Introduction
Lung cancer remains one of the most common types of
fatal malignancy. Non-small cell lung cancer (NSCLC), characterized
by its high incidence, is the leading cause of cancer-associated
mortality worldwide (1). NSCLC
accounts for approximately 80–85% of all lung cancer cases
(2) and the majority of patients
are diagnosed with local advanced or metastatic disease (3). Although the epidermal growth factor
receptor-tyrosine kinase inhibitors (EGFR-TKIs) are recommended as
first-line treatment for patients whose tumors harbor activating
EGFR mutations (4),
platinum-based, double-agent chemotherapy represents the standard
of care for unselected patients with advanced NSCLC (5). Cisplatin (DDP) is a commonly used
drug with a high curative effect on lung cancer (6). However, in chemotherapy-treated
NSCLC, the duration of response is relatively short due to primary
or acquired resistance to chemotherapy (7,8).
Therefore, it is considered to be urgent to improve the efficacy of
DDP-based chemotherapy and to develop novel treatment strategies to
overcome DDP resistance for advanced NSCLC.
Niclosamide, a teniacide in the anthelmintic family,
which is particularly effective against cestodes, has been approved
for use in humans for many years (9). A recent study reported that
niclosamide was a multi-functional agent, performing anti-obesity
(10), anti-diabetic (11), anti-viral (12,13)
and anti-sclerotic (14)
activities. Additionally, niclosamide has been identified as a
potent anticancer agent using various high-throughput screening
assays (15). Niclosamide inhibits
the Wnt/β-catenin, mammalian target of rapamycin complex 1, signal
transducer and activator of transcription 3 (STAT3), nuclear
factor-κB and Notch signaling pathways, and targets mitochondria in
cancer cells to induce cell cycle arrest, growth inhibition and
apoptosis. A host of studies have established the anticancer
activities of niclosamide in in vitro and in vivo
models. Furthermore, Li et al (16) identified that niclosamide overcame
acquired resistance to erlotinib via suppression of STAT3 in NSCLC.
Liu et al (17)
demonstrated that niclosamide alone or in combination with DDP
significantly inhibited MDA-MB-231/DDP-sensitive (CS) and
MDA-MB-231-DDP-resistant (CR) cell proliferation in vitro.
However, the effect of niclosamide on cisplatin-resistant human
lung cancer cells remains unknown.
In the present study, whether niclosamide could
enhance the cytotoxic effects of DDP in cisplatin-resistant
A549/DDP lung cancer cells was investigated, and the underlying
mechanisms were evaluated further.
Materials and methods
Cell culture
Human A549 lung carcinoma cells and
cisplatin-resistant human A549/DDP lung carcinoma cells were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China) and cultured in GibcoRPMI-1640 medium
(Thermo Fisher Scientific, Inc, Waltham, MA, USA), supplemented
with 10% (v/v) dialyzed heat-inactivated bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in 5% CO2.
Cell viability assay
Cell viability was determined using the Cell
Counting kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). CCK-8 allows very convenient assays by utilizing
Dojindo's highly water-soluble tetrazolium salt. WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt] produces a water-soluble formazan dye upon
reduction in the presence of an electron carrier. CCK-8 allows
sensitive colorimetric assays for the determination of the number
of viable cells in cell proliferation and cytotoxicity assays.
WST-8 is reduced by dehydrogenases in cells to give an orange
colored product (formazan), which is soluble in the tissue culture
medium. The quantity of formazan dye generated by the activity of
dehydrogenases in cells is directly proportional to the number of
living cells. Briefly, cells in the early log phase were
trypsinized and plated in 96-well plate at a density of
5×103 cells per well. Cells were treated with various
concentrations of niclosamide (Sigma-Aldrich; Merck KGa, Darmstadt,
Germany) and DDP (Haosen Medicine Corp., Liangyungang, China) for
24 h at 37°C. Cell density was measured using the CCK-8 assay
according to the manufacturer's instructions. The absorbance of
each well was determined at a wavelength of 450 nm using a
microplate reader (Thermo Electron Corp., Shanghai, China). The
inhibition rate was calculated as follows: Inhibition rate
(%)=[1-(T-B)/(U-B)]x100%; where T is the treated cell absorbance, U
is the untreated cell absorbance and B is the background absorbance
when neither drug nor CCK-8 was added. All experiments were
repeated at least three times independently.
Combination index (CI) analysis
To evaluate whether the antitumor effects of
niclosamide combined with DDP were synergistic, additive or
antagonistic, combination index (CI) value for drug synergy was
calculated using the CompuSyn software (Version 2.1, ComboSyn,
Inc., Paramus, NJ, USA) as previously described (14). Using data obtained from CCK-8
assays and CompuSyn software, the dose-effect curves for single
agents and their combinations were generated, and the CI values for
each dose and the corresponding effect level, referred to as the
fraction affected (Fa; the fraction of cells inhibited following
drug exposure, for example 0.5 when cell growth is inhibited by
50%), were calculated. CI values <1 indicated a synergistic
effect, values equal to 1 indicated an additive effect and values
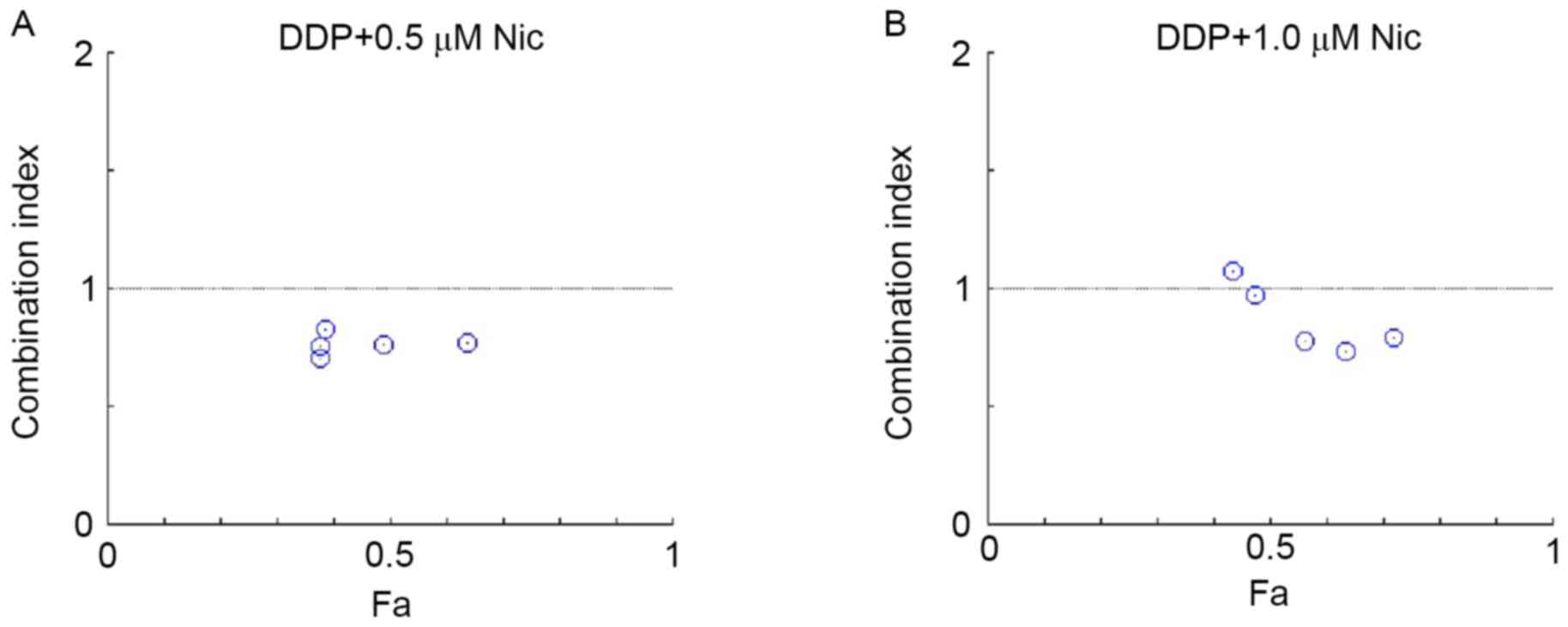
>1 indicated an antagonistic effect. Then, to provide a visual
illustration of drug interactions, the Fa-CI plot was constructed
by simulating CI values over a range of Fa levels from 0.1 to 0.95
(18,19).
Analysis of apoptosis by Annexin
V/propidium iodide (PI) staining
Apoptosis was assessed by Annexin V/PI detection as
described previously (20). The
A549/DDP cells were plated at a density of 1×105 cells
per well in six-well plates. The next day, cells were treated with
DDP (5 µg/ml), niclosamide (1 µM) or cisplatin (5 µg/ml) combined
with niclosamide (1 µM) for 36h. The cells were harvested, and
washed three times with phosphate-buffered saline (PBS) at 4°C.
Cells were then incubated with 5 µl Annexin V-fluorescein
isothiocyanate for 3 min and with 20 ng/ml PI in the dark for 15
min. The suspension was then analyzed by flow cytometry (BD
Biosciences, San Jose, CA, USA). All data were collected and
analyzed by FACSDiva version 6.1.3 (BD Biosciences). The
experiments were repeated three times independently and the results
were presented as the mean ± standard deviation.
Western blot analysis
Subsequent to treatments, the cells were collected
and lysed. Lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1%
Triton X-100, sodium pyrophosphate, β-glycerophosphate, EDTA,
Na3VO4 and leupeptin was purchased from
Beyotime Institute of Biotechnology (Shanghai, China). Total
protein (~20 µg) was separated on a 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis gel and transferred to a
polyvinylidene fluoride membrane. After blocking with 5% non-fat
milk in PBS + 0.1% Tween-20 for 1 h, the membrane was incubated
with the appropriate primary antibody: Anti-caspase-3 (#14220; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-LRP (sc-23916;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-c-myc (D84C12, 1:1,000; Cell Signaling Technology, Inc.) and
anti-β-tubulin (AT819, 1:2,000; Beyotime Institute of
Biotechnology) overnight at 4°C. After washing with PBS three times
(10 min each), the membrane was incubated with goat anti-rabbit
(A0208; 1:2,000; Beyotime Institute of Biotechnology) or anti-mouse
(A0216; 1:2,000; Beyotime Institute of Biotechnology)
IgG-horseradish peroxidase-conjugated secondary antibody for 1 h at
room temperature and washed with PBS three times. The ECL system
(Applygen Technologies, Inc., Beijing, China) was used to detect
blotting signals according to the manufacturer's instructions.
Detection of β-tubulin served as a loading control.
Statistical analysis
Continuous data are expressed as the mean ± standard
deviation. For two-group comparison, the Student's t-test method
was used. SPSS 13.0 software was used to perform all statistical
analyses (SPSS, Inc., Chicago, IL, USA). For more than two-group
comparison, one-way ANOVA was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
Niclosamide inhibits the growth of
A549 and A549/DDP cells
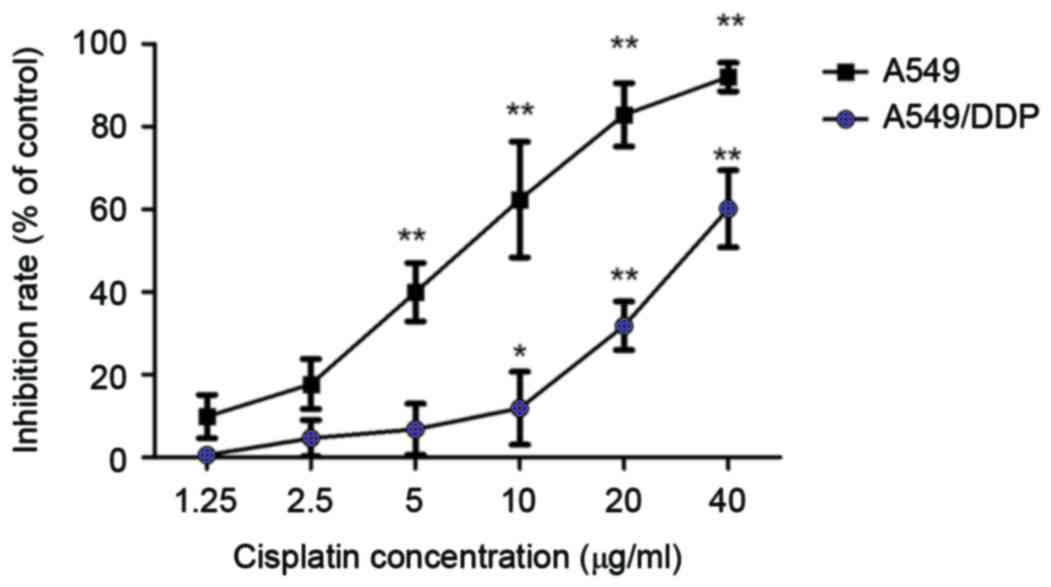
In order to confirm the differential sensitivity of
A549 and its derivative cisplatin-resistant cell line, A549/DDP, to
DDP, cells were treated with different concentrations of DDP as
indicated for 24 h, and cell viability was measured by CCK-8 assay.
IC50 values were calculated using GraphPad Prism 5.0
(GraphPad Software Inc., La Jolla, CA, USA). The data demonstrated
that the IC50 values of DDP in A549 and A549/DDP cells
were 6.81±0.78 and 32.5±0.21 µg/ml, respectively (Fig. 1). The resistance index of A549/DDP
cells to DDP was the IC50 of A549/DDP cells divided by
IC50 of the A549 cells or 32.5/6.81 µg/ml=4.77. These
data demonstrate that the A549/DDP cell line has a certain
resistance to DDP, which is suitable for drug resistance study.
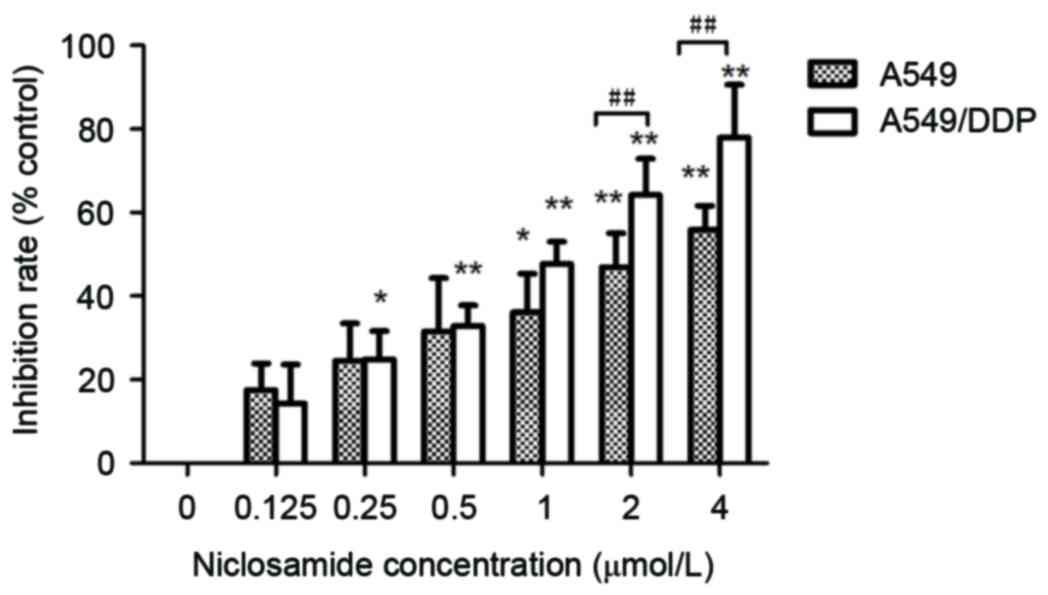
The A549 and A549/DDP cells were treated with
various concentrations of niclosamide as indicated (0, 0.125,
0.25,0.5, 1.0, 2.0 and 4.0 µM) for 24 h. Cell viability was
measured using CCK-8 assay. The current results demonstrated that
niclosamide significantly suppressed cell growth in a
dose-dependent manner in A549 and A549/DDP cells (Fig. 2; P<0.05). The IC50
values after 24 h of niclosamide in the A549 and A549/DDP cells
were 2.60±0.21 and 1.15±0.18 µM, respectively. Niclosamide appears
to exert a markedly greater inhibitory effect on A549/DDP cells
when compared with parental A549 cells.
Niclosamide enhances the inhibitory
effect of DDP on A549/DDP cells
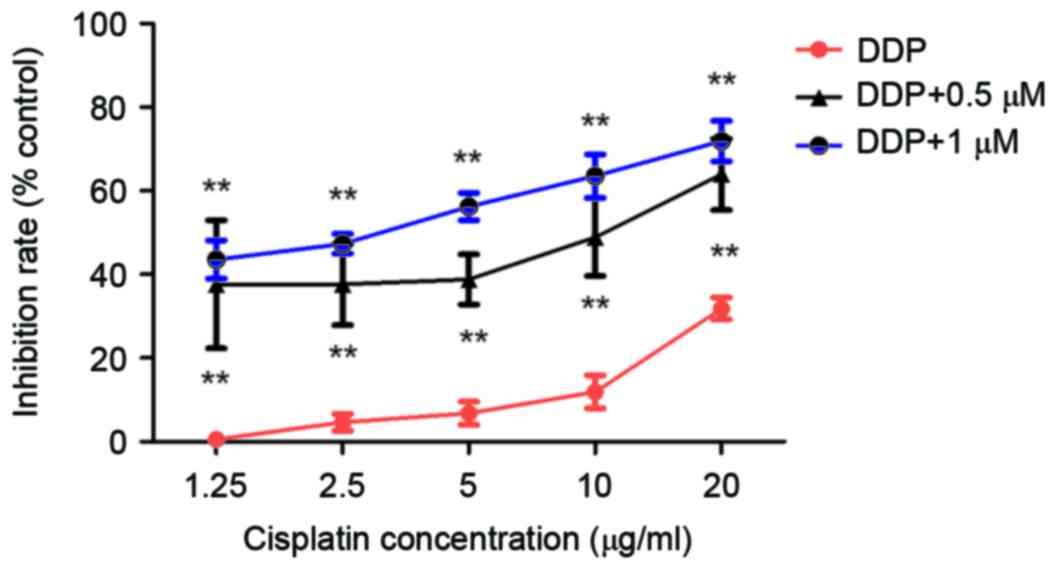
To examine whether niclosamide combined with DDP
exhibits enhanced antitumor effects in cisplatin-resistant lung
cancer, A549/DDP cells were treated with 0.5 or 1 µM niclosamide
along with 1.25, 2.5, 5, 10 or 20 µg/ml DDP for 24 h. The
niclosamide-treated cells demonstrated increased sensitivity to DDP
at all concentrations (Fig. 3;
P<0.05). According to the combined index calculated with
CompuSyn software, the CI value of DDP in combination with
niclosamide was <1, indicating that DDP combined with
niclosamide exerts a synergistic effect on A549/DDP cells (Fig. 4).
Niclosamide combined with DDP enhances
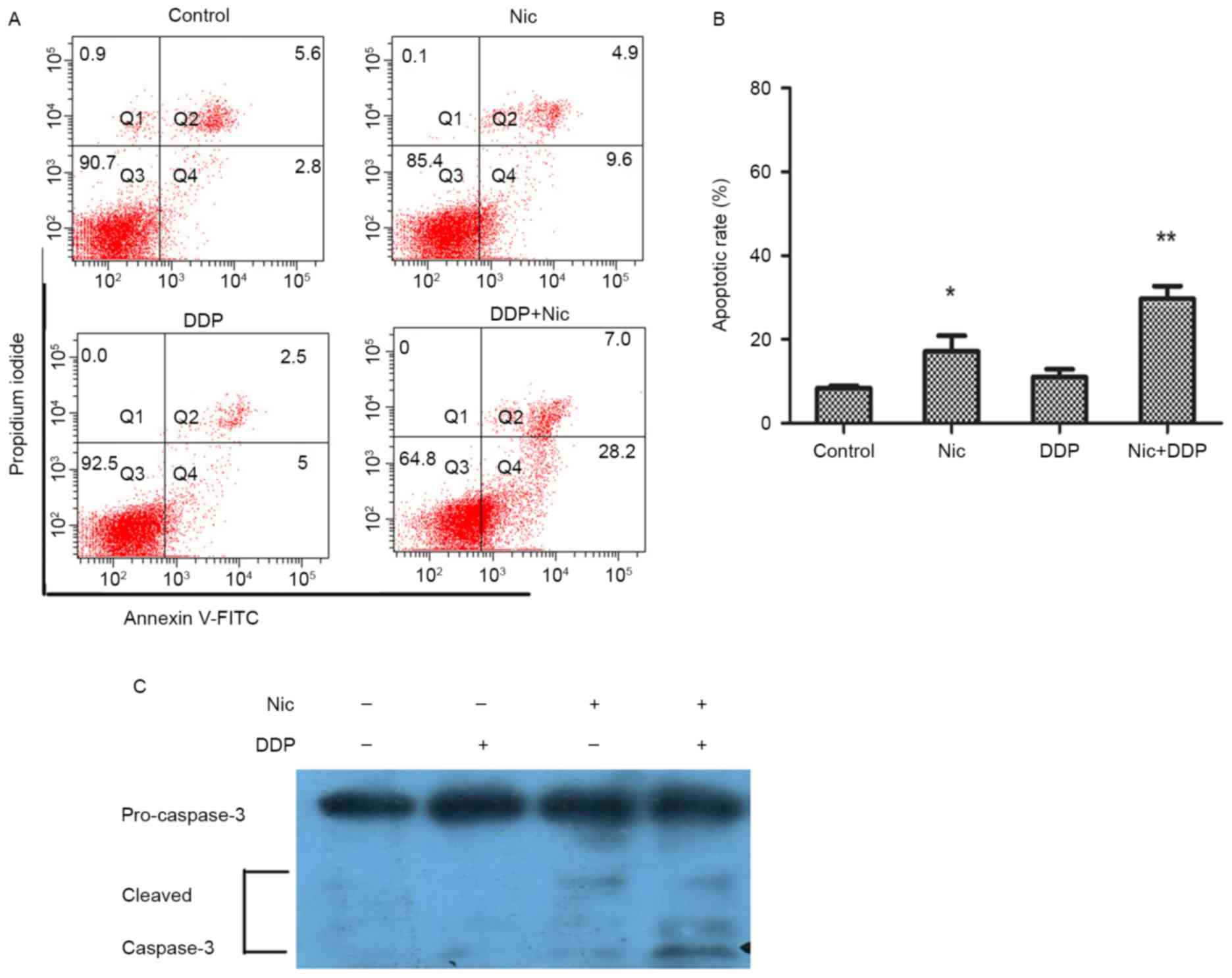
the apoptosis of A549/DDP cells
Subsequently, the apoptosis of A549/DDP cells after
niclosamide (1 µM) and DDP (5 µg/ml) treatment was evaluated using
flow cytometry. Apoptotic cells were detected following treatment
with 1 µM niclosamide and/or 5 µg/ml DDP for 36 h. Annexin V/PI
analysis indicated that the apoptotic ratios of the control group,
niclosamide, DDP and combined treatment group in the A549/DDP cells
were 8.36±1.05, 11.0±3.18, 16.5±5.25 and 30.36±4.36%, respectively
(Fig. 5A and B). The current data
demonstrated that niclosamide in combination with DDP significantly
enhanced the tumor killing effect by inducing apoptosis.
Furthermore, western blotting was used to detect the activation of
caspase-3 protein in A549/DDP cells after the same treatment. The
cleavage of caspase-3 was observed to be markedly increased in the
combined treatment group compared with the mono-treatment group
(Fig. 5C). Therefore, the current
results indicate that niclosamide combined with DDP may enhance
cytotoxic effects by inducing apoptosis in A549/DDP cells.
Niclosamide sensitizes A549/DDP cells
to DDP by downregulating LRP and c-myc
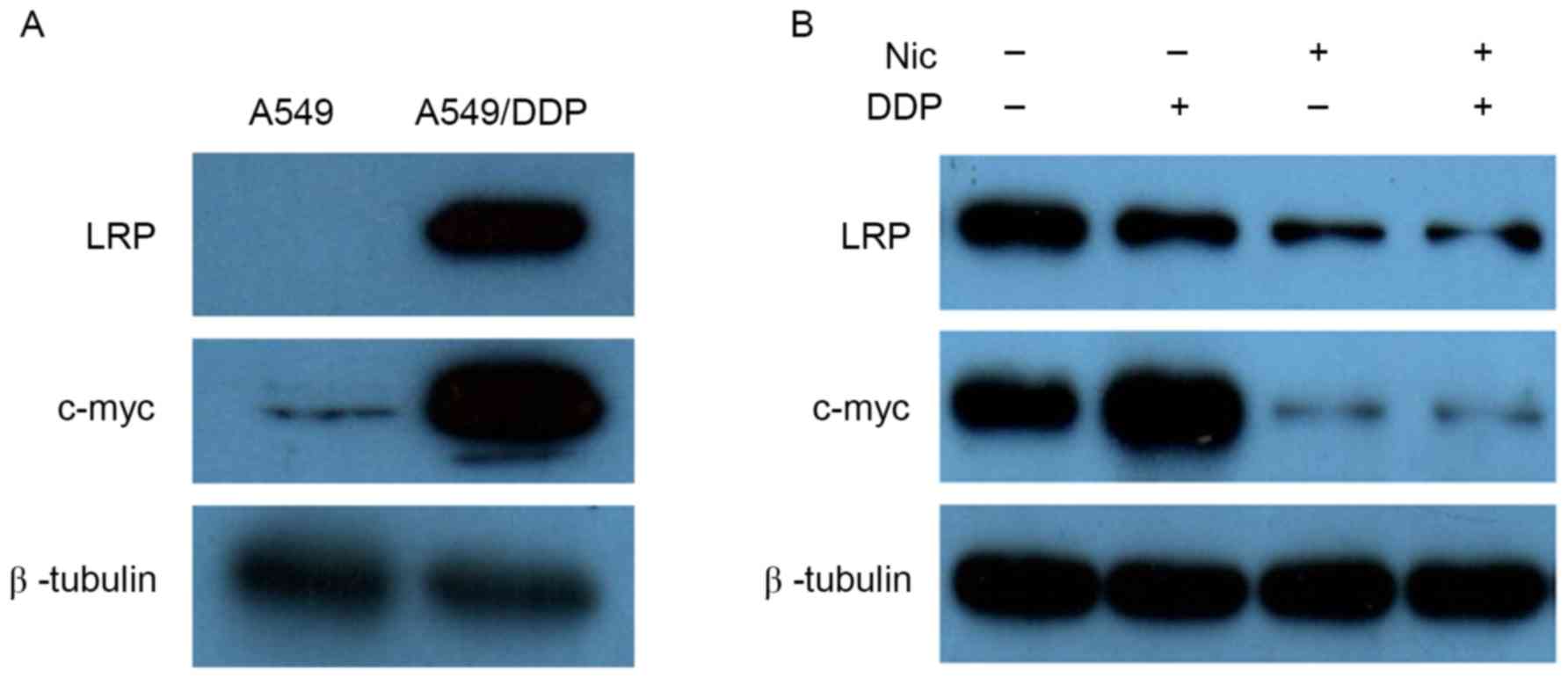
To further document the underlying mechanisms by
which niclosamide enhanced the inhibitory effect of DDP in A549/DDP
cells, western blotting was used to detect the impact of
niclosamide on DDP-resistant associated proteins. Initially, the
basic expression levels of LRP and c-myc proteins were evaluated in
A549 and A549/DDP cells. The expression levels of LRP and c-myc
proteins in A549/DDP cells were significantly higher than those of
the A549 cells (Fig. 6A).
Subsequently, the changes of LRP and c-myc protein expression
levels were investigated after treatment with niclosamide and/or
DDP in A549/DDP cells. Following treatment with 1.0 µM niclosamide
alone, A549/DDP cells demonstrated downregulation of LRP and c-myc
protein expression levels, while DDP alone exerted no effect on LRP
and c-myc protein expression levels. However, upon the combination
treatment of niclosamide and DDP, the expression levels of the two
proteins were significantly decreased compared with the controls
(Fig. 6B). Due to the roles of LRP
and c-myc protein on DDP resistance, it was inferred that
niclosamide may enhance the cytotoxic effect of DDP on A549/DDP
cells by downregulating the expression level of c-myc protein.
Discussion
DDP treatment often results in the development of
chemoresistance, leading to therapeutic failure (21). Establishing drugs that may overcome
DDP resistance is a promising strategy for improving the
therapeutic effects of lung cancer treatment. However, drug
development, from the initial lead discovery to the final
medication, is an expensive and lengthy process (22). By contrast, identifying novel
indications for old drugs is considerably faster and more
economical than inventing a novel drug altogether, as existing
drugs have known pharmacokinetics and safety profiles, and have
often been approved for human use (23). In the present study, niclosamide
markedly suppressed the proliferation of cisplatin-resistant human
A549/DDP lung cancer cells in vitro (Fig. 2). Furthermore, the current study
demonstrated that niclosamide in combination with DDP resulted in a
synergistic effect in A549/DDP cells (Fig. 3).
Previous studies have reported that niclosamide
exhibited synergistic effects when combined with chemotherapeutic
agents, oxaliplatin (24),
cytarabine, etoposide, daunorubicin and temozolomide (25), and DDP (17). Additionally, niclosamide reversed
the resistance of human head and neck cancer cells, and non-small
cell lung cancer cells to erlotinib (16,26).
Similarly, Liu et al (17)
identified that niclosamide alone or in combination with DDP
significantly inhibited MDA-MB-231/CS and MDA-MB-231/CR cell
proliferation in vitro. Therefore, niclosamide reduces the
proliferation of cisplatin-resistant lung cancer cells, indicating
that niclosamide may serve as a novel therapeutic strategy, either
alone or in combination with DDP, for lung cancer treatment,
particularly those resistant to DDP.
The development of DDP resistance arises due to
changes in the biochemical pharmacology of DDP. To elucidate
whether niclosamide enhances the antitumor effect in A549/DDP
cells, the expression levels of LRP and c-myc proteins were
examined, and found to be associated with DDP-resistance. Notably,
LRP and c-myc were significantly overexpressed in A549/DDP cells
compared with A549 cells, and niclosamide reduced the expression
levels of LRP and c-myc proteins (Fig.
6). LRP is the predominant human vault protein (27). It reduces the drug concentration in
the nucleus and decreases the drug effect on DNA targets (28). A previous study demonstrated that
vaults, including LRP, are overexpressed in multidrug-resistant
cancer cell lines (29).
Consistently, the level of LRP expression is significantly higher
in cisplatin-resistant A549/DDP cells than that in parental A549
cells. Furthermore, clinical studies have reported that LRP
expression levels predict drug resistance and poor outcome in
various types of cancer (30,31).
LRP was identified as an independent prognostic factor for overall
survival in advanced NSCLC treated with DDP-based chemotherapy
(32–34).
C-myc is an important proto-oncogene associated with
tumor occurrence and development; its abnormal expression is
significant in promoting cell division and proliferation (35). C-myc has been demonstrated to
function in numerous cellular processes, including cell
proliferation, differentiation and transformation. In addition,
c-myc influences cellular sensitivity to DDP (36). Analysis of a panel of ovarian
cancer cell lines demonstrated that c-myc protein expression levels
were higher in cisplatin-resistant cells when compared with their
cisplatin-resistant counterparts. Furthermore, silencing of c-myc
by siRNA significantly reduced the tumor growth of
cisplatin-resistant cell xenografts (37). Xie et al (38) demonstrated that c-myc was important
in regulating DDP resistance in A549/DDP lung cancer cells.
Consequently, the current data indicates that niclosamide may
enhance the antitumor effect of DDP via suppression of LRP and
c-myc proteins, and niclosamide may be a potentially useful
therapeutic agent for the treatment of cisplatin-resistant human
lung cancer.
On the basis of our findings, combined treatment
with niclosamide and DDP may represent a novel and effective
strategy for treatment of NSCLC, including for those patients who
have already developed resistance to platinum-based therapy. An
in vivo animal model would assist in further investigating
the efficacy prior to clinical assessment.
Acknowledgements
The present study was supported by funding from the
National Natural Science Foundation of China (grant no. 81201736),
Natural Science Foundation of Guangdong Province, China (grant no.
2015A030310460), The Research Fund of Guangdong Medical University
(grant no. 2X14031).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al:
Metastatic non-small-cell lung cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
suppl 5:v1–v27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crinò L, Weder W, van Meerbeeck J and
Felip E; ESMO Guidelines Working Group, : Early stage and locally
advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21 Suppl 5:v103–v115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schuette WH, Gröschel A, Sebastian M,
Andreas S, Müller T, Schneller F, Guetz S, Eschbach C, Bohnet S,
Leschinger MI and Reck M: A randomized phase II study of pemetrexed
in combination with cisplatin or carboplatin as first-line therapy
for patients with locally advanced or metastatic non-small-cell
lung cancer. Clin Lung Cancer. 14:215–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lo Iacono M, Monica V, Vavalà T, Gisabella
M, Saviozzi S, Bracco E, Novello S, Papotti M and Scagliotti GV:
ATF2 contributes to cisplatin resistance in non-small cell lung
cancer and celastrol induces cisplatin resensitization through
inhibition of JNK/ATF2 pathway. Int J Cancer. 136:2598–2609. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang F, Duan S, Tsai Y, Keng PC and Chen
Y, Lee SO and Chen Y: Cisplatin treatment increases stemness
through upregulation of hypoxia-inducible factors by interleukin-6
in non-small cell lung cancer. Cancer Sci. 107:746–754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Hadiya BM: Niclosamide: Comprehensive
profile. Profiles Drug Subst Excip Relat Methodol. 32:67–96. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Gareeb AI, Aljubory KD and Alkuraishy
HM: Niclosamide as an anti-obesity drug: An experimental study. Eat
Weight Disord. 22:339–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chowdhury MK, Turner N, Bentley NL, Das A,
Wu LE, Richani D, Bustamante S, Gilchrist RB, Morris MJ, Shepherd
PR and Smith GC: Niclosamide reduces glucagon sensitivity via
hepatic PKA inhibition in obese mice: Implications for glucose
metabolism improvements in type 2 diabetes. Sci Rep. 7:401592017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang L, Yang M, Yuan Y, Li X and Kuang E:
Niclosamide inhibits lytic replication of Epstein-Barr virus by
disrupting mTOR activation. Antiviral Res. 138:68–78. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YM, Lu JW, Lin CC, Chin YF, Wu TY,
Lin LI, Lai ZZ, Kuo SC and Ho YJ: Antiviral activities of
niclosamide and nitazoxanide against chikungunya virus entry and
transmission. Antiviral Res. 135:81–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morin F, Kavian N, Nicco C, Cerles O,
Chéreau C and Batteux F: Niclosamide prevents systemic sclerosis in
a reactive oxygen species-induced mouse model. J Immunol.
197:3018–3028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Li PK, Roberts MJ, Arend RC, Samant
RS and Buchsbaum DJ: Multi-targeted therapy of cancer by
niclosamide: A new application for an old drug. Cancer Lett.
349:8–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li R, Hu Z, Sun SY, Chen ZG, Owonikoko TK,
Sica GL, Ramalingam SS, Curran WJ, Khuri FR and Deng X: Niclosamide
overcomes acquired resistance to erlotinib through suppression of
STAT3 in non-small cell lung cancer. Mol Cancer Ther. 12:2200–2212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Chen X, Ward T, Pegram M and Shen
K: Combined niclosamide with cisplatin inhibits
epithelial-mesenchymal transition and tumor growth in
cisplatin-resistant triple-negative breast cancer. Tumour Biol.
37:9825–9835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klimaszewska-Wisniewska A,
Halas-Wisniewska M, Tadrowski T, Gagat M, Grzanka D and Grzanka A:
Paclitaxel and the dietary flavonoid fisetin: A synergistic
combination that induces mitotic catastrophe and autophagic cell
death in A549 non-small cell lung cancer cells. Cancer Cell Int.
16:102016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang MH, Moon SU, Sung JH, Kim JW, Lee KW,
Lee HS, Lee JS and Kim JH: Antitumor activity of HM781-36B, alone
or in combination with chemotherapeutic agents, in colorectal
cancer cells. Cancer Res Treat. 48:355–364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Zhou H, Yu Y, Li J, Li H, Jiang
D, Chen Z, Yang D, Xu Z and Yu Z: Combination of gambogic acid with
cisplatin enhances the antitumor effects on cisplatin-resistant
lung cancer cells by downregulating MRP2 and LRP expression. Onco
Targets Ther. 9:3359–3368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tessari M, Pilla M, Andreoli M, Hutcheson
DM and Heidbreder CA: Antagonism at metabotropic glutamate 5
receptors inhibits nicotine- and cocaine-taking behaviours and
prevents nicotine-triggered relapse to nicotine-seeking. Eur J
Pharmacol. 499:121–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chong CR and Sullivan DJ Jr: New uses for
old drugs. Nature. 448:645–646. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Osada T, Chen M, Yang XY, Spasojevic I,
Vandeusen JB, Hsu D, Clary BM, Clay TM, Chen W, Morse MA and Lyerly
HK: Antihelminth compound niclosamide downregulatesWnt signaling
and elicits antitumor responses in tumors with activating APC
mutations. Cancer Res. 71:4172–4182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin Y, Lu Z, Ding K, Li J, Du X, Chen C,
Sun X, Wu Y, Zhou J and Pan J: Antineoplastic mechanisms of
niclosamide in acute myelogenous leukemia stem cells: Inactivation
of the NF-kappaB pathway and generation of reactive oxygen species.
Cancer Res. 70:2516–2527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li R, You S, Hu Z, Chen ZG, Sica GL, Khuri
FR, Curran WJ, Shin DM and Deng X: Inhibition of STAT3 by
niclosamide synergizes with erlotinib against head and neck cancer.
PLos One. 8:e746702013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scheffer GL, Wijngaard PL, Flens MJ,
Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC and
Scheper RJ: The drug resistance-related protein LRP is the human
major vault protein. Nat Med. 1:578–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Filipits M, Pohl G, Stranzl T, Suchomel
RW, Scheper RJ, Jäger U, Geissler K, Lechner K and Pirker R:
Expression of the lung resistance protein predicts poor outcome in
de novo acute myeloid leukemia. Blood. 91:1508–1513.
1998.PubMed/NCBI
|
|
29
|
Dalton WS and Scheper RJ: Lung
resistance-related protein: Determining its role in multidrug
resistance. J Natl Cancer Inst. 91:1604–1605. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kerr EH, Frederick PJ, Egger ME, Stockard
CR, Sellers J, DellaManna D, Oelschlager DK, Amm HM, Eltoum IE,
Straughn JM, et al: Lung resistance-related protein (LRP)
expression in malignant ascitic cells as a prognostic marker for
advanced ovarian serous carcinoma. Ann Surg Oncol. 20:3059–3065.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuji K, Wang YH, Takanashi M, Odajima T,
Lee GA, Sugimori H and Motoji T: Overexpression of lung
resistance-related protein and P-glycoprotein and response to
induction chemotherapy in acute myelogenous leukemia. Hematol Rep.
4:e182012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Li ZN, Du YJ, Li XQ, Bao QL and Chen
P: Expression of MRP1, BCRP, LRP, and ERCC1 in advanced
non-small-cell lung cancer: Correlation with response to
chemotherapy and survival. Clin Lung Cancer. 10:414–421. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Li ZN, Yu LC, Bao QL, Wu JR, Shi SB
and Li XQ: Association of expression of MRP1, BCRP, LRP and ERCC1
with outcome of patients with locally advanced non-small cell lung
cancer who received neoadjuvant chemotherapy. Lung Cancer.
69:116–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang W, Mao Y, Zhan Y, Huang J, Wang X,
Luo P, Li LI, Mo D, Liu Q, Xu H and Huang C: Prognostic
implications of survivin and lung resistance protein in advanced
non-small cell lung cancer treated with platinum-based
chemotherapy. Oncol Lett. 11:723–730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biliran H Jr, Banerjee S, Thakur A, Sarkar
FH, Bollig A, Ahmed F, Wu J, Sun Y and Liao JD: c-Myc-induced
chemosensitization is mediated by suppression of cyclin D1
expression and nuclear factor-kappa B activity in pancreatic cancer
cells. Clin Cancer Res. 13:2811–2821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Torigoe T, Izumi H, Ishiguchi H, Yoshida
Y, Tanabe M, Yoshida T, Igarashi T, Niina I, Wakasugi T, Imaizumi
T, et al: Cisplatin resistance and transcription factors. Curr Med
Chem Anticancer Agents. 5:15–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reyes-González JM, Armaiz-Peña GN, Mangala
LS, Valiyeva F, Ivan C, Pradeep S, Echevarría-Vargas IM,
Rivera-Reyes A, Sood AK and Vivas-Mejía PE: Targeting c-MYC in
platinum-resistant ovarian cancer. Mol Cancer Ther. 14:2260–2269.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie C, Pan Y, Hao F, Gao Y, Liu Z, Zhang
X, Xie L, Jiang G, Li Q and Wang E: C-Myc participates in
β-catenin-mediated drug resistance in A549/DDP lung adenocarcinoma
cells. APMIS. 122:1251–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|