Introduction
Hepatocellular carcinoma (HCC) is the most common
cancer worldwide, and it is a major cause of cancer-associated
mortality (1,2). HCC occurs due to multifactorial
causes such as smoking, alcohol, human hepatitis virus, fatigue and
obesity (3,4). The current therapy for HCC includes
surgery, chemotherapy and radiotherapy. However, the therapeutic
outcomes are unsatisfactory (3).
Cancer cells need an energy source such as glucose and lipids for
proliferation and division, and then, they spread to another part
of the body from the primary site (5). A previous study reported that
bioactive compounds from natural plant extracts play a crucial role
as potential therapeutic agents (6). Many plant extracts induce anticancer
effects such as anti-proliferative and anti-metastatic effects and
cell cycle arrest in various cancer cell lines (7–9).
Moreover, the anticancer activity of natural plant extracts has
been investigated in HepG2 and Hep3B hepatocellular carcinoma cells
(3,10).
Protein kinase B (PKB, also known as Akt) plays a
significant role in various cellular functions such as cell
survival, proliferation and metabolism (11). The phosphorylation of Akt at serine
473 is known to regulate Glycogen Synthase kinase-3β (GSK-3β)
inactivation through phosphorylation of GSK-3β at serine 9
(12). GSK-3β is one of the two
GSK-3 isoforms, and it regulates cell growth, differentiation and
cell survival (13). Inactive
GSK-3β induces MDM2-mediated p53 ubiquitination and degradation
(13). Tumor protein p53 (p53)
induces cell cycle arrest and apoptosis through transcriptional
regulation of p21Cip1/Waf1, Mouse double minute 2
homolog (MDM2) and Bcl-2-associated X protein (Bax) genes, and also
regulates the activation of Bak and caspase-3 (14,15).
The p21 protein, one of the cyclin-dependent kinases (CDKs), can
arrest cell cycle at G1 phase through inhibition of cyclin E-CDK2
complex (16). However, previous
studies have suggested that p21 can induce apoptosis and cell cycle
arrest via p53-dependent and p53-independent signaling pathways
(16–18).
Cnidium monnieri (L.) Cusson is a well-known
Chinese medicinal herb that is commonly used for treating
gynecological diseases, carbuncles, ringworm and nephritis
(19–21). According to previous study, the
ethanol extracts from C. monnieri contains several bioactive
compounds such as osthole and xanthotoxol (22). In the present study, we
investigated wether ethanol extracts from C. monnieri (CME)
have an influence on cell cycle arrest and apoptosis in
hepatocellular carcinoma cells. Furthermore, to determine whether
CME-induced cell cycle arrest and apoptosis occurred via a
p53-dependent or p53-independent mechanism, we confirmed the
regulation of Akt/GSK-3β and p53 signaling pathway by CME in HepG2
(wild-type p53) and Hep3B (p53-null) hepatocellular carcinoma
cells.
Materials and methods
Reagent
Cnidium monnieri (L.) Cusson were purchased
from Dong Kyung PHARM Co., Ltd. (Seoul, Korea). The 100 g of CME
was soaked in 800 ml of 99.9% ethanol, and then stirring for 48 h
at room temperature. The extract was filtered through filter paper
(qualitative filter paper no. 1; Toyo Roshi Kaisha, Ltd., Tokyo,
Japan) and concentrated with a rotary evaporator to remove the
ethanol. The ethanol extracts of Cnidium monnieri (L.)
Cusson (CME) was dissolved in dimethyl sulfoxide (DMSO; stock
solution, 10–100 mg/ml) and refrigerated at −20°C for long storage.
The final concentration of CME in the culture medium was controlled
at 10–100 µg/ml. LY294002 (PI3K/Akt inhibitor) was purchased from
Calbiochem (EMD Millipore, Billerica, MA, USA) and Pifithrin-α (p53
inhibitor) was purchased from Sigma-Aldrich (St Louis, MO,
USA).
Cell culture
The human hepatocellular carcinoma cell lines HepG2
and Hep3B were obtained from the American Type Culture Collection
(ATCC, Rockville, MD, USA). The cells were grown in DMEM medium
(Hyclone, Laboratories Inc., Logan, UT, USA) containing 10% fetal
bovine serum and 1% antibiotics (both Hyclone Laboratories Inc.) at
37°C in a 5% CO2 atmosphere. The cells were suspended by
Trypsin-EDTA (Hyclone, Laboratories Inc.) and separated at
1×106 cells/ml per 100-mm plate, every 48 h.
Determination of cell viability using
MTT assay
The cells were seeded at 1×104 cells/ml
in 12-well plate and incubated for 24 h. Following incubation, the
cells were treated with the CME (10–100 µg/ml) for 24 or 48 h at
37°C in a 5% CO2 atmosphere. The inhibitor was
pre-treated for 30 min before treating CME. The respective medium
was removed, and cells were incubated with 20 µl of MTT solution (5
mg/ml) in phosphate-buffered saline (PBS) for 1 h. Converted purple
formazan from MTT was solubilized in dimethyl sulfoxide (DMSO). The
absorbance of the solution in each well was determined using a
microplate reader (model 680, Bio-Rad Laboratories, Inc., Tokyo,
Japan) at 595 nm.
Measurement of cell cycle arrest
The cells were seeded at 1×106 cells/ml
in 60-mm plate and incubated for 24 h. Following incubation, the
cells were treated with the CME for 24 h at 37°C in a 5%
CO2 atmosphere. The inhibitor was pre-treated for 30 min
before treating CME. Total cells were harvested by trypsinization,
collected by centrifugation, washed with 3 ml of PBS (twice). The
supernatant was removed and discarded. The pellet were resuspended
in 1 ml of cold 70% ethanol and freezed at −20°C for at least 3 h.
Ethanol-fixed cells were centrifuged at 800 × g for 5 min and
washed with 1 ml of PBS. The supernatant was removed, and
ethanol-fixed cells were resuspended 500 µl of PBS. The cells were
stained with 4 µl of PI (5 mg/ml) and 10 µl of RNase (10 mg/ml) for
20 min at room temperature. Fluorescence intensity was analyzed
using a Flow cytometry-FACS Canto (Becton-Dickinson Biosciences,
Drive Frankline Lage, NJ, USA).
Identification of apoptosis by Hoechst
33342
The cells were seeded at 1×104 cells/ml
in 12-well plate and incubated for 24 h after put microscope cover
glass (Marlenfeld GmbH & Co., Lauda-Königshofen, Germany) into
well. Following incubation, the cells were treated with the CME
(20, 40, 60 µg/ml) for 24 h at 37°C in a 5% CO2
atmosphere. After 24 h, the cells were treated with 0.7 µM Hoechst
33342 and incubated for 30 min. Cells were fixed with 3.5%
formaldehyde 500 µl for 20 min and then were gently washed thrice
with 150 µl of PBS for 5 min. Placed 10 µl of the mounting solution
(50% glycerol) on a slide glass and covered with a cover glass. The
chromatin was observed using fluorescence microscope
(magnification, ×200; Axioskop 50, Carl Zeiss, Thornwood, NY,
USA).
Western blot analysis
The cells were seeded at 1×105 cells/ml
in 6-well plate and incubated for 24 h. Then, the cells were
treated with the concentration of CME for 24 h at 37°C in a 5%
CO2 atmosphere. The inhibitor was pre-treated for 30 min
before treating CME. The cells were then rinsed twice with ice-cold
PBS and scraped with RIPA lysis buffer (50 mM Tris-HCl pH 8.0, 150
mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mM PMSF) and
subjected to western blot analysis. Protein quantification was
performed by Bradford assay. 30 µg of protein was loaded per lane.
The Nitrocellulose membranes (0.45 µm; cat. no. 10600003; GE
Healthcare Life Science, Freiburg, Germany) were blocked with 2%
bovine serum albumin (BSA, Bovogen, Melbourne, Australia) in 1X
TBST (24.7 mM Tris-HCl, pH 8.0, 137 mM NaCl, 0.05% Tween-20) for 1
h 30 min and incubated overnight at 4°C with primary antibodies
targeting mouse monoclonal-p-Akt (Ser473) (1:2,000; cat. no. 4051),
rabbit monoclonal-Akt (1:1,000; cat. no. 4685), rabbit
monoclonal-GSK-3β (1:1,000; cat. no. 9315), rabbit monoclonal-Bax
(1:1,000; cat. no. 5023), rabbit monoclonal-Bak (1:1,000; cat. no.
6947), rabbit monoclonal-caspase-3 (1:1,000; cat. no. 9665), rabbit
polyclonal-Bcl-2 (1:2,000; cat. no. 2876), and rabbit
polyclonal-β-actin (1:2,000; cat. no. 4967); all purchased from
Cell Signaling Technology, Inc. (Beverly, MA, USA) and rabbit
polyclonal-p-MDM2 (Ser166) (1:3,000; cat. no. ab131355), rabbit
monoclonal-MDM2 (1:3,000; cat. no. ab178938), rabbit monoclonal-p21
(1:3,000; cat. no. ab109520), mouse monoclonal-cyclin E (1:3,000;
cat. no. ab3927), rabbit monoclonal-p-cdk2 (Tyr15) (1:3,000; cat.
no. ab76146), and rabbit monoclonal-p-cdk2 (Thr14) (1:3,000; cat.
no. ab68265); all purchased from Abcam Inc. (Cambridge, MA, USA)
and rabbit polyclonal-p-GSK-3β (Ser9) (1:1,000; cat. no.
sc-11757-R), and mouse monoclonal-p53 (1:1,000; cat. no. sc-126)
were purchased from Santa Cruz Inc. (Santa Cruz, CA, USA). After
primary antibody incubation, the membranes were washed 4 times for
5 min each with 1X TBST at room temperature. Following the addition
of the secondary antibody; goat polyclonal-anti-mouse antibody
conjugated with HRP (1:10,000; cat. no. PA1-30126; Thermo
Scientific Rockford, IL, USA) and goat anti-rabbit antibody
conjugated with HRP (1:10,000; cat. no. 166-2408; Bio-Rad
Laboratories, Inc., Tokyo, Japan), the membrane were reacted for 1
h 30 min at room temperature with gentle agitation. After secondary
antibody incubation, the membranes were washed 4 times for 10 min
each with 1X TBST at room temperature. Proteins were detected using
SuperSignal West Pico Chemiluminescent Substrate (cat. no. PI34080;
Thermo Scientific Rockford, IL, USA) and visualized on CP-BU new
X-ray film (Agfa HealthCare, Inc., Mortsel, Belgium).
Statistics
MTT assay were statistically analyzed using an
unpaired an independent sample t-test (IBM SPSS Statistics 20.0,
SPSS Inc., Chicago, IL, USA). A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
CME treatment suppresses cell
proliferation and induces cell morphology change in HepG2
cells
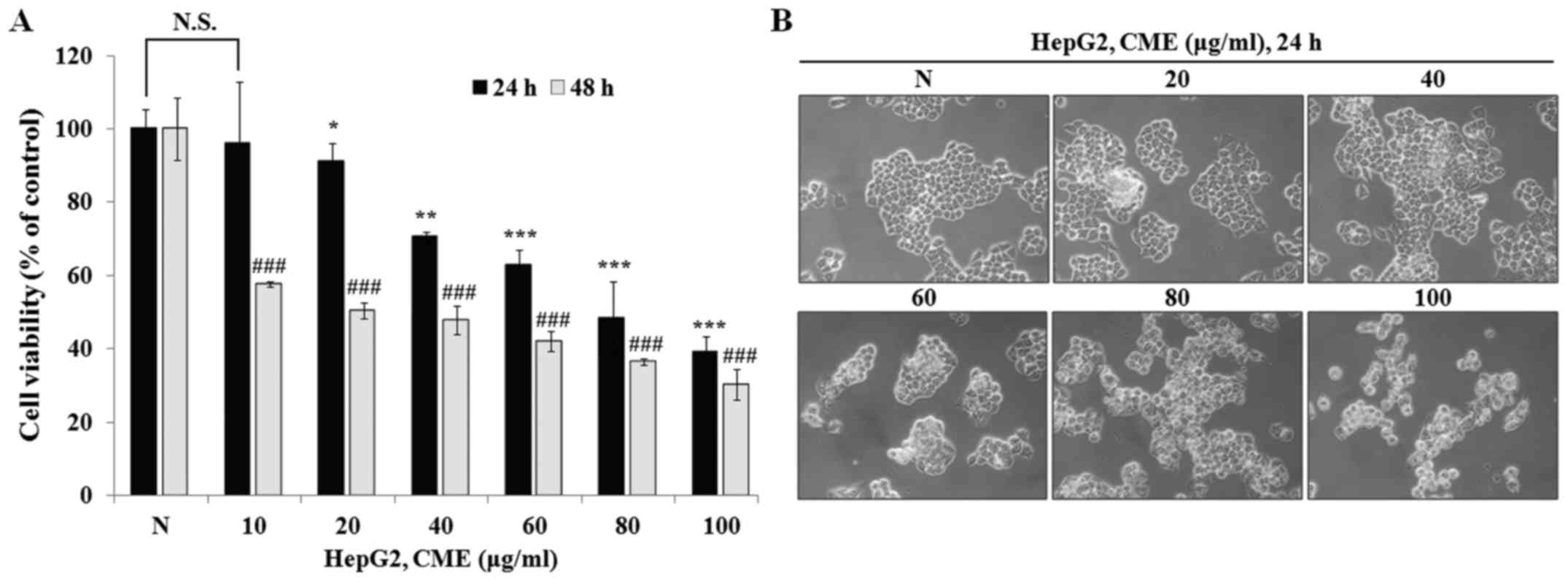
To determine the anti-proliferative effect of CME,
we performed MTT assay after treatment with various concentrations
of CME (10–100 µg/ml) for 24 and 48 h in HepG2 cells. As shown in
Fig. 1A, CME inhibited cell
proliferation in a dose-dependent manner (IC50 value=88.76 µg/ml in
24 h and 30.93 µg/ml in 48 h). Also, we observed the cell
morphology change after treatment with CME for 24 h. As shown in
Fig. 1B, cell morphology was
changed by CME in a dose-dependent manner. Cell shrinkage and loss
of proliferation potential were increased when cells were treated
with CME (20–100 µg/ml). Furthermore, we confirmed that the number
of cells that were detached from the cell culture plate was
increased. These characteristics were observed during the process
of apoptosis. These results indicated that CME has
anti-proliferative and cell morphology change-inducing effects in
HepG2 hepatoma cells.
CME treatment induces apoptosis in
HepG2 cells
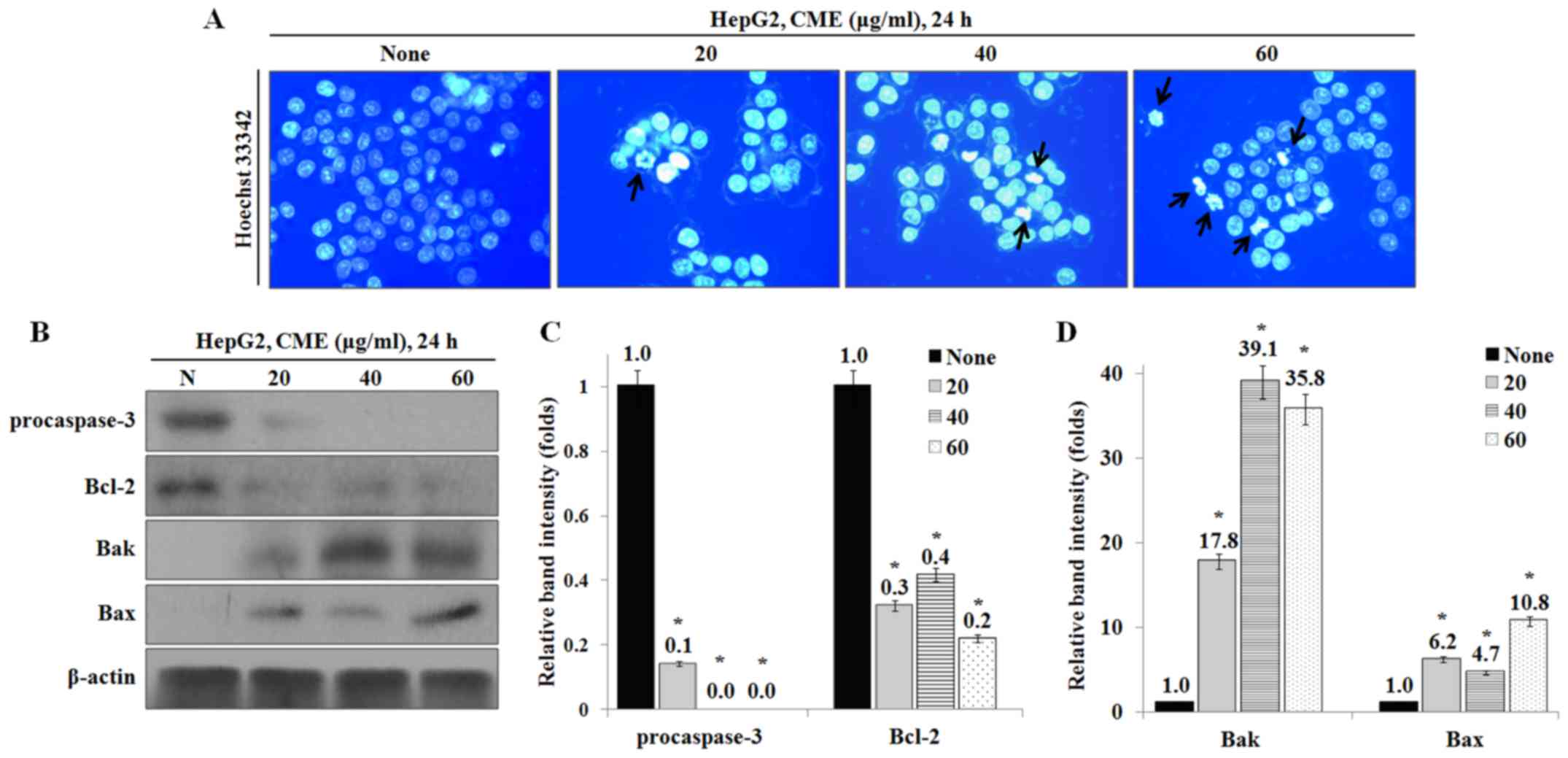
To identify whether CME-induced effects on cell
growth and morphology were caused by apoptosis, we performed
Hoechst 33342 staining and western blot analysis. As a result, the
apoptotic DNA fragmentation was increased by CME in a
dose-dependent manner (Fig. 2A).
Also, the expression levels of apoptosis-associated proteins, Bax
and Bak, were increased and the levels of procaspase-3 and
anti-apoptotic protein Bcl-2 were decreased by CME (Fig. 2B-D).
CME treatment induces cell cycle
arrest at G1 phase
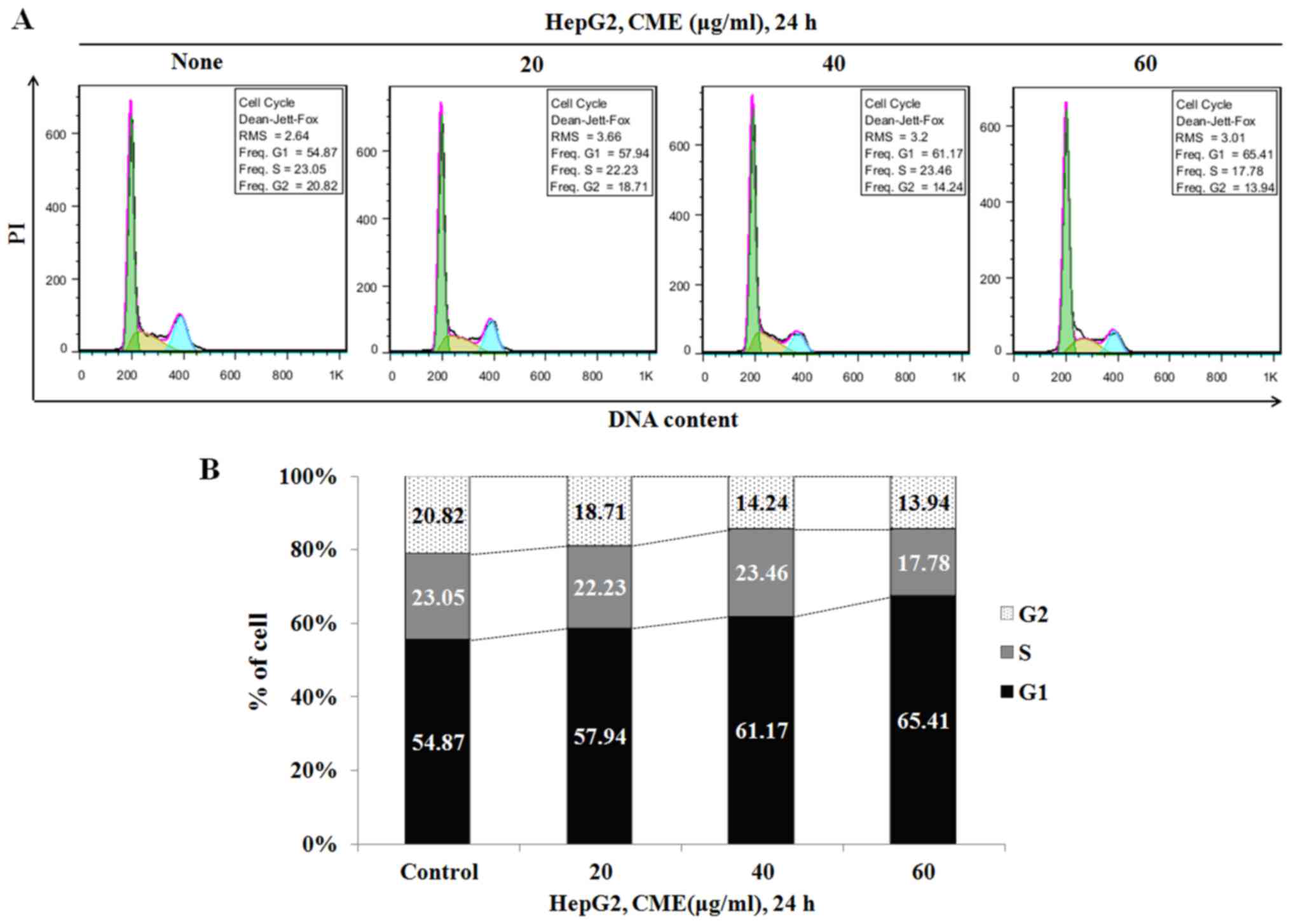
To confirm whether the reduced cell viability was
due to cell cycle arrest, we analyzed the cell cycle after
treatment with various concentrations of CME (20–60 µg/ml) for 6 h.
As indicated in Fig. 3, the
percentage of G1 phase in HepG2 cells was increased to 54.87%
(control group), 57.94% (20 µg/ml), 61.17% (40 µg/ml) and 65.41%
(60 µg/ml).
CME treatment regulates the expression
levels of cell cycle-related proteins in HepG2 cells
We examined the effect of CME on the expression
levels of cell cycle-related proteins by CME using western blot
analysis. The results showed that the expression levels of p-Akt,
p-GSK-3β, p-MDM2 and cyclin E were decreased, while the levels of
p53, p21, p-CDK2 (T14) and p-CDK2 (Y15) were increased in a
dose-dependent manner (Fig.
4).
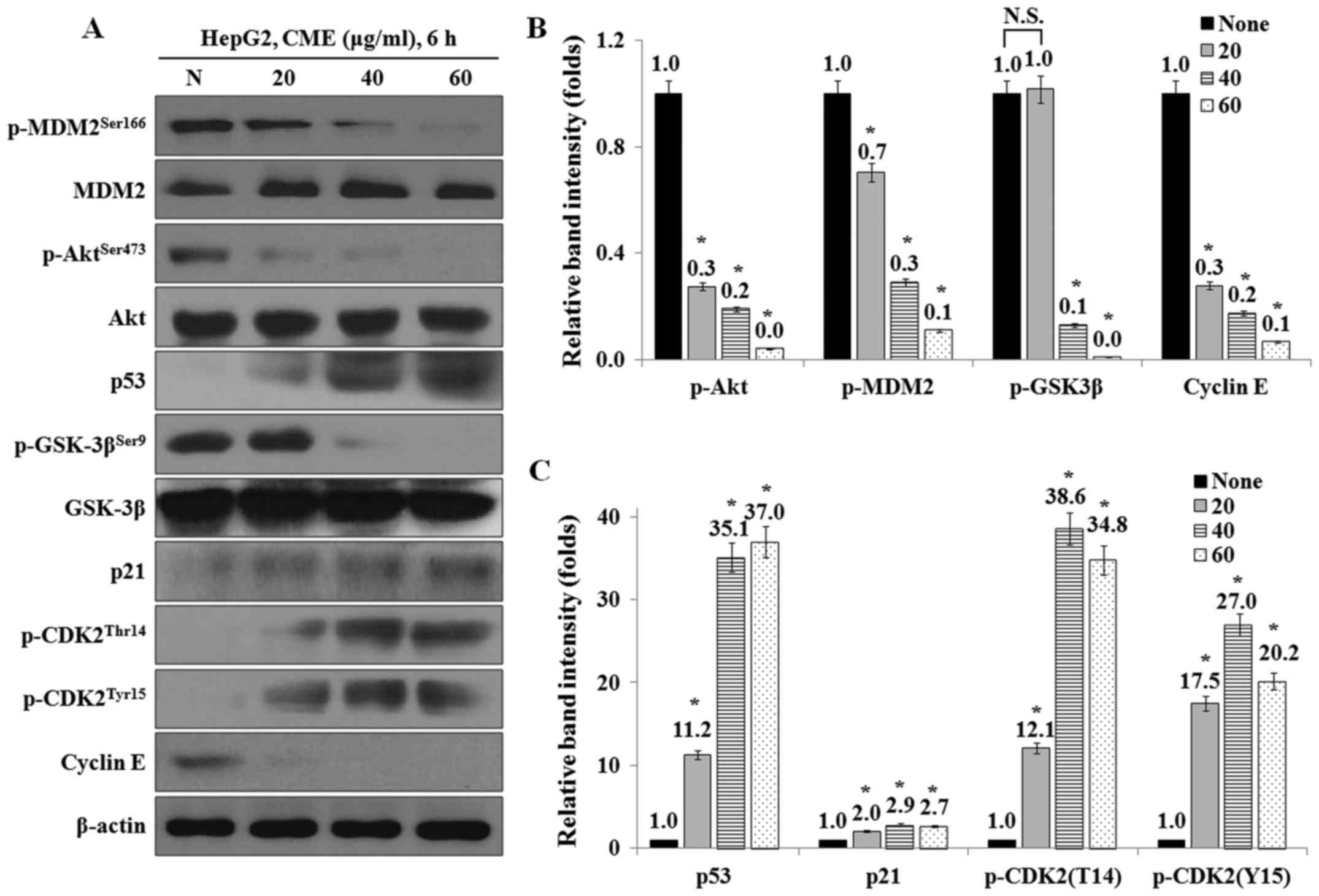 | Figure 4.CME treatment regulates the expression
levels of cell cycle-mediated proteins in HepG2 cells. (A) CME
effects on p-Akt, p-GSK-3β, p-MDM2, p53, p21, p-CDK2 (Thr14),
p-CDK2 (Tyr15) and cyclin E. Cells were treated 20–60 µg/ml of CME
for 6 h. Protein levels were determined by Western blot analysis.
The β-actin probe served as protein-loading control. (B and C)
Relative band intensity of cell cycle-mediated proteins. The
statistical analysis of the data was carried out by use of an
independent sample t-test. *P<0.001 vs. con (each experiment,
n=3). CME, Cnidium monnieri (L.) Cusson extract; p,
phosphorylated; Akt, protein kinase B; GSK-3β, glycogen synthase
kinase-3β; MDM2, Mouse double minute 2 homolog; p53, Tumor protein
p53; p21, cyclin-dependent kinase inhibitor 1; CDK2,
cyclin-dependent kinase 2. |
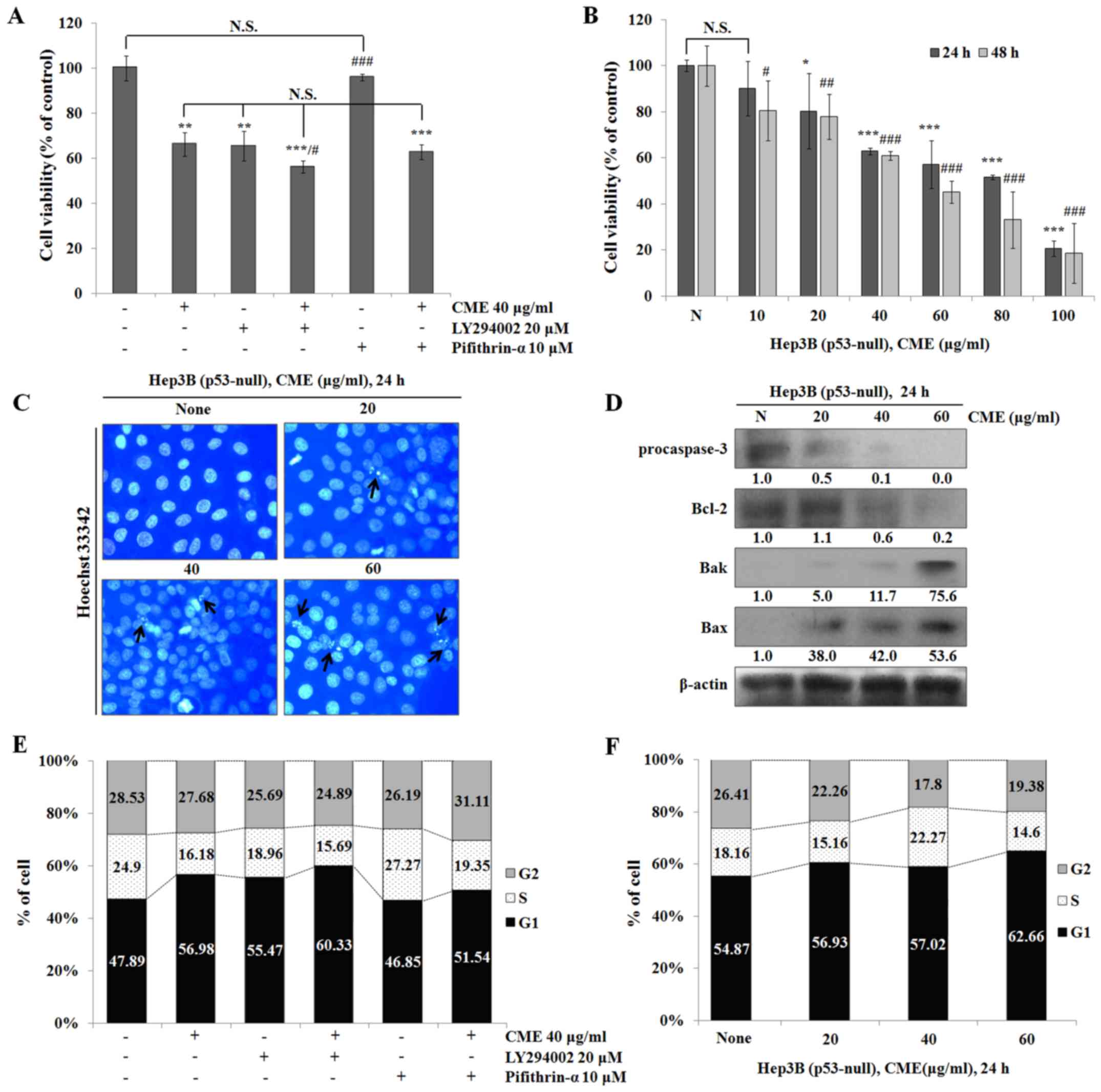
CME-induced apoptosis and cell cycle arrest were
occurred through regulation of Akt/GSK-3β signaling pathway in a
p53-independent manner. To determine the association between
CME-induced apoptosis and cell cycle arrest and the Akt/p53
signaling pathway, we used specific inhibitors such as LY294002
(PI3K/Akt inhibitor) and pifithrin-α (p53 inhibitor). We co-treated
the cells with LY294002 or pifithrin-α and CME and performed the
MTT assay. As shown in Fig. 5A,
cell viabilities in the CME-treated group and the LY294002-treated
group were decreased compared to that in the control group. Also,
the cell viability in the CME/LY294002 co-treated group was
decreased more than that in the CME-treated group. Furthermore,
cell viabilities in the CME/pifithrin-α co-treated group and the
CME-treated group did not differ. To confirm whether CME-induced
anti-proliferative and apoptotic effects occur in a p53-independent
manner, we performed the MTT assay, Hoechst 33342 staining and
western blot analysis in Hep3B (p53-null) cells. As a result, CME
suppressed Hep3B cell proliferation (Fig. 5B) (IC50 value=64.46 µg/ml in 24 h
and 46.32 µg/ml in 48 h), and induced apoptotic DNA fragmentation
in a dose-dependent manner (Fig.
5C). Also, the expression levels of apoptosis-associated
proteins were regulated by CME in Hep3B cells (Fig. 5D). In the cell cycle analysis,
treatment with CME or LY294002 induced cell cycle arrest at G1
phase and co-treatment with CME/LY294002 and CME/pifithrin-α also
induced G1 arrest in HepG2 cells (Fig.
5E). Moreover, the percentage of G1 phase was increased by CME
in Hep3B cells (Fig. 5F). These
results supported the claim that CME induces apoptosis and G1 cell
cycle arrest in a p53-independent manner.
CME treatment induces G1 arrest
through regulation of Akt/GSK-3β signaling pathway and increase of
p21 expression in a p53-independent manner
To investigate whether the CME-induced cell cycle
arrest occurred through p53-independent pathway, we performed
western blot analysis after treatment with LY294002 (PI3K/Akt
inhibitor) and pifithrin-α (p53 inhibitor). As a result, the
expression levels of p-Akt, p-GSK-3β, p-MDM2 and cyclin E were
decreased in the CME-treated group compared to the control group
and the expression levels in the CME/LY294002 co-treated group were
also decreased (Fig. 6A).
Especially, when we co-treated cells with CME and pifithrin-α, the
expression levels of p-Akt, p-GSK-3β, p-MDM2 and cyclin E were
decreased and the expression levels of p53, p21, p-CDK (T14) and
p-CDK (Y15) were increased by CME although pifithrin-α-treatment
was performed. Additionally, the expression levels of cell
cycle-related proteins were regulated by CME in Hep3B (p53-null)
cells (Fig. 6B).
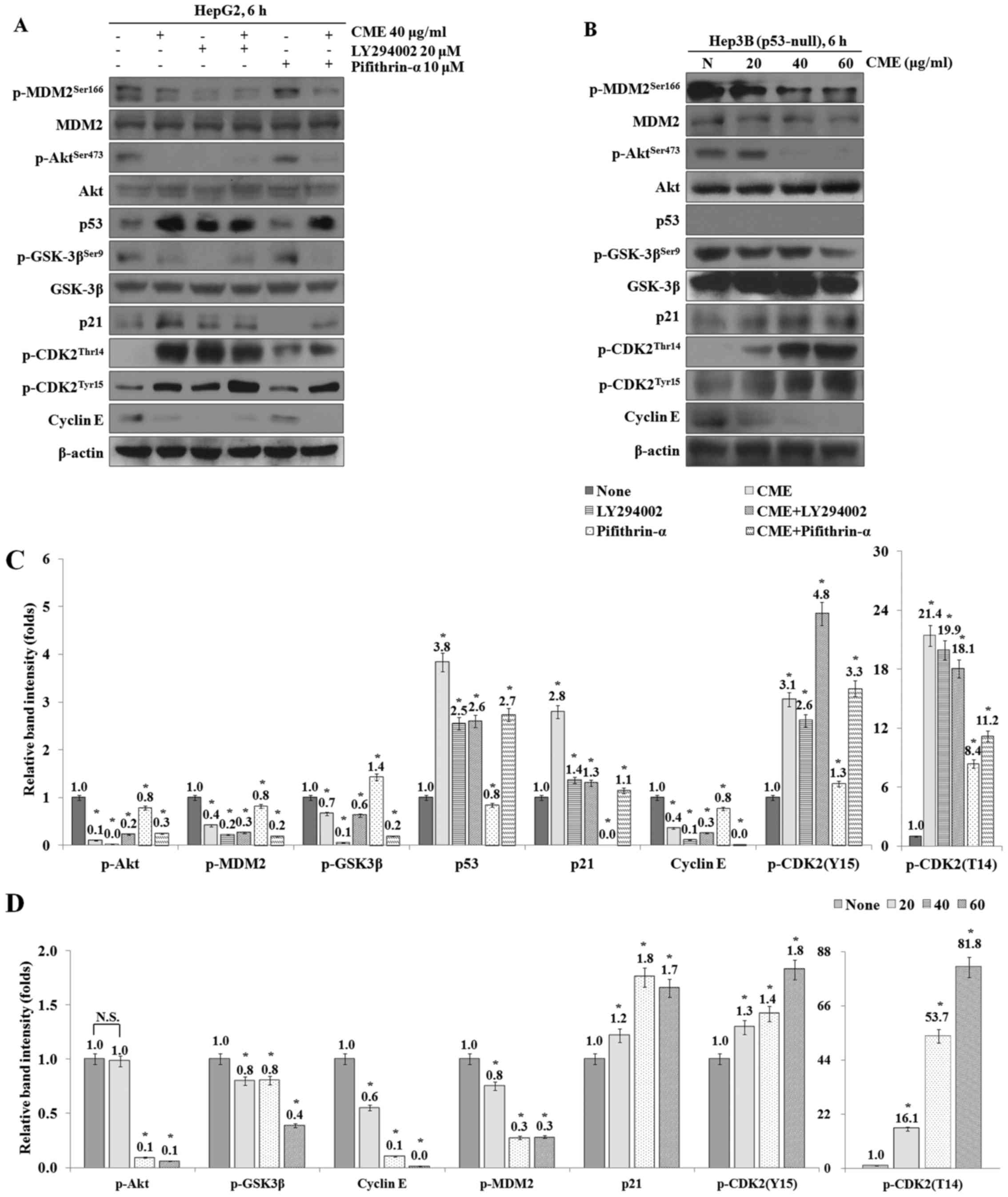 | Figure 6.CME induces G1 arrest through
regulation of Akt/GSK-3β signaling pathway in p53-independent
manner. (A) Co-treatment of LY294002 or Pifithrin-α with CME
regulates cell cycle-mediated proteins in HepG2 cells. (B) Cells
were treated with variable concentrations of CME (40–60 µg/ml) for
6 h in Hep3B cells. Protein levels were determined by western blot
analysis. The β-actin probe served as protein-loading control. (C
and D) Relative band intensity of cell cycle-mediated proteins in
HepG2 (C) and Hep3B (D) cells. The statistical analysis of the data
was carried out by use of an independent sample t-test. *P<0.001
vs. con (each experiment, n=3). NS, not significant; CME,
Cnidium monnieri (L.) Cusson extract; p, phosphorylated;
Akt, protein kinase B; GSK-3β, glycogen synthase kinase-3β; MDM2,
Mouse double minute 2 homolog; p53, tumor protein p53; p21,
cyclin-dependent kinase inhibitor 1; CDK2, cyclin-dependent kinase
2. |
Discussion
Chinese medicinal herbs have been used for the
treatment of various diseases in China and Southeast Asia (23). Also, many studies have focused on
the anticancer effects of phytochemicals which is contained in
natural plant (3,7–10).
In the present study, we examined whether ethanol extracts from C.
monnieri (CME)-induced apoptotic and cell cycle arrest
effects were occurred by p53-dependent or p53-independent mechanism
in HepG2 (wild-type p53) and Hep3B (p53-null) hepatocellular
carcinoma cells.
Preferentially, we confirmed the anti-proliferative
effect and cell morphological change induced by CME through the MTT
assay and observation of cell morphology. HepG2 cell viability was
decreased by CME in a dose- and time-dependent manner (Fig. 1A). Also, when we treated the cells
with CME, the cell shrinkage and DNA fragmentation were increased
and cell density was decreased in a dose-dependent manner (Fig. 1B). To determine whether the
anti-proliferative effect and these characteristics occurred due to
apoptosis and cell cycle arrest, we performed Hoechst 33342
staining and cell cycle analysis. Furthermore, we examined the
expression levels of apoptosis and cell cycle arrest-associated
proteins. In a previous study, the number of apoptotic bodies was
increased by natural plant extracts in a dose-dependent manner
(5,6,24).
Our results showed that CME induces an increase in the number of
apoptotic bodies in a dose-dependent manner (Fig. 2A). The Bcl-2 family, including
Bcl-2, Bax and Bak, plays an essential role in
mitochondria-dependent apoptosis (25). Bax and Bak activation induces
caspase-3 activity through formation of mitochondrial membrane pore
and apoptosome (26). In our
study, we confirmed that CME treatment inhibited the expression of
Bcl-2 and pro-caspase-3 and induced the expression of Bax and Bak
proteins (Fig. 2B-D).
Additionally, we determined the cell cycle arrest effect of CME by
performing cell cycle analysis. When we treated the cells with CME
(20–60 µg/ml), the number of G1 phase cells was increased in a
dose-dependent manner (Fig. 3).
Akt induces inhibition of GSK-3β through phosphorylation at Ser9
(27). Inhibited form of GSK-3β
regulates p53 ubiquitination by controlling MDM2 phosphorylation
(14,28). Activated p53 was translocated to
the nucleus and then it induced transcription of p21 and Bax
(15). p21 is a major inhibitor of
Cdks which control cell cycle progression (29). Cell cycle at G1 phase is regulated
by the cyclin E/CDK2 complex (16). Therefore, we determined the
expression levels of cell cycle-related proteins by preforming
western blot analysis. As shown in Fig. 4, the protein levels of p-Akt,
p-GSK-3β, p-MDM2 and cyclin E were decreased and the protein levels
of p53, p21, p-CDK2 (Thr14) and p-CDK2 (Tyr15) were increased by
CME treatment.
To confirm whether CME induces apoptosis and cell
cycle arrest via regulating Akt/p53 signaling pathway, we performed
the experiments after pre-treatment with LY294002 (PI3K/Akt
inhibitor) and Pifithrin-α (p53 inhibitor). When we treated the
cells with LY294002, HepG2 cell viability was decreased compared to
that in the control group (Fig.
4A). Also, cell viability in the CME/LY294002 co-treated group
was decreased more than that in the CME-treated group and the
Pifithrin-α-treated group was not significantly different compared
to the control group. However, cell viability in the
CME/Pifithrin-α-co-treated group was decreased similar to that in
the CME-treated group. These results suggested that the CME-induced
anti-proliferative effect occurred in a p53-independent manner.
Therefore, to determine the role of p53 in the CME-induced
anti-proliferative effect, we examined cell viability in Hep3B
(p53-null) cells. The results showed that CME treatment reduces
cell growth in a dose- and time-dependent manner (Fig. 5B).
Additionally, by performing Hoechst 33342 staining
and western blot analysis in Hep3B cells, we determined that CME
induces apoptotic DNA fragmentation and regulates the expression of
apoptotic proteins (Fig. 5C-D). In
the cell cycle analysis, the number of G1 phase cells in the
LY294002-treated group and the CME/LY294002 co-treated group was
increased in HepG2 cells. Moreover, co-treatment with CME and
Pifithrin-α induced G1 cell cycle arrest (Fig. 5E). In Hep3B cells, CME also induced
G1 cell cycle arrest (Fig. 5F).
Our results determined that CME-induced apoptosis and G1 cell cycle
arrest effects occurred in a p53-independent manner.
To investigate the CME-induced G1 cell cycle arrest
which occurred via p53-independent signaling pathway, we identified
the expression of cell cycle regulation proteins after treatment
with CME and LY294002 or Pifithrin-α. The results showed that the
expression levels of p-Akt, p-GSK-3β, p-MDM2 and cyclin E were
decreased and the expression levels of p53, p21, p-CDK2 (Thr14) and
p-CDK2 (Tyr15) were increased in the CME/LY294002 and the
CME/Pifithrin-α co-treated groups (Fig. 6A and C). Furthermore, cell
cycle-related proteins including p-Akt, p-GSK-3β, p-MDM2, p21,
cyclin E, p-CDK2 (Thr14) and p-CDK2 (Tyr15) were regulated except
for p53 protein (Fig. 6B and D).
According to a previous study, GSK-3β phosphorylates MDM2 protein
and overexpression of MDM2 promotes degradation of p21 through
proteasome-mediated degradation in a p53-independent manner
(30,31). Also, many of the studies have
reported that natural extracts induce apoptosis and cell cycle
arrest through controlling the p53-independent signaling pathway
(32–34). Moreover, overexpression of p21
induces the expression of pro-apoptotic protein Bax and modulates
the Bcl-2:Bax ratio in Hep3B cells (35).
In conclusion, CME treatment induces apoptosis and
cell cycle arrest at G1 phase in HepG2 and Hep3B hepatocellular
carcinoma cells. Also, these anticancer effects occurred through
increase of p21 protein expression by Akt/GSK-3β/MDM2 signaling
pathway in a p53-independent manner.
References
|
1
|
Wang Y, Nie H, Zhao X, Qin Y and Gong X:
Bicyclol induces cell cycle arrest and autophagy in HepG2 human
hepatocellular carcinoma cells through the PI3K/AKT and
Ras/Raf/MEK/ERK pathways. BMC Cancer. 16:7422016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y, Tao C, Huang X, He H, Shi H, Zhang
Q and Wu H: Metformin induces apoptosis of human hepatocellular
carcinoma HepG2 cells by activating an AMPK/p53/miR-23a/FOXA1
pathway. Onco Targets Ther. 9:2845–2853. 2016.PubMed/NCBI
|
|
3
|
Hsu SC, Kuo CL, Lin JP, Lee JH, Lin CC, Su
CC, Lin HJ and Chung JG: Crude extracts of Euchresta formosana
radix induce cytotoxicity and apoptosis in human hepatocellular
carcinoma cell line (Hep3B). Anticancer Res. 27:2415–2425.
2007.PubMed/NCBI
|
|
4
|
Grizzi F, Franceschini B, Hamrick C,
Frezza EE, Cobos E and Chiriva-Internati M: Usefulness of
cancer-testis antigens as biomarkers for the diagnosis and
treatment of hepatocellular carcinoma. J Transl Med. 5:32007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SH, Jung DW, Kim GT, Park SY, Kim SY,
Park OJ and Kim YM: Quercetin of plants extracts regulates Sestrin2
and induces apoptosis in HT-29 colon cancer cells. J Cancer Prev.
17:244–250. 2012.
|
|
6
|
Parkash O, Kumar A and Kumar P Ajeet:
Anticancer Potential of Plants and Natural Products: A review. Am J
Pharmacol Sci. 1:104–115. 2013.
|
|
7
|
Tai Y, Sun YM, Zou X, Pan Q, Lan YD, Huo
Q, Zhu JW, Guo F, Zheng CQ, Wu CZ and Liu H: Effect of polygonatum
odoratum extract on human breast cancer MDA-MB-231 cell
proliferation and apoptosis. Exp Ther Med. 12:2681–2687. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park C, Jeong JS, Jeong JW, Kim SO, Kim
YJ, Kim GY, Hong SH and Choi YH: Ethanol extract of Kalopanax
septemlobus leaf inhibits HepG2 human hepatocellular carcinoma cell
proliferation via inducing cell cycle arrest at G1 phase. Asian Pac
J Trop Med. 9:344–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byambaragchaa M, de la Cruz J, Yang SH and
Hwang SG: Anti-metastatic potential of ethanol extract of Saussurea
involucrata against hepatic cancer in vitro. Asian Pac J Cancer
Prev. 14:5397–5402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi EJ and Kim GH: Antioxidant and
anticancer activity of Artemisia princeps var. orientalis extract
in HepG2 and Hep3B hepatocellular carcinoma cells. Chin J Cancer
Res. 25:536–543. 2013.PubMed/NCBI
|
|
11
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy cellular
signaling. Cell Signal. 14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Romorini L, Garate X, Neiman G, Luzzani C,
Furmento VA, Guberman AS, Sevlever GE, Scassa ME and Miriuka SG:
AKT/GSK3β signaling pathway is critically involved in human
pluripotent stem cell survival. Sci Rep. 6:356602016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thotala DK, Hallahan DE and Yazlovitskaya
EM: Glycogen synthase kinase 3β inhibitors protect hippocampal
neurons from radiation-induced apoptosis by regulating MDM2-p53
pathway. Cell Death Differ. 19:387–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watcharasit P, Bijur GN, Song L, Zhu J,
Chen X and Jope RS: Glycogen synthase kinase-3beta (GSK3beta) binds
to and promotes the actions of p53. J Biol Chem. 278:48872–48879.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan J, Zhuang L, Leong HS, Iyer NG, Liu ET
and Yu Q: Pharmacologic Mmodulation of glycogen synthase
kinase-3beta promotes p53-dependent apoptosis through a direct
Bax-mediated mitochondrial pathway in colorectal cancer cells.
Cancer Res. 65:9012–9020. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karimian A, Ahmadi Y and Yousefi B:
Multiple functions of p21 in cell cycle, apoptosis and
transcriptional regulation after DNA damage. DNA Repair (Amst).
42:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng J, Zhang HH, Zhou CX, Li C, Zhang F
and Mei QB: The histone deacetylase inhibitor trichostatin A
induces cell cycle arrest and apoptosis in colorectal cancer cells
via p53-dependent and -independent pathways. Oncol Rep. 28:384–388.
2012.PubMed/NCBI
|
|
18
|
Zhang R, Wang Y, Li J, Jin H, Song S and
Huang S: The Chinese herb isolate yuanhuacine (YHL-14) induces G2/M
arrest in human cancer cells by up-regulating p21 protein
expression through an p53 protein-independent cascade. J Biol Chem.
289:6394–6403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang G, Liu J, Ren B, Tang Y, Owusu L, Li
M, Zhang J, Liu L and Li W: Anti-tumor effects of osthole on
ovarian cancer cells in vitro. J Ethnopharmacol. 193:368–376. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang LL, Wang MC, Chen LG and Wang CC:
Cytotoxic activity of coumarins from the fruits of Cnidium monnieri
on leukemia cell lines. Planta Med. 69:1091–1095. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu YP: Chinese Material Medica:
Chemistry, Pharmacology, and Applications. Harwood Academic
Publishers; Amsterdam: pp. 6241998
|
|
22
|
Li HB and Chen F: Simultaneous separation
and purification of five bioactive coumarins from the Chinese
medicinal plant Cnidium monnieri by high-speed counter-current
chromatography. J Sep Sci. 28:268–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao T, Pan H, Feng Y, Li H and Zhao Y:
Petroleum ether extract of Chenopodium album L. prevents cell
growth and induces apoptosis of human lung cancer cells. Exp Ther
Med. 12:3301–3307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang R, Zhang Q, Peng X, Zhou C, Zhong Y,
Chen X, Qiu Y, Jin M, Gong M and Kong D: Stellettin B induces G1
arrest, apoptosis and autophagy in Hhuman non-small cell lung
cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep.
6:270712016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chien SY, Wu YC, Chung JG, Yang JS, Lu HF,
Tsou MF, Wood WG, Kuo SJ and Chen DR: Quercetin-induced apoptosis
acts through mitochondrial- and caspase-3-dependent pathways in
human breast cancer MDA-MB-231 cells. Hum Exp Toxicol. 28:493–503.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Degli Esposti M and Dive C: Mitochondrial
membrane permeabilisation by Bax/Bak. Biochem Biophys Res Commun.
304:455–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rössig L, Badorff C, Holzmann Y, Zeiher AM
and Dimmeler S: Glycogen synthase kinase-3 couples AKT-dependent
signaling to the regulation of p21Cip1 degradation. J Biol Chem.
277:9684–9689. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kulikov R, Boehme KA and Blattner C:
Glycogen synthase kinase 3-dependent phosphorylation of Mdm2
regulates p53 abundance. Mol Cell Biol. 25:7170–7180. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Z, Wang H, Li M, Agrawal S, Chen X
and Zhang R: MDM2 is a negative regulator of p21WAF1/CIP1,
independent of p53. J Biol Chem. 279:16000–16006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sutherland C: What are the bona fide GSK3
substrates? Int J Alzheimers Dis 2011. 5056072011.
|
|
32
|
Yuan L, Zhang Y, Xia J, Liu B, Zhang Q,
Liu J, Luo L, Peng Z, Song Z and Zhu R: Resveratrol induces cell
cycle arrest via a p53-independent pathway in A549 cells. Mol Med
Rep. 11:2459–2464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen G, Xu C, Chen C, Hebbar V and Kong
AN: p53-independent G1 cell cycle arrest of human colon carcinoma
cells HT-29 by sulforaphane is associated with induction of p21CIP1
and inhibition of expression of cyclin D1. Cancer Chemother
Pharmacol. 57:317–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim EJ, Kim GT, Kim BM, Lim EG, Kim SY, Ha
SH, Kim YM and Yoo JG: Cell cycle arrest effects by artemisia annua
linné in Hep3B liver cancer cell. KSBB J. 30:175–181. 2015.
View Article : Google Scholar
|
|
35
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|