Introduction
Obesity is a global public health problem due to its
close positive association with several severe chronic diseases,
including type 2 diabetes, cardiovascular disease, and several
types of cancer (1). Obesity
involves abnormal or excessive fat accumulation in adipose tissue,
and occurs as a result of adipocyte hyperplasia and hypertrophy
(2). Although the mass of adipose
tissue generally depends on the balance between adipogenesis and
lipolysis, adipocyte apoptosis is an additional important
contributor to the reduction of body fat (3,4). The
reduction in the number of adipocytes via induction of adipocyte
apoptosis is therefore a potential strategy to attenuate
obesity.
Sulforaphane (SFN), an isothiocyanate enriched in
cruciferous vegetables, such as broccoli and broccoli sprouts,
possesses a wide range of properties, including antioxidant
(5), anticholesterol (6) and anticancer effects (7). SFN exerts an anticancer effect by
inducing tumor cell apoptosis (8,9). A
previous study demonstrated that SFN treatment reduced the body
weight of obese mice (10). In
addition, SFN was observed to inhibit adipogenesis and stimulate
lipolysis in pre-adipocytes and adipocytes in vitro
(11,12). In a previous study, treatment of
mature 3T3-L1 adipocytes with 60 µM SFN induced apoptosis by
inhibiting the AKT serine/threonine kinase 1 (Akt) signaling
pathway (13). Therefore, SFN may
be a promising agent for the treatment or prevention of obesity via
the induction of adipocyte apoptosis.
Myricetin (Myr;
3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone) is a
major dietary flavonoid, commonly present in tea, berries and
medicinal herbs. It has been demonstrated to possess antioxidant,
anti-inflammatory, and anticancer properties (14–17).
Myr functions as a potent anti-apoptotic inhibitor in cancer cell
lines by regulating phosphatidylinositol 3 kinase (PI3K) and
extracellular mitogen-activated protein kinase (18). These pathways affect cancer-cell
growth and survival (19,20). Previous studies have demonstrated
that Myr exhibits anti-obesity activities in vivo and in
vitro (21,22). In 3T3-L1 adipocytes, treatment with
100 µM Myr induced a significant decrease in intracellular
triglyceride accumulation, inhibited pre-adipocyte differentiation,
and enhanced mature-adipocyte lipolysis; however, it did not reduce
cell viability (22). There is a
growing body of evidence from studies investigating drug discovery
from natural products, which are increasing in popularity.
Administration of a combination of natural compounds has become
increasingly attractive for the treatment of obesity, as there is
some evidence to suggest that multi-drug combinations lead to
pharmacological potentiation, which optimistically leads to lower
doses, fewer adverse side effects, and an extended treatment window
(23–26). Although SFN and Myr are well
tolerated, it is not yet clear whether Myr induces apoptosis in
adipocytes, or whether the combination of Myr and SFN may exert a
stronger effect than either compound alone. Therefore, the effect
of combined treatment of 40 µM SFN (S40) and 100 µM Myr (M100) on
3T3-L1 adipocyte apoptosis was investigated in the present study.
The results demonstrated that combined treatment with SFN and Myr
significantly increased the level of adipocyte apoptosis induction,
when compared with either agent alone. In addition, this enhanced
effect on the level of apoptosis was associated with activation of
the mitochondrial apoptotic pathway mediated by Akt. Therefore, SFN
plus Myr treatment may be advantageous for induction of adipocyte
apoptosis, as the two compounds appear to target the same
pharmacological pathway.
Materials and methods
Cell culture and reagents
The mouse 3T3-L1 pre-adipocyte cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA) and was maintained at 37°C and 5% CO2 in Dulbecco's
modified Eagle's medium (DMEM; GE Healthcare Life Sciences,
Chalfont, UK) supplemented with 10% calf serum (CS) or 10% fetal
bovine serum (FBS; Zhejiang Tianhang Biotechnology Co., Ltd.,
Huzhou, China), as indicated for each procedure, plus 100 U/ml
penicillin, and 100 mg/ml streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Myr, SFN, insulin (INS),
3-isobutyl-1-methylxanthine (IBMX), dexamethasone (DEX), and Oil
Red O dye were purchased from Merck KGaA. Primary antibodies
against Akt (catalog no. 9272), phosphorylated (p)-Akt at Ser473
(p-AktSer473; catalog no. 4060), ribosomal protein S6
kinase β-1 (alternatively known as p70S6K1; catalog no. 2708),
p-p70S6K1 (catalog no. 9208), caspase 3 (catalog no. 9662),
poly-ADP-ribose-polymerase (PARP; catalog no. 9532), the B-cell
lymphoma-2 (Bcl-2; catalog no. 3498) apoptosis regulator,
Bcl-2-associated death promoter (Bad; catalog no. 9292), and p-Bad
at Ser112 (p-Badser112; catalog no. 9291) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Primary
antibodies against Bcl-2 associated X (Bax) apoptosis regulator
(catalog no. AB026) was purchased from Beyotime Institute of
Biotechnology (Haimen, China). The primary antibody against β-actin
was purchased from Santa Cruz Biotechnology, Inc., Dallas, TX, USA
(catalog no. sc-130656). The horseradish peroxidase-conjugated
secondary antibodies (catalog no. BM 2006 and BA1025) were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China).
Adipocyte differentiation
3T3-L1 pre-adipocytes cultured until ~90% confluent
(D0) following 2 days of incubation with 10% CS/DMEM
growth medium. The medium was then replaced with 10% FBS/DMEM
supplemented with 10 µg/ml INS, 1 µM DEX and 0.5 mM IBMX to induce
differentiation. Following 2 days (D2), the medium was
replaced with 10% FBS/DMEM containing 10 µg/ml INS for a further 2
days (D4). This was followed by culture in the 10%
CS/DMEM growth medium for an additional 6 days; during which time
the medium was refreshed every other day. At 10 days following
induction of differentiation (D10), >90% of the cells
consisted of mature adipocytes, which contained an abundance of
lipid droplets and were ready for treatment.
Oil red O staining
At the end of differentiation (D10), the
mature adipocytes were washed with phosphate-buffered saline (PBS)
and fixed with 10% neutral buffered formalin for 30 min at room
temperature. The cells were then washed with PBS and subsequently
stained with filtered 0.5% (w/v) Oil red O solution for 30 min at
room temperature in the dark. Cells were then washed three times
with distilled water. Photographs of the Oil Red O-stained cells
were captured using a Nikon digital camera system (Nikon
Corporation, Tokyo, Japan). A total of 99% (v/v) isopropanol was
then added to the cells and incubated for 5 min at room temperature
to remove the dye, and the lipid content of the cells was
quantified by spectrophotometry at 570 nm.
MTT assay
The viability of mature adipocytes was determined
using an MTT assay kit (Beyotime Institute of Biotechnology,
Haimen, China), which measures the production of formazan by
catalytically active cells and therefore provides a measure of the
number of viable cells. Pre-adipocytes were seeded onto a 96-well
plate at a density of 2.5×103 cells/well and induced to
differentiate into mature adipocytes using the aforementioned
methods. At D10 the mature adipocytes were treated with
S40 and/or M100 for 24 h. Following treatment, the cells were
incubated with 0.5 mg/ml MTT reagent for 3 h at 37°C to allow the
formation of formazan crystals. Dimethyl sulfoxide (150 µl) was
subsequently added to each well to dissolve the formazan crystals.
The optical density (OD) was determined by measuring the absorbance
of wells at 570 nm using a microplate reader. Inhibition of cell
viability was calculated as follows: Inhibition (%) =
[1-(ODtreated adipocytes /ODuntreated
adipocytes)] ×100.
Measurement of adipocyte
apoptosis
Pre-adipocytes were seeded in 6-cm culture dishes
(4×104 cells/dish) coated with poly-lysine, and induced
to differentiate into mature adipocytes using the aforementioned
methods. Mature adipocytes were then treated with S40 and/or M100
for 24 h. The cells were subsequently fixed with 4%
paraformaldehyde at room temperature for 10 min, washed twice with
PBS and stained with 10 µg/ml Hoechst 33258 solution for 5 min in
the dark. Cells were washed with cold PBS and a drop of anti-fade
mounting medium (Beyotime Institute of Biotechnology) was added.
Alterations in nuclear morphology were observed under an inverted
fluorescence microscope (Olympus IX51 Inverted Microscope; Olympus
Corporation, Tokyo, Japan).
The ApoStrand™ ELISA Apoptosis Detection kit (Enzo
Life Sciences, Inc., Farmingdale, NY, USA) was used to quantify the
levels of single-stranded DNA (ssDNA) generated during apoptosis,
according to the manufacturer's instructions. Briefly, mature
3T3-L1 adipocytes (2.5×103 cells/well) were transferred
to 96-well plates following treatment with S40 and/or M100 for 24
h. The cells were fixed at room temperature for 30 min with 4%
formamide, and then the ssDNA in apoptotic cells was stained with a
mixture of the primary antibody and the peroxidase-labeled
secondary antibody provided with the kit. The cells were
subsequently incubated with peroxidase substrate at room
temperature for 45 min, and the absorbance at 405 nm was read.
Western blotting
Following treatment with S40 and/or M100 for 24 h,
~4×107 mature adipocytes were harvested for extraction
of mitochondrial and cytoplasmic protein, and ~1×107
cells were used to extract total protein. Briefly, 2 ml
mitochondrial separation reagent containing 1 mM PMSF (Beyotime
Institute of Biotechnology) was added, and then shaken at 4°C for 3
min followed by centrifugation at 600 × g for 10 min at 4°C for 10
min. The supernatant (cytoplasm) and the precipitation
(mitochondria) were separated and lysed in Nonidet P-40 lysis
buffer on ice for 1 h. The supernatant was collected by
centrifugation at 12,000 × g for 15 min at 4°C. The protein
concentration was quantified using an enhanced bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). Protein
samples (50 µg/lane) were electrophoresed by 10% SDS-PAGE and
transferred to a polyvinylidene fluoride membrane. The membranes
were blocked in blocking buffer containing 5% nonfat dry milk in
Tris-buffered saline with 0.05% Tween 20 (TBST) for 1 h at room
temperature. The membranes were then probed with the specific
primary antibodies (diluted 1:1,000) overnight at 4°C in TBST. The
membranes were subsequently washed with TBST three times for 5 min
each time, and incubated with the horseradish peroxidase-conjugated
secondary antibodies (diluted 1:10,000) for 1.5 h at room
temperature. Membranes were washed again with TBST, developed with
an enhanced chemiluminescence kit (Roche Applied Science, Penzberg,
Germany) and exposed to X-ray film in a dark room. The protein
bands were analyzed using a Gel Doc™ XR+ imaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
of at least three independent experiments. SPSS version 10.0 (SPSS,
Inc., Chicago, IL, USA) was used for statistical analysis. One-way
analysis of variance and the Student-Newman-Keuls post hoc test
were used to evaluate statistical differences among groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Mature adipocyte determination
At D10 of adipocyte differentiation, the
vast majority of 3T3-L1 cells appeared to contain Oil Red O-stained
lipid droplets, which indicated that adipocyte maturation had
occurred (Fig. 1).
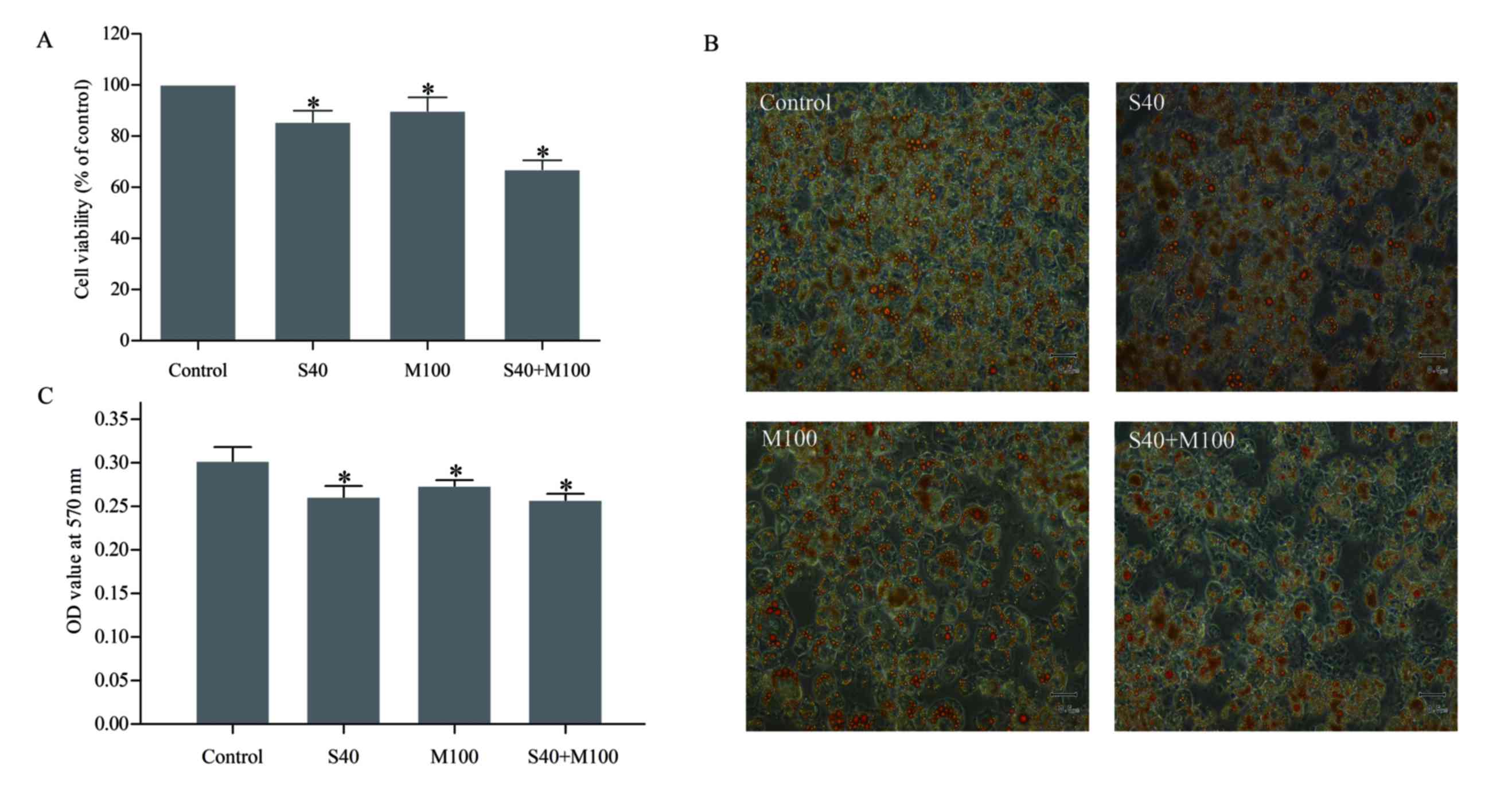
SFN and Myr combined decreased
adipocyte viability
The results of the MTT assay revealed that treatment
with S40 or M100 reduced the viability of mature adipocytes by
14.74±0.02 and 9.36±0.03%, respectively, when compared with
untreated cells (P<0.05; Fig.
2A, Table I). However, when
S40 and M100 were applied in combination, cell viability was
reduced by 33.07±0.02% when compared with the untreated cells
(P<0.05; Table I). Treatment
with these agents together did not demonstrate an additive effect,
as this would have led to a decrease in cell viability of
24.10±0.05% (P<0.05; Table I),
which suggests that the observed effect was synergistic.
 | Table I.Percentage change in viability,
apoptosis and cellular lipid of 3T3-L1 mature adipocytes treated
with 40 µM SFN (S40) plus 100 µM Myr (M100). |
Table I.
Percentage change in viability,
apoptosis and cellular lipid of 3T3-L1 mature adipocytes treated
with 40 µM SFN (S40) plus 100 µM Myr (M100).
|
| Treatment (%
change) |
|---|
|
|
|
|---|
|
| Inhibition of cell
viabilitya | Increase of
ssDNAb | Decrease of
intracellular lipidc |
|---|
| Control |
0.00±0.03u |
0.00±0.05u |
0.00±1.61u |
| S40 |
14.74±0.02uv |
27.38±0.08uv |
13.58±1.26uv |
| M100 |
9.36±0.03uvw |
9.74±0.08uvw |
9.44±0.68uvw |
| S40+M100 |
33.07±0.02x |
108.04±0.18x |
14.83±0.73uv |
| Calculated additive
responsed |
24.10±0.05xy |
37.12±0.16xy |
23.02±1.94x |
Oil Red O staining revealed that treatment with SFN
and/or Myr decreased the number of lipid droplets that accumulated
in mature adipocytes (Fig. 2B).
Quantitative spectrophotometry analysis revealed that S40 and/or
M100 treatment significantly reduced the lipid content of the cells
when compared with untreated cells (P<0.05; Fig. 2C, Table I). Combined treatment with S40 and
M100 was not associated with a significant reduction in cellular
lipid content when compared with S40 or M100 treatment alone
(Table I).
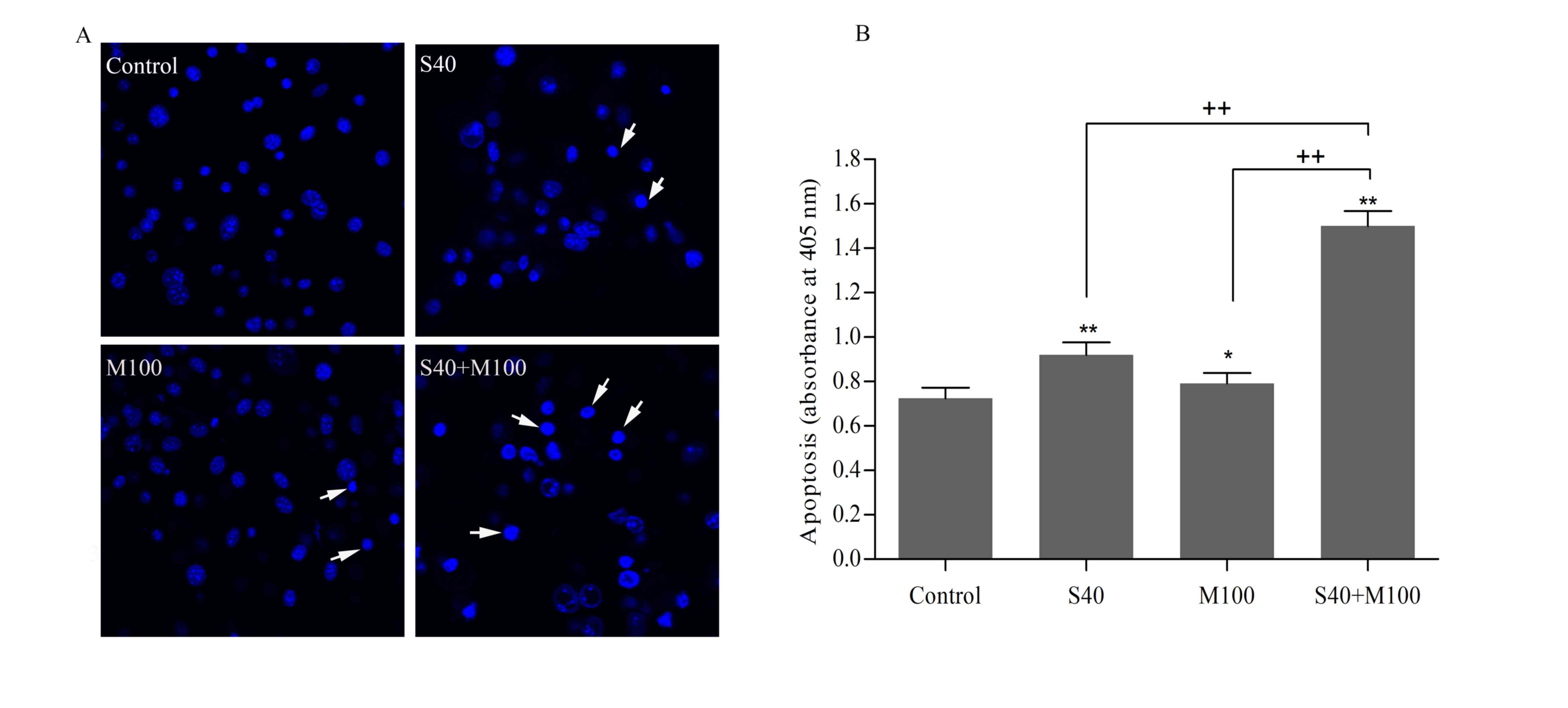
Combined SFN and Myr treatment induced
apoptosis
Hoechst 33258 nuclear staining of mature adipocytes
revealed that cells displayed characteristic features of apoptosis,
including shrinkage, nuclear fragmentation, and chromatin
condensation following treatment with SFN and/or Myr for 24 h
(Fig. 3A). Apoptosis was further
assessed by quantifying the level of ssDNA in cells, which may be
used as a marker of apoptotic cells. The ssDNA levels indicated
that, while SFN and Myr treatment alone induced apoptosis in
adipocytes when compared with the untreated control cells, the
effect of combined treatment was significantly greater than that of
each compound alone (P<0.05; Fig.
3B). Compared with the control, S40 and M100 increased the
levels of apoptosis by 27.38±0.08 and 9.74±0.08%, respectively
(P<0.05; Table I). However,
combined treatment with S40 and M100 increased apoptosis by
108.04±0.18% when compared with the control (P<0.05; Table I). The calculated additive effect
would have led to an increase in apoptosis levels of 37.11±0.16%
when compared with the controls (P<0.05; Table I), which therefore suggests that
SFN and Myr act synergistically to induce apoptosis in
adipocytes.
Involvement of the mitochondrial
apoptotic pathway
Bcl-2 family proteins are critical regulators of the
mitochondrial apoptotic pathway. The ratio of Bax to Bcl-2 is
important in determining cell fate (25). In the present study, SFN treatment
markedly increased Bax protein expression levels, while Myr
demonstrated no observable effect when compared with the control
(Fig. 4A). Neither SFN nor Myr
treatment demonstrated any observable effect on Bcl-2 expression
levels at the concentrations tested (Fig. 4A). However, treatment with SFN and
Myr combined increased Bax expression and decreased Bcl-2
expression levels when compared with the control, thus altering the
Bax/Bcl-2 ratio in favor of apoptosis (Fig. 4A). In addition, combined treatment
markedly increased Bad expression in the mitochondria when compared
with the control, which suggests that Bad translocation from the
cytoplasm to the mitochondria was activated, thus promoting
apoptosis (Fig. 4B). Furthermore,
phosphorylation of Bad at Ser112, which is thought to be necessary
and sufficient to promote cell survival, was markedly decreased in
the cells treated with SFN plus Myr compared with control cells or
cells treated with each compound alone (Fig. 4B). Caspase 3 is the main executor
caspase of the majority of programmed cell-death processes
(27). As demonstrated in Fig. 4A, treatment with either SFN or Myr
for 24 h led to an increase in the levels of cleaved caspase 3 and
increased cleavage of the downstream DNA-repair enzyme, PARP, from
its native 116 kDa form to the inactive 89 kDa form. As expected,
combined treatment additionally induced caspase 3 cleavage and PARP
inactivation (Fig. 4A).
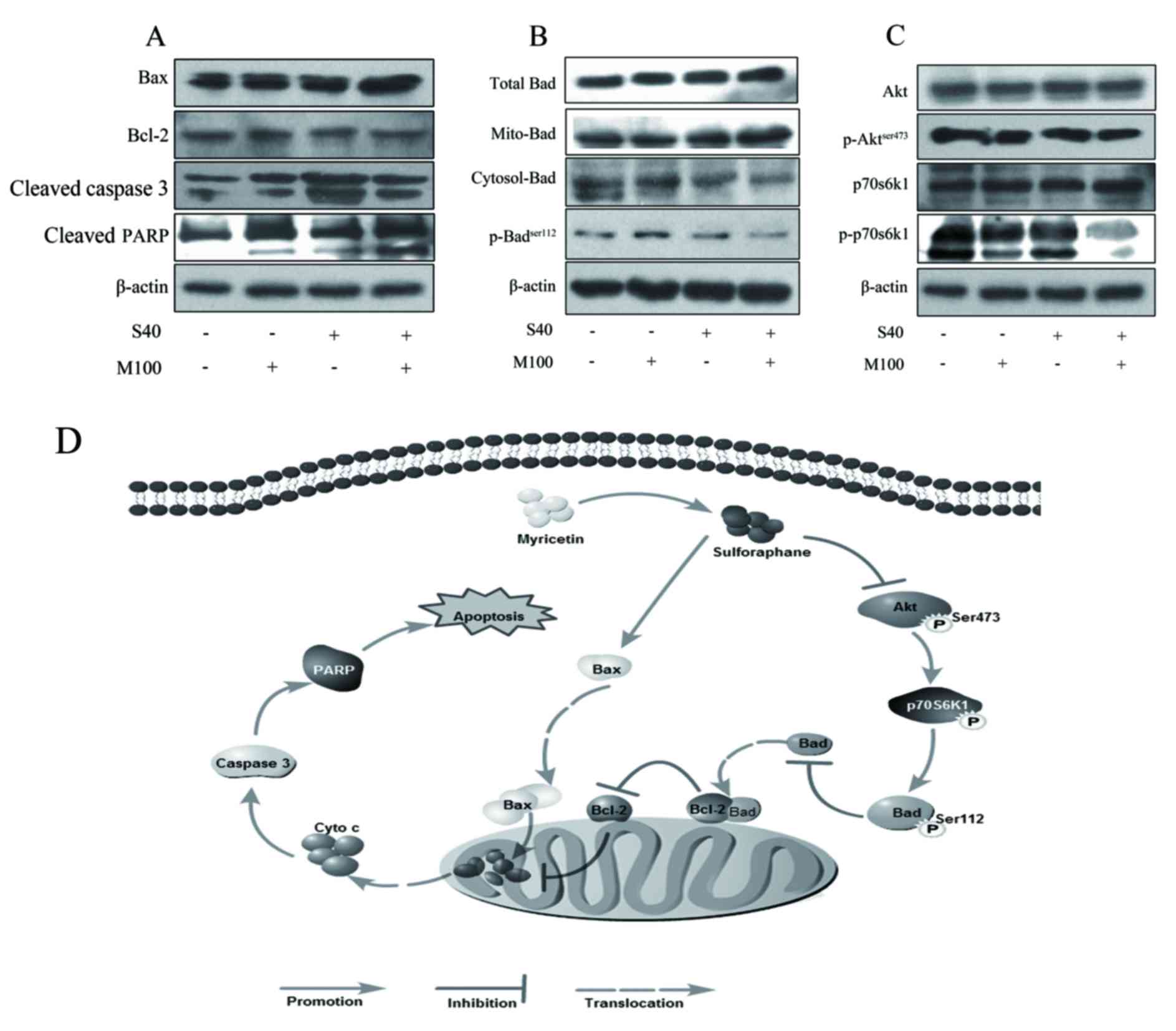 | Figure 4.Expression of apoptosis-associated
proteins in 3T3-L1 adipocytes treated with SFN and/or Myr for 24 h,
as determined by western blot analysis. Images of blots showing the
protein expression levels of (A) Bax, Bcl-2, cleaved caspase 3, and
cleaved PARP. (B) Total, cytoplasmic and mitochondrial Bad, and
p-BadSer112. (C) Akt, p-AktSer473, p70S6K1,
and p-p70S6K1. β-actin was used as a loading control.
Representative blots of three independent experiments are shown.
(D) Proposed schematic diagram of a combination of Myr and
SFN-induced apoptosis of adipocyte. Myr enhanced the inhibitory
effect of SFN on the Akt signaling pathway, increased SFN-induced
upregulation of the Bax/Bcl-2 ratio and activation of caspase-3.
SFN, sulforaphane; Myr, myricetin; Bax, Bcl-2 associated X; Bcl-2,
B-cell lymphoma-2; PARP, poly-ADP-ribose-polymerase; Bad,
Bcl-2-associated death promoter; p-, phosphorylated; Akt, AKT
serine/threonine kinase 1; p70S6K1, ribosomal protein S6 kinase
β-1; S40, 40 µM SFN treatment; M100, 100 µM Myr treatment. |
Myr potentiated the effect of SFN on
Akt phosphorylation
Western blot analysis revealed that SFN or Myr
treatment alone did not demonstrate any observable effect on Akt
phosphorylation at Ser473; however, combined treatment with these
agents markedly inhibited Akt phosphorylation when compared with
the controls (Fig. 4C). Total Akt
expression levels were unaffected in cells treated with SFN and/or
Myr (Fig. 4C). As expected,
combined SFN and Myr treatment was associated with an observable
decrease in the levels of p-p70S6K1 when compared with controls
(Fig. 4C). SFN treatment alone
decreased p70S6K1 phosphorylation; however, this effect was not as
pronounced as combined SFN and Myr treatment (Fig. 4C). The expression levels of total
p70S6K1 in adipocytes were not altered in response to SFN and/or
Myr treatment (Fig. 4C).
Discussion
A number of natural compounds have been reported to
induce apoptosis in 3T3-L1 pre-adipocytes or adipocytes, indicating
that they may be promising agents for the treatment of obesity
(28). It has become evident that
the combined use of several natural products may be more effective
in the prevention and treatment of obesity than conventional,
single-agent regimens, due to synergistic effects and fewer adverse
side effects (29). In the present
study, combined treatment of 3T3-L1 adipocytes with SFN and Myr
decreased cell viability to a greater extent than the additive
effect of each compound alone. In addition, the SFN plus Myr
treatment induced significantly higher levels of apoptosis in
adipocytes when compared with each compound alone. This was
determined by measuring the characteristic phenotypic alterations
associated with apoptosis, including nuclear fragmentation,
chromatin condensation, and ssDNA levels.
The Bcl-2 family proteins, including the
anti-apoptotic proteins Bax, Bcl-2 antagonist/killer 1 and Bad, as
well as pro-apoptotic proteins, Bcl-2, Bcl-2-like 1, and Bcl-2 like
2, are important components in the regulation of apoptosis. Bcl-2
is located on the surface of mitochondria where it maintains the
integrity of the mitochondrial membrane and functions as a negative
regulator of apoptosis (30). By
contrast, Bax usually resides in the cytosol. Its translocation to
the mitochondria leads to the activation of an apoptotic cascade in
response to apoptotic stimuli (31). Therefore, the relative Bax/Bcl-2
ratio is an important determinant of whether cells will undergo
apoptosis. Increased Bax and downregulated Bcl-2 expression has
been reported in capsaicin-induced apoptosis of 3T3-L1 adipocytes
(32). In addition,
curcumin-induced apoptosis in SW872 human adipocytes is accompanied
by an increase in the Bax/Bcl-2 ratio (33). In the present study, combined
treatment with SFN and Myr significantly increased the Bax/Bcl-2
ratio in adipocytes when compared with either agent alone. A
previous study demonstrated that, at doses of ≥60 µM, Bad was
involved in the pro-apoptotic effects of SFN (13). In the present study, combined
treatment of adipocytes with 40 µM SFN and 100 µM Myr induced the
translocation of Bad to the mitochondria and the de-phosphorylation
of Bad at Ser112.
Caspase 3 is a crucial mediator of apoptosis and is
indispensable for apoptosis in numerous cell types (27). Activated caspase 3 cleaves the
downstream cell death-associated substrate, PARP, into 89 and 28 kD
fragments. The cleaved PARP fragments are hallmarks of the progress
of apoptosis (34). A number of
previous studies have reported that several natural products
activate caspase 3 and induce apoptosis in 3T3-L1 adipocytes
(28,33). The present study demonstrated that
the treatment of adipocytes with SFN and Myr combined, induced
caspase 3 activation and PARP cleavage. These results support the
hypothesis that the enhanced effects of combined SFN and Myr
treatment on adipocyte apoptosis are associated with activation of
the mitochondrial apoptosis pathway.
The Akt signaling pathway serves an important role
in the regulation of cell death and cell survival (19). SFN has been reported to induce
programmed death in Caco-2 colorectal cancer cells via activation
of PI3K/Akt (35). A previous
study demonstrated that the Akt/p70S6K1/Bad signaling pathway may
be involved in Myr-induced apoptosis in HepG2 hepatocellular
carcinoma cells (36). In the
present study, treatment of adipocytes with SFN and Myr combined
significantly inhibited the phosphorylation of Akt and its
downstream target, p70S6K1, which suggests that the Akt signaling
pathway may have been involved in the observed increase in
apoptosis levels induced by the combination treatment (Fig. 4D).
In conclusion, treatment with Myr and SFN combined
was associated with synergistic pro-apoptotic effects in
adipocytes. This increase in apoptosis may have been due to an
increase in the Bax/Bcl-2 ratio, the mitochondrial translocation of
Bad, the activation of caspase 3, and inactivation of the Akt
signaling pathway. The results suggest that administration of Myr
and SFN combined may be beneficial for the treatment or prevention
of obesity, as it provides the advantage of lowering the dose
required to gain a therapeutic effect, thereby potentially
minimizing side effects. Further studies investigating the effects
of different Myr and SFN combination regimens in adipocytes are
therefore warranted.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81673163 and
81450048), the Zhejiang Provincial Natural Science Foundation of
China (grant no. LY14H260001), the Zhejiang Provincial Key
Laboratory of Pathological and Physiological Technology (grant no.
20012E10018), Ningbo Scientific Innovation Team for Environmental
Hazardous Factor Control and Prevention (2016C51001) and the School
of Medicine, Ningbo Civil Outstanding Talent and Leadership Fund.
The present study was partly sponsored by the K.C. Wong Magna Fund
in Ningbo University and the Graduate Research Innovation Fund in
Ningbo University (grant no. G16026).
References
|
1
|
World Health Organization, . Fact sheet no
311. 2015, http://www.who.int/mediacentre/factsheets/fs311/en/print.htmlDec.
4–2015
|
|
2
|
Jo J, Gavrilova O, Pack S, Jou W, Mullen
S, Sumner AE, Cushman SW and Periwal V: Hypertrophy and/or
Hyperplasia: Dynamics of adipose tissue growth. PLoS Comput Biol.
5:e10003242009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prins JB, Walker NI, Winterford CM and
Cameron DP: Human adipocyte apoptosis occurs in malignancy. Biochem
Biophys Res Commun. 205:625–630. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prins JB, Walker NI, Winterford CM and
Cameron DP: Apoptosis of human adipocytes in vitro. Biochem Biophys
Res Commun. 201:500–507. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee YJ and Lee SH: Sulforaphane induces
antioxidative and antiproliferative responses by generating
reactive oxygen species in human bronchial epithelial BEAS-2B
cells. J Korean Med Sci. 26:1474–1482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Juengel E, Maxeiner S, Rutz J, Justin S,
Roos F, Khoder W, Tsaur I, Nelson K, Bechstein WO, Haferkamp A and
Blaheta RA: Sulforaphane inhibits proliferation and invasive
activity of everolimus-resistant kidney cancer cells in vitro.
Oncotarget. 7:85208–85219. 2016.PubMed/NCBI
|
|
7
|
Shehatou GS and Suddek GM: Sulforaphane
attenuates the development of atherosclerosis and improves
endothelial dysfunction in hypercholesterolemic rabbits. Exp Biol
Med (Maywood). 241:426–436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Zhang T, Korkaya H, Liu S, Lee HF,
Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS and Sun D:
Sulforaphane, a dietary component of broccoli/broccoli sprouts,
inhibits breast cancer stem cells. Clin Cancer Res. 16:2580–2590.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarkar R, Mukherjee S, Biswas J and Roy M:
Sulphoraphane, a naturally occurring isothiocyanate induces
apoptosis in breast cancer cells by targeting heat shock proteins.
Biochem Biophys Res Commun. 427:80–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi KM, Lee YS, Kim W, Kim SJ, Shin KO,
Yu JY, Lee MK, Lee YM, Hong JT, Yun YP and Yoo HS: Sulforaphane
attenuates obesity by inhibiting adipogenesis and activating the
AMPK pathway in obese mice. J Nutr Biochem. 25:201–207. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JH, Moon MH, Jeong JK, Park YG, Lee
YJ, Seol JW and Park SY: Sulforaphane induced adipolysis via
hormone sensitive lipase activation, regulated by AMPK signaling
pathway. Biochem Biophys Res Commun. 426:492–497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi KM, Lee YS, Sin DM, Lee S, Lee MK,
Lee YM, Hong JT, Yun YP and Yoo HS: Sulforaphane inhibits mitotic
clonal expansion during adipogenesis through cell cycle arrest.
Obesity (Silver Spring). 20:1365–1371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao A, Shen Y, Wang A, Chen S, Zhang H,
Chen F, Chen Z, Wei H, Zou Z, Shan Y and Zhang X: Sulforaphane
induces apoptosis in adipocytes via Akt/p70s6k1/Bad inhibition and
ERK activation. Biochem Biophys Res Commun. 465:696–701. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ong KC and Khoo HE: Biological effects of
myricetin. Gen Pharmacol. 29:121–126. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ross JA and Kasum CM: Dietary flavonoids:
Bioavailability, metabolic effects, and safety. Annu Rev Nutr.
22:19–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weng CJ and Yen GC: Flavonoids, a
ubiquitous dietary phenolic subclass, exert extensive in vitro
anti-invasive and in vivo anti-metastatic activities. Cancer
Metastasis Rev. 31:323–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun F, Zheng XY, Ye J, Wu TT, Wang J and
Chen W: Potential anticancer activity of myricetin in human T24
bladder cancer cells both in vitro and in vivo. Nutr Cancer.
64:599–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phillips PA, Sangwan V, Borja-Cacho D,
Dudeja V, Vickers SM and Saluja AK: Myricetin induces pancreatic
cancer cell death via the induction of apoptosis and inhibition of
the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer
Lett. 308:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonni A, Brunet A, West AE, Datta SR,
Takasu MA and Greenberg ME: Cell survival promoted by the Ras-MAPK
signaling pathway by transcription-dependent and -independent
mechanisms. Science. 286:1358–1362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang CJ, Tzeng TF, Liou SS, Chang YS and
Liu IM: Myricetin increases hepatic peroxisome
proliferator-activated receptor α protein expression and decreases
plasma lipids and adiposity in rats. Evid Based Complement Alternat
Med. 2012:7871522012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Q, Wang ST, Yang X, You PP and Zhang
W: Myricetin suppresses differentiation of 3 T3-L1 preadipocytes
and enhances lipolysis in adipocytes. Nut Rese. 35:317–327. 2015.
View Article : Google Scholar
|
|
23
|
Yang JY, Della-Fera MA, Rayalam S and
Baile CA: Enhanced effects of xanthohumol plus honokiol on
apoptosis in 3T3-L1 adipocytes. Obesity (Silver Spring, Md).
16:1232–1238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang JY, Della-Fera MA, Rayalam S, Ambati
S, Hartzell DL, Park HJ and Baile CA: Enhanced inhibition of
adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with
combinations of resveratrol and quercetin. Life Sci. 82:1032–1039.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rayalam S, Yang JY, Della-Fera MA, Park
HJ, Ambati S and Baile CA: Anti-obesity effects of xanthohumol plus
guggulsterone in 3T3-L1 adipocytes. J Med Food. 12:846–853. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baile CA, Yang JY, Rayalam S, Hartzell DL,
Lai CY, Andersen C and Della-Fera MA: Effect of resveratrol on fat
mobilization. Ann N Y Acad Sci. 1215:40–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rayalam S, Della-Fera MA and Baile CA:
Phytochemicals and regulation of the adipocyte life cycle. J Nutr
Biochem. 19:717–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rayalam S, Della-Fera MA, Yang JY, Park
HJ, Ambati S and Baile CA: Resveratrol potentiates genistein's
antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J
Nutr. 137:2668–2673. 2007.PubMed/NCBI
|
|
30
|
Vander Heiden MG and Thompson CB: Bcl-2
proteins: Regulators of apoptosis or of mitochondrial homeostasis?
Nat Cell Biol. 1:E209–E216. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu CL and Yen GC: Effects of capsaicin on
induction of apoptosis and inhibition of adipogenesis in 3T3-L1
cells. J Agric Food Chem. 55:1730–1736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu L, Han MB, Gao Y, Wang H, Dai L, Wen Y
and Na LX: Curcumin triggers apoptosis via upregulation of
Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes.
Mol Med Rep. 12:1151–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agarwal A, Mahfouz RZ, Sharma RK, Sarkar
O, Mangrola D and Mathur PP: Potential biological role of poly
(ADP-ribose) polymerase (PARP) in male gametes. Reprod Biol
Endocrinol. 7:1432009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jakubíková J, Sedlák J, Mithen R and Bao
Y: Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane-
and erucin-induced phase II enzymes and MRP2 transcription, G2/M
arrest and cell death in Caco-2 cells. Biochem Pharmacol.
69:1543–1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang XH, Chen SY, Tang L, Shen YZ, Luo L,
Xu CW, Liu Q and Li D: Myricetin induces apoptosis in HepG2 cells
through Akt/p70S6K/bad signaling and mitochondrial apoptotic
pathway. Anticancer Agents Med Chem. 13:1575–1581. 2013. View Article : Google Scholar : PubMed/NCBI
|