Introduction
Kawasaki disease (KD) is a systemic vasculitis
syndrome of unknown etiology. The vasculitis mainly attacks
coronary arteries, and cardiac sequelae, such as coronary aneurysms
and coronary insufficiencies, are some of the most serious
manifestations of this disease (1–3). It
is particularly prevalent in infants and young children (4). Although the clinical features,
diagnosis and treatment of KD are well established, its
pathogenesis has not been identified yet. Several lines of evidence
suggest that an interplay between microbial infection and genetic
predisposition serve a role in the development of the disease
(5–7).
MicroRNAs (miRNAs) are endogenous single-strand,
non-coding RNAs of 18–25 nucleotides in length, that
post-transcriptionally regulate gene expression through
sequence-specific interaction with target messenger RNAs (mRNAs)
(8,9). miRNAs are highly conserved, and their
expression is time specific (10).
miRNAs exhibit powerful regulatory roles in many biological
processes, including cell metabolism, proliferation,
differentiation and apoptosis (11). Aberrant expression of miRNAs has
been confirmed to be associated with various human diseases
including cancers, cardiovascular diseases and inflammatory
conditions (12–14). Blood circulating miRNA levels are
stable (15) and their unique
expression patterns may be used as a novel, non-invasive biomarker
for disease diagnosis (16).
Recent studies identified circulating miRNAs as biomarkers for many
disorders, such as cardiovascular disease (17,18)
and inflammatory diseases (19).
However, previous reports on the expression of circulating miRNAs
in KD are limited. Additional studies are required to determine
whether there is differential miRNA expression in the circulating
plasma in patients with KD and the functions of target genes.
The present study aimed to identify a panel of
plasma miRNAs that are differentially expressed in patients with KD
and to provide a possible direction for studying the
pathophysiological mechanisms of KD.
Materials and methods
Specimen source
Plasma specimens for miRNA microarray hybridization
were obtained from children with KD (n=6) and from healthy control
children (n=6) between May 2013 and August 2013; plasma specimens
for reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) were obtained from children with KD (n=8) and from
healthy control children (n=8) between September 2013 and October
2013 at the Children's Hospital of Soochow University (Suzhou,
China). Patients in the control group underwent regular health
examinations and had no infections. KD was defined according to the
criteria established by the American Heart Association in 2004
(20). Venous blood (4 ml) was
collected from the patients in EDTA-containing tubes on the day of
diagnosis for KD. The blood samples were first centrifuged at 820 ×
g for 10 min at 4°C, and then at 16,000 × g for 10 min at 4°C.
Plasma was collected in 1.5 ml eppendorf tubes and stored at −80°C.
The 12 biologically independent plasma samples were analyzed
individually, rather than pooling the samples. The plasma samples
were labeled K or C for KD and control, respectively, followed by a
coding number, to protect the privacy of the participants during
all molecular studies. All parents of participants provided written
informed consent for participation in this study, and the samples
were processed under the approval of the Ethics Committee of
Children's Hospital of Soochow University (Suzhou China).
RNA extraction and quantification
Total RNA was extracted from plasma (400 µl/sample)
using a mirVana PARIS RNA and Native Protein Purification
kit (cat. no. AM1556; Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
Briefly, 10 volumes of lysis/binding buffer and 1/10 volume of
miRNA homogenate was added to the plasma and mixed well. A 1:1
ratio of acid phenol:chloroform equal to the lysate volume was
added to the miRNA homogenate additive. The mixture was centrifuged
for 5 min at 1,000 × g at room temperature, the aqueous upper phase
was removed and transferred to a fresh tube. Following the addition
of 1.25 volumes 100% ethanol, the lysate/ethanol mixture was passed
through a filter cartridge, which was subsequently washed with 700
µl miRNA wash solution 1 and 500 µl wash solution 2/3. RNA was
eluted from the filters with 100 µl elution solution that was
warmed to 95°C. Subsequently, the eluate, which contained the RNA,
was collected and stored at −80°C. Total RNA was quantified using a
NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.).
RNA sample quality was evaluated by an Agilent 2100 Bioanalyzer
(Agilent Technologies, Inc., Santa Clara, CA, USA). An RNA ≥7.0 was
accepted for microarray analysis and RT-qPCR.
miRNA microarray hybridization
miRNA profile analysis of the plasma samples was
performed using a miRNA Microarray Chip V2.4 (Agilent Technologies,
Inc.), which contains probes for 2,549 human miRNAs with a sample
input of 100 ng total RNA. Briefly, dephosphorylation was performed
by gently mixing the total RNA with 2 µl calf intestinal alkaline
phosphatase master mix (Agilent Technologies, Inc.) and incubating
the mixture at 37°C in a circulating water bath for 30 min.
Subsequently, 2.8 µl 100% dimethyl sulfoxide was added to each
sample and incubated at 100°C in a circulating water bath for 5–10
min for denaturation. The samples were labeled using a miRNA
Complete Labeling and Hybridization kit and hybridized on an
Agilent SureHyb Microarray Hybridization Chamber (both from Agilent
Technologies, Inc.). Following hybridization, the chip was washed
using GE wash buffer 1 and GE wash buffer 2 (Gene Expression Wash
Buffer kit; cat. no. 5188–5327; Agilent Technologies, Inc.). The
chip was scanned and the data were extracted using Agilent Feature
Extraction Software version 10.7.1.1 (Agilent Technologies, Inc.).
Data were standardized using GeneSpring Software version 13.1
(Agilent Technologies, Inc.). Fold change ≥2 and P<0.05 were
used to indicate significant differences in gene expression, and
cluster analysis was performed using Genespring software version
14.8 (Agilent Technologies, Inc.).
RT-qPCR
The total RNA extracted from the samples met the
quality control requirements and qualified for RT-qPCR analysis.
Each 20 µl RT reaction was performed according the manufacturer's
protocol of the miScript II Reverse Transcriptase kit (Qiagen GmbH,
Hilden, Germany) in a GeneAmp PCR system 9700 (Applied Biosystems)
for 60 min at 37°C, followed by heat inactivation of the RT for 5
min at 95°C. qPCR was performed using a LightCycler 480 II (Roche
Diagnostics, Basel, Switzerland) with the 10 µl reaction mixtures
comprising cDNA (1 µl), 2X LightCycler 480 SYBR-Green I Master mix
(5 µl), universal primer (0.2 µl), miRNA-specific primer (0.2 µl)
and nuclease-free water (3.6 µl). The upstream primer of hsa-miR-16
was 5′TAG CAG CAC GTA AAT ATT GGC G3′. The upstream primer of
hsa-miR-765 was 5′TGG AGG AGA AGG AAG GTG ATG3′. The upstream
primer of hsa-miR-33b-3p was 5′CAG TGC CTC GGC AGT GCA GCC C3′. The
upstream primer of has-miR-223-3p was 5′TGT CAG TTT GTC AAA TAC CCC
A3′. Reactions were incubated in a 384-well plate at 95°C for 10
min, followed by 40 cycles at 95°C for 10 sec and at 60°C for 30
sec. Each sample was run in triplicate. miRNA expression levels
were normalized to the internal reference hsa-miR-16 and external
reference cel-miR-39 and were determined using the comparative
threshold cycle 2−ΔΔCq method (21).
miRNA target gene prediction
miRNA target genes were predicted using GeneSpring
version 13.1 software (Agilent Technologies, Inc.). TargetScan
(www.targetscan.org), PITA (genie.weizmann.ac.il/pubs/mir07/mir07_data.html) and
microRNA.org (www.microrna.org/microrna/home.do) databases were used
to predict the intersectional miRNA target genes. The data were
analyzed using Venny software version2.1
(bioinfogp.cnb.csic.es/tools/venny). Common target genes were
analyzed for gene ontology (GO) functional term enrichment, such as
biological process (BP), cellular component (CC) and molecular
function (MF); GeneSpring and Kyoto Encyclopedia of Genes and
Genomes (KEGG) were used in the pathway analysis.
Statistical analysis
The patients with KD and control patient sample data
were compared using the Wilcoxon rank sum test. Statistical
analysis was performed using SPSS version 18.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features
Patient samples used for both microarray and RT-qPCR
analyses exhibited no differences in sex and age distributions
among the patients and controls (Tables I and II).
 | Table I.Clinical features of patients with KD
and control patients used in the microarray analysis. |
Table I.
Clinical features of patients with KD
and control patients used in the microarray analysis.
|
| Age (months) | Sex |
|---|
|
|
|
|
|---|
| Sample no. | KD | Control | KD | Control |
|---|
| 1 | 25 | 19 | Female | Male |
| 2 | 46 | 17 | Female | Female |
| 3 | 24 | 17 | Female | Female |
| 4 | 8 | 7 | Male | Male |
| 5 | 36 | 37 | Female | Female |
| 6 | 7 | 8 | Male | Female |
 | Table II.Clinical features of patients with KD
and control patients used for reverse transcription-quantitative
polymerase chain reaction analysis. |
Table II.
Clinical features of patients with KD
and control patients used for reverse transcription-quantitative
polymerase chain reaction analysis.
|
| Age (months) | Sex |
|---|
|
|
|
|
|---|
| Sample no. | KD | Control | KD | Control |
|---|
| 1 | 13 | 15 | Male | Male |
| 2 | 24 | 22 | Male | Male |
| 3 | 34 | 32 | Male | Female |
| 4 | 36 | 31 | Male | Female |
| 5 | 22 | 17 | Female | Male |
| 6 | 11 | 15 | Female | Male |
| 7 | 56 | 49 | Male | Male |
| 8 | 22 | 39 | Female | Male |
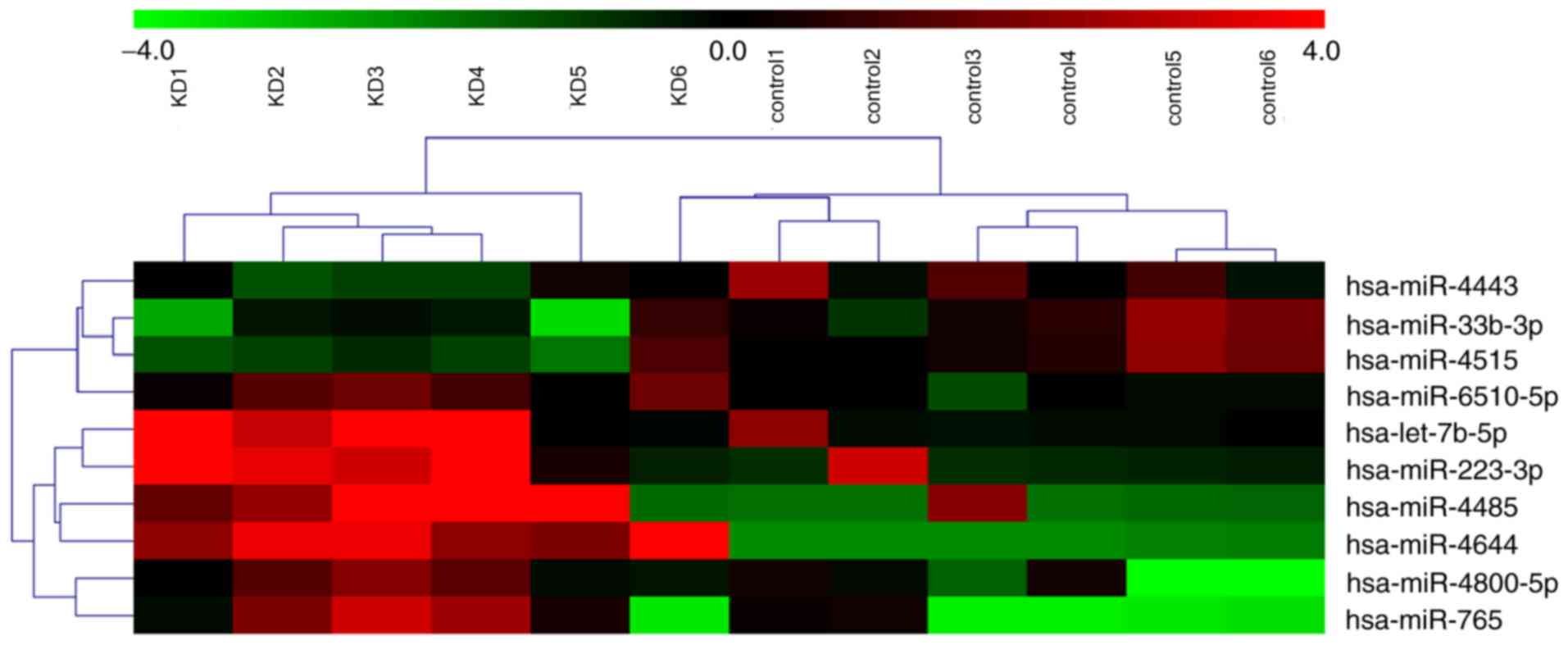
Differential miRNA expression
Microarray analysis of the plasma samples from the
KD and control groups revealed that seven miRNAs were significantly
upregulated (hsa-let-7b-5p, hsa-miR-223-3p, hsa-miR-4485,
hsa-miR-4644, hsa-miR-4800-5p, hsa-miR-6510-5p and hsa-miR-765) and
three were significantly downregulated (hsa-miR-33b-3p,
hsa-miR-4443 and hsa-miR-4515) in the KD plasma samples compared
with the control group (Fig. 1;
Table III).
 | Table III.Differentially expressed microRNAs of
the two groups. |
Table III.
Differentially expressed microRNAs of
the two groups.
| miRNA | P-value | FC | Trend | Sequence | Chr | miRBase ID |
|---|
| hsa-let-7b-5p |
3.15×10−2 | 8.448197 | Up |
AACCACACAACCTACTACC | 22 | MIMAT000003 |
| hsa-miR-223-3p |
3.71×10−2 | 8.468529 | Up |
TGGGGTATTTGACAAACTGAC | X | MIMAT000020 |
| hsa-miR-33b-3p |
4.90×10−2 | 3.495833 | Down | GGGCTGCACTGCCG | 17 | MIMAT000481 |
| hsa-miR-4443 |
4.26×10−2 | 2.347132 | Down |
AAAACCCACGCCTCC | 3 | MIMAT001891 |
| hsa-miR-4485 |
1.05×10−2 | 1.699564 | Up |
TTAGGGTACCGCGGC | 11 | MIMAT001909 |
| hsa-miR-4515 |
2.21×10−2 | 2.975258 | Down | GGGCTGCCGGGA | 15 | MIMAT001902 |
| hsa-miR-4644 |
1.00×10−7 | 34.0109 | Up |
CTTCTGTCTCTTTTCTCTC | 6 | MIMAT001974 |
|
hsa-miR-4800-5p |
4.78×10−2 | 7.431324 | Up |
TCCTTCCTTCCTCGG | 4 | MIMAT001998 |
|
hsa-miR-6510-5p |
5.53×10−3 | 2.450577 | Up |
GACTCCTCTCTCTCCC | 17 | MIMAT002546 |
| hsa-miR-765 |
4.13×10−2 | 8.229987 | Up |
CATCACCTTCCTTCTCCT | 1 | MIMAT000395 |
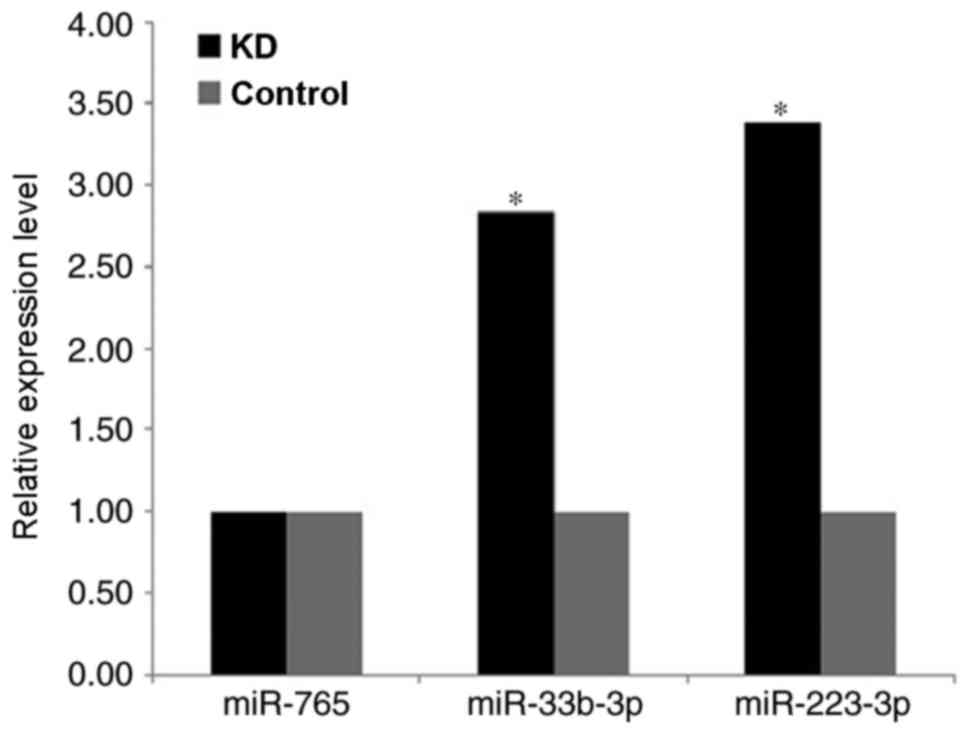
RT-qPCR
The selected miRNAs hsa-miR-765, hsa-miR-223-3p and
hsa-miR-33b-3p underwent RT-qPCR which have been reported in
previous studies (22–25) and their target genes can be found
in miRNA databases, such as TargetScan (www.targetscan.org) and PITA (genie.weizmann.ac.il/pubs/mir07/mir07_data.html).
Automated RT-qPCR determination of the three miRNAs was performed
using hsa-miR-16 as the internal reference. The melting curves
indicated good PCR amplification specificity, with one perfect
single peak for each miRNA. The relative expression levels of
hsa-miR-223-3p and hsa-miR-33b-3p were significantly higher in the
KD group compared to the control group (P<0.05; Fig. 2). The relative expression level of
hsa-miR-765 between the two groups was not significantly different
(P>0.5).
Target genes
A total of 62 common target genes of hsa-miR-223-3p
were identified by comparing three different target gene
predictions and was detected by both RT-qPCR and microarray
analysis (Fig. 3 and Table IV).
 | Table IV.Predicted target genes of
hsa-miR-223-3p. |
Table IV.
Predicted target genes of
hsa-miR-223-3p.
| GeneID | Symbol | GeneID | Symbol | GeneID | Symbol |
|---|
| 6477 | SIAH1 |
2872 | MKNK2 |
10600 | USP16 |
| 84133 | ZNRF3 |
5997 | RGS2 |
8763 | CD164 |
| 2034 | EPAS1 |
4848 | CNOT2 |
9962 | SLC23A2 |
| 538 | ATP7A |
1080 | CFTR |
57835 | SLC4A5 |
| 10890 | RAB10 | 160518 | DENND5B |
84312 | BRMS1L |
| 6925 | TCF4 |
3836 | KPNA1 |
26118 | WSB1 |
| 84255 | SLC37A3 |
10492 | SYNCRIP | 143098 | MPP7 |
| 255488 | RNF144B |
1010 | CDH12 |
4774 | NFIA |
| 9852 | EPM2AIP1 |
23250 | ATP11A |
463 | ZFHX3 |
| 64145 | RBSN |
6383 | SDC2 |
23220 | DTX4 |
| 92 | ACVR2A |
91860 | CALML4 | 284403 | WDR62 |
| 9472 | AKAP6 |
23435 | TARDBP |
3131 | HLF |
| 55156 | ARMC1 |
29789 | OLA1 |
4628 | MYH10 |
| 27154 | BRPF3 |
214 | ALCAM |
3572 | IL6ST |
| 9882 | TBC1D4 |
55602 | CDKN2AIP |
2309 | FOXO3 |
| 22883 | CLSTN1 |
5898 | RALA |
9868 | TOMM70 |
| 55588 | MED29 |
490 | ATP2B1 |
26269 | FBXO8 |
| 5581 | PRKCE | 125950 | RAVER1 |
5814 | PURB |
| 154796 | AMOT |
11221 | DUSP10 |
51105 | PHF20L1 |
| 2308 | FOXO1 |
8939 | FUBP3 |
5617 | PRL |
| 149018 | LELP1 |
54842 | MFSD6 |
|
|
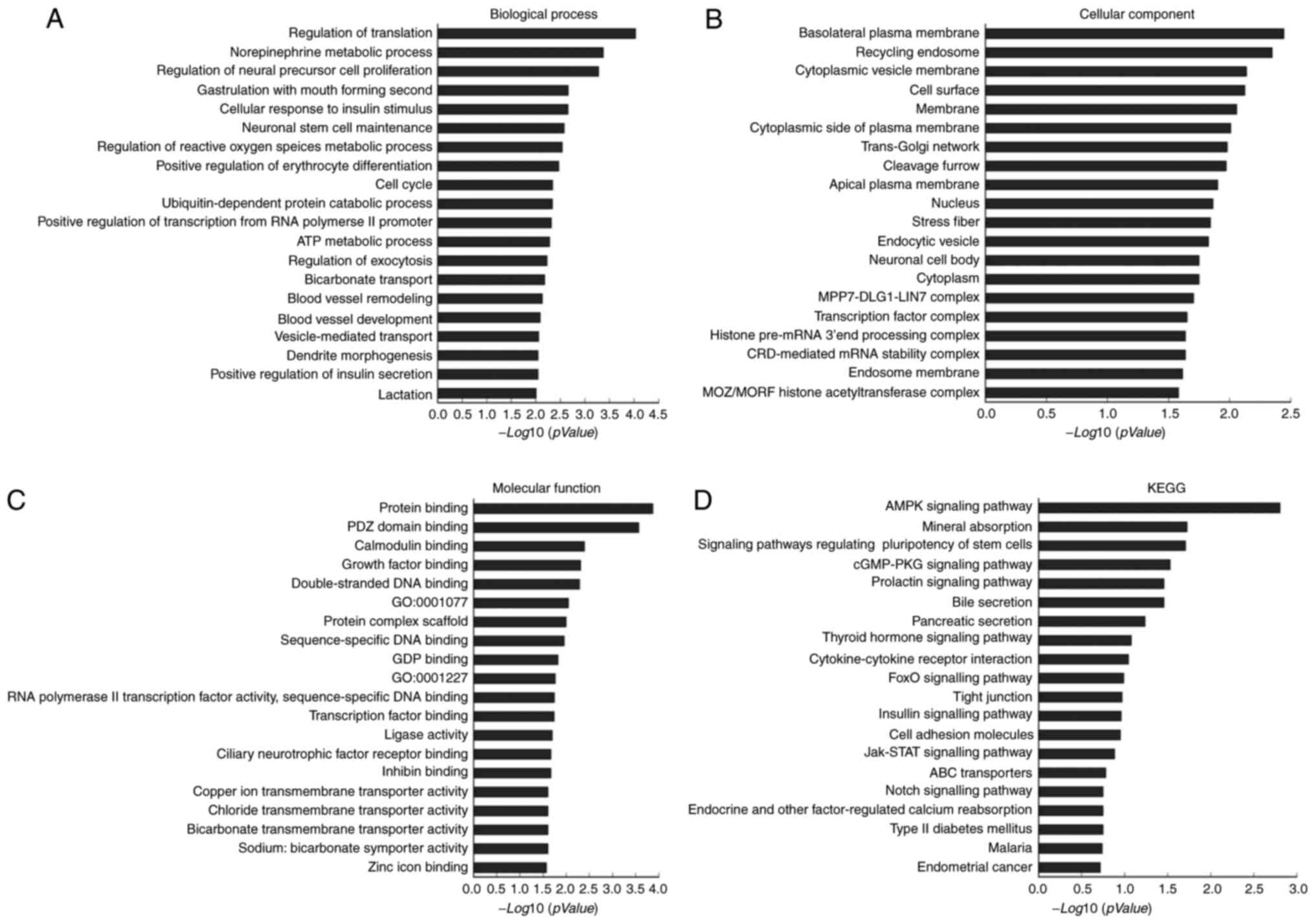
GO analysis
The 62 predicted target genes of hsa-miR-223-3p were
enriched in BPs (including regulation of translation,
norepinephrine metabolic process and regulation of neural precursor
cell proliferation; Fig. 4A), CCs
(including, basolateral plasma membrane, recycling endosome and
cytoplasmic vesicle membrane; Fig.
4B) and MFs (including, protein binding, PDZ-domain binding and
calmodulin binding; Fig. 4C).
KEGG pathway analysis
The biological pathway enrichment analysis of the 62
predicted target genes showed that hsa-miR-223-3p was significantly
enriched in the AMP-activated protein kinase (AMPK) signaling
pathway, mineral absorption pathway and signaling pathways
regulating pluripotency of stem cell (Fig. 4D).
Discussion
KD is a childhood multisystemic vasculitis; the
mechanisms involved in the pathogenesis of vasculitis are poorly
understood. Necrotizing arteritis, subacute chronic vasculitis and
luminal myofibroblastic proliferation have been previously
identified as the three basic processes of KD pathogenesis
(26). Necrotizing arteritis is an
acute process that may be responsible for saccular aneurysms.
Following the onset of KD, both subacute chronic vasculitis and
luminal myofibroblastic proliferation persist for months to
years.
The present study identified 10 differentially
expressed miRNAs, a number of which have been reported previously,
such as hsa-miR-765, hsa-miR-33b-3p and hsa-miR-223-3p. hsa-miR-765
has been reported in coronary disease (22) and cancer (23). hsa-miR-33b-3p has also been
reported in cancer (24). And
hsa-miR-223-3p has been reported in diabetes mellitus (25), and KD (27). Therefore, RT-qPCR was performed to
verify these three miRNAs. As most patients with KD are newborns,
the amount of blood that can be withdrawn is limited and that is
why different plasma samples were used in microarray analysis and
RT-qPCR, which is a limitation of the current study. Given miRNAs
strong regulatory roles in cellular metabolism, proliferation,
differentiation, apoptosis and stress (reviewed in 11), they may
provide clues for understanding the pathophysiology of KD and may
be potentially useful in future diagnostic and therapeutic
strategies. As they exist in a very stable state in the serum or
plasma (28,29), miRNAs are suitable as biological
markers for KD diagnosis and follow-up (30). As the results of microarray are not
always stable, the present study used RT-qPCR to validate the
results of microarray. RT-qPCR validation revealed no difference in
hsa-miR-765 expression levels and increased hsa-miR-33b-3p levels
in the plasma of acute KD, which was inconsistent with the
microarray results. The difference in results between the
microarray and RT-qPCR may be due to detection sensitivity
differences and sample heterogeneity. In addition, one of the
differentially expressed miRNAs, hsa-miR-223-3p, for which both
microarray and RT-qPCR revealed increased expression, was selected
for target gene prediction.
Currently, few studies (27,31,32)
have focused on circulating miRNAs in patients with KD. One
previous study used high-throughput sequencing in the peripheral
blood in patients with acute and convalescent KD to identify six
differentially expressed miRNAs, including miR-143, miR-199b-5p,
miR-618, miR-223, miR-145 and miR-145* (27). Using a group of febrile patients
with KD as the control, another study reported elevated serum
levels of miRNA-200c and miR-371-5p in patients with KD (31). High levels of miR-182 and
miR-296-5p have been reported during the acute febrile phase,
whereas high levels of miR-93, miR-145*, miR-145 and miR-150-3p
were detected in the defervescence stage (32). It has been suggested that miR-93
may regulate vascular endothelial growth factor (VEGF-A) expression
and may contribute to the understanding of the pathogenesis of
arteritis in acute KD. A recent study demonstrated significantly
higher serum miR-92a-3p expression levels were detected in children
with KD compared with febrile children (33); however, a different study reported
that no miRNAs in coronary artery tissues were diagnostic for KD
(34). The present study
hypothesized that the different sample sources of in vivo
circulating blood and in vitro coronary artery tissues
contributed to the wholly opposite results. For example, the study
by Rowley et al examined miRNA expression in coronary artery
tissue from patients who had succumbed to KD (death within weeks
after onset), which differed from the study by Rong et al
that used circulating blood from living patients with KD (33,34).
He et al revealed that KD sera suppressed the Krüppel-like
factor 4/miR-483 axis in human umbilical vein endothelial cells,
and increased the expression of connective tissue growth factor and
induction of endothelial-to-mesenchymal transition. This
detrimental process in the endothelium may contribute to coronary
artery abnormalities in KD patients (35).
Previous studies have demonstrated that miR-223 is
expressed in monocytes and macrophages, and may be the key to
regulating inflammation (36).
miR-223 was also reported to be transported in plasma and delivered
to recipient cells by high-density lipoproteins (HDLs) in patients
or mice with hypercholesterolemia (37), and it was demonstrated that the
anti-inflammatory properties of HDL may be conferred, in part,
through HDL-miR-223 delivery and the repression of intercellular
adhesion molecule-1 translation in endothelial cells (38). miR-223 was previously demonstrated
to target β1 integrin to antagonize angiogenesis and prevent growth
factor signaling in endothelial cells (39). miR-223 was suggested to be a
potential biomarker of type 2 diabetes (25). In addition, platelets were
demonstrated to remotely modulate vascular endothelial cell
apoptosis by releasing microvesicles that contain miR-223, which
targets insulin-like growth factor 1 receptor and promotes advanced
glycation end product-induced vascular endothelial cell apoptosis
(40). One recent study revealed
that high miR-223 expression levels in vascular endothelial cells
may function as a novel endocrine genetic signal and participate in
vascular injury of KD (41);
however, the exact mechanism was not determined.
The present study identified 62 putative target
genes of hsa-miR-223-3p. GO term enrichment analysis identified a
number of biological processes, cellular components and molecular
functions that may be related to KD; KEGG pathway analysis
indicated that the target genes were enriched in AMPK signaling,
mineral absorption and signaling pathways regulating stem cell
pluripotency, whose role in KD needs to be defined. Previous
studies have indicated the existence of specific signals or
pathways in KD. For example, it was predicted that, along with
other differentially expressed miRNAs, miR-145 may participate in
regulating the expression of genes in the transforming growth
factor β (TGF-β) pathway of arterial wall myofibroblasts (27), and miR-93 may participate in
regulating VEGF-A expression in the pathogenesis of arteritis in
acute KD (32); signaling pathways
regulating stem cell pluripotency and cytokine-cytokine receptor
interaction pathways may individually include TGF-β and VEGF-A.
In conclusion, 10 differentially expressed miRNAs
were detected by microarray chip in the plasma of patients with
acute KD, of which 3 miRNAs were verified by RT-qPCR, but only
hsa-miR-223-3p was found to be consistently detected by both. A
total of 62 potential target genes of hsa-miR-223-3p were
identified. Previous studies have reported that miR-223 may
regulate inflammation of vascular endothelial cells. Therefore,
hsa-miR-223-3p may participate in the pathogenesis of KD, and
determination of its functions and mechanisms in KD require further
verification.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (grant nos. 81370217 and
81570455), the Natural Science Foundation of Young (grant nos.
81400222 and 81300124) and the Suzhou Science and Technology Bureau
(grant nos. KJXW2014015 and SYS201633).
References
|
1
|
Sudo D, Monobe Y, Yashiro M, Mieno NM,
Uehara R, Tsuchiya K, Tsuchiya K, Sonobe T and Nakamura Y: Coronary
artery lesions of incomplete Kawasaki disease: A nationwide survey
in Japan. Eur J Pediatr. 171:651–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukazawa R: Long-term prognosis of
Kawasaki disease: Increased cardiovascular risk? Curr Opin Pediatr.
22:587–592. 2010.PubMed/NCBI
|
|
3
|
Tsuda E, Abe T and Tamaki W: Acute
coronary syndrome in adult patients with coronary artery lesions
caused by Kawasaki disease: Review of case reports. Cardiol Young.
21:74–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Makino N, Nakamura Y, Yashiro M, Ae R,
Tsuboi S, Aoyama Y, Kojo T, Uehara R, Kotani K and Yanagawa H:
Descriptive epidemiology of Kawasaki disease in Japan, 2011 2012:
From the results of the 22nd nationwide survey. J Epidemiol.
25:239–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Del Principe D, Pietraforte D, Gambardella
L, Marchesi A, de Jacobis Tarissi I, Villani A, Malorni W and
Straface E: Pathogenetic determinants in Kawasaki disease: The
haematological point of view. J Cell Mol Med. 21:632–639. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shulman ST and Rowley AH: Kawasaki
disease: Insights into pathogenesis and approaches to treatment.
Nat Rev Rheumatol. 11:475–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon KL: Update of genetic susceptibility
in patients with Kawasaki disease. Korean J Pediatr. 58:84–88.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sunderland N, Skroblin P, Barwari T,
Huntley RP, Lu R, Joshi A, Lovering RC and Mayr M: MicroRNA
biomarkers and platelet reactivity: The clot thickens. Circ Res.
120:418–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Song Y, Yao L, Song G and Teng C:
Circulating microRNAs: Promising biomarkers involved in several
cancers and other diseases. DNA Cell Biol. 36:77–94. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tijsen AJ, Pinto YM and Creemers EE:
Circulating microRNAs as diagnostic biomarkers for cardiovascular
diseases. Am J Physiol Heart Circ Physiol. 303:H1085–H1095. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Rooij E and Olson EN: MicroRNA:
Powerful new regulators of heart disease and provocative
therapeutic targets. J Clin Invest. 117:2369–2376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Connell RM, Taganov KD, Boldin MP, Cheng
G and Baltimore D: MicroRNA-155 is induced during the macrophage
inflammatory response. Proc Natl Acad Sci USA. 104:1604–1609. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reid G, Kirschner MB and van Zandwijk N:
Circulating microRNAs: Association with disease and potential use
as biomarkers. Crit Rev Oncol Hematol. 80:193–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He
J, Qin YW and Jing Q: Circulating microRNA: A novel potential
biomarker for early diagnosis of acute myocardial infarction in
humans. Eur Heart J. 31:659–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji X, Takahashi R, Hiura Y, Hirokawa G,
Fukushima Y and Iwai N: Plasma miR-208 as a biomarker of myocardial
injury. Clin Chem. 55:1944–1949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akbas F, Coskunpinar E, Aynaci E, Oltulu Y
and Yildiz P: Analysis of serum micro-RNAs as potential biomarker
in chronic obstructive pulmonary disease. Exp Lung Res. 38:286–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Newburger JW, Takahashi M, Gerber MA,
Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P,
Baltimore RS, et al: Diagnosis, treatment, and long-term management
of Kawasaki disease: A statement for health professionals from the
committee on rheumatic fever, endocarditis, and Kawasaki disease,
council on cardiovascular disease in the young, American heart
association. Circulation. 110:2747–2771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sheikh Ali MS, Xia K, Li F, Deng X, Salma
U, Deng H, Wei Wei L, Yang TL and Peng J: Circulating miR-765 and
miR-149: Potential noninvasive diagnostic biomarkers for geriatric
coronary artery disease patients. Biomed Res Int.
2015:7403012015.PubMed/NCBI
|
|
23
|
Tömböl Z, Eder K, Kovács A, Szabó PM,
Kulka J, Likó I, Zalatnai A, Rácz G, Tóth M, Patócs A, et al:
MicroRNA expression profiling in benign (sporadic and hereditary)
and recurring adrenal pheochromocytomas. Mod Pathol. 23:1583–1595.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu N, Li Z, Yu Z, Yan F, Liu Y, Lu X and
Yang W: MicroRNA-33b suppresses migration and invasion by targeting
c-Myc in osteosarcoma cells. PLoS One. 9:e1153002014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu H and Leung SW: Identification of
microRNA biomarkers in type 2 diabetes: A meta-analysis of
controlled profiling studies. Diabetologia. 58:900–911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Orenstein JM, Shulman ST, Fox LM, Baker
SC, Takahashi M, Bhatti TR, Russo PA, Mierau GW, de Chadarévian JP,
Perlman EJ, et al: Three linked vasculopathic processes
characterize Kawasaki disease: A light and transmission electron
microscopic study. PLoS One. 7:e389982012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimizu C, Kim J, Stepanowsky P, Trinh C,
Lau HD, Akers JC, Chen C, Kanegaye JT, Tremoulet A, Ohno-Machado L
and Burns JC: Differential expression of miR-145 in children with
Kawasaki disease. PLoS One. 8:e581592013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2015. View Article : Google Scholar
|
|
29
|
Fichtlscherer S, Zeiher AM and Dimmeler S:
Circulating microRNAs: Biomarkers or mediators of cardiovascular
diseases? Arterioscler Thromb Vasc Biol. 31:2383–2390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gilad S, Meiri E, Yogev Y, Yogev Y,
Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M,
Cholakh H, et al: Serum microRNAs are promising novel biomarkers.
PLoS One. 3:e31482008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yun KW, Lee JY, Yun SW, Lim IS and Choi
ES: Elevated serum level of microRNA (miRNA)-200c and miRNA-371-5p
in children with Kawasaki disease. Pediatr Cardiol. 35:745–752.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saito K, Nakaoka H, Takasaki I, Hirono K,
Yamamoto S, Kinoshita K, Miyao N, Ibuki K, Ozawa S, Watanabe K, et
al: MicroRNA-93 may control vascular endothelial growth factor A in
circulating peripheral blood mononuclear cells in acute Kawasaki
disease. Pediatr Res. 80:425–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rong X, Jia L, Hong L, Pan L, Xue X, Zhang
C, Lu J, Jin Z, Qiu H, Wu R and Chu M: Serum miR-92a-3p as a new
potential biomarker for diagnosis of Kawasaki disease with coronary
artery lesions. J Cardiovasc Transl Res. 10:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rowley AH, Pink AJ, Reindel R, Innocentini
N, Baker SC, Shulman ST and Kim KY: A study of cardiovascular miRNA
biomarkers for Kawasaki disease. Pediatr Infect Dis J.
33:1296–1299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He M, Chen Z, Martin M, Zhang J, Sangwung
P, Woo B, Tremoulet AH, Shimizu C, Jain MK, Burns JC and Shyy JY:
miR-483 Targeting of CTGF suppresses endothelial-to-mesenchymal
transition: Therapeutic implications in Kawasaki disease. Circ Res.
120:354–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnnidis JB, Harris MH, Wheeler RT,
Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD and
Camargo FD: Regulation of progenitor cell proliferation and
granulocyte function by microRNA-223. Nature. 451:1125–1129. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vickers KC, Palmisano BT, Shoucri BM,
Shamburek RD and Remaley AT: MicroRNAs are transported in plasma
and delivered to recipient cells by high-density lipoproteins. Nat
Cell Biol. 13:423–433. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tabet F, Vickers KC, Torres Cuesta LF,
Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado-Lourenco L,
Levin MG, Thacker S, et al: HDL-transferred microRNA-223 regulates
ICAM-1 expression in endothelial cells. Nat Commun. 5:32922014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thum T: MicroRNA-223 made its way into
vascular research. Circ Res. 113:1270–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pan Y, Liang H, Liu H, Li D, Chen X, Li L,
Zhang CY and Zen K: Platelet-secreted microRNA-223 promotes
endothelial cell apoptosis induced by advanced glycation end
products via targeting the insulin-like growth factor 1 receptor. J
Immunol. 192:437–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chu M, Wu R, Qin S, Hua W, Shan Z, Rong X,
Zeng J, Hong L, Sun Y, Liu Y, et al: Bone marrow-derived
microRNA-223 works as an endocrine genetic signal in vascular
endothelial cells and participates in vascular injury from Kawasaki
disease. J Am Heart Assoc. 6:pii: e004878. 2017. View Article : Google Scholar
|