Introduction
Heart failure (HF) results from impaired ventricular
filling and/or ejection function caused by a variety of cardiac
structural or functional diseases (1). As cardiac output does not meet the
requirements of body tissues for metabolism, HF behaves similarly
to a series of syndromes that are characterized by having a
clinical manifestation of pulmonary circulation deficiency and/or
congestion of systemic circulation, and organ-tissue blood
perfusion insufficiency (2). The
main symptoms of HF include difficulty in breathing which limits
physical activity and fluid retention. Previously, chronic HF (CHF)
has been considered to be a clinical syndrome characterized by
chronic inflammation combined with a neuroendocrine disorder
(3). It is well documented that
the systemic insulin resistance phenomenon exhibited in patients
with CHF is caused by a variety of factors (4).
The mechanism of insulin resistance in HF is rather
complex. The current hypothesis is that activation of the
renin-angiotensin aldosterone system, sympathetic nervous system
activation, cytokine and nerve endocrine hormone alterations (e.g.,
interleukin-6), inflammatory factor release caused by reduced
insulin secretion, and insulin sensitivity decreases are the main
causes of insulin resistance (5).
Additionally, there may be other functional mechanisms (4,6,7).
It has been reported that androgen functions in
vivo and androgen receptors occurin cardiac tissue (8,9).
Marsh et al (10)
demonstrated the presence of a functional androgen receptor, which
regulates gene expression in myocardial cells, indicating that the
cardiovascular system is a target of androgens. Sex hormones and
insulin are known to interact in the body. Testosterone (T) and
estradiol (E2) are considered to be useful in maintaining normal
insulin sensitivity in the physiological concentration range;
however, outside this range, these hormones are able to promote
insulin resistance (11). It has
been demonstrated that there is a gender difference in terms of the
effect of sex hormones on insulin sensitivity. For instance, T can
lead to insulin resistance in females, whereas T significantly
improves insulin sensitivity in peripheral tissues in males
(12). T therapy may improve
insulin sensitivity in both normal and diseased populations,
including in obese men or diabetics (13). However, the level of androgens in
HF, and whether androgen supplementation could improve insulin
resistance in HF, remains to be elucidated.
The aim of the present study was to analyze the
effect of androgens on myocardial apoptosis and the expression of
the insulin receptor (IR) and insulin receptor substrate-1 (IRS-1)
in a CHF rat model. In addition, the theoretical foundation of the
pathophysiological factors influencing the prognosis and outcome of
CHF was investigated.
Materials and methods
Animal modeling
A total of 120 Sprague Dawley male rats (7 weeks
old) weighing 230±20 g were enrolled in the present study. The rats
were randomly divided into five groups, the sham group contained 20
rats whereas the other four groups contained 25 rats. The five
groups were named as follows: (A) the sham operation group (20
rats), (B) castrated group, (C) HF group, (D) castrated + HF group,
and (E) castrated + HF + T replacement therapy group. The rats were
kept at 25°C, 0.03% CO2 and 12/12 h light/dark cycle
with free access to food and water.
Animal models were prepared as follows. The rats
were fed normally for 1 week, and testectomy was performed on rats
in groups B, D and E. First, the rats were anesthetized with ether
and fixed in dorsal recumbency. Lidocaine was then injected locally
at the midpoint of the line connecting the two knees in front of
the genitals. A 1-1.5 cm incision was made using ophthalmic
scissors, and forceps were inserted between the skin and muscles
for elevation of the skin to expose the muscles of the abdominal
wall. The incision was extended through the body wall to penetrate
the abdominal cavity and enlarge the surgical window. In the
surgical field, the bladder was located. Fat tissue is present on
the two sides of the bladder along with the testicular artery and
vein converging at the head of the testicle. The fat tissue was
elevated and the testicles were extracted. The testicles and
epididymal artery and vein were ligated using a suture and an
incision was made through the vascular bundle and testicular neck.
Following surgery, the incision was disinfected with an iodophor,
but no antibiotics were administered. Starting from the second day
following surgery, 2 mg/kg testosterone propionate was injected
subcutaneously once every 2 days to group E for a total of 30 days.
The rats in groups C, D and E received intraperitoneal injections
of doxorubicin hydrochloride at a dose of 2.5 mg/kg once every 5
days, 6 times in total. The cumulative dose was 15 mg/kg. Following
the last administration, food and water intake, mobility, shedding
of hair, and mortality of the rats were observed for 3 weeks. For
groups A and B, an equal volume of 0.9% sodium chloride was
injected intraperitoneally once every 5 days, 6 times in total.
After 1 week of normal diet, and the second day after castration in
groups B, D, and E, group A was given subcutaneous injection of
peanut oil until the end of the study. The current study was
approved by the Medical Ethics Committee of Yan'an Hospital
Affiliated to Kunming Medical University.
Echocardiography
Echocardiography was performed at the beginning and
the end of the experiment, using a Mindray M7 portable ultrasound
machine. The following indicators were detected: Left ventricular
diastolic diameter, left ventricular end systolic diameter, the
thickness of the interventricular septum and posterior wall, left
ventricular end diastolic volume and left ventricular end systolic
volume. Ejection fraction (EF), left ventricular fraction
shortening (FS) and left ventricular mass (LVM) were calculated
using the Devereux formula (14).
Sample collection and storage
At the end of experiment, venous blood was sampled
and the rats were sacrificed. The hearts were harvested and the
left ventricles were preserved at −80°C. Fasting plasma glucose
(FPG), fasting insulin (FIS) and T were measured at the beginning
and the end of the experiment. Blood samples were collected from
the tail vein. FPG was detected by the glucose oxidase method, with
the OneTouch® UltraVue™ blood glucose
monitoring system and glucose strips (Johnson & Johnson, New
Brunswick, NJ, USA). FIS was measured using Human Insulin ELISA kit
(catalog no. ab200011; Abcam, Cambridge, UK). The insulin
sensitivity index (ISI) was calculated as follows:
ISI=1/(FPGxFIS).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA of left ventricular myocytes in rats was
extracted with TRIzol® reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) from the left ventricular
myocardial tissue of rats, followed by 1.5% agarose gel
electrophoresis. The samples with clear 28S and 18S rRNA and no
obvious degradation were subjected to RT-PCR using an
All-in-One™ First-Strand cDNA Synthesis kit
(GeneCopoeia, Inc., Rockville, MD, USA). Using the RT-PCR products
as templates, semiquantitative PCR amplification was performed
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The primers of IR (the PCR product was 154
bp) were: Forward, 5′-GTTCTGGAATTGCGTGCTTT-3′ and reverse,
5′-TGCTTCATGAGCCAAGTCAC-3′. The primers of IRS-1 (the PCR product
was 160 bp) were: Forward, 5′-TTGATGTCCAGCTGAGTYCCT-3′ and reverse,
5′-CTTGAAGGGATCGCTCATGT-3′. β-actin was used as the internal
control and the primers of β-actin were forward,
5′-TGGGTATGGAATCCTGTGGCA-3′ and reverse,
5′-TGTTGGCATAGAGGTCTTTACGG-3′. The cycling program was as follows:
95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 5
sec, and annealing at 56°C for 30 sec and extension at 68°C for 30
sec. The products were subjected to 1.0% agarose gel
electrophoresis and visualized with ethidium bromide. Intensity of
the bands was visualized on the Tanon-5200 Chemiluminescent Imaging
system (Tanon Science and Technology, Co., Ltd., Shanghai, China)
and the relative PCR amounts of IR and IRS-1 were recorded as
I/I(β-actin).
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) detection
TUNEL method was used to detect cell apoptosis using
a commercial kit according to manufacturer's protocol (Roche
Applied Science, Penzberg, Germany). Briefly, sections were
de-waxed in xylene (5 min), rehydrated in graded ethanol (2 min)
and then rinsed in PBS for 5 min three times. Sections were
digested with Proteinase K (20 µg/ml; Sigma Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature for 15 min and then soaked
in PBS for 5 min. Endogenous peroxidase was quenched with 3%
H2O2 in PBS for 5 min. After washing with
PBS, sections were then incubated with working strength Tdt enzyme
in a humidified chamber at 37°C for 1 h. Once the enzymatic
reaction was stopped, the slides were rinsed with PBS, and the
samples were incubated in peroxidase substrate for 30 min at RT in
an humidified chamber. Then, sections were incubated in TBS with
0.05% diaminobenzidine for 5 min. The slides were counterstained
with 5% methyl green solution for 10min and mounted with permanent
mounting media DPX (Panreac SA, Barcelona, Spain). Images were
captured under a BX53TR research microscope (Olympus, Tokyo,
Japan). The cells with brown granules were considered to be
apoptotic cells. A total of six rats in each group were taken and
five images were captured for each specimen. The total number of
cardiac cells and apoptotic cells were recorded and the mean values
were calculated. The formula for calculating the apoptotic index
was as follows: Apoptosis index (AI)=number of apoptotic
cells/total number of myocardial cells ×100%.
Statistical analysis
All statistical analyses were performed using SPSS
for Windows software (version 17.0; SPSS, Inc., Chicago, IL, USA).
The data were expressed as the mean ± standard deviation. Multiple
comparisons were performed using one-way analysis of variance and
Student-Newman-Keuls test was used to examine the differences
between the two groups when a significant result (P<0.05) was
identified. The counted data are expressed by the constituent ratio
or percentage. The experiments were repeated ≥ three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Model suitability
To assess the suitability of the animal model, the
number of rats that survived in each group was recorded. As
demonstrated in Table I, the
survival rate of groups A and B were 100 and 96%, respectively. The
survival rate of the rats in group D was 72%, which was the lowest
out of the five groups, whereas the survival rate of group C (80%)
was lower compared with group E. The results demonstrated that the
animal model was successfully prepared.
 | Table I.Survival of the five groups of Sprague
Dawley rats following modeling. |
Table I.
Survival of the five groups of Sprague
Dawley rats following modeling.
|
| Group |
|---|
|
|
|
|---|
| Variable | A | B | C | D | E |
|---|
| Prior to
modeling | 20 | 25 | 25 | 25 | 25 |
| Following
modeling | 20 | 24 | 20 | 18 | 22 |
| Survival, % | 100 | 96 | 80 | 72 | 88 |
Results of the serological
indices
To investigate the effect of androgen on insulin
resistance in rats with CHF, the FPG, FIS and ISI were measured in
all groups. The results are illustrated in Fig. 1 and Table II. Multiple comparison tests
demonstrated that there were no differences in the five groups in
FPG, FIS, and ISI prior to animal modeling. Following modeling,
however, ISI of the group B was decreased compared with group A,
and FPG and FIS in group D was increased compared with group E
(P<0.01; Fig. 1A and B).
However, the ISI in group D was significantly lower than that in
group E (P<0.01; Fig. 1C).
These results demonstrated that androgens could improve insulin
resistance in peripheral blood of rats with HF.
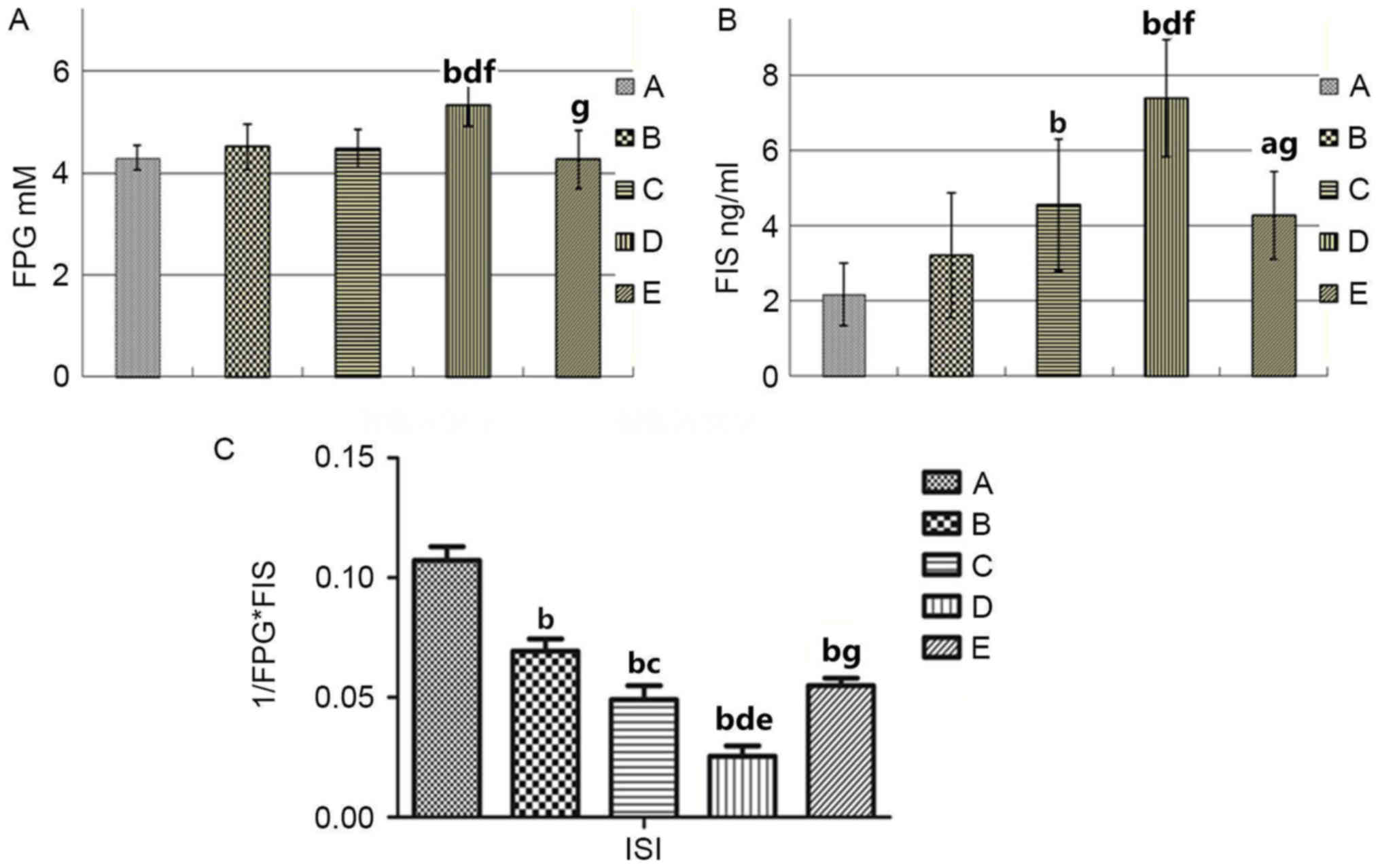 | Figure 1.Comparison of the serological indices
in group A (sham operation), B (castrated), C (HF), D (castrated +
HF) and E (castrated + HF + testosterone therapy) replacement
Sprague Dawley rats. (A) Fasting serum glucose levels. (B) Fasting
serum insulin levels. (C) ISI. aP<0.05,
bP<0.01 vs. group A; cP<0.05,
dP<0.01 vs. group B; eP<0.05,
fP<0.01 vs. group C; gP<0.01 vs. group
D. FPG, fasting plasma glucose; FIS, fasting insulin, ISI, insulin
sensitivity index; HF, heart failure. |
 | Table II.Comparison of FPG, FIS and ISI in the
5 groups of Sprague Dawley rats. |
Table II.
Comparison of FPG, FIS and ISI in the
5 groups of Sprague Dawley rats.
|
| Group |
|---|
|
|
|
|---|
| Variable | A | B | C | D | E |
|---|
| FPG, mmol/l |
4.30±0.24 |
4.51±0.44 |
4.48±0.37 |
5.33±0.41a–c |
4.26±0.58d |
| FIS, ng/ml |
2.17±0.84 |
3.2±1.664 |
4.546±1.74a |
7.39±1.55a–c |
4.276±1.17d,e |
| ISI |
0.107±0.018 |
0.069±0.017a |
0.049±0.017a,f |
0.025±0.015a,b,g |
0.055±0.011a,d |
Results of heart Doppler
ultrasound
To characterize the effect of androgen on cardiac
structure and function, color Doppler ultrasound was performed and
echocardiographic indices (EF, FS and LVM) prior to and following
modeling were observed. As demonstrated in Fig. 2 and Table III, EF and FS in group B were
decreased compared with group A. EF and FS in group D were
significantly decreased compared with group E, whereas the LVM in
group D was significantly increased compared with group E
(P<0.01). In conclusion, the results of the present study
demonstrated that androgen supplementation could improve cardiac
function and ventricular hypertrophy in rats with HF.
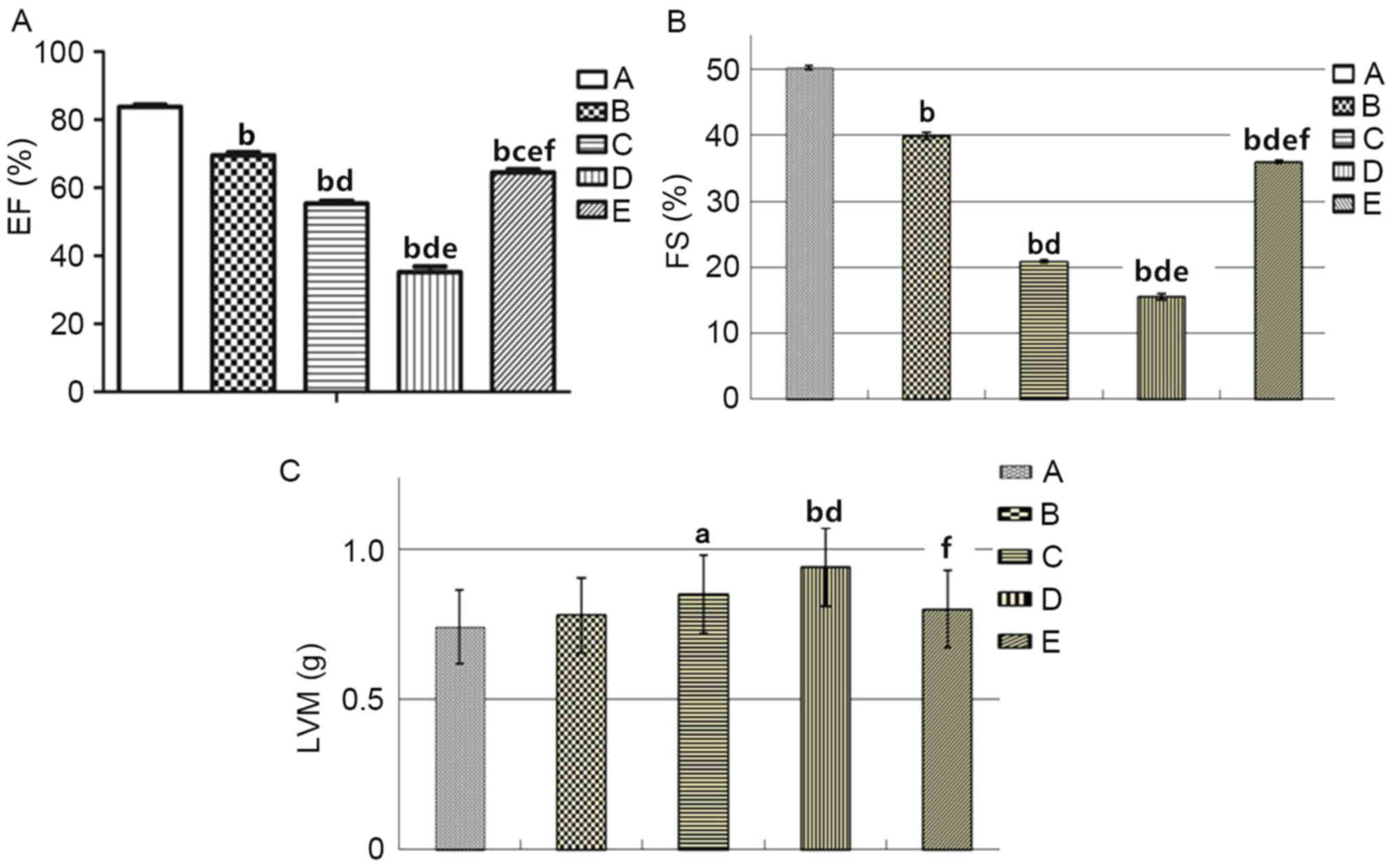 | Figure 2.Comparison of color Doppler
echocardiography indices of the heart in: Group A, sham operation;
group B, castrated; group C, HF; group D, castrated + HF; and group
E, castrated + HF + testosterone therapy replacement Sprague Dawley
rats. (A) EF. (B) Left ventricular FS. (C) LVM.
aP<0.05, bP<0.01 vs. group A;
cP<0.05; dP<0.01 vs. group B;
eP<0.01 vs. group C; fP<0.01 vs. group
D. HF, heart failure; EF, ejection fraction; FS, fractional
shortening; LVM, left ventricular mass. |
 | Table III.Comparison of heart color Doppler
echocardiography indexes (EF, FS, LVM) in the 5 groups of Sprague
Dawley rats. |
Table III.
Comparison of heart color Doppler
echocardiography indexes (EF, FS, LVM) in the 5 groups of Sprague
Dawley rats.
|
| Group |
|---|
|
|
|
|---|
|
| A | B | C | D | E |
|---|
| EF, % |
83.78±0.674 |
69.53±0.903a |
55.42±0.905a,b |
35.23±1.66a–c |
64.55±0.88a,c,d,e |
| FS, % |
50.29±0.35 |
39.87±0.59a |
20.86±0.26a,b |
15.5±0.43a–c |
35.98±0.25a–c,e |
| LVM, g |
0.74±0.122 |
0.78±0.125 |
0.85±0.130f |
0.94±0.129a,b |
0.80±0.129e |
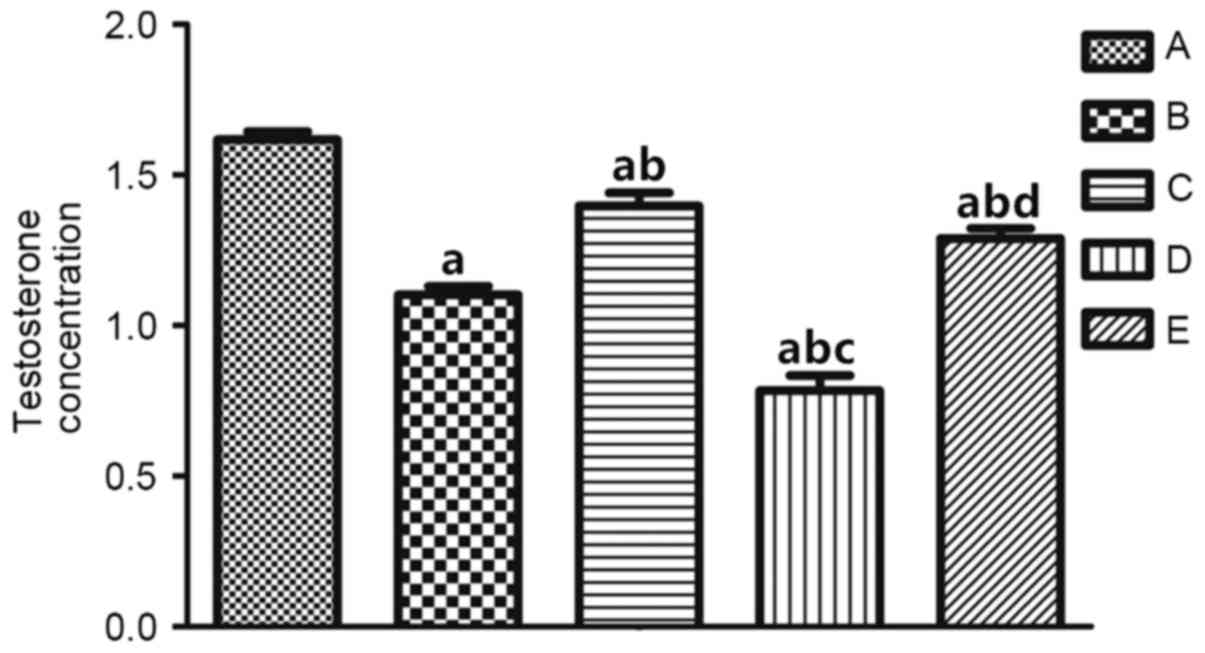
Comparison of T levels
In order to analyze the alterations in androgen
levels in rats with CHF, the T level in rats of the different
groups was measured and compared, and these results are shown in
Fig. 3. The T level in CHF rats
(group C) was decreased compared with group A and increased
compared with group B, whereas the T level of group D was
significantly decreased compared with group A (P<0.01; Table IV). These observations
demonstrated that androgen levels are decreased in CHF rats.
 | Table IV.Comparison of T levels in the 5
groups of Sprague Dawley rats. |
Table IV.
Comparison of T levels in the 5
groups of Sprague Dawley rats.
|
| Group |
|---|
|
|
|
|---|
| Variable | A | B | C | D | E |
|---|
| T, ng/ml |
1.62±0.08 |
1.10±0.09a |
1.40±0.13a,b |
0.79±0.18a–c |
1.29±0.12a,b,d |
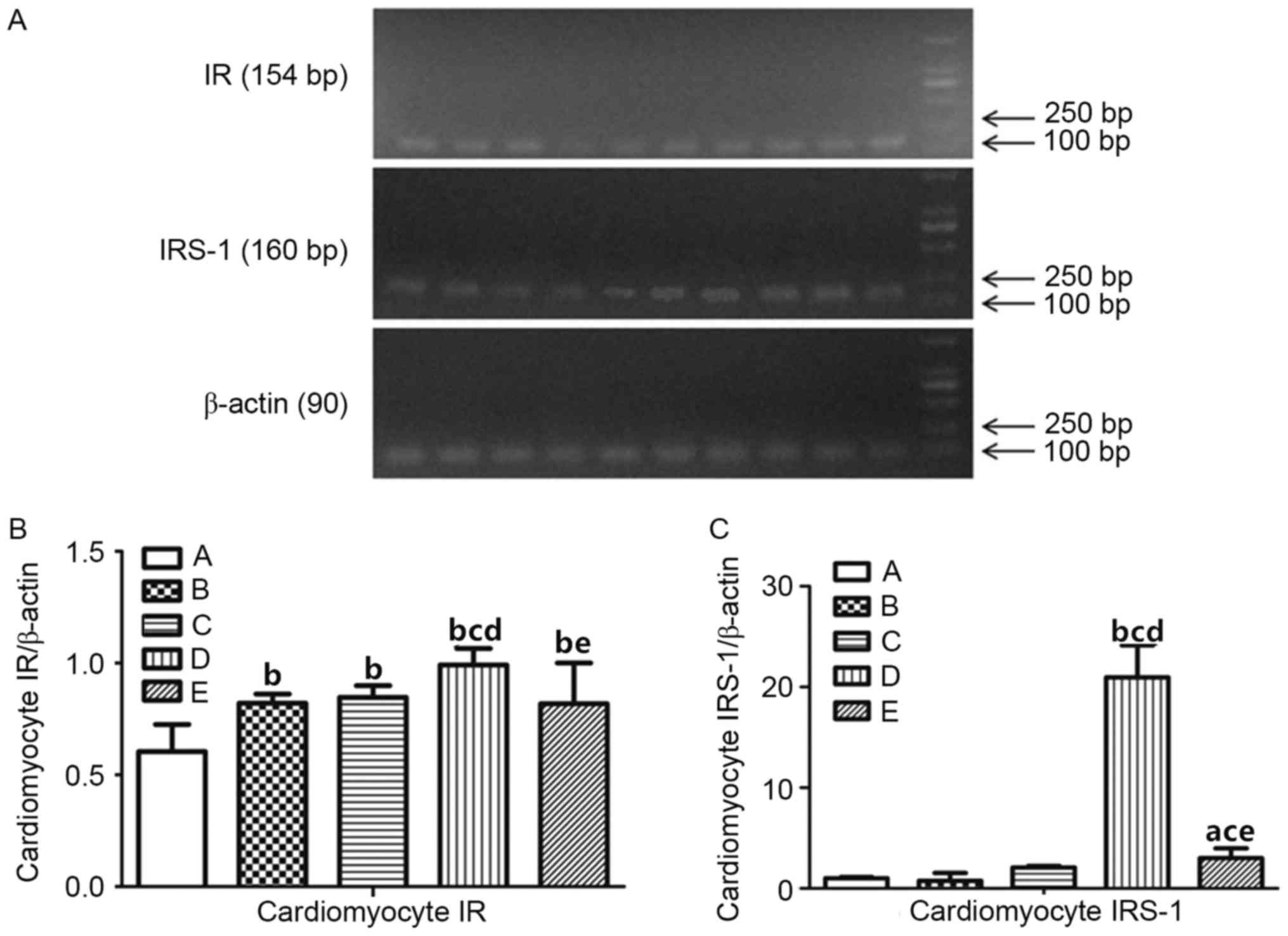
Expression of IR and IRS-1
To verify the underlying mechanism of androgen on
insulin resistance in rats with CHF, the expression levels of IR
and IRS-1 in different groups were tested by RT-PCR (Fig. 4A). The results are exhibited in
Fig. 4B and C. Following
statistical analysis, myocardial IR expression levels in groups B,
C, D and E were significantly increased compared with group A
(P<0.01; Table V). Compared
with group B, myocardial IR expression level in group D (P<0.01)
increased, whereas no significant differences were demonstrated in
groups C and E (P>0.05). Compared with group C, myocardial IR
expression level in group D was significantly increased
(P<0.01), whereas no statistically significant difference was
demonstrated compared with group E (P>0.05). Compared with group
D, myocardial IR expression level in group E decreased
significantly (P<0.01). These results indicated that the
expression levels of the IR and IRS-1 in cardiac muscle cells of
CHF rats with androgen deficiency increased, and insulin resistance
occurred.
 | Table V.Comparison of IR and IRS-1 expression
levels investigated using RT-PCR in cardiac muscle cells in Sprague
Dawley rats. |
Table V.
Comparison of IR and IRS-1 expression
levels investigated using RT-PCR in cardiac muscle cells in Sprague
Dawley rats.
|
| Group |
|---|
|
|
|
|---|
| Variable | A | B | C | D | E |
|---|
| IR |
0.60±0.12 |
0.82±0.04a |
0.847±0.05a |
0.99±0.073a–c |
0.82±0.18a,d |
| IRS-1 |
1.008±0.13 |
0.756±0.78 |
2.095±0.16 |
20.97±3.16a–c |
3.001±0.99b,d,e |
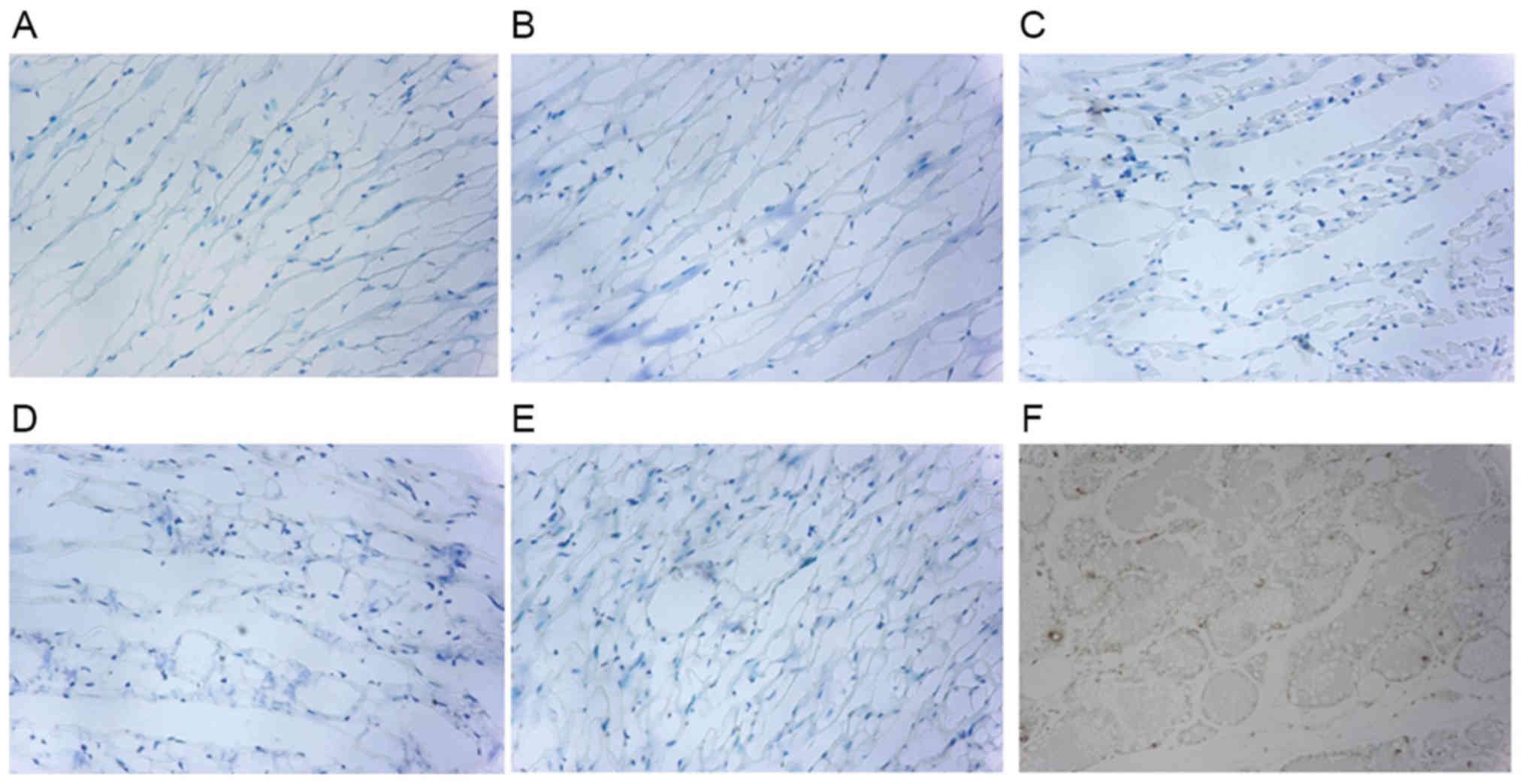
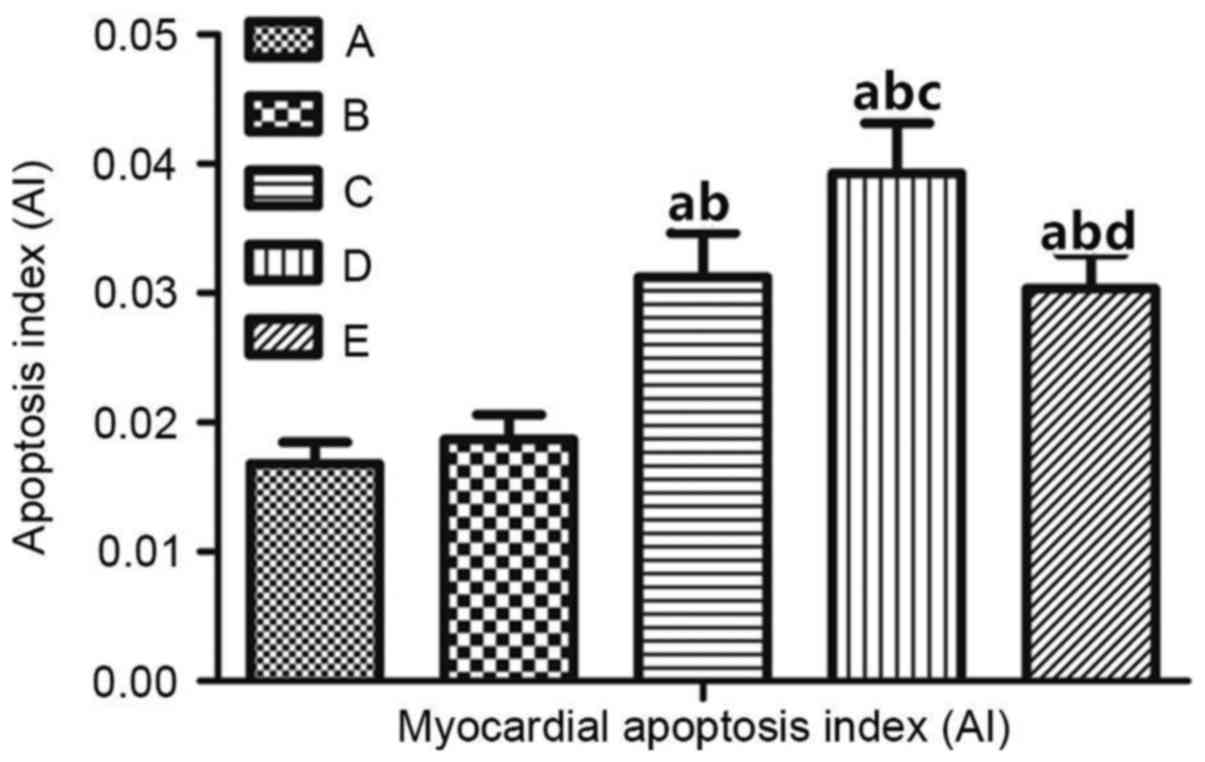
Apoptosis of rat myocardial cells
In order to investigate the underlying effect of
androgens on the apoptosis of cardiac cells in rats, a TUNEL assay
was performed. The results are demonstrated in Fig. 5. The apoptosis of myocardial cells
in CHF rats (group C) was significantly increased compared with
groups A and B (Table VI). In
rats with androgen deficiency and HF (group D), myocardial
apoptosis was significantly increased compared with group E
(P<0.01; Fig. 6). These
observations suggested that apoptosis of myocardial cells in rats
with androgen deficiency and HF occurs, and that apoptosis is
improved in rats with CHF following T supplementation.
 | Table VI.Comparison of cardiac muscle cell AI
in Sprague Dawley rats. |
Table VI.
Comparison of cardiac muscle cell AI
in Sprague Dawley rats.
|
| Group |
|---|
|
|
|
|---|
| Variable | A | B | C | D | E |
|---|
| AI |
0.017±0.002 |
0.019±0.002 |
0.031±0.003a,b |
0.039±0.004a,b |
0.03±0.003a–c |
Discussion
In the present study, compared with the sham
operation group (group A), the biochemical indicators and cardiac
indices demonstrated that, when CHF occurred, the androgen levels
were reduced, the insulin sensitivity decreased, and insulin
resistance developed in the body. Additionally, the results of the
present study demonstrated that ventricular remodeling occurred,
myocardial IR expression increased, and cardiac insulin resistance
developed. Doehner et al (15) demonstrated that
dehydroepiandrosterone (DHEA) levels in 53 cases of male patients
with CHF were significantly decreased compared with healthy
controls. Kontoleon et al (16) demonstrated that T levels in
patients with CHF was significantly decreased, and the T level is
positively correlated with heart index. Zhou et al (17) argued that, in CHF male castrated
rats, the decreased T level aggravated cardiac insufficiency,
whereas treatment with physiological T was able to protect the
contractile function of the heart. A previous study demonstrated
that insulin resistance exists in patients with HF, resulting in
normal glucose levels and insulin hyperlipidemia during fasting or
glucose load (18). These results
all are consistent with the results of the present study.
When insulin resistance occurs in the body, the
heart muscle also exhibits insulin resistance. According to a
previous study, when insulin resistance occurs, the myocardial
glucose uptake is reduced and fatty acid uptake increases, which
leads to an increase in myocardial lipotoxicity (4). Simultaneously, the free fatty acid
level increases, which in turn causes insulin-mediated glucose
utilization barriers, aggravating insulin resistance (19). In the present study, it was
demonstrated that IRs in group C were increased compared with group
A, but decreased compared with group D. These results indicated
that during CHF, the expression of IRs increased and insulin
resistance occurred in the cardiac myocytes. The IR may also be
associated with an increased intake of fatty acids and a decreased
uptake of glucose by myocytes. In the present study, however, no
significant alterations in the expression of IRS-1 in myocardial
cells of group C was demonstrated compared with group A. The
possible reasons may be that, when CHF occurs, insulin resistance
may arise in the receptor itself, or the number of insulin
receptors in cardiac cells is reduced, or a mutation on the
receptor gene will occur. These underlying mechanisms remain to be
confirmed.
Myocardial cell programmed death is alternatively
referred to as myocyte apoptosis, which not only may affect heart
development, but also serves an important pathophysiological role
in primary hypertension, HF, arrhythmia, and other cardiovascular
diseases. In the present study it was demonstrated that, compared
with group A, the expression of myocardial IR increased in groups C
and D. The myocardial cell apoptosis index in group C increased
compared with group A, whereas group D was increased compared with
group C. These results indicated that myocardial insulin resistance
during CHF could induce the apoptosis of myocardial cells.
Therefore, it was hypothesized that improvement in insulin
resistance in patients with CHF could increase the protective
effect of insulin on the myocardium and reduce apoptosis of the
myocardial cells, which, in turn, could delay the progression of
HF. With the recent advances in the understanding of the mechanisms
of signal transduction, it has been demonstrated that insulin as a
mitogenic compound is able to promote proliferation and
differentiation. When combined with the IR, insulin activates the
tyrosine kinase in the β-subunit of the IR, thereby causing
phosphorylation of IRS-1 (20).
Additionally, insulin is able to connect with downstream proteins
containing SH2 domains and thereby generate signaling cascades in
the phosphoinositide 3-kinase/protein kinase B signaling pathway,
which initiates insulin-resistant cell apoptosis (21).
In the present study, it was demonstrated that
myocardial IR and IRS-1 expression, and myocardial cell apoptosis
indexes were decreased in group E compared with group D. This
indicates that androgen treatment is able to improve the
insulin-resistant state during CHF by reducing myocardial cell IR
and IRS-1 expression, which leads to a decrease in myocardial cell
apoptosis, thereby leading to an improvement in cardiac function.
Androgens are mainly produced in the testes of male animals,
although a small amount of androgen is secreted by the adrenal
glands (22). Androgens serve
their physiological role via their receptors. Previous studies have
demonstrated that androgens serve a wide range of functions in
vivo, and androgen receptors have been demonstrated in cardiac
tissue (8,9). This indicates that androgens are
likely to produce cardiovascular benefits by acting on receptors in
the myocardium.
In light of the results of the present study a CHF
rat model was created, and data were obtained to aid the
elucidation of the underlying mechanism of androgens in CHF
therapy. It may be hypothesized that androgen levels and insulin
sensitivity decreased in CHF, whereas the expression of the IR and
IRS-1 in cardiac muscle cells increased, and insulin resistance
occurred. Supplementation with androgens could improve insulin
resistance, reduce the expression of the IR and the IRS-1 in
cardiac muscle cells, and thereby reduce the apoptosis of
myocardial cells in CHF. Additionally, androgen may reduce the
apoptosis of cardiac muscle cells in HF by improving insulin
resistance. However, there are several limitations associated with
the present study. The study sample size was small, and a larger
sample size would be required for further study. In addition, the
present study is based on an animal model, and further studies are
required in a clinical setting to confirm these conclusions.
Acknowledgements
The present study was supported by the Basic
Research Project of Yunnan Province (grant no. 2013FZ139).
References
|
1
|
Erdei T, Smiseth OA, Marino P and Fraser
AG: A systematic review of diastolic stress tests in heart failure
with preserved ejection fraction, with proposals from the EU-FP7
MEDIA study group. Eur J Heart Fail. 16:1345–1361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tohmo H, Karanko M, Korpilahti K, Scheinin
M, Viinamäki O and Neuvonen P: Enalaprilat in acute intractable
heart failure after myocardial infarction: A prospective,
consecutive sample, before-after trial. Crit Care Med. 22:965–973.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pugh PJ, Jones RD, Jones TH and Channer
KS: Heart failure as an inflammatory condition: Potential role for
androgens as immune modulators. Eur J Heart Fail. 4:673–680. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van de Weijer T, Schrauwen-Hinderling VB
and Schrauwen P: Lipotoxicity in type 2 diabetic cardiomyopathy.
Cardiovasc Res. 92:10–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Papadimitriou L and Kalogeropoulos AP:
Inflammatory biomarkers and therapeutic targets in heart failure.
Curr Med Chem. 22:2716–2726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashrafian H, Frenneaux MP and Opie LH:
Metabolic mechanisms in heart failure. Circulation. 116:434–448.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schroeder ET, Vallejo AF, Zheng L, Stewart
Y, Flores C, Nakao S, Martinez C and Sattler FR: Six-week
improvements in muscle mass and strength during androgen therapy in
older men. J Gerontol A Biol Sci Med Sci. 60:1586–1592. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang CK, Lee SO, Chang E, Pang H and
Chang C: Androgen receptor (AR) in cardiovascular diseases. J
Endocrinol. 229:R1–R16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Smet MA, Lapauw B and De Backer T: Sex
steroids in relation to cardiac structure and function in men.
Andrologia. 49:2017.doi: 10.1111/and.12610. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marsh JD, Lehmann MH, Ritchie RH, Gwathmey
JK, Green GE and Schiebinger RJ: Androgen receptors mediate
hypertrophy in cardiac myocytes. Circulation. 98:256–261. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johansson A, Ahrén B, Forsberg H and
Olsson T: Testosterone and diurnal rhythmicity of leptin, TNF-alpha
and TNF-II receptor in insulin-resistant myotonic dystrophy
patients. Int J Obes Relat Metab Disord. 26:1386–1392. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boyanov MA, Boneva Z and Christov VG:
Testosterone supplementation in men with type 2 diabetes, visceral
obesity and partial androgen deficiency. Aging Male. 6:1–7. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borissova AM, Tankova T, Kamenova P,
Dakovska L, Kovacheva R, Kirilov G, Genov N, Milcheva B and Koev D:
Effect of hormone replacement therapy on insulin secretion and
insulin sensitivity in postmenopausal diabetic women. Gynecol
Endocrinol. 16:67–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Simone G, Devereux RB and Wallerson DC:
Echocardiographic assessment of left ventricular hypertrophy in
rats using a simplified approach. Am J Hypertens. 7:555–558. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doehner W, von Haehling S and Anker SD:
Insulin resistance in chronic heart failure. J Am Coll Cardiol.
52:239–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kontoleon PE, Anastasiou-Nana MI,
Papapetrou PD, Alexopoulos G, Ktenas V, Rapti AC, Tsagalou EP and
Nanas JN: Hormonal profile in patients with congestive heart
failure. Int J Cardiol. 87:179–183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Q, Liu JH, Ke J, CheN B and MA YX:
Effects of testosterone on left ventricular function of male rats
with chronic heart failure. China J Modern Med. 15:2104–2106.
2005.
|
|
18
|
Paolisso G, De Riu S, Marrazzo G, Verza M,
Varricchio M and D'Onofrio F: Insulin resistance and
hyperinsulinemia in patients with chronic congestive heart failure.
Metabolism. 40:972–977. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong AK, AlZadjali MA, Choy AM and Lang
CC: Insulin resistance: A potential new target for therapy in
patients with heart failure. Cardiovasc Ther. 26:203–213. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Witteles RM and Fowler MB:
Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and
treatment options. J Am Coll Cardiol. 51:93–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao F, Gao E, Yue TL, Ohlstein EH, Lopez
BL, Christopher TA and Ma XL: Nitric oxide mediates the
antiapoptotic effect of insulin in myocardial ischemia-reperfusion:
The roles of PI3-kinase, Akt, and endothelial nitric oxide synthase
phosphorylation. Circulation. 105:1497–1502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonagura TW, Zhou H, Babischkin JS, Pepe
GJ and Albrecht ED: Expression of P-450 aromatase, estrogen
receptor α and β, and α-inhibin in the fetal baboon testis after
estrogen suppression during the second half of gestation.
Endocrine. 39:75–82. 2011. View Article : Google Scholar : PubMed/NCBI
|