guangzhou, China) or
TNF-α (50 ng/ml) and ox-LDL (20 µg/ml) co-treatment for 24–48 h. In
the experiment of autophagy inhibition, chondrocytes at 70–80%
confluency were pre-treated with or without 3-MA (10 mM) for 24 h
before processing for vehicle control or TNF-α (50 ng/ml) and
ox-LDL (20 µg/ml) co-treatment for 24 h.
Lenti-virus mediated autophagy protein
5 (ATG5) knockdown
ATG5 siRNA lentivirus and control lentivirus were
obtained from Shanghai Hanbio Co. Ltd., Shanghai, China.
Chondrocytes cultured in a 6 well plate at 50% confluence were
infected with 50 µl ATG5 siRNA and control lentivirus in 3 ml serum
free human chondrocyte medium containing 8 µg/ml puromycin for 24
h. Medium change was performed after 24 h and the efficacy of ATG-5
knockdown was verified by western blotting. Chondrocytes were
collected at 48 h after lentivirus infection, followed by TNF-α (50
ng/ml) and ox-LDL (20 µg/ml) co-treatment for 24 h.
Cell viability
Cell viability was determined by trypan blue
exclusion assay.
Immunofluorescence
Cells were collected onto a clean glass slide by
using centrifugation at 1,000 rpm for 5 min. The slide was then
incubated with LC3 primary antibody for 1 h at 37°C, washed 3 times
with PBS, incubated with FITC-conjugated secondary antibody for 1 h
at room temperature and finally dropped with DAPI containing
anti-quench reagents. Fluorescence images were observed and
analyzed using Zeiss LSM 510 laser-scanning confocal microscope
(Goettingen, Germany).
DNA fragmentation assay
After serum-starvation for 12–24 h, the medium was
changed to serum-free medium containing Ox-LDL (20 µg/ml) or
vehicle control for 24–48 h. Cells were washed with ice-cold PBS
and lysed in a buffer containing 10 mM Tris-HCl, pH 7.4, 150 mM
NaCl, 0.3% SDS and 20 mM EDTA at 4°C for 30 min. After incubation
with RNase A (20 µg/ml) and proteinase K (0.4 mg/ml), DNA was
extracted with phenol-chloroform-isoamyl alcohol (25:24:1),
dissolved in 30 µl Tris-EDTA buffer, and then subjected to 3%
agarose gel electrophoresis. Fragmented DNA was visualized with the
SYBR green I DNA staining system.
Western blot analysis
Cells treated according to abovementioned procedure
were lysed in RIPA buffer, followed by high speed centrifugation
and protein quantification using a bicinchoninic acid assay.
Cellular proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidenedifluoride membranes. After blocking, the membranes
were incubated with primary monoclonal antibodies against LC3,
Caspase-8 and Caspase-3 (Cell Signaling Technology, Cambridge, MA,
USA). β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was
used as the loading control. Horseradish peroxidase-conjugated
secondary antibodies were applied to detect labeled proteins.
Protein bands were developed with SuperSignal Ultra
Chemiluminescent Substrate (Pierce, Rockford, IL, USA) on X-ray
films (Kodak, Tokyo, Japan).
Statistical analysis
Statistical analysis was carried out using SPSS v18
(SPSS, Chicago, IL, USA). Data were reported as means ± standard
deviation (SD). Student's t-test or one-way analysis of
variance was used to determine the significance of difference
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased level of ox-LDL and Lox-1 in
OA patients
In order to clarify the possible role of ox-LDL and
Lox-1 during the presence of osteoarthritis, we firstly collected
SF and cartilage samples from 40 OA patients and 40 normal control
subjects (please see Table II
about the demographic data of these subjects) and determined the
ox-LDL level in SF and Lox-1 expression in cartilage tissues by
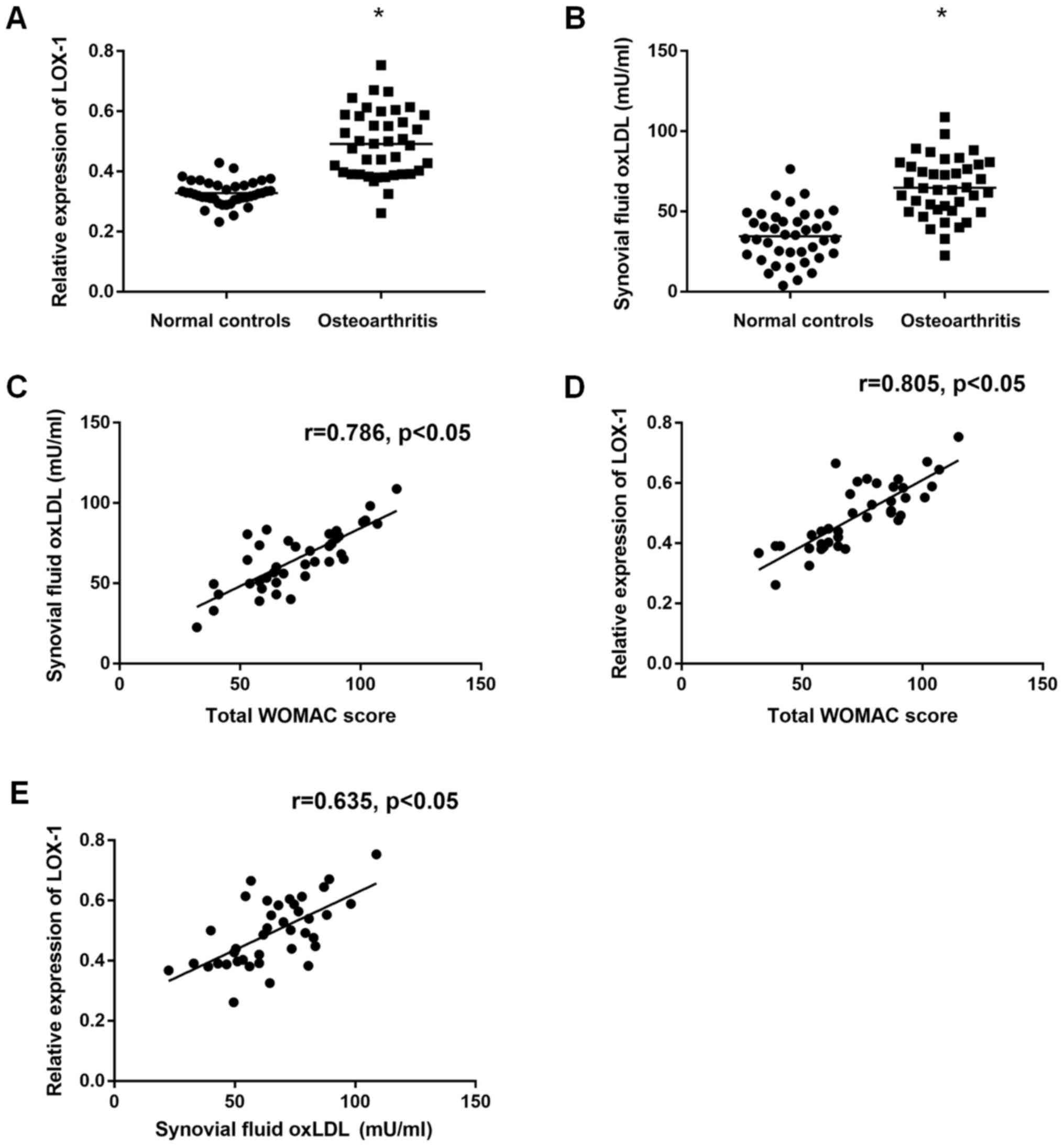
ELISA and qRT-PCR, respectively. The results showed that
significantly increased ox-LDL level [(64.8±18.3) mU/ml vs.
(34.5±15.7) mU/ml, P<0.05] (Fig.
1A) and Lox-1 expression (0.49±0.11 vs. 0.33±0.04, P<0.05)
could be found in OA patients compared to normal controls (Fig. 1B). Moreover, we also found that
correlations could be found between the ox-LDL (r=0.786, P<0.01)
(Fig. 1C) or Lox-1 (r=0.805,
P<0.01) (Fig. 1D) and the total
WOMAC score, which is a common disease severity indexes used in OA.
In addition, the association between ox-LDL and LOX-1 was also
observed according to the correlation analyses (r=0.635, P<0.05)
(Fig. 1E). These results suggested
that increased level of ox-LDL and Lox-1 was presented in OA
patients and according to the results from correlation analyses,
ox-LDL and Lox-1 may be involved in the pathogenesis of OA.
Facilitation of TNF-α-mediated
chondrocyte death by ox-LDL
According to the previous description (15), low-grade inflammation with
increased expression of proinflammatory cytokines (eg. TNF-α) in
articular cartilage and synovium result in chondrocyte death and
contribute to disease progression of OA. Since the increased ox-LDL
level and Lox-1 expression was found in OA patients and they were
considered to be involved in the disease process of OA, we further
established an in vitro TNF-α mediate chondrocyte
inflammation model to clarify the role of ox-LDL and LOX-1 during
OA process. As shown in Fig. 2A,
TNF-α and ox-LDL alone did not affect the cell death obviously
compared to the control (TNF-α vs. ox-LDL vs. Control: 4.65±2.21%
vs. 6.86±4.65% vs.3.60±1.04%, P>0.05), however, combine use
TNF-α and ox-LDL could result in significantly increased
chondrocyte death and these effects could be reversed by Lox-1
monoclonal antibody (TNF+oxLDL vs. control: 66.90±6.98% vs.
3.60±1.04%; TNF+oxLDL vs. TNF+oxLDL+Lox-1 Ab: 66.90±6.98% vs.
11.40±1.50%, P>0.05). In order to further confirmed the cell
phenomenon during the TNF-α and ox-LDL combined treatment, we
employed DNA fragmentation assay and flow cytometry assay to
verification. Our results demonstrated obvious DNA fragmentation
phenotype could be found during the combine use TNF-α and ox-LDL,
but not in the condition of TNF-α and ox-LDL alone, and DNA
fragmentation could be abolished by Lox-1 antibody (Fig. 2B); moreover, the flow cytometry
results of cell apoptosis showed similar trend and the cell
apoptosis rate under control, TNF-α, ox-LDL, TNF+oxLDL and
TNF+oxLDL+Lox-1 Ab was 3.0, 4.0, 6.4, 62.7 and 13.7%, respectively
(Fig. 2C). In addition, we also
employed western-blotting to examine the apoptosis initiate caspase
(caspase-8) and executioner caspase (caspase-3) and the results
showed that increased level of cleaved caspase-8 and caspase-3 were
found in the setting of TNF-α and ox-LDL combination, which could
be abolished by adding in Lox-1 antibody (Fig. 2D). These results indicated that
ox-LDL could facilitate the TNF-α effects on chondrocyte death by
increasing cell apoptosis. Autophagy is involved in the cell death
process mediated by TNF-α and ox-LDL.
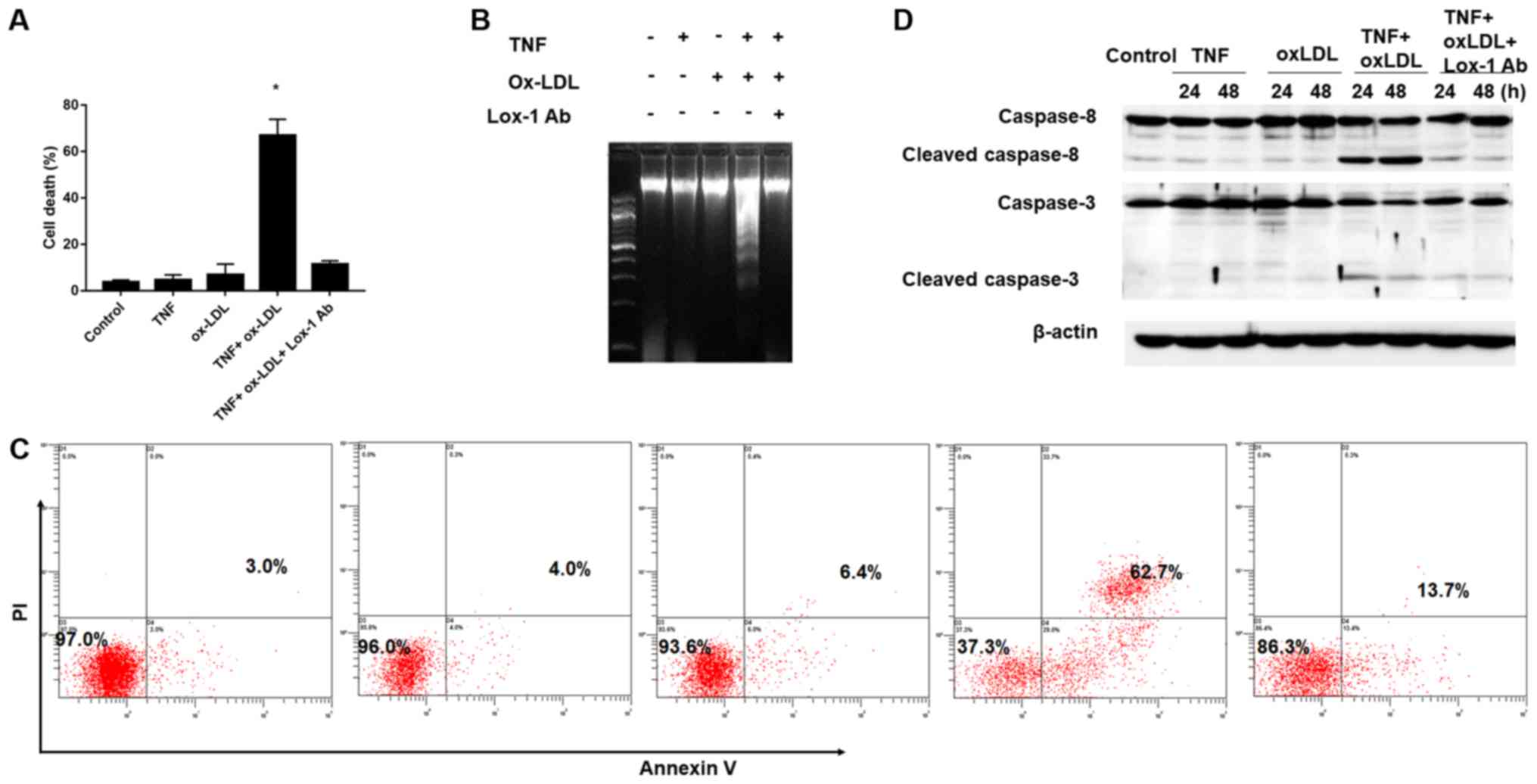 | Figure 2.Facilitation of TNF-α-mediated
chondrocyte death by oxidized low density lipoprotein (ox-LDL).
After 30 min preincubation with or without anti-LOX-1 antibody (10
μg/ml), Chondrocytes were co-treated with TNF-α (50 ng/ml) and
ox-LDL (20 μg/ml), and harvested at 24 or 48 h for (A) cell death,
(B) DNA fragmentation assay, (C) Flow cytometry analysis of the
chrondrocyte apoptosis by Annexin V/PI staining and (D) western
blot analysis of apoptosis related proteins. (A, B and C) Ox-LDL
co-treatment could facilitate TNF-α-mediated chondrocyte death and
this process could be blocked by Lox-1 monoclonal antibody
pretreatment. (D) Western-blot analysis revealed that increased
level of cleaved caspase-8 and caspase-3 in TNF-α and ox-LDL
co-treated chondrocytes, and that this effect could be blocked by
Lox-1 antibody pretreatment.*P<0.05 compared to control. ox-LDL,
oxidized low density lipoprotein; Lox-1, lectin-like ox-LDL
receptor-1; TNF-α, tumor necrosis factor α; Ab, antibody; PI,
propidium iodide. |
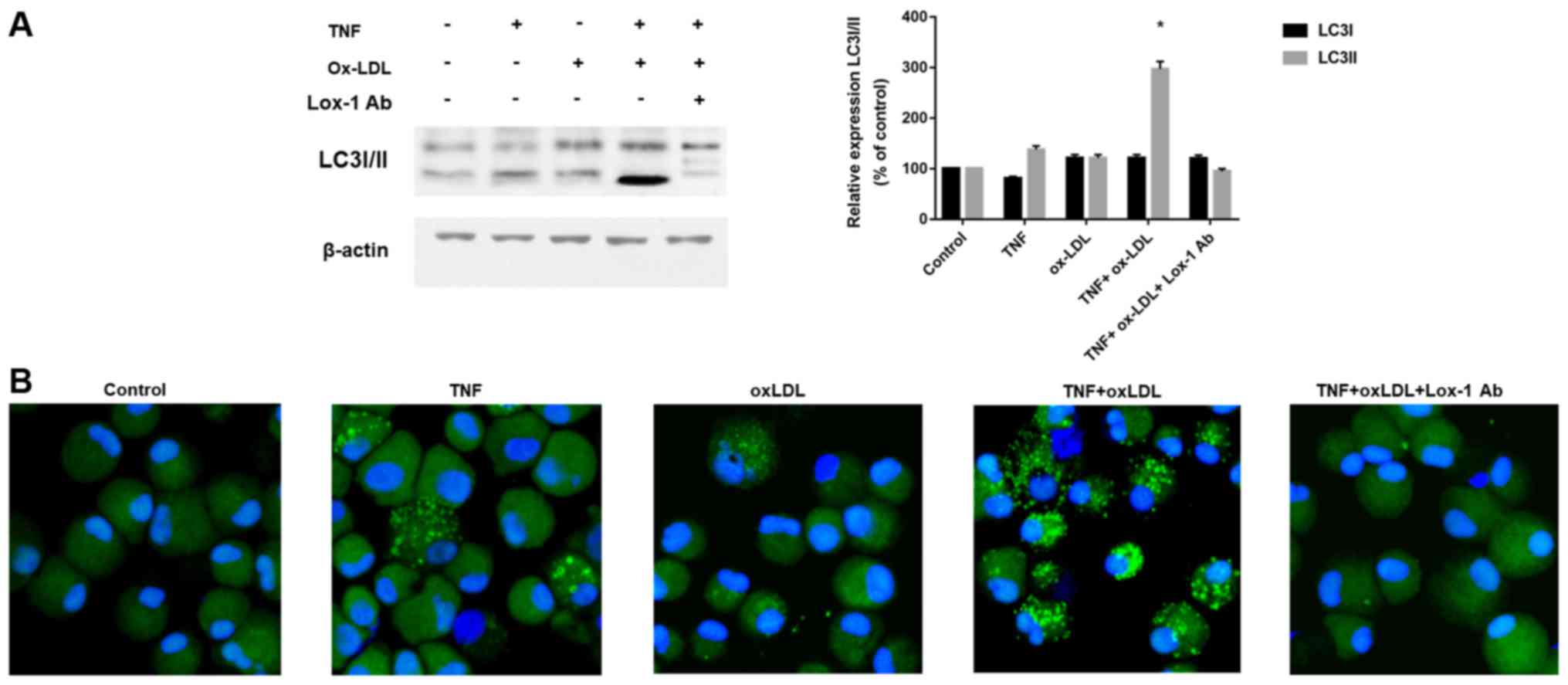
According to previous description (16), both apoptotic as well as
non-apoptotic mechanisms were involved in the cell death affected
by OA and autophagy related cell death was considered as one of
novel mechanism involved in the chondrocyte death. We therefore
examined the autophagy classical marker LC3 by western-blotting and
immunofluorescence. As shown in Fig.
3A, we found that increased LC3-II, which is an indicator of
autophagy activation, was found after TNF-α and ox-LDL combination
treatment, and this process could be blocked by Lox-1 antibody
neutralization. The immunofluorescence results in Fig. 3B also confirmed the effects of
TNF-α and ox-LDL combination on autophagy activation by LC3 green
fluorescence and Lox-1 antibody treatment could inhibit this
process. These results indicated autophagy is involved in the cell
death process mediated by TNF-α and ox-LDL.
Autophagy inhibition reverse the cell
death process mediated by TNF-α and ox-LDL
Since the above results suggested the involvement
and enhancement of autophagy process in the cell death process
mediated by TNF-α and ox-LDL, we here further determined the cell
death by induction of autophagy inhibition by classical autophagy
inhibitor 3-MA and ATG5 siRNA. According to the results, both 3-MA
and ATG5 siRNA treatment could result in the decreased level of
LC3II, which was verified by western-blotting (Fig. 4A for 3-MA; Fig. 4E for ATG5 siRNA) and
immunofluorescence (Fig. 4B for
3-MA; Fig. 4F for ATG5 siRNA). We
further employed the cell viability counting to verify the cell
death phenotype, both 3-MA and ATG5 siRNA treatment could result in
decreased cell death as shown in Fig.
4C and G, respectively. In addition, the western-blotting
analysis of caspase-8 and caspase-3 also verified that both 3-MA
and ATG5 siRNA treatment could decreased the level of cleaved
caspase-8 and capase-3 (Fig. 4D
for 3-MA; Fig. 4H for ATG5 siRNA).
Taken together, by using small molecule inhibitor 3-MA and gene
manipulating ATG5 knockdown, we confirmed the autophagy inhibition
could reverse the cell death process mediated by TNF-α and
ox-LDL.
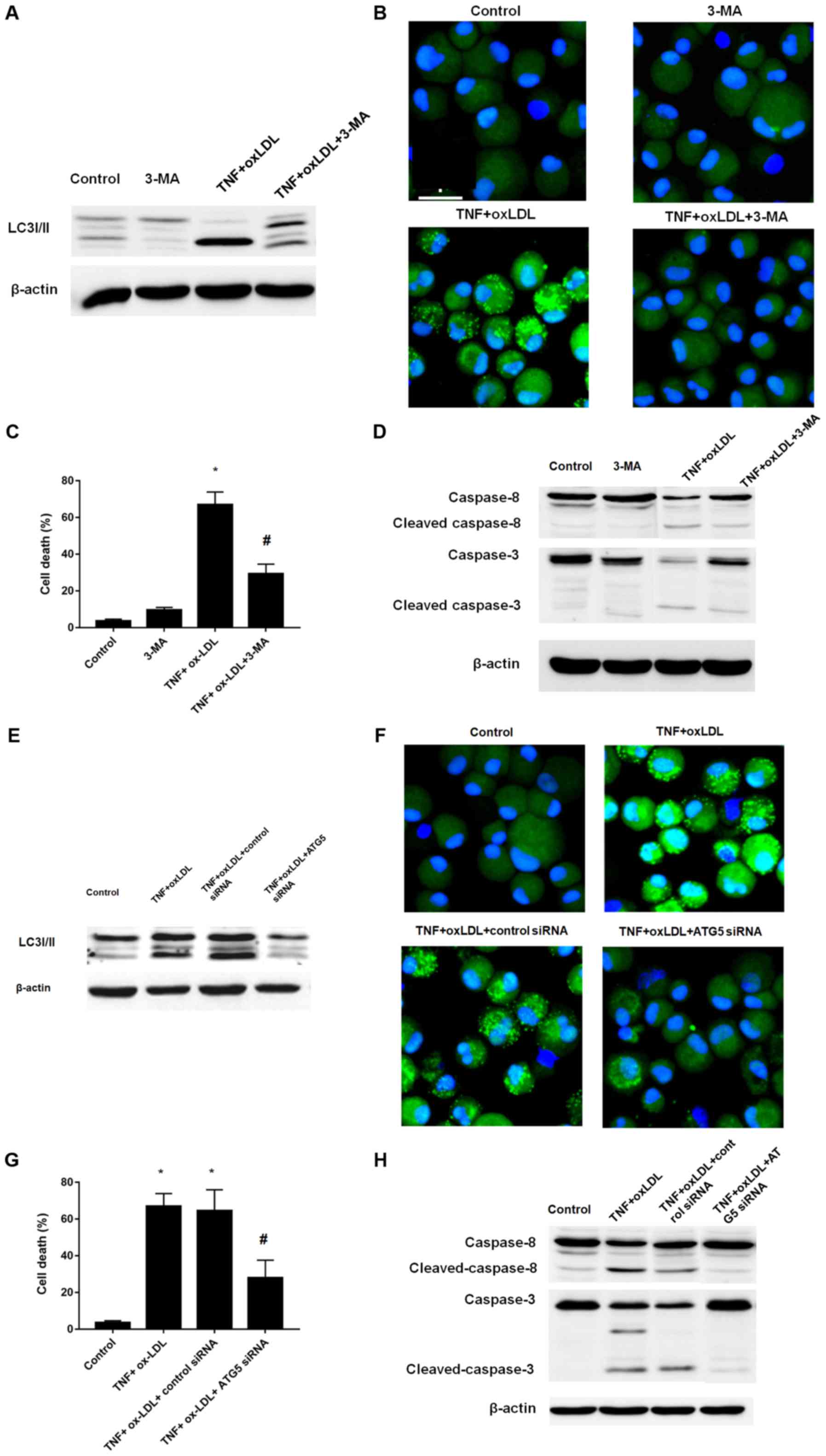 | Figure 4.Autophagy inhibition by 3-MA or Atg-5
siRNA could reverse the effects mediated by TNF-α and ox-LDL
co-treatment on chondrocytes. (A) Western blot analysis of the
expression of LC3I/II. (B) LC3 pattern analysis by confocal
microscopy. (C) Cell death analysis. (D) Western blot analysis of
apoptosis related protein cleaved caspase-3 and caspase-8. 3-MA (5
mM) treatment could reverse the LC3II enhancement, punctuate LC3
pattern, increased cell death and cleaved caspase-3 and caspase-8
mediated TNF-α and ox-LDL co-treatment in chondrocytes. (E) Western
blot analysis of the expression of LC3I/II. (F) LC3 pattern
analysis by confocal microscopy. (G) Cell death analysis. (H)
Western blot analysis of apoptosis related protein cleaved
caspase-3 and caspase-8. Atg-5 siRNA treatment could reverse the
LC3II enhancement, punctuate LC3 pattern, increased cell death and
cleaved caspase-3 and caspase-8 mediated TNF-α and ox-LDL
co-treatment in chondrocytes. *P<0.05 compared to control,
#P<0.05 compared to TNF-α + ox-LDL group. ox-LDL,
oxidized low density lipoprotein; Lox-1, lectin-like ox-LDL
receptor-1; si, small interfering; TNF-α, tumor necrosis factor α;
LC3, microtubule-associated proteins 1A/1B light chain 3B; 3-MA,
3-methyladenine autophagy inhibitor; Atg-5, autophagy protein
5. |
Discussion
In present study, we first found that significantly
increased ox-LDL level in SF sample and LOX-1 expression level in
cartilage tissue was found in OA patients compared to those
corresponding samples from control subjects. Based on this
phenotype, we further explored the effect of ox-LDL on TNF-α
mediated chondrocyte death, and results showed that ox-LDL could
facilitate TNF-α mediated chondrocyte death and this effect could
be blocked by LOX-1 antibody neutralization. Moreover, we also
found that autophagy inhibition by 3-MA and Atg-5 siRNA could
reverse the cell death effect mediated by TNF-α and ox-LDL
co-treatment on chondrocytes. Therefore, we concluded that ox-LDL
facilitates tumor necrosis factor-α mediated chondrocyte death via
its interaction with LOX-1 and autophagy is involved as the
mechanisms. To the best of our knowledge, this the first report to
link ox-LDL, chondrocyte death and autophagy.
According to recent studies, peroxidation of serum
LDL was observed during the inflammation and infection process
(17). During the osteoarthritis
or rheumatoid arthritis, accelerated vascular porosity could be
initiated by inflammation, thereby resulting in invasion of various
inflammatory cells and permeation of the biological activators,
such as oxLDL, into joints. Furthermore, ox-LDL has suggested to
play an important role in the pathogenesis of some ageing related
disorders (eg. atherosclerosis) (18–20).
In addition, both hypercholesterolemia and hypertension was
identified as the risk factor for OA (9,10).
Taken together, these evidences suggested that ox-LDL may be
involved in the OA. Therefore, we first collected the SF to perform
the quantitative analysis of ox-LDL and significantly elevated
ox-LDL was found in OA patients compared to normal controls.
LOX-1, firstly cloned from cultured bovine aortic
endothelial cells, is identified as the receptor of ox-LDL
(21). Previous studies have shown
that the expression of LOX-1 on non-phagocytes, such as vascular
endothelial cells, smooth muscle cells, platelets and
cardiomyocytes (22,23), and inducible expression of LOX-1
could be found during the inflammation. Notably, it has been
reported that expression of LOX and association of ox-LDL was found
in chondrocytes of a rat model of arthritis (24). Moreover, treatment of these
arthritis rats with anti-LOX-1 monoclonal antibody could suppress
articular cartilage degeneration. In vitro chondrocytes
model showed that enhanced LOX-1 expression could be found by
treatment with ox-LDL and pro-inflammatory cytokine
(eg.interleukin-1β) (25). Here,
we examined the expression of LOX-1 in cartilage tissue and our
results showed that increased level of LOX-1 in cartilage tissue
from OA patients compared to normal controls.
Two mechanisms are proposed to be involved in the
TNF-α mediated chondrocyte apoptosis, including direct apoptosis
induction and indirect apoptosis priming by Fas ligand presentation
(26,27). The proto-oncogenes of Bcl-2/Bax
family are involved in the cellular signaling pathway of TNF-α
mediated chondrocyte apoptosis, and activation of effector caspases
(such as caspase-3 and caspase-8) are proposed as the downstream
signaling events (28). We here
found that ox-LDL could enhance the TNF-α mediated chondrocyte
apoptosis as evidenced by increased level of cleaved caspases.
Here, our found that cell death under the treatment of TNF-α is not
significant higher than the control (Fig. 2A) and this result was consistent
with several previous studies (29–32).
However, the further investigate might be needed to prove the exact
role of TNF-α on chondrocyte biology. Autophagy is a considered as
the catabolic pathways for intracellular macromolecules
degradation. At the beginning of autophagy, autophagosome was
formed by sequestration of cytoplasmic organelles in a membrane
vacuole. Then, fusion of the autophagosomes with lysosomes could
result in degradation and recycling of the cellular materials.
Recent studies have shown that increased autophagic activity could
induce cell death (33,34). According to previous studies,
autophagy is an important cell survival mechanism under various
forms of stress (35). Autophagy
serves not only to regulate the final stages of the chondrocyte
lifecycle, but also the rate at which chondrocytes enter the
maturation process (36).
Autophagy in normal adult articular cartilage is an important
mechanism for cellular homeostasis (16). Catabolic and nutritional stresses
could also increase autophagy in OA, and during the initial
degenerative phase at least, autophagy is increased in OA
chondrocytes and cartilage, with increased level of autophagy
related molecules, including LC3 and Beclin-1 in OA chondrocytes
(37). Here, we used TNF to induce
inflammation to mimic the OA chondrocytes. Moreover, ox-LDL was
shown to induce apoptosis in multiple cells (such as endothelial
cells (38) and macrophages)
(39) and we observed increased
level of ox-LDL in OA patients, therefore, we add ox-LDL to the OA
chondrocyte model and examined its effects. Increased LC3
represents the increased autophagy level, while increased caspase-3
and caspase-8 represents the executioner caspase and initiator
caspase, respectively, during the cell apoptosis. Our results was
consistent with the conclusion that both apoptotic as well as
non-apoptotic mechanisms were involved in the cell death affected
by OA, which were found by previous studies (16,40–42).
According to previous description (40–42),
both apoptotic as well as non-apoptotic mechanisms were involved in
the cell death affected by OA and they also concluded that
demonstrated that cell death of chondrocytes within OA undergoes
changes different from the classical apoptosis and they considered
that this type of death is a combination between the classical
apoptosis and autophagy. Our results here supported these
descriptions. Moreover, although the role of autophagy in cell
death has been elucidated in multiple experimental and
physiological system, controversial are existed on the positive or
negative role of autophagy on cell death due to both cytoprotective
and cell death functions are implicated during autophagy process
(43–45). The results here suggested that
autophagy inhibition could facilitate the chondrocytes survival
during the challenge of TNF-α and ox-LDL. Here, we did not examine
the effects of TNF-α inhibitors on change of LOX-1 expression level
because we are unable to obtain the monoclonal antibodies for
TNF-alpha inhibitor (46), but
these examinations could be the potential future work.
In conclusion, we demonstrated here that ox-LDL
could interaction with LOX-1 on chondrocyte and promote TNF-α
mediated chondrocyte death via autophagy related mechanisms.
Acknowledgements
The present study was funded by National Nature
Science Foundation of China to Yaozeng Xu (81472077 and
81672238).
References
|
1
|
Palazzo C, Nguyen C, Lefevre-Colau MM,
Rannou F and Poiraudeau S: Risk factors and burden of
osteoarthritis. Ann Phys Rehabil Med. 59:134–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prieto-Alhambra D, Judge A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lohmander LS and Roos EM: Clinical update:
Treating osteoarthritis. Lancet. 370:2082–2084. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Mani SB, He Y, Hall AM, Xu L, Li
Y, Zurakowski D, Jay GD and Warman ML: Induced superficial
chondrocyte death reduces catabolic cartilage damage in murine
posttraumatic osteoarthritis. J Clin Invest. 126:2893–2902. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Q, Sun Y, Zhang P and Zheng J:
Simultaneously blocking necrosis and apoptosis to protect TMJ
chondrocytes from TNF-alpha induced death: A preliminary study. Int
J Clin Experiment Med. 9:2202–2210. 2016.
|
|
7
|
Jang KW, Buckwalter JA and Martin JA:
Inhibition of cell-matrix adhesions prevents cartilage chondrocyte
death following impact injury. J Orthop Res. 32:448–454. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Intekhab-Alam NY, White OB, Getting SJ,
Petsa A, Knight RA, Chowdrey HS, Townsend PA, Lawrence KM and Locke
IC: Urocortin protects chondrocytes from NO-induced apoptosis: A
future therapy for osteoarthritis? Cell Death Dis. 4:e7172013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu J, Clancy M, Aliabadi P, Vasan R and
Felson DT: Metabolic syndrome, its components, and knee
osteoarthritis: The framingham Osteoarthritis study. Arthritis
Rheumatol. 69:1194–1203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haugen IK, Ramachandran VS, Misra D, Neogi
T, Niu J, Yang T, Zhang Y and Felson DT: Hand osteoarthritis in
relation to mortality and incidence of cardiovascular disease: Data
from the Framingham Heart Study. Ann Rheum Dis. 74:74–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hussain S Monira, Wang Y, Cicuttini FM,
Simpson JA, Giles GG, Graves S and Wluka AE: Incidence of total
knee and hip replacement for osteoarthritis in relation to the
metabolic syndrome and its components: A prospective cohort study.
Semin Arthritis Rheum. 43:429–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellamy N, Buchanan WW, Goldsmith CH,
Campbell J and Stitt LW: Validation study of WOMAC: A health status
instrument for measuring clinically important patient relevant
outcomes to antirheumatic drug therapy in patients with
osteoarthritis of the hip or knee. J Rheumatol. 15:1833–1840.
1988.PubMed/NCBI
|
|
13
|
Roorda LD, Jones CA, Waltz M, Lankhorst
GJ, Bouter LM, Van der Eijken JW, Willems WJ, Heyligers IC,
Voaklander DC, Kelly KD and Suarez-Almazor ME: Satisfactory cross
cultural equivalence of the Dutch WOMAC in patients with hip
osteoarthritis waiting for arthroplasty. Ann Rheum Dis. 63:36–42.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji Y, Strawn TL, Grunz EA, Stevenson MJ,
Lohman AW, Lawrence DA and Fay WP: Multifaceted role of plasminogen
activator inhibitor-1 in regulating early remodeling of vein bypass
grafts. Arterioscler Thromb Vasc Biol. 31:1781–1787. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Almonte-Becerril M, Navarro-Garcia F,
Gonzalez-Robles A, Vega-Lopez MA, Lavalle C and Kouri JB: Cell
death of chondrocytes is a combination between apoptosis and
autophagy during the pathogenesis of Osteoarthritis within an
experimental model. Apoptosis. 15:631–638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balci B: The modification of serum lipids
after acute coronary syndrome and importance in clinical practice.
Curr Cardiol Rev. 7:272–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Libby P and Hansson GK: Inflammation and
immunity in diseases of the arterial tree: Players and layers. Circ
Res. 116:307–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perrins CJ and Bobryshev YV: Current
advances in understanding of immunopathology of atherosclerosis.
Virchows Archiv. 458:117–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Twardowski L, Cheng F, Michaelsen J,
Winter S, Hofmann U, Schaeffeler E, Müller S, Sonnenberg M, Steuer
K, Ott G, et al: Enzymatically modified low-density lipoprotein is
present in all stages of aortic valve sclerosis: Implications for
pathogenesis of the disease. J Am Heart Assoc. 4:e0021562015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawamura T, Kume N, Aoyama T, Moriwaki H,
Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T and Masaki
T: An endothelial receptor for oxidized low-density lipoprotein.
Nature. 386:73–77. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu S, Ogura S, Chen J, Little PJ, Moss J
and Liu P: LOX-1 in atherosclerosis: Biological functions and
pharmacological modifiers. Cell Mol Life Sci. 70:2859–2872. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Misaka T, Suzuki S, Sakamoto N, Yamaki T,
Sugimoto K, Kunii H, Nakazato K, Saitoh S, Sawamura T, Ishibashi T
and Takeishi Y: Significance of soluble lectin-like oxidized LDL
receptor-1 levels in systemic and coronary circulation in acute
coronary syndrome. Biomed Res Int. 2014:6491852014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagawa T, Akagi M, Hoshikawa H, Chen M,
Yasuda T, Mukai S, Ohsawa K, Masaki T, Nakamura T and Sawamura T:
Lectin-like oxidized low-density lipoprotein receptor 1 mediates
leukocyte infiltration and articular cartilage destruction in rat
zymosan-induced arthritis. Arthritis Rheum. 46:2486–2494. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishimura S, Akagi M, Yoshida K, Hayakawa
S, Sawamura T, Munakata H and Hamanishi C: Oxidized low-density
lipoprotein (ox-LDL) binding to lectin-like ox-LDL receptor-1
(LOX-1) in cultured bovine articular chondrocytes increases
production of intracellular reactive oxygen species (ROS) resulting
in the activation of NF-kappaB. Osteoarthritis Cartilage.
12:568–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yumoto K, Nifuji A, Rittling SR, Tsuchiya
Y, Kon S, Uede T, Denhardt DT, Hemmi H, Notomi T, Hayata T, et al:
Osteopontin deficiency suppresses tumor necrosis factor-α-induced
apoptosis in chondrocytes. Cartilage. 3:79–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim J, Xu M, Xo R, Mates A, Wilson GL, AW
IV Pearsall and Grishko V: Mitochondrial DNA damage is involved in
apoptosis caused by pro-inflammatory cytokines in human OA
chondrocytes. Osteoarthritis Cartilage. 18:424–432. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Csaki C, Mobasheri A and Shakibaei M:
Synergistic chondroprotective effects of curcumin and resveratrol
in human articular chondrocytes: Inhibition of IL-1beta-induced
NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther.
11:R1652009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caramés B, López-Armada MJ, Cillero-Pastor
B, Lires-Dean M, Vaamonde C, Galdo F and Blanco FJ: Differential
effects of tumor necrosis factor-alpha and interleukin-1beta on
cell death in human articular chondrocytes. Osteoarthritis
Cartilage. 16:715–722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
López-Armada MJ, Caramés B, Lires-Deán M,
Cillero-Pastor B, Ruiz-Romero C, Galdo F and Blanco FJ: Cytokines,
tumor necrosis factor-alpha and interleukin-1beta, differentially
regulate apoptosis in osteoarthritis cultured human chondrocytes.
Osteoarthritis Cartilage. 14:660–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SW, Song YS, Lee SY, Yoon YG, Lee SH,
Park BS, Yun I, Choi H, Kim K, Chung WT and Yoo YH: Downregulation
of protein kinase CK2 activity facilitates tumor necrosis
factor-α-mediated chondrocyte death through apoptosis and
autophagy. PLoS One. 6:e191632011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang LB, Meng DH, Lee SM, Liu SH, Xu QT,
Wang Y and Zhang J: Dihydroartemisinin inhibits catabolism in rat
chondrocytes by activating autophagy via inhibition of the NF-κB
pathway. Sci Rep. 6:389792016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang K: Autophagy and apoptosis in liver
injury. Cell Cycle. 14:1631–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia J, Yao W, Guan M, Dai W, Shahnazari M,
Kar R, Bonewald L, Jiang JX and Lane NE: Glucocorticoid dose
determines osteocyte cell fate. FASEB J. 25:3366–3376. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mizushima N: Physiological functions of
autophagy. Curr Top Microbiol Immunol. 335:71–84. 2009.PubMed/NCBI
|
|
36
|
Shapiro IM, Layfield R, Lotz M, Settembre
C and Whitehouse C: Boning up on autophagy: The role of autophagy
in skeletal biology. Autophagy. 10:7–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sasaki H, Takayama K, Matsushita T, Ishida
K, Kubo S, Matsumoto T, Fujita N, Oka S, Kurosaka M and Kuroda R:
Autophagy modulates osteoarthritis-related gene expression in human
chondrocytes. Arthritis Rheum. 64:1920–1928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hong D, Bai YP, Gao HC, Wang X, Li LF,
Zhang GG and Hu CP: Ox-LDL induces endothelial cell apoptosis via
the LOX-1-dependent endoplasmic reticulum stress pathway.
Atherosclerosis. 235:310–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng H, Cui D, Quan X, Yang W, Li Y,
Zhang L and Liu E: Lp-PLA2 silencing protects against
ox-LDL-induced oxidative stress and cell apoptosis via Akt/mTOR
signaling pathway in human THP1 macrophages. Biochem Biophys Res
Commun. 477:1017–1023. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Del Carlo M Jr and Loeser RF: Cell death
in osteoarthritis. Curr Rheumatol Rep. 10:37–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
D'Lima D, Hermida J, Hashimoto S, Colwell
C and Lotz M: Caspase inhibitors reduce severity of cartilage
lesions in experimental osteoarthritis. Arthritis Rheum.
54:1814–1821. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kuhn K, D'Lima DD, Hashimoto S and Lotz M:
Cell death in cartilage. Osteoarthritis Cartilage. 12:1–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mathew R and White E: Autophagy in
tumorigenesis and energy metabolism: Friend by day, foe by night.
Curr Opin Genet Dev. 21:113–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bhutia SK, Mukhopadhyay S, Sinha N, Das
DN, Panda PK, Patra SK, Maiti TK, Mandal M, Dent P, Wang XY, et al:
Autophagy: Cancer's friend or foe? Adv Cancer Res. 118:61–95. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Marx J: Autophagy: Is it cancer's friend
or foe? Science. 312:1160–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lis K, Kuzawinska O and Bałkowiec-Iskra E:
Tumor necrosis factor inhibitors-state of knowledge. Arch Med Sci.
10:1175–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|