Introduction
Ischemic stroke has high morbidity and mortality
rates and is a substantial burden for patients and society
(1). A degree of progress has been
made, including treatment with intravenous recombinant tissue
plasminogen activator (rt-PA) (2,3) and
recombinant T cell receptor ligand combined with rt-PA; however,
the majority of clinical cases are treated with rt-PA thrombolytic
therapy as the primary method. Due to the narrow therapeutic window
of 4.5 h, therapeutic strategies for ischemic stroke remain
unsatisfactory. A number of studies have reported that autophagy
servesan important rolein cerebral ischemic injury in animal models
and cellular models, by causing progressive degeneration of the
brain (4,5).
Autophagy is a lysosomal degradation pathway which
is essential for cell survival, proliferation, differentiation and
homeostasis (6,7). Autophagy may be activated by potent
extracellular stimuli, including starvation, viral infection,
ischemia and hypoxia (8). However,
excessive autophagy may induce cell death via direct autophagic
cell death or indirect crosstalk with apoptosis (9,10).
Protein kinase mTOR (mTOR) is an atypical serine/threonine protein
kinase, and is important for growth regulation (11). mTOR is primarily regulated by the
phosphatidylinositol 3-kinase (PI3K)/RAC-α serine/threonine-protein
kinase (Akt)/mTOR signaling pathway, which serves important roles
in the inhibition of cellular apoptosis, and the promotion of cell
proliferation and cell survival (12,13).
A number studies have demonstrated that the process of autophagy is
negatively regulated by the activation of mTOR. It has been
reported that autophagy may protect against toxicity and promote
neuronal survival and plasticity, thereby leading to learning
rescue and memory enhancement by activating mTOR protein
biosynthesis (14,15). Therefore, retaining the
PI3K/Akt/mTOR signaling pathway and inhibiting autophagy maybe a
potential approach for the treatment ofischemic stroke.
Cornin is an iridoid glycoside isolated from the
fruit of Verbena officinalis L. that has protective
potential against cerebral ischemia injury and induces angiogenesis
in vitro (16–18). However, the effect and molecular
mechanism of cornin on autophagy in stroke remains unclear. The
present study investigated whether cornin was able to inhibit
autophagy through upregulation of the PI3K/Akt/mTOR signaling
pathway in SH-SY5Y cells, and aimed to provide a novel theoretical
basis for the treatment of ischemic stroke with cornin.
Materials and methods
Drugs and reagents
Cornin (purity >99.0%; CAS no. 548-37-8;
molecular formula, C17H24O10;
molecular weight, 388.37) was provided by Shandong Engineering
Research Center for Nature Drug (Shangdong, China) was dissolved in
sterile physiological (0.9%) saline to make a stock solution.
Dilutions were prepared according to the different administration
doses.
The Akt (cat. no. 8805; 1:1,000 dilution),
phosphorylated (p)-Akt (cat. no. 38449; 1:500 dilution), mTOR (cat.
no. 2732; 1:2,000 dilution), p-mTOR (cat. no. 109268; 1:1,000
dilution), apoptosis regulator Bcl-2 (cat. no. 32124; 1:1,000
dilution), apoptosis regulator BAX (Bax) (cat. no. 32503; 1:1,000
dilution), β-actin (cat. no. 8226; 1:500 dilution),
microtubule-associated proteins 1A/1B light chain 3B (LC3) (cat.
no. 128025; 1:1,000 dilution) and Beclin-1 (cat. no. 62557; 1:2,000
dilution) antibodies were purchased from Abcam (Cambridge, UK). The
following pharmacological agents were used: The PI3K/Akt inhibitor
LY294002 and the mTOR inhibitor rapamycin (both Merck KGaA,
Darmstadt, Germany).
Cell culture
The human neuroblastoma SH-SY5Y cell line was
obtained from the Institute of Basic Medical Sciences of Chinese
Academy of Medical Sciences (Beijing, China). Short-tandem repeat
analysis was performed by Shanghai Saily Biotechnologies Co., Ltd.
(Shanghai, China) to ascertain that the cell line used in the
present study was of human origin (sample no. 20160921-01). SH-SY5Y
cells were maintained in Dulbecco's modified Eagle's medium (DMEM;
HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life
Sciences) and antibiotics (100 U/ml penicillin G and 100 µg/ml
streptomycin; Beijing Solarbio Science and Technology Co., Ltd.,
Beijing, China) in a humidified atmosphere of 5% CO2 at
37°C. The control group was treated with dimethyl sulfoxide (DMSO).
In order to study the mechanism of the effect of cornin on
autophagy, SH-SY5Y cells were incubated with cornin (9 µM) for 24 h
prior to OGD, followed by 10 µM LY294002 or 10 µM rapamycin for 6
h.
In vitro OGD model
In order to simulate OGD in vitro, SH-SY5Y
cells were incubated in a hypoxia solution for 6 h. The hypoxia
solution contained 0.9 mM NaH2PO4, 6.0 mM
NaHCO3, 1.0 mM CaCl2, 1.2 mM
MgSO4, 40 mM sodium lactate, 20 mM HEPES, 98.5 mM NaCl
and 10.0 mM KCl (pH adjusted to 6.8), and was bubbled with
N2 for 30 min prior to application. The O2
pressure of the hypoxia solution was adjusted to 64.0 kPa. Hypoxic
conditions were produced by placing the plates of cultured SH-SY5Y
cells in a hypoxic incubator (Kendro; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with the oxygen adjusted to 1.0% and the
CO2 to 5.0%. Prior to hypoxia, SH-SY5Y cells were
pretreated with various concentrations (3, 9 and 27 µM) of cornin
for 24 h. Normal culturing (DMEM containing 2% FBS under 20% oxygen
and 5% CO2) served as the negative control, and the
hypoxia solution culture served as the control.
Determination of cell viability and
lactate dehydrogenase (LDH) leakage
SH-SY5Y cells were incubated with or without cornin
in the hypoxia solution for 6 h and cell viability was assessed
using an MTT assay. MTT solution (5 mg/ml; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added into the cells and the cells
were incubated for 4 h in 37°C. Following the removal of the
medium, DMSO was added to dissolve the blue-colored formazan
product. The absorbance was measured at a wavelength of 490 nm to
determine the optical density value of each well. LDH, an indicator
of cellular injury, was detected according to manufacturer's
protocol of the LDH assay kit (Beijing Zhongsheng Bioreagent,
Beijing, China). The formula LDH leakage rate (%)=Ae/At ×100 was
used, in which Ae indicated extracellular LDH (cell culture fluid)
and At indicated intracellular and extracellular LDH (cell
lysate).
Western blot analysis
SH-SY5Y cells were cultured for 24 h, washed twice
with ice-cold PBS and lysed in NP40 lysis buffer (BioSource
International, Inc., Camarillo, CA, USA) (50 mM Tris, pH 7.4; 250
mM NaCl; 5 mM EDTA; 50 mM NaF; 1 mM Na3VO4;
1% NP-40; and 0.02% NaN3) supplemented with 1 mM
phenylmethylsulfonyl fluoride and 1X protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA). Equal amounts of cellular protein (40
µg) were separated by 12 or 10% SDS-PAGE and electrophoretically
transferred to polyvinylidene difluoride membranes. The membranes
were blocked in 5% (w/v) skimmed milk for 2 h at room temperature.
The membranes were then incubated with the following specific
antibodies: Anti-Akt (cat. no. 8805; 1:1,000 dilution), anti-p-Akt
(cat. no. 38449; 1:500 dilution), anti-mTOR (cat. no. 2732; 1:2,000
dilution), anti-p-mTOR (cat. no. 109268; 1:1,000 dilution),
anti-LC3 (cat. no. 128025; 1:1,000 dilution), anti-beclin-1 (cat.
no. 62557; 1:2000 dilution), anti-Bcl-2 (cat. no. 32124; 1:1,000
dilution), anti-Bax (cat. no. 32503; 1:1,000 dilution),
anti-caspase-3 (cat. no. 13847; 1:1,000 dilution) and anti-β-actin
(cat. no. 8226; 1:500 dilution) as a loading control at 4°C
overnight. Subsequently, they were incubated for 2 h at room
temperature with goat anti-rabbit horseradish peroxidase-conjugated
secondary antibody (cat no. A0208; 1:5,000 dilution; Beyotime
Institute of Biotechnology). Protein bands were visualized using
enhanced chemiluminescence regent (EMD Millipore, Billerica, MA,
USA). The optical densities of the bands were scanned and
quantified using a Gel Doc2000 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Data were normalized against those of the
corresponding β-actin bands. Results are expressed as a fold
increase compared with the control.
Statistical analysis
All of the experiments were performed in triplicate.
Quantitative data from experiments are expressed as the mean ±
standard deviation. Significance was determined by one-way analysis
of variance followed by Dunnett's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of cornin on cultured SH-SY5Y
cells against OGD-induced cell death
The cell viability of OGD-treated SH-SY5Y cells was
markedly decreased compared with the normal cultured cells.
However, the cell viability of OGD-treated cells was markedly
increased following treatment with cornin (3–27 µM), as presented
in Table I. In order to further
investigate the protective effect of cornin, the LDH leakage rate
was estimated. A significant increase in the LDH leakage rate in
SH-SY5Y cells was observed following OGD. Incubation with various
concentrations of cornin significantly inhibited the OGD-induced
LDH release in a concentration-dependent manner.
 | Table I.Effects of cornin on viability and LDH
leakage in SH-SY5Y cells exposed to oxygen-glucose deprivation. |
Table I.
Effects of cornin on viability and LDH
leakage in SH-SY5Y cells exposed to oxygen-glucose deprivation.
| Groups | OGD | Content, µM | Cell viability,
% | LDH leakage, % |
|---|
| Normal | − | − |
91.1±2.9 |
3.0±0.9 |
| Control | + | _ |
51.1±3.5a |
22.9±3.2a |
|
| + | 3 |
63.7±4.0b |
18.6±1.6b |
| Cornin | + | 9 |
72.2±2.7b |
16.7±1.7b |
|
| + | 27 |
62.2±3.2b |
19.2±2.2b |
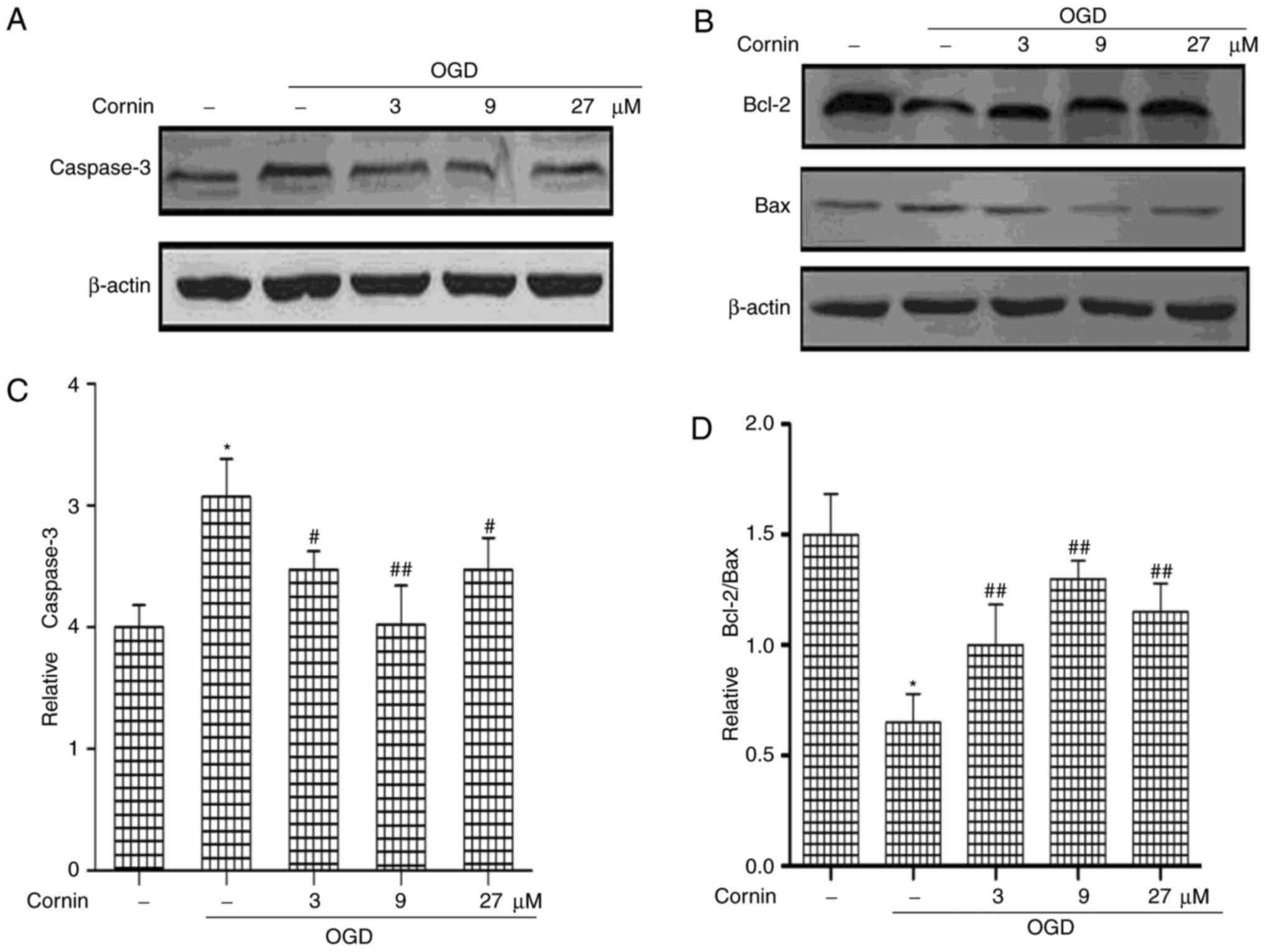
Effect of cornin on the expression of
cellular apoptosis-associated proteins in SH-SY5Y cells
The Bcl-2 and caspase families are the important
mediators of apoptosis. Bcl-2, caspase-3, and Bax protein levels
were determined to elucidate whether cornin was able to protect
SH-SY5Y cells from OGD-induced apoptosis. The protein levels of
Caspase-3 and Bax were significantly increased, and Bcl-2 was
decreased, by treatment with OGD, compared with normal cells.
However, cornin increased Bcl-2, and decreased Bax and caspase-3
levels significantly in OGD-treated cells (Fig. 1).
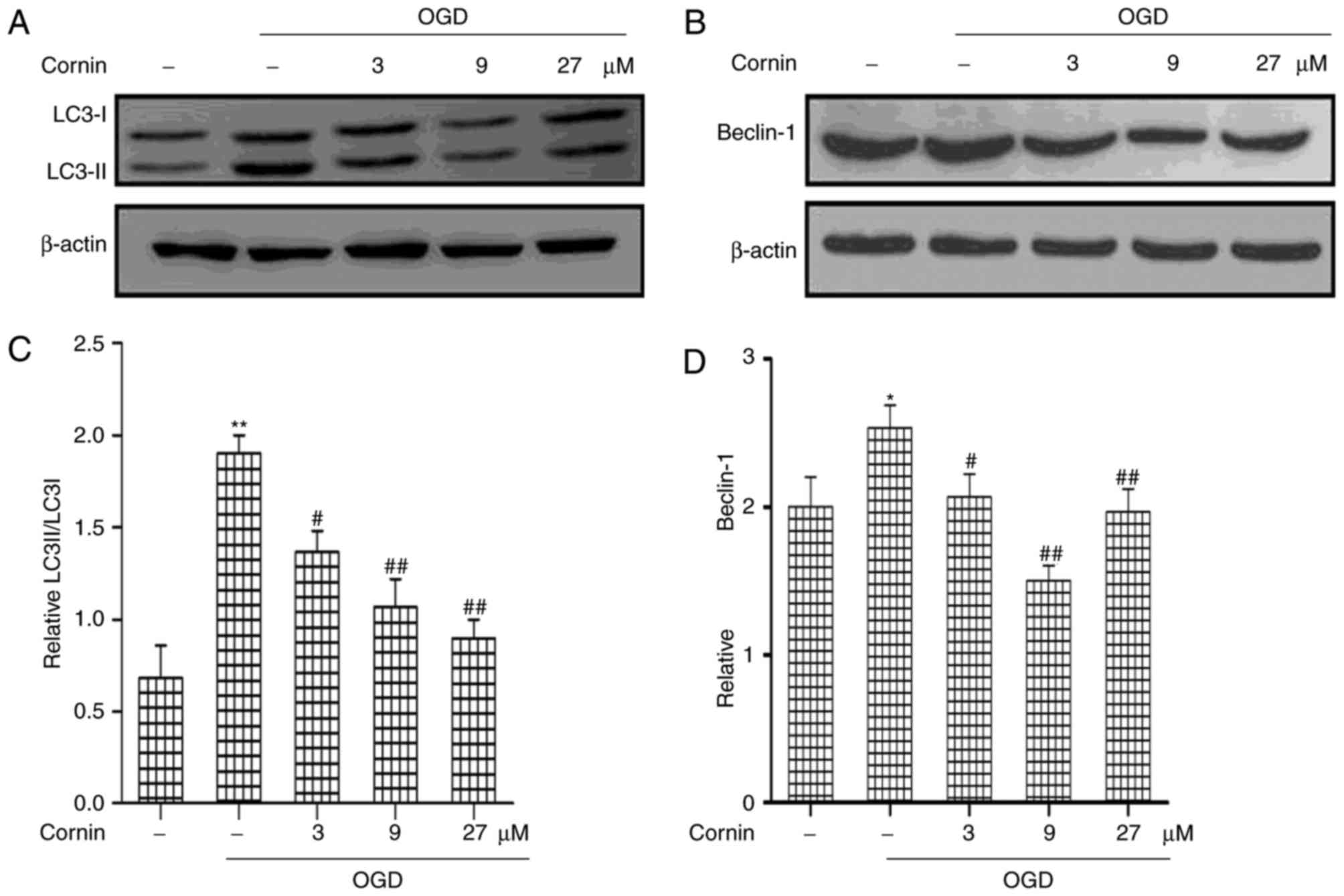
Protective effect of cornin is
associated with inhibited autophagy in OGD-treated SH-SY5Y
cells
In order to determine the protective mechanisms of
cornin in OGD-treated cells, the present study analyzed the effects
of cornin on autophagy in the protection of SH-SY5Y cells. Previous
studies have demonstrated that autophagy activation is involved in
ischemic stroke (19,20); however, the role of cornin in
regulating autophagy in ischemic stroke has not been clearly
defined. In the model used in the present study, the regulatory
effect of cornin on LC3 and beclin-1, which are frequently used as
indicators of autophagy, was investigated. LC3 and beclin-1
expression in the OGD model were detected by western blot analysis.
As presented in (Fig. 2), beclin-1
and the ratio of LC3-II/LC3-I increased in the OGD model compared
with the control group. This effect was significantly inhibited by
treatment with cornin for 24 h prior to OGD, in a
concentration-dependent manner.
Cornin affects OGD-induced autophagy,
involving the activation of PI3K/Akt/mTOR pathway
Previous studies demonstrated that cornin inhibited
OGD-induced cell damages by activating PI3K/Akt signaling (16,18).
Therefore, the present study aimed to further examine whether
cornin may affect OGD-induced autophagy through the Akt/mTOR
pathway. SH-SY5Y cells were treated with 3, 9, or 27 µM cornin, and
control groups were treated with DMSO. A total of 24 h
subsequently, the phosphorylation levels of Akt and mTOR were
examined using western blotting. The results demonstrated that
cornin was able to upregulate the protein levels of p-Akt, and
p-mTOR under OGD, as presented in Fig.
3.
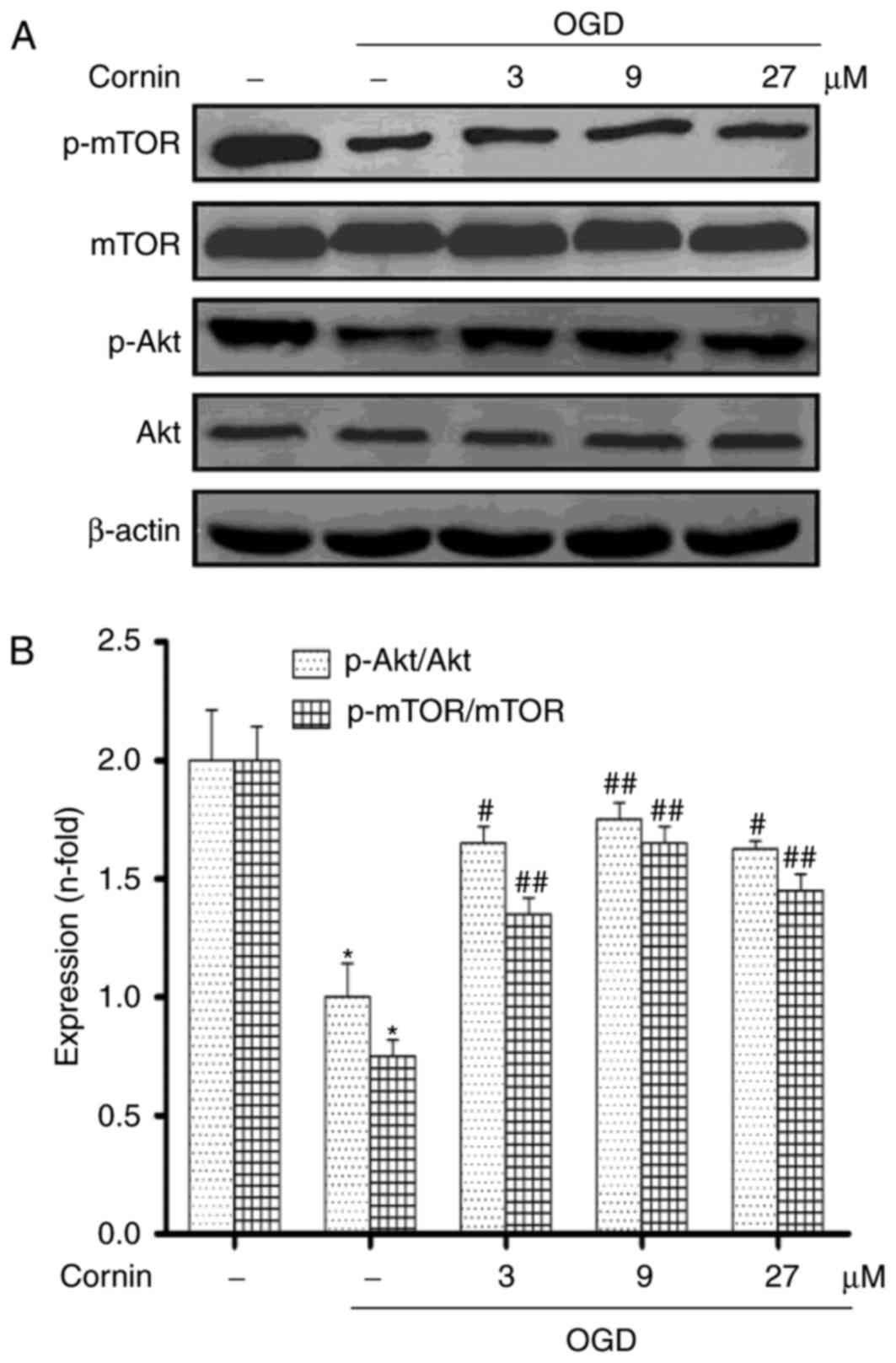 | Figure 3.Effect of cornin on the protein
expression of Akt, p-Akt, mTOR and p-mTOR. SH-SY5Y cells were
treated with cornin (3, 9 and 27 µM) for 24 h and cell lysates were
subjected to immunoblot analysis for detecting the levels of (A)
AKT, p-Akt, mTOR, p-mTOR. β-actin was used as the cell lysate
loading control. (B) Densitometric analysis was performed. The
results are expressed as the mean ± standard deviation. n=3.
*P<0.01 vs. control group; #P<0.05,
##P<0.01 vs. OGD group. OGD, oxygen-glucose
deprivation; Akt, RAC-α serine/threonine-protein kinase; mTOR,
protein kinase mTOR; p, phosphorylated. |
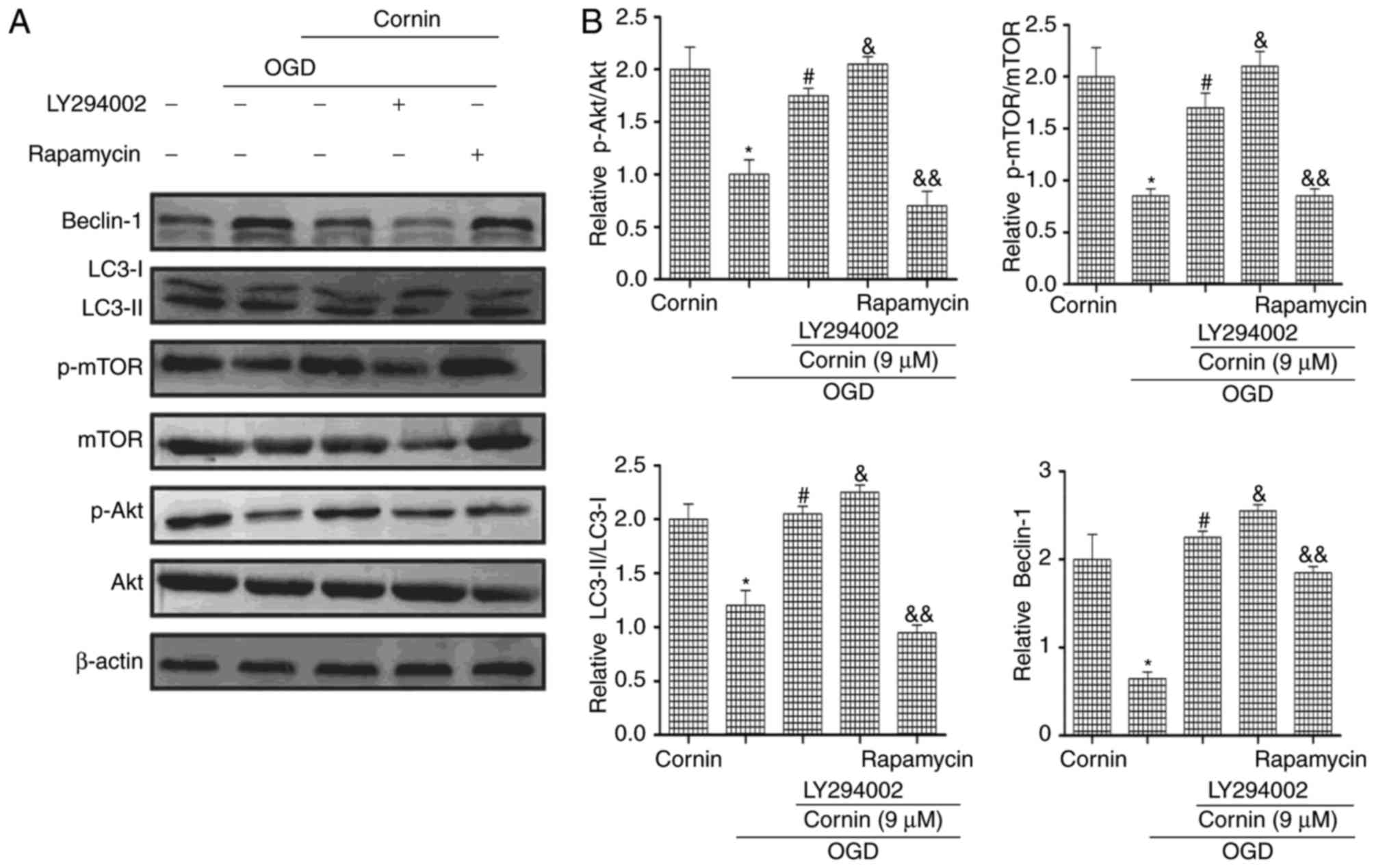
To investigate whether cornin affected OGD-induced
autophagy via the PI3K/Akt/mTOR signaling pathway, the PI3K/Akt
inhibitor LY294002 and the mTOR inhibitor rapamycin were used in
western blotting. The protein expression levels of p-Akt, p-mTOR,
LC3-II/LC3-I and beclin-1 were decreased by treatment with
LY294002. Rapamycin increased mTOR phosphorylation, and increased
LC3-II/LC3-I and beclin-1 protein expression, as presented in
(Fig. 4). These results of the
present study indicated that cornin reduced OGD-induced autophagy
by affecting Akt and mTOR phosphorylation levels.
Discussion
The principal findings of the present study
indicated that cornin exerted protective effects against
OGD-induced autophagy in SH-SY5Y cells. Additionally, treatment
with cornin markedly activated the expression of p-Akt and p-mTOR.
The increased levels of LC3II/LC3I and beclin-1 were decreased
following treatment with cornin. Following inhibition of PI3K/Akt
with LY294002, p-Akt, p-mTOR, LC3II/LC3I and beclin-1 were
significantly decreased in SH-SY5Y cells. Similarly, following
inhibition of mTOR with rapamycin, LC3II/LC3I and beclin-1 were
significantly increased in SH-SY5Y cells. The results of the
present study suggested that cornin was involved in the modulation
of OGD-induced autophagy on PI3K/Akt/mTOR signaling.
It is known that autophagy is a highly conserved
pathway for degradation and servesan important role in cerebral
ischemic injury. However, excessive autophagy may exacerbate
cellular damage and result in autophagic cell death or apoptosis
(21,22). The present study demonstrated
reduced cell viability, increased LDH leakage, and upregulation of
caspase-3 protein in the OGD model, which indicated that cornin may
exert protective effects on OGD-induced apoptosis.
LC3 is a an important indicator of the occurrence of
autophagy. During autophagy, the cytosolic form LC3-I is converted
to the phosphatidylethanolamine-conjugated form LC3-II to promote
autophagosome formation (23).
Therefore, an increase in the expression of LC3-II has been used to
indicate the activation of autophagy (24). Beclin-1, an autophagy-associated
protein, may mediate other autophagicproteins attached to
autophagosome membranes and decrease LC3-II accumulation. Beclin-1
is an additional import indicator of the degree of autophagy
(25,26). In the present study, LC3-II/ LC3-I
and Beclin-1 were increased in SH-SY5Y cells subjected to 6 h of
OGD, whereas cornin reduced them in a concentration-dependent
manner. These results indicated that the anti-apoptotic protective
effect of cornin may be associated with a decreased in the
autophagy induced by OGD.
In order to elucidate the mechanisms underlying the
regulatory effect of cornin on OGD-induced autophagy in SH-SY5Y
cells, the present study investigated the effects of cornin on the
activation of the PI3K/Akt/mTOR pathway. mTOR, one of the
downstream targets of the PI3K/Akt signaling pathway, is a key
regulator of cell growth, proliferation, autophagy and survival
(27,28). Previous studies have suggested that
PI3K/Akt/mTOR signaling is important for nerve vascular unit
survival during ischemia (29,30).
The present study demonstrated that treatment with cornin markedly
activated the phosphorylationof Akt and mTOR. The PI3K/Akt
inhibitor LY294002 significantly abrogated the increased
phosphorylation of Akt and mTOR, and reduced the increased levels
of LC3-II/LC3-I and beclin-1 following treatment with cornin.
Additionally, the inhibition of mTOR by rapamycin strengthened the
occurrence of autophagy.
In conclusion, the results of the present study
suggested that cornin may be a novelway to regulate cerebral
ischemia-induced autophagy in neurons. Although the data from the
present study provided cellular and pharmacological proof of
principle for the use of cornin in vitro, in vivo
validation of these mechanisms remains to be obtained. The present
findings provided novel evidence for the regulatory mechanism of
autophagy and may provide a theoretical basis for the development
of cornin as a treatment for cerebral ischemia.
Acknowledgements
The present study was supported by Binzhou Medical
University Science and Technology Program (grant no. BY2016KJ11),
and financially supported in part by the National Natural Science
Foundation of China (grant no. 31570352).
References
|
1
|
Endres M and Dirnagl U: Ischemia and
stroke. Adv Exp Med Biol. 513:455–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bas DF, Ozdemir AO, Colak E and Kebapci N:
Higher insulin resistance level is associated with worse clinical
response in acute ischemic stroke patients treated with intravenous
thrombolysis. Transl Stroke Res. 7:167–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mandava P, Shah SD, Sarma AK and Kent TA:
An outcome model for intravenous rt-pa in acute ischemic stroke.
Transl Stroke Res. 6:451–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu Q, Harris VA, Kumar S, Mansour HM and
Black SM: Autophagy in neonatal hypoxia ischemic brain is
associated with oxidative stress. Redox Biol. 6:516–523. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kroemer G and Levine B: Autophagic cell
death: The story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Puyal J and Clarke PG: Targeting autophagy
to prevent neonatal stroke damage. Autophagy. 5:1060–1061. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang ZQ, Yang Y, Lu T, Luo P, Li J, Wu JP,
Tang ZZ, Lu QP and Duan QH: Protective effect of autophagy
inhibition on ischemia-reperfusion-induced injury of N2a cells. J
Huazhong Univ Sci Technolog Med Sci. 33:810–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uchiyama Y, Koike M and Shibata M:
Autophagic neuron death in neonatal brain ischemia/hypoxia.
Autophagy. 4:404–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L,
Su L and Zhang Y: Inhibition of autophagy contributes to ischemic
postconditioning-induced neuroprotection against focal cerebral
ischemia in rats. PLoS One. 7:e460922012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maiese K: Targeting molecules to medicine
with mTOR, autophagy and neurodegenerative disorders. Br J Clin
Pharmacol. 82:1245–1266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei H, Li Y, Han S, Liu S, Zhang N, Zhao
L, Li S and Li J: cPKCgamma-modulated autophagy in neurons
alleviates ischemic injury in brain of mice with ischemic stroke
through Akt-mTOR pathway. Transl Stroke Res. 7:497–511. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mao XY, Zhou HH, Li X and Liu ZQ:
Huperzine A alleviates oxidative glutamate toxicity in hippocampal
ht22 cells via activating bdnf/trkb-dependent PI3K/akt/mtor
signaling pathway. Cell Mol Neurobiol. 36:915–925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao C, Cai Y, Zhang X, Huang H, Wang J,
Wang Y, Tong X, Wang J and Wu J: Ischemic preconditioning mediates
neuroprotection against ischemia in mouse hippocampal CA1 neurons
by inducing autophagy. PLoS One. 10:e01371462015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L,
Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al: bFGF regulates
autophagy and ubiquitinated protein accumulation induced by
myocardial ischemia/reperfusion via the activation of the
PI3K/Akt/mTOR pathway. Sci Rep. 5:92872015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang Z, Jiang W, Luan H, Zhao F and Zhang
S: Cornin induces angiogenesis through PI3K-Akt-eNOS-VEGF signaling
pathway. Food Chem Toxicol. 58:340–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, Zhang G, Kang Z, Xu Y, Jiang W and
Zhang S: Cornin increases angiogenesis and improves functional
recovery after stroke via the Ang1/Tie2 axis and the Wnt/β-catenin
pathway. Arch Pharm Res. 39:133–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang WL, Zhang SP, Zhu HB, Jian Hou and
Tian JW: Cornin ameliorates cerebral infarction in rats by
antioxidant action and stabilization of mitochondrial function.
Phytother Res. 24:547–552. 2010.PubMed/NCBI
|
|
19
|
Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T,
Fu W, Zhang J, Wu W, Zhang X and Chen YG: Autophagy negatively
regulates Wnt signalling by promoting Dishevelled degradation. Nat
Cell Biol. 12:781–790. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xingyong C, Xicui S, Huanxing S, Jingsong
O, Yi H, Xu Z, Ruxun H and Zhong P: Upregulation of myeloid cell
leukemia-1 potentially modulates beclin-1-dependent autophagy in
ischemic stroke in rats. BMC Neurosci. 14:562013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi R, Weng J, Zhao L, Li XM, Gao TM and
Kong J: Excessive autophagy contributes to neuron death in cerebral
ischemia. CNS Neurosci Ther. 18:250–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fimia GM, Stoykova A, Romagnoli A, Giunta
L, Di Bartolomeo S, Nardacci R, Corazzari M, Fuoco C, Ucar A,
Schwartz P, et al: Ambra1 regulates autophagy and development of
the nervous system. Nature. 447:1121–1125. 2007.PubMed/NCBI
|
|
23
|
Larsen KE and Sulzer D: Autophagy in
neurons: A review. Histol Histopathol. 17:897–908. 2002.PubMed/NCBI
|
|
24
|
Zhang Z, Singh R and Aschner M: Methods
for the detection of autophagy in Mammalian cells. Curr Protoc
Toxicol. 69(20): 12.1–20.12.26. 2016.
|
|
25
|
Cao Y and Klionsky DJ: Physiological
functions of Atg6/Beclin 1: A unique autophagy-related protein.
Cell Res. 17:839–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mei Y, Glover K, Su M and Sinha SC:
Conformational flexibility of BECN1: Essential to its key role in
autophagy and beyond. Protein Sci. 25:1767–1785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Widlund AL, Baur JA and Vang O: mTOR: More
targets of resveratrol? Expert Rev Mol Med. 15:e102013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Shen L, Wang Z, Jiang HP and Liu LX:
Tanshinone IIA protects against myocardial ischemia reperfusion
injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed
Pharmacother. 84:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang C, Zhang X, Teng Z, Zhang T and Li Y:
Downregulation of PI3K/Akt/mTOR signaling pathway in
curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J
Pharmacol. 740:312–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W, Yang Y, Hu Z, Ling S and Fang M:
Neuroprotective effects of DAHP and Triptolide in focal cerebral
ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway
activation. Front Neuroanat. 9:482015. View Article : Google Scholar : PubMed/NCBI
|