Introduction
Lung cancer is the most common type of cancer
worldwide; the incidence and mortality rates of lung cancer are the
highest among all human malignant tumors (1,2). At
present, lung cancer is the leading cause of cancer-associated
mortality among men and women in China and western countries. It is
estimated that the number of new cases of lung cancer reached
651,053 with a mortality rate of 529,153, in 2011 (3).
Tobacco smoke has an important role in the
initiation and development of lung cancer. It is estimated that
~90% of lung cancer cases are associated with tobacco smoke
(4), including 80% of all female
and 90% of all male lung cancer cases (5). As a complex mixture, tobacco smoke
contains >6,000 individual chemical constituents, among which
150 have been reported to exert toxicological effects, including
the induction of free radicals and carcinogenic activities
(6,7). These ingredients are responsible for
the carcinogenic potential of tobacco smoke, including the
transformation and progression of cancer. Although progress has
been made concerning the molecular mechanisms responsible for
tobacco smoke-induced lung cancer development, the molecular
pathogenesis requires further investigation.
Epithelial-mesenchymal-transition (EMT) is involved
in embryonic development and tumorigenesis (8,9). EMT
is decisive in cancer invasion and metastasis. Evidence has
indicated that EMT contributes to tumor progression by allowing
cancer cells to avoid apoptosis and cellular senescence (10). During this process, cells
progressively lose expression of membranous epithelial markers and
acquire mesenchymal features (11). Tobacco smoke has been reported to
promote EMT (12,13). It has been identified that tobacco
smoke-induced EMT may regulate the early events in carcinogenesis,
including deprivation of cell-cell adhesion and apical-basal
polarity, reduced expression of epithelial cadherin and
upregulation of cell mobility (13). However, the potential mechanisms of
tobacco smoke-induced EMT remain unclear.
The mitogen activated protein kinase (MAPK) cascade
is a crucial signaling cascade involved in the transmission of
stress-associated stimuli (14).
This pathway also serves a key role in the development and
progression of cancer (15). The
classical MAPK pathway includes extracellular-signal-regulated
kinase (ERK), c-Jun N-terminal kinase (JNK) and p38. Recent studies
have reported that EMT may be controlled by ERK1/2, JNKs, p38 and
ERK5 (16,17). Results from our previous studies
have demonstrated that MAPK pathways are involved in tobacco
smoke-induced EMT (18–21).
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,
6-hepadiene-3,5-dione] is the principal active component of the
plant Curcuma longa. For centuries curcumin has been used
widely throughout India and South Asia in Ayurvedic medicine due to
its nontoxic and beneficial properties, which include
anti-inflammatory, antioxidant, wound healing and antiseptic
properties (22–25). In addition, evidence has indicated
that curcumin exhibits anticancer properties by affecting various
biological pathways involved in apoptosis, mutagenesis, cell cycle
regulation, oncogene expression, metastasis and angiogenesis
(26–28). The ability of curcumin to inhibit
carcinogenesis has been reported in vivo and in vitro
(29). Our previous studies have
demonstrated that tobacco smoke-induced alterations in EMT in the
bladder and stomach tissues of mice are effectively attenuated by
curcumin (18,19). However, to the best of our
knowledge, whether curcumin exhibits a protective effect on tobacco
smoke-induced lung EMT is yet to be determined.
The present study aimed to investigate the effect of
tobacco smoke on the activation of MAPK pathways and EMT in the
lung in vivo, and further investigate the protective effects
of curcumin against tobacco smoke-exposure. The results confirmed
that curcumin exhibits a chemopreventive effect on tobacco
smoke-induced lung EMT.
Materials and methods
Chemicals and reagents
Primary antibodies against phosphorylated (p)-ERK1/2
(cat. no. 4370S; 1:1,000), (p)-p38 (cat. no. 4511S; 1:1,000),
(p)-JNK (cat. no. 9251S; 1:1,000), (p)-ERK5 (cat. no. 3371S;
1:500), (p)-c-Jun (cat. no. 9164S; 1:500), (p)-c-Fos (cat. no.
5348S; 1:500), Fos-like 2 activator protein-1 (AP-1) transcription
factor subunit (Fra-2) (cat. no. 19967S; 1:1,000), E-cadherin (cat.
no. 3195S; 1:1,000), N-cadherin (cat. no. 4061S; 1:500) and
vimentin (cat. no. 3932S; 1:1,000) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Zona occludens
(ZO)-1 (cat. no. sc-8146; 1:500) primary antibody was purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The primary
antibody for GAPDH (cat. no. 5014; 1:3,000) was from Biogot
Technology Co., Ltd. (Nanjing, China). E-cadherin, ZO-1,
N-cadherin, vimentin and GAPDH primers for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) were
synthesized according to published sequences from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Curcumin
(purity, >99%) was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany).
Animal and tobacco smoke exposure
A total of 52 male BALB/c mice (4-weeks-old; weight,
18–22 g) were purchased from the Animal Research Center of Nanjing
Medical University (Nanjing, China). All mice were allowed one week
acclimatize to circumstances prior to experimental exposure. Mice
were raised in polypropylene cages at 22±0.5°C and 40–60% relative
humidity with a 12-h light/dark cycle, and water and basal diet
were provided ad libitum. All mice procedure protocols were
approved by the Animal Care and Welfare Committee of Nanjing
Medical University.
Mice were randomly divided into tobacco
smoke-exposure and control groups (n=10/group). The tobacco
smoke-exposure group was treated with tobacco smoke for 6 h daily
for 12 weeks. The control group animals were exposed to filtered
air. Mice in the tobacco smoke group were exposed to tobacco smoke
in a smoking apparatus designed by the authors. A smoke machine was
used to combust filterless commercial cigarettes (12 mg tar and 1.1
mg nicotine per cigarette, Hongtashan brand; Hongta Group, Yuxi,
China) to generate tobacco smoke. The smoke machine pumped the
cigarette smoke regularly from burning filterless cigarettes (5
min/cigarette). The target concentration of smoke was delivered to
whole-body exposure chambers in total particulate matter of 85
mg/m3. The components were monitored and characterized
as: Carbon monoxide (14.72±2.89 mg/m3) and total
particulate matter TPM (0 mg/m3) for the control group;
and carbon monoxide (184.07±23.51 mg/m3) and TPM
(83.53±5.63 mg/m3) for the tobacco smoke exposure group.
Mice were sacrificed at the end of the final tobacco smoke
exposure, and the lung tissues were isolated, frozen and stored at
−80°C until analysis.
Curcumin treatment of mice
Doses were selected based on those employed by
previous studies involving the treatment of mice with curcumin,
which were 50 or 100 mg/kg body weight (BW) per day in animal
models (30,31). BALB/c mice were randomly assigned
into the following four groups (n=8 per group): Filtered air group,
in which mice were exposed to filtered air and under a controlled
diet; a tobacco smoke-exposed group, in which mice were exposed to
tobacco smoke and under a controlled diet; tobacco smoke + curcumin
50 mg group, in which mice were exposed to tobacco smoke and under
a controlled diet supplemented with curcumin at a dose of 50 mg/kg
BW/day; and tobacco smoke + curcumin 100 mg group, in which mice
were exposed to tobacco smoke and under a controlled diet
supplemented with curcumin at a dose of 100 mg/kg BW/day. The mice
were exposed to filtered air or tobacco smoke at a target
concentration of 85 mg/m3 total particulate matter for 6
h daily for 12 weeks. Dietary consumption levels and body weight
were measured and recorded every 3 days to determine the
administration dosages of curcumin. The diets were prepared weekly
for each group. At the end of the exposure, mice were sacrificed
and the lung tissues were frozen and stored at-80°C until
analysis.
Western blot analysis
Western blot analyses were performed following
standard procedures. Briefly, lung tissues were lysed (lysate
buffer, 5 mmol/l EDTA; 50 mmol/l Tris pH 7.5, 1% sodium dodecyl
sulfate (SDS), 10 µg/ml aprotinin, 1% sodium deoxycholate, 1%
NP-40, 1% Triton-X 100, 1 mM phenylmethylsulfonyl fluoride, 10
µg/ml leupeptin) and the lysate supernatants were obtained by
centrifugation at 12,000 × g for 20 min at 4°C and the pellets were
discarded. A BCA Protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.) was used to measure protein concentrations.
Electrophoresis was performed to fractionate 50 µg proteins by
7.5–10% SDS-PAGE. Subsequently, proteins were transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% non-fat milk for 1 h at room
temperature, membranes were incubated with primary antibodies
overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated secondary antibody (cat. no. 00001-2;
1:5,000) from Biogot Technology Co., Ltd. (Nanjing, China) for 1 h
at room temperature. The membranes were developed with SignalFire
Elite ECL Reagent (Cell Signaling Technology, Inc.). GAPDH served
as the loading control. ImageJ k 1.45 (National Institutes of
Health, Bethesda, MD, USA) was used to perform densitometric
analysis on protein bands.
RT-qPCR analysis
Total RNA was extracted from the mouse lung tissues
using RNAiso Plus (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. Subsequently, 2 µg purified total RNA was
reverse transcribed to cDNA using PrimeScript RT Master mix (Takara
Bio, Inc.) according to the manufacturer's protocol. The RT
reaction was performed as follows: 37°C 15 min, 85°C 5 sec, and
hold at 4°C, using AMV Reverse Transcriptase (Promega Corporation,
Madison, WI, USA). qPCR was performed according to the
manufacturer's protocol: Initial denaturation at 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec, 72°C for
30 sec using the SYBR® Premix Ex Taq™ II
(Takara Bio, Inc.) and an ABI prism 7300 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primers
(Invitrogen; Thermo Fisher Scientific, Inc.) used were as follows:
E-cadherin forward, 5′-TCGACACCCGATTCAAAGTGG−3′ and reverse,
5′-TTCCAGAAACGGAGGCCTGAT−3′; ZO-1 forward,
5′-GCAGCCACAACCAATTCATAG−3′ and reverse,
5′-GCAGACGATGTTCATAGTTTC-3′; vimentin forward,
5′-CCTTGACATTGAGATTGCCA-3′ and reverse, 5′-GTATCAACCAGAGGGAGTGA-3′;
N-cadherin forward, 5′-ATCAAGTGCCATTAGCCAAG-3′ and reverse,
5′-CTGAGCAGTGAATGTTGTCA-3′; GAPDH forward,
5′-GCTGCCCAACGCACCGAATA-3′ and reverse, 5′-GAGTCAACGGATTTGGTCGT-3′.
The specificity of the PCR products was confirmed using melting
curve analysis. Relative gene expression levels, normalized to
GAPDH expression, were calculated by a comparative threshold cycle
(Cq) method using the formula 2−(ΔΔCq) (32). Each sample was performed in
triplicate.
Statistical analysis
Statistical differences were analyzed using one-way
analysis of variance for comparison of statistical differences
among multiple groups and the Fisher's least significant difference
test. Unpaired Student's t-test was also used for comparisons
between two groups. SPSS software version 16.0 (SPSS, Inc.,
Chicago, IL, USA) was used to perform statistical analysis. Data
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Tobacco smoke induces EMT alterations
in lung tissues of mice
Tobacco smoke is a major risk factor for lung
cancer. EMT has important functions in various types of cancer,
including lung cancer, and tobacco smoke-induced EMT is implicated
in tobacco smoke-associated malignant transformations. To determine
whether alterations in EMT were induced by tobacco smoke in an
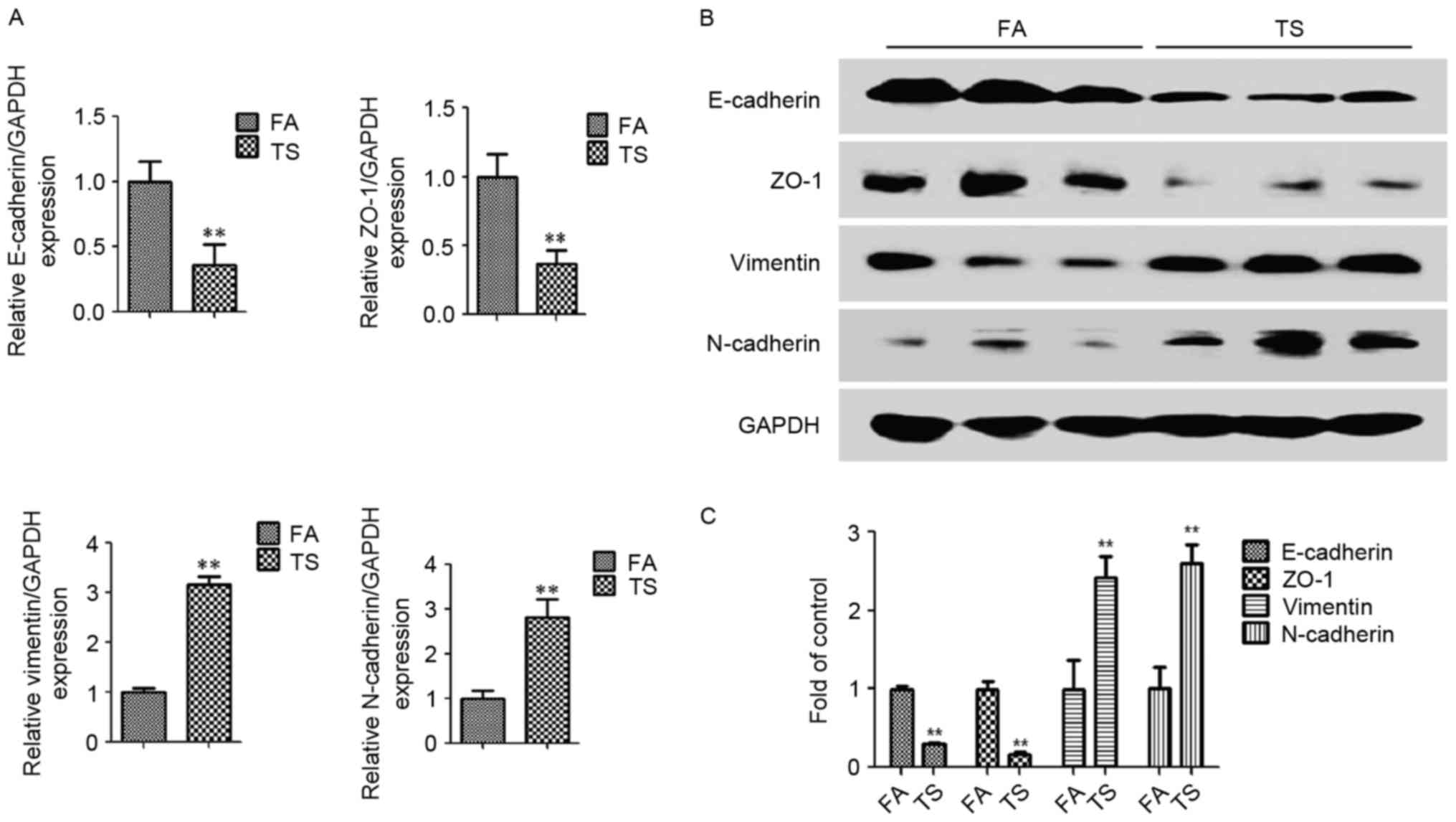
animal model, mice were exposed to tobacco smoke for 12 weeks, and
the expression of the epithelial markers E-cadherin and ZO-1, and
the mesenchymal markers vimentin and N-cadherin, in lung tissues
was examined. Tobacco smoke exposure led to reduced E-cadherin and
ZO-1 mRNA levels in mice lungs, and the mRNA expression of vimentin
and N-cadherin was increased, compared with the filtered
air-treated group, as determined by RT-qPCR (Fig. 1A). Western blot analysis further
revealed that tobacco smoke exposure also led to similar effects at
the protein level, with downregulated E-cadherin and ZO-1 protein
expression levels, and upregulated N-cadherin and vimentin protein
expression levels compared with the filtered air-treated group
(Fig. 1B and C).
Tobacco smoke activates pulmonary
MAPK/AP-1 pathways in mice
The expression levels of p-ERK1/2, p-JNK, p-p38 and
p-ERK5 were also measured to establish whether the observed tobacco
smoke-induced pulmonary EMT alterations are associated with changes
in MAPK activation. The results demonstrated that tobacco smoke
increased the activation of ERK1/2, JNK and p38 pathways, while it
suppressed the ERK5 MAPK pathway (Fig.
2A). Additionally, tobacco smoke exposure upregulated the
levels of p-c-Fos, p-c-Jun and Fra-2, which are indicators of the
AP-1 activation status (Fig.
2B).
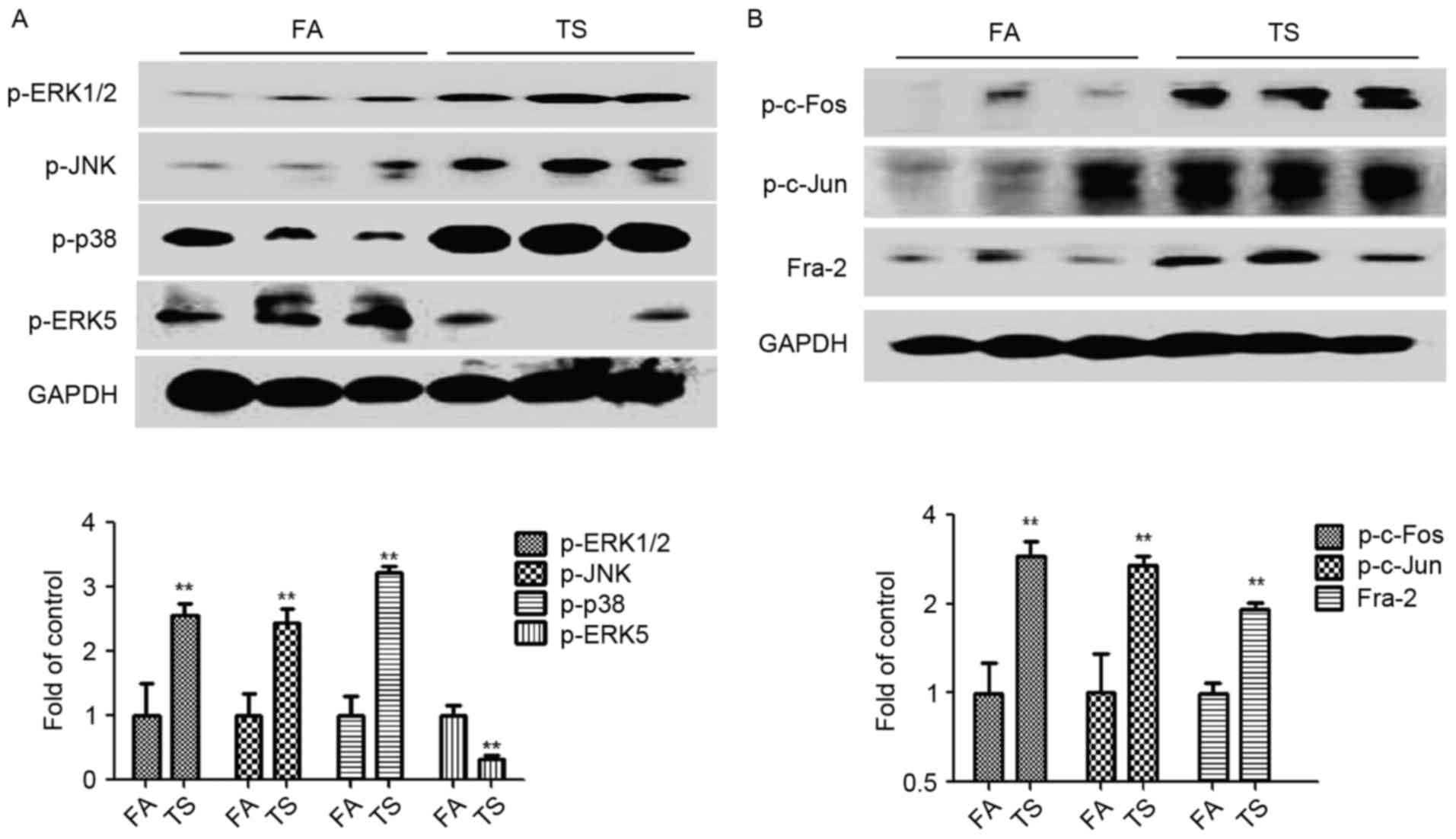 | Figure 2.TS increased MAPK activation in the
lungs of mice. (A) p-ERK1/2, p-JNK, p-p38 and p-ERK5 expression by
western blotting. (B) Western blotting results for p-c-Fos, p-c-Jun
and Fra-2. Data are presented as the mean ± standard deviation.
**P<0.01 vs. FA. GAPDH was used as a loading control. TS,
tobacco smoke; MAPK, mitogen-activated protein kinase; p-,
phosphorylated-; ERK, extracellular-signal-regulated kinase; JNK,
c-Jun N-terminal kinase; Fra-2, Fos-like 2 activator protein-1
transcription factor subunit; FA, filtered air. |
Curcumin reverses tobacco
smoke-induced EMT alterations in the lungs of mice
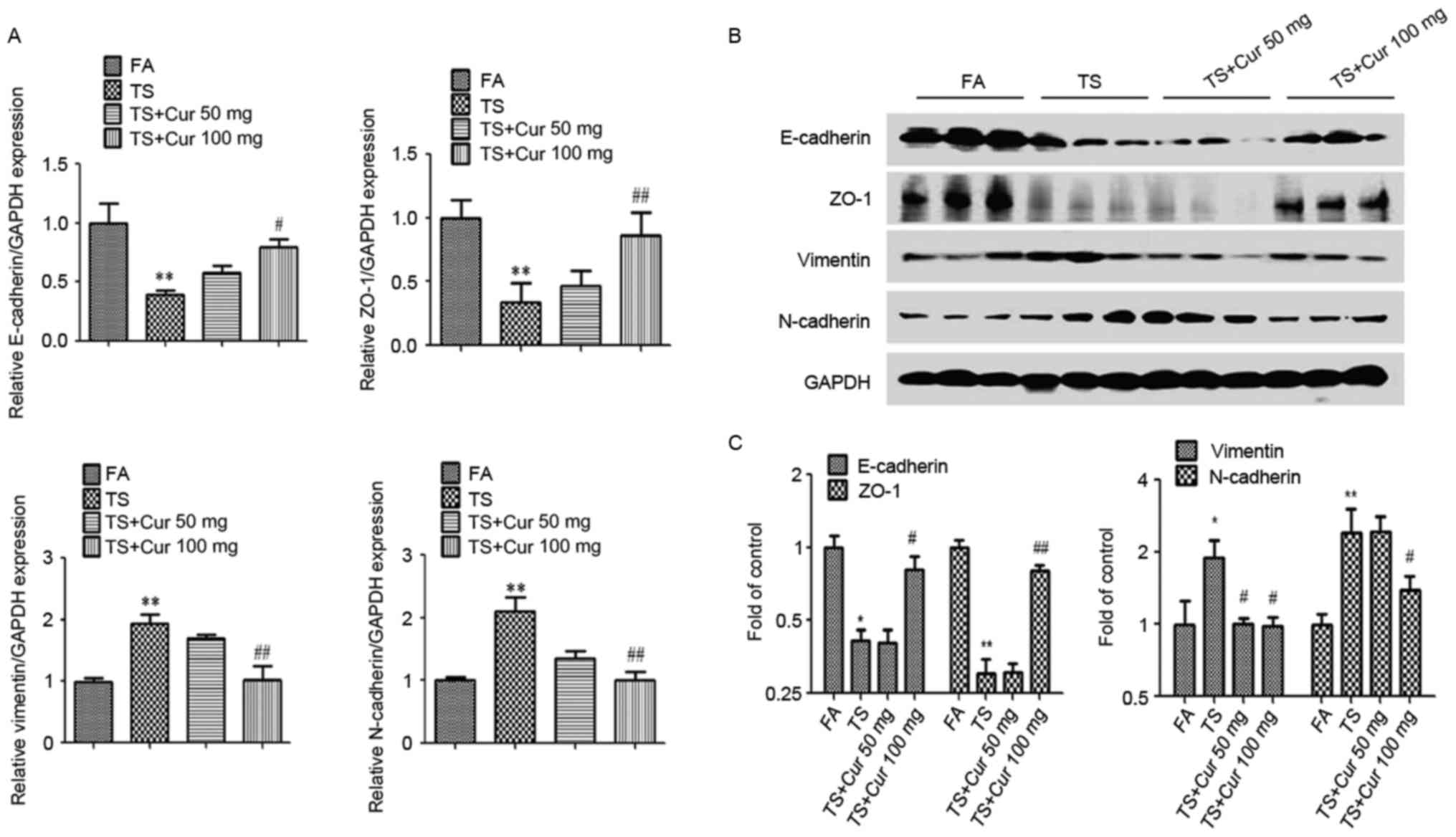
To investigate whether curcumin may reverse tobacco
smoke-mediated EMT in the mouse lung, mice were treated with
curcumin (50 or 100 mg/kg BW) and exposed to tobacco smoke for 12
weeks. Tobacco smoke-induced alterations in the mRNA and protein
expression EMT markers were subsequently investigated. As expected,
the results demonstrated that curcumin, particularly 100 mg/kg BW
curcumin, treatment attenuated the tobacco smoke-induced decreases
in E-cadherin and ZO-1 expression, and increases in vimentin and
N-cadherin expression. These results indicate that curcumin
reversed tobacco smoke-induced alterations in pulmonary EMT in
vivo (Fig. 3).
Curcumin attenuates tobacco
smoke-induced pulmonary MAPK alterations
To gain an improved understanding of the influence
of curcumin on tobacco smoke-mediated pulmonary activation of the
MAPK/AP-1 pathways, the changes in MAPK/AP-1 activation following
curcumin treatment were examined. Western blot analysis revealed
that curcumin (100 mg/kg BW) suppressed tobacco smoke-induced
ERK1/2, p38 and JNK activation, and increased ERK5 activation in
the lung tissues of mice exposed to tobacco smoke (Fig. 4A). The results also demonstrated
that treatment with 100 mg/kg BW curcumin significantly decreased
tobacco smoke-induced AP-1 activation, as indicated by reduced
expression of p-c-Fos, p-c-Jun and Fra-2 compared with the tobacco
smoke-treated group without curcumin treatment (Fig. 4B).
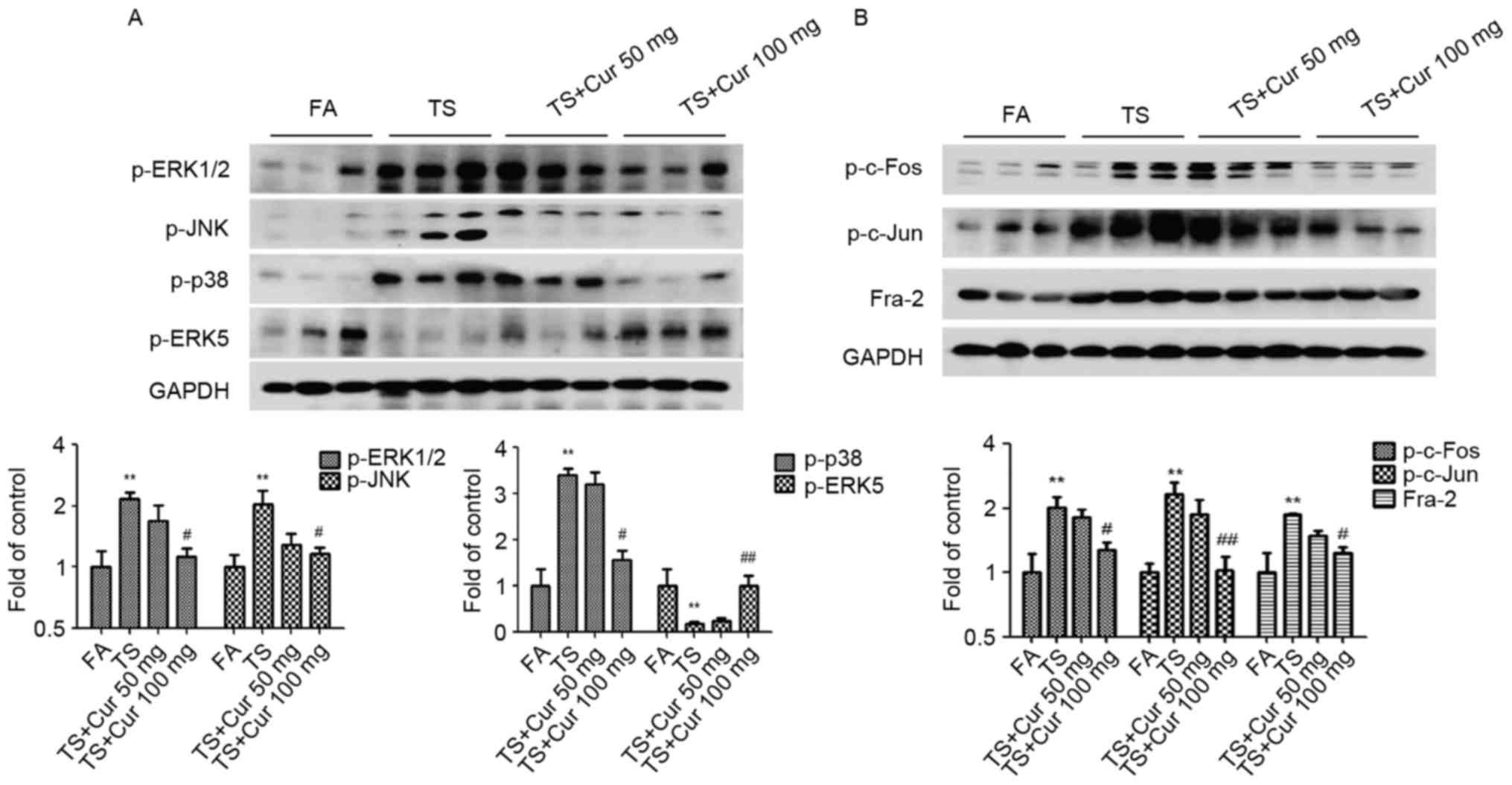 | Figure 4.Curcumin altered TS-induced MAPK/AP-1
activation in the lungs of mice. (A) p-ERK1/2, p-JNK, p-p38 and
p-ERK5 protein expression by western blotting. (B) Western blot
analysis of p-c-Fos, p-c-Jun and Fra-2. Data are presented as the
mean ± standard deviation. **P<0.01 vs. FA;
#P<0.05 and ##P<0.01 vs. TS-only group.
TS, tobacco smoke; MAPK, mitogen-activated protein kinase; AP-1,
activator protein-1; p-, phosphorylated-; ERK,
extracellular-signal-regulated kinase; JNK, c-Jun N-terminal
kinase; Fra-2, Fos-like 2 AP-1 transcription factor subunit; FA,
filtered air; Cur, curcumin. |
Discussion
Lung cancer is the leading cause of
cancer-associated mortality globally. Studies have confirmed the
association between the occurrence of lung cancer and tobacco smoke
(33,34). As a major contributor to lung
cancer, tobacco smoke promotes the initiation and progression of
lung cancer. However, further investigation of the mechanisms
involved in the initiation and development of lung cancer induced
by tobacco smoke is required. The present study demonstrated that
EMT alterations were induced by tobacco smoke in the lungs of mice.
It further revealed that the activation of MAPK pathways was
implicated in tobacco smoke-induced lung EMT, in addition to AP-1
activation. Furthermore, the results indicated that curcumin
treatment prevented tobacco smoke-induced lung EMT changes and
MAPK/AP-1 activation in vivo.
EMT serves an important role in cancer initiation
and development. The exposure of cells to carcinogens has been
reported to induce EMT during transformation and tumor formation
(35–39), indicating that EMT, by promoting
cell malignancy transformation, may be associated with the
initiation of tumorigenesis. The results of the present study were
consistent with these reports, as they demonstrated that, following
exposure to tobacco smoke for 12 weeks, tobacco alterations in EMT
were observed in the lungs of mice. Tobacco smoke exposure
decreased the expression of the epithelial markers E-cadherin and
ZO-1, and increased the expression of the mesenchymal markers
vimentin and N-cadherin. These results indicate that tobacco smoke
may induce lung EMT in vivo.
EMT alterations are associated with various cell
signaling pathways. MAPK pathways are reported to promote the
initiation and progression of cancer, and are therefore important
in the tumorigenic process (40).
Studies have demonstrated that p38, ERK1/2 and JNK promote EMT
(16,17,41).
However, the role of ERK5 in EMT regulation has not been well
characterized. The present study identified that tobacco smoke
upregulated p38, ERK1/2 and JNK, thus inducing lung EMT in
vivo. However, it was also demonstrated that tobacco smoke
downregulated ERK5 activation, which was consistent with our
previous findings (20,21). Furthermore, the present study also
demonstrated that tobacco smoke-induced EMT was associated with
increased activation of AP-1. These results indicate that tobacco
smoke-induced lung EMT may be associated with MAPK/AP-1
activation.
Curcumin is a turmeric-derived active component that
has been widely used in medicine in India and Southeast Asia
(42). It has been used as a
chemopreventive agent against a number of tumors due to its
anticancer functions. The safety and anticancer activities of
curcumin have been reported in various cancers (43,44).
In the present study, BALB/c mice were treated with curcumin at 50
and 100 mg/kg BW/day. These concentrations were selected as they
proved to be efficient in other animal studies (31,45).
These doses are equivalent to a human dose of 3–6 g/day per adult
(46). Following curcumin and
tobacco smoke treatment for 12 weeks, changes in the mRNA and
protein expression of EMT markers were examined, and the results
indicated that the expression of the epithelial markers ZO-1 and
E-cadherin was downregulated, while the expression of the
mesenchymal markers N-cadherin and vimentin was upregulated, in
mice treated with tobacco smoke compared with those treated with
filtered air. Furthermore, curcumin (100 mg/kg BW/day) markedly
attenuated tobacco smoke-induced EMT alterations in the lungs of
mice. It was also demonstrated that treatment with curcumin at 100
mg/kg BW suppressed tobacco smoke-induced activation of JNK, ERK1/2
and p38 MAPK pathways, in addition to AP-1 activation. Thus, the
results of the present study indicate that the curcumin-mediated
protective effect against tobacco smoke-induced pulmonary EMT
changes may occur via MAPK/AP-1 pathways.
In conclusion, the present study demonstrated that
curcumin treatment effectively attenuated tobacco smoke-induced
MAPK activation and EMT changes in lung tissue. These data may
provide novel insights into the molecular pathogenesis and
chemoprevention of tobacco smoke-associated lung cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81602883,
81373005 and 81072330), the National Basic Research Program of
China (973 Program; grant no. 2013CB910303) and the China
Postdoctoral Science Foundation Funded Project (grant no.
2016M591792).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
3
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar :
|
|
4
|
Agudo A, Bonet C, Travier N, González CA,
Vineis P, Bueno-de-Mesquita HB, Trichopoulos D, Boffetta P,
Clavel-Chapelon F, Boutron-Ruault MC, et al: Impact of cigarette
smoking on cancer risk in the European prospective investigation
into cancer and nutrition study. J Clin Oncol. 30:4550–4557. 2012.
View Article : Google Scholar
|
|
5
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans: Tobacco smoke and involuntary
smoking. IARC Monogr Eval Carcinog Risks Hum. 83:1–1438. 2004.
|
|
6
|
Breheny D, Cunningham F, Kilford J, Payne
R, Dillon D and Meredith C: Application of a modified gaseous
exposure system to the in vitro toxicological assessment of tobacco
smoke toxicants. Environ Mol Mutagen. 55:662–672. 2014. View Article : Google Scholar
|
|
7
|
Li LF, Chan RL, Lu L, Shen J, Zhang L, Wu
WK, Wang L, Hu T, Li MX and Cho CH: Cigarette smoking and
gastrointestinal diseases: The causal relationship and underlying
molecular mechanisms (Review). Int J Mol Med. 34:372–380. 2014.
View Article : Google Scholar
|
|
8
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View
Article : Google Scholar :
|
|
9
|
Nieto MA: Epithelial plasticity: A common
theme in embryonic and cancer cells. Science. 342:12348502013.
View Article : Google Scholar
|
|
10
|
Rhim AD: Epithelial to mesenchymal
transition and the generation of stem-like cells in pancreatic
cancer. Pancreatology. 13:114–117. 2013. View Article : Google Scholar :
|
|
11
|
Pinto CA, Widodo E, Waltham M and Thompson
EW: Breast cancer stem cells and epithelial mesenchymal
plasticity-implications for chemoresistance. Cancer Lett.
341:56–62. 2013. View Article : Google Scholar
|
|
12
|
Wang Q, Wang Y, Zhang Y, Zhang Y and Xiao
W: Involvement of urokinase in cigarette smoke extract-induced
epithelial-mesenchymal transition in human small airway epithelial
cells. Lab Invest. 95:469–479. 2015. View Article : Google Scholar
|
|
13
|
Eurlings IM, Reynaert NL, van den Beucken
T, Gosker HR, de Theije CC, Verhamme FM, Bracke KR, Wouters EF and
Dentener MA: Cigarette smoke extract induces a phenotypic shift in
epithelial cells; involvement of HIF1α in mesenchymal transition.
PLoS One. 9:e1077572014. View Article : Google Scholar :
|
|
14
|
Jalmi SK and Sinha AK: ROS mediated MAPK
signaling in abiotic and biotic stress-striking similarities and
differences. Front Plant Sci. 6:7692015. View Article : Google Scholar :
|
|
15
|
Khavari TA and Rinn J: Ras/Erk MAPK
signaling in epidermal homeostasis and neoplasia. Cell Cycle.
6:2928–2931. 2007. View Article : Google Scholar
|
|
16
|
Wang J, Li JZ, Lu AX, Zhang KF and Li BJ:
Anticancer effect of salidroside on A549 lung cancer cells through
inhibition of oxidative stress and phospho-p38 expression. Oncol
Lett. 7:1159–1164. 2014.
|
|
17
|
Li T, Zhang C, Ding Y, Zhai W, Liu K, Bu
F, Tu T, Sun L, Zhu W, Zhou F, et al: Umbilical cord-derived
mesenchymal stem cells promote proliferation and migration in MCF-7
and MDA-MB-231 breast cancer cells through activation of the ERK
pathway. Oncol Rep. 34:1469–1477. 2015. View Article : Google Scholar
|
|
18
|
Liang Z, Xie W, Wu R, Geng H, Zhao L, Xie
C, Li X, Zhu M, Zhu W, Zhu J, et al: Inhibition of tobacco
smoke-induced bladder MAPK activation and epithelial-mesenchymal
transition in mice by curcumin. Int J Clin Exp Pathol. 8:4503–4513.
2015.
|
|
19
|
Liang Z, Wu R, Xie W, Geng H, Zhao L, Xie
C, Wu J, Geng S, Li X, Zhu M, et al: Curcumin suppresses MAPK
pathways to reverse tobacco smoke-induced gastric
epithelial-mesenchymal transition in mice. Phytother Res.
29:1665–1671. 2015. View
Article : Google Scholar
|
|
20
|
Liang Z, Xie W, Wu R, Geng H, Zhao L, Xie
C, Li X, Huang C, Zhu J, Zhu M, et al: ERK5 negatively regulates
tobacco smoke-induced pulmonary epithelial-mesenchymal transition.
Oncotarget. 6:19605–19618. 2015. View Article : Google Scholar :
|
|
21
|
Lu L, Chen J, Tang H, Bai L, Lu C, Wang K,
Li M, Yan Y, Tang L, Wu R, et al: EGCG suppresses ERK5 activation
to reverse tobacco smoke-triggered gastric epithelial-mesenchymal
transition in BALB/c mice. Nutrients. 8:pii: E380. 2016. View Article : Google Scholar
|
|
22
|
Toda S, Miyase T, Arichi H, Tanizawa H and
Takino Y: Natural antioxidants. III. Antioxidative components
isolated from rhizome of Curcuma longa L. Chem Pharm Bull
(Tokyo). 33:1725–1728. 1985. View Article : Google Scholar
|
|
23
|
Satoskar RR, Shah SJ and Shenoy SG:
Evaluation of anti-inflammatory property of curcumin (diferuloyl
methane) in patients with postoperative inflammation. Int J Clin
Pharmacol Ther Toxicol. 24:651–654. 1986.
|
|
24
|
Sidhu GS, Singh AK, Thaloor D, Banaudha
KK, Patnaik GK, Srimal RC and Maheshwari RK: Enhancement of wound
healing by curcumin in animals. Wound Repair Regen. 6:167–177.
1998. View Article : Google Scholar
|
|
25
|
Negi PS, Jayaprakasha GK, Rao Jagan Mohan
L and Sakariah KK: Antibacterial activity of turmeric oil: A
byproduct from curcumin manufacture. J Agric Food Chem.
47:4297–4300. 1999. View Article : Google Scholar
|
|
26
|
Aggarwal BB, Sundaram C, Malani N and
Ichikawa H: Curcumin: The indian solid gold. Adv Exp Med Biol.
595:1–75. 2007. View Article : Google Scholar
|
|
27
|
Saha S, Adhikary A, Bhattacharyya P, Das T
and Sa G: Death by design: Where curcumin sensitizes drug-resistant
tumours. Anticancer Res. 32:2567–2584. 2012.
|
|
28
|
Aggarwal BB and Harikumar KB: Potential
therapeutic effects of curcumin, the anti-inflammatory agent,
against neurodegenerative, cardiovascular, pulmonary, metabolic,
autoimmune and neoplastic diseases. Int J Biochem Cell Biol.
41:40–59. 2009. View Article : Google Scholar
|
|
29
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: A short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar
|
|
30
|
Gonçalves Vde P, Ortega AA, Guimarães MR,
Curylofo FA, Junior Rossa C, Ribeiro DA and Spolidorio LC:
Chemopreventive activity of systemically administered curcumin on
oral cancer in the 4-nitroquinoline 1-oxide model. J Cell Biochem.
116:787–796. 2015. View Article : Google Scholar
|
|
31
|
Okamoto Y, Pehlivan D, Wiszniewski W, Beck
CR, Snipes GJ, Lupski JR and Khajavi M: Curcumin facilitates a
transitory cellular stress response in Trembler-J mice. Hum Mol
Genet. 22:4698–4705. 2013. View Article : Google Scholar :
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Chen Z, Peto R, Zhou M, Iona A, Smith M,
Yang L, Guo Y, Chen Y, Bian Z, Lancaster G, et al: Contrasting male
and female trends in tobacco-attributed mortality in China:
Evidence from successive nationwide prospective cohort studies.
Lancet. 386:1447–1456. 2015. View Article : Google Scholar :
|
|
34
|
Pandeya N, Wilson LF, Bain CJ, Martin KL,
Webb PM and Whiteman DC: Cancers in Australia in 2010 attributable
to tobacco smoke. Aust N Z J Public Health. 39:464–470. 2015.
View Article : Google Scholar :
|
|
35
|
Yue H, Yun Y, Gao R, Li G and Sang N:
Winter polycyclic aromatic hydrocarbon-bound particulate matter
from peri-urban North China promotes lung cancer cell metastasis.
Environ Sci Technol. 49:14484–14493. 2015. View Article : Google Scholar
|
|
36
|
Yeo CD, Kim JW, Ha JH, Kim SJ, Lee SH, Kim
IK and Kim YK: Chemopreventive effect of phosphodieasterase-4
inhibition in benzo (a)pyrene-induced murine lung cancer model. Exp
Lung Res. 40:500–506. 2014. View Article : Google Scholar
|
|
37
|
Luo F, Ji J, Liu Y, Xu Y, Zheng G, Jing J,
Wang B, Xu W, Shi L, Lu X and Liu Q: MicroRNA-21, up-regulated by
arsenite, directs the epithelial-mesenchymal transition and
enhances the invasive potential of transformed human bronchial
epithelial cells by targeting PDCD4. Toxicol Lett. 232:301–309.
2015. View Article : Google Scholar
|
|
38
|
Chen ZJ, Yang XL, Liu H, Wei W, Zhang KS,
Huang HB, Giesy JP, Liu HL, Du J and Wang HS: Bisphenol A modulates
colorectal cancer protein profile and promotes the metastasis via
induction of epithelial to mesenchymal transitions. Arch Toxicol.
89:1371–1381. 2015. View Article : Google Scholar
|
|
39
|
Mennecier G, Torres LN, Cogliati B,
Sanches DS, Mori CM, Latorre AO, Chaible LM, Mackowiak II, Nagamine
MK, Da Silva TC, et al: Chronic exposure of lung alveolar
epithelial type II cells to tobacco-specific carcinogen NNK results
in malignant transformation: A new in vitro lung carcinogenesis
model. Mol Carcinog. 53:392–402. 2014. View
Article : Google Scholar
|
|
40
|
Masliah-Planchon J, Garinet S and Pasmant
E: RAS-MAPK pathway epigenetic activation in cancer: miRNAs in
action. Oncotarget. 7:38892–38907. 2016. View Article : Google Scholar
|
|
41
|
Cellurale C, Sabio G, Kennedy NJ, Das M,
Barlow M, Sandy P, Jacks T and Davis RJ: Requirement of c-Jun NH
(2)-terminal kinase for Ras-initiated tumor formation. Mol Cell
Biol. 31:1565–1576. 2011. View Article : Google Scholar :
|
|
42
|
Patel PB, Thakkar VR and Patel JS:
Cellular effect of curcumin and citral combination on breast cancer
cells: Induction of apoptosis and cell cycle arrest. J Breast
Cancer. 18:225–234. 2015. View Article : Google Scholar :
|
|
43
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
44
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: An ‘old-age’
disease with an ‘age-old’ solution. Cancer Lett. 267:133–164. 2008.
View Article : Google Scholar
|
|
45
|
Sun LN, Chen ZX, Liu XC, Liu HY, Guan GJ
and Liu G: Curcumin ameliorates epithelial-to-mesenchymal
transition of podocytes in vivo and in vitro via regulating
caveolin-1. Biomed Pharmacother. 68:1079–1088. 2014. View Article : Google Scholar
|
|
46
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar
|