Introduction
Osteoporosis is a major public health problem,
affecting over 200 million individuals worldwide (1). It is reported that ~40% of Caucasian
postmenopausal women exhibit osteoporosis and the prevalence is
expected to continue to escalate in the near future with an
increasingly elderly population (2). In postmenopausal women, there is
inordinate bone resorption relative to bone redeposition, caused by
hormone deficiencies (3).
Postmenopausal osteoporosis is directly linked to the decline in
estrogen (E2), which can increase the generation and activity of
osteoclasts (4).
As E2 is a well-documented factor for bone
maintenance and hormone replacement therapy (HRT) has been
demonstrated to possess beneficial effects on postmenopausal
osteoporosis (5). HRT
significantly decreased vertebral fracture risk and increased bone
mineral density (BMD) in postmenopausal women with osteoporosis
(6). However, severe adverse
effects of long-term HRT have been reported, including breast and
endometrial cancer (7,8). Depending on the effects of suppressed
bone turnover, bisphosphonates are widely used for osteoporosis
following marked reductions in HRT prescribing. Despite showing
marked anti-resorptive effects, troublesome side effects of
bisphosphonates have been reported, including atrial fibrillation,
esophageal cancer, atypical femoral fractures and osteonecrosis of
the jaw (9–11). Due to these limitations of
osteoporosis therapies, there has been growing interest in
alternative therapies from natural sources (12).
Cynanchum wilfordii root has been used in
traditional Korean medicine for the treatment of geriatric and
musculoskeletal disease including hair graying, impotence and
muscle/bone weakness (13).
Several studies have suggested that C. wilfordii has
ameliorative effects on hypertension, hypercholesterolemia, gastric
disorders and tumors (14–17). A study (18) identified that a herbal formula
containing extracts of C. wilfordii attenuates various
menopausal symptoms including hot flushes, insomnia, night sweats
and vaginal dryness without any influence on the female hormone
levels.
However, the anti-osteoporotic effects of C.
wilfordii have not been established. The present study
investigated the anti-osteoporotic effects of C. wilfordii
water extract (CW) and its mechanisms in ovariectomized
(OVX)-induced osteoporosis mice and in human osteoblast-like Saos-2
cells.
Materials and methods
Preparation of CW
The roots of C. wilfordii Hemsley was
obtained from Jung-do Herb, Co. Ltd. (Guri, Korea). A total of 20 g
of C. wilfordii were extracted with 200 ml distilled water
for 24 h at room temperature (RT). The extract was concentrated in
a rotary vacuum evaporator, and designated CW (yield: 13.7%). A
voucher specimen (CW-W100) was deposited at the department of
Convergence Korean Medical Science, Kyung Hee University.
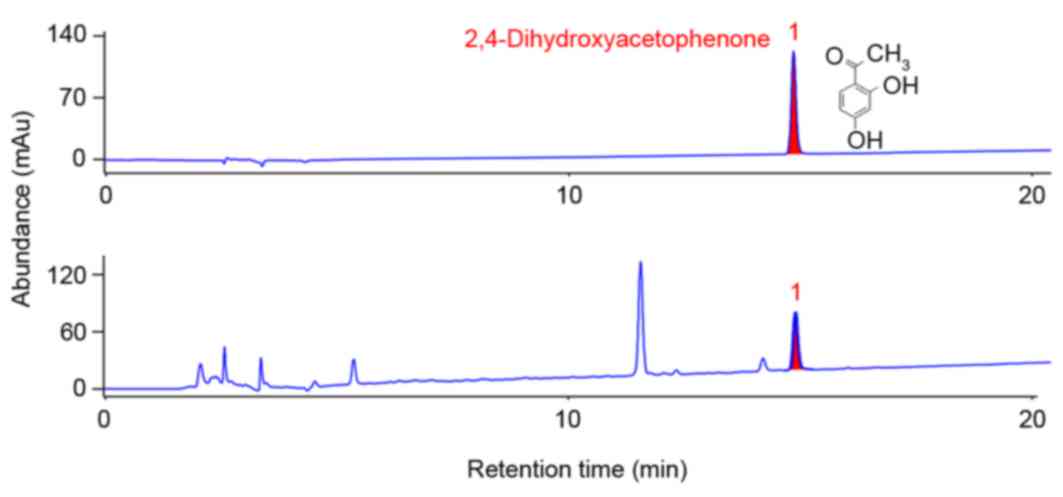
For quantification of CW, 2,4-dihydroxyacetophenone
was used as a standard. The content of 2,4-dihydroxyacetophenone
was measured using a high-performance liquid
chromatography-evaporative light scattering detector (HPLC-ELSD;
Agilent 1100 series; Agilent Technologies, Inc., Santa Clara, CA,
USA). The extract was dissolved in 70% methanol and sonicated for
30 min. After filtering through a 0.45 µm filter membrane, an
aliquot was injected in HPLC analysis. The column used was a
Shiseido Capcell Pak C18 (250×4.6 mm, 5 µm; Shiseido Co., Ltd.,
Tokyo, Japan). The mobile phase consisted of 0.05% acetic acid in
water and acetonitrile with 1.0 ml/min of flow rate at 30°C. As
demonstrated in Fig. 1, the
concentration of 2,4-dihydroxyacetophenone in CW was 13.689 µg/ml
(0.091%).
Ovariectomy-induced animals and in
vivo treatment
A total of 36 female ICR mice aged 6 weeks (Raon Bio
Animal, Inc., Yong-in, Korea) were provided free access to a
standard chow diet (Orient Co. Ltd., Seongnam, Korea) and tap
water. They were housed in a controlled environment (22±2°C, a
relative humidity of 50±5% and a 12 h light:dark cycle). The animal
studies were conducted in accordance with the rules and regulations
established by the Institutional Animal Ethics Committee of the
Kyung Hee University [KHUASP (SE) −15-079].
After acclimatization for 1 week, the mice, with the
exception of the normal group, were surgically ovariectomized
(OVX), then recovered and osteoporosis induced for 9 weeks. They
were randomly divided into three groups (OVX + vehicle, OVX + E2,
and OVX + CW). 17β-estradiol (E2; 10 µg/kg/day) was injected
intraperitoneally to the OVX + E2 group as a positive control and 1
mg/kg/day CW orally administrated to OVX + CW group. Normal and OVX
+ vehicle mice were orally administrated vehicle (in PBS containing
1% DMSO). All mice were treated 5 times per week for 3 weeks, then
sacrificed. The body weight was measured weekly. The blood sample
was collected by cardiac puncture.
Bone histopathology for hematoxylin
and eosin (H&E) and tartrate resistant acid phosphatase (TRAP)
staining
The epicondyles were removed and immediately fixed
in 10% formalin for 18 h. Prior to dehydration, bone tissues were
demineralized in 0.1 M ethylenediaminetetraacetic acid for 1 month.
The sections of epicondyle were cut at a 5 µm thickness and stained
with H&E or an Acid Phosphatase, Leukocyte TRAP kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Stained tissues
were observed using Leica Application Suite microscope software
(version 3.2.276.2; Leica Microsystems, Buffalo Grove, IL,
USA).
Measurement of bone mineral content
(BMC) and BMD
Subsequent to sacrifice, the proximal femur was
collected and cleaned without attached muscles and connective
tissue. The sample was stored at −80°C in PBS until analysis.
Dual-energy X-ray absorptiometry with a PIXImus instrument (Lunar
Corp., Madison, WI, USA) was used for the determination of BMC and
BMD.
Serum analysis
The collected blood was centrifuged at 12,000 × g
for 30 min at RT and the supernatant stored at −80°C until use. The
concentration of serum osteocalcin was measured using Mouse
Gla-osteocalcin high sensitive EIA kit (TaKaRa Bio, Inc., Otsu,
Japan) according to the manufacturer's protocol.
Cell culture and treatment
Saos-2 cells (human osteosarcoma cell line; Korean
Cell Line Bank, Seoul, Korea) were cultured with Dulbecco's
modified Eagle's medium (DMEM) with 10% fetal bovine serum and
antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in 5%
humidified CO2 atmosphere. Saos-2 cells were plated in
6-well culture plates at 0.8×105cells/well. CW (1, 10
and 100 µg/ml) in FBS-free DMEM medium was administered for 24
h.
Western blot analysis
RIPA buffer (50 mM Tris-HCl; pH 7.4, 1% Nonidet
P-40, 0.5% sodium deoxycholate, 150 mM NaCl) containing protease
inhibitors (Roche Diagnostics, Indianapolis, IN, USA) was used for
uterus and Saos-2 cell protein extraction. The lysate (30 µg) was
denatured with 2X loading buffer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and separated on a 10% sodium dodecyl sulfate
(SDS)-polyacrylamide gel, and then electrotransferred onto a PVDF
membrane (Bio-Rad Laboratories, Inc.). Primary antibodies targeting
osterix (cat. no. Ab94744; Abcam, Cambridge, UK), osteoprotegerin
(cat. no. sc-11383; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and β-actin (cat. no. sc-47778; Santa Cruz Biotechnology,
Inc.) in TBS-T (1:1,000 dilution) were incubated overnight at 4°C
and secondary antibody anti-mouse IgG (1:2,000 dilution; Cell
Signaling Technology, Inc.) in TBS-T was incubated for 1 h at RT.
The proteins were visualized using an enhanced chemiluminescence
detection system (GE Healthcare Life Sciences, Uppsala, Sweden).
Visualized bands were quantified using a computerized densitometry
system ImageJ (version 1.38e; National Institutes of Health,
Bethesda, MD, USA). All samples were analyzed in triplicate.
Statistical analysis
Significance was determined by one-way analysis of
variance and Dunnett's multiple comparison tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
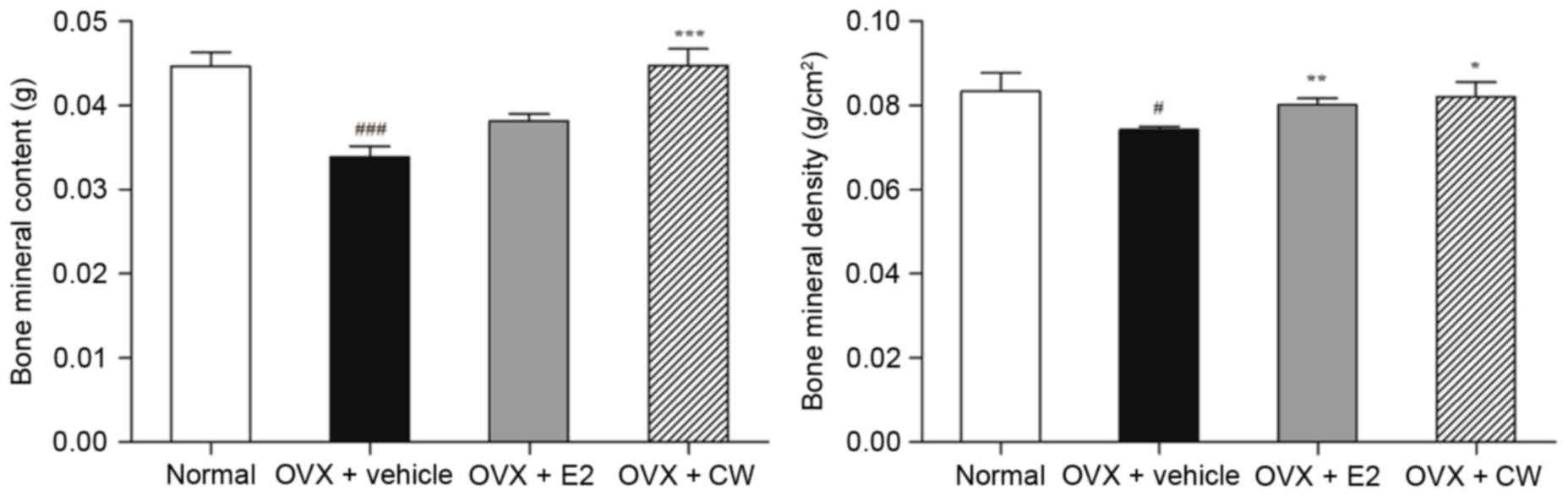
Effect of CW on BMC and BMD
The BMC level in the OVX + vehicle group was
significantly decreased to 0.034±0.003 g compared with the normal
group (0.045±0.004 g). CW treatment significantly increased the
level of BMC ~32% (0.045±0.004 g; Fig.
2). In addition, the BMD level of the OVX + vehicle group
(0.074±0.001 g/cm2) was significantly lowered ~10.8%
compared with the normal group (0.083±0.008 g/cm2).
There was a 10.5% increase in BMD level following treatment of CW
(0.082±0.006 g/cm2; Fig.
2). Taken together, CW treatment demonstrated recoveries of BMC
in addition to BMD levels.
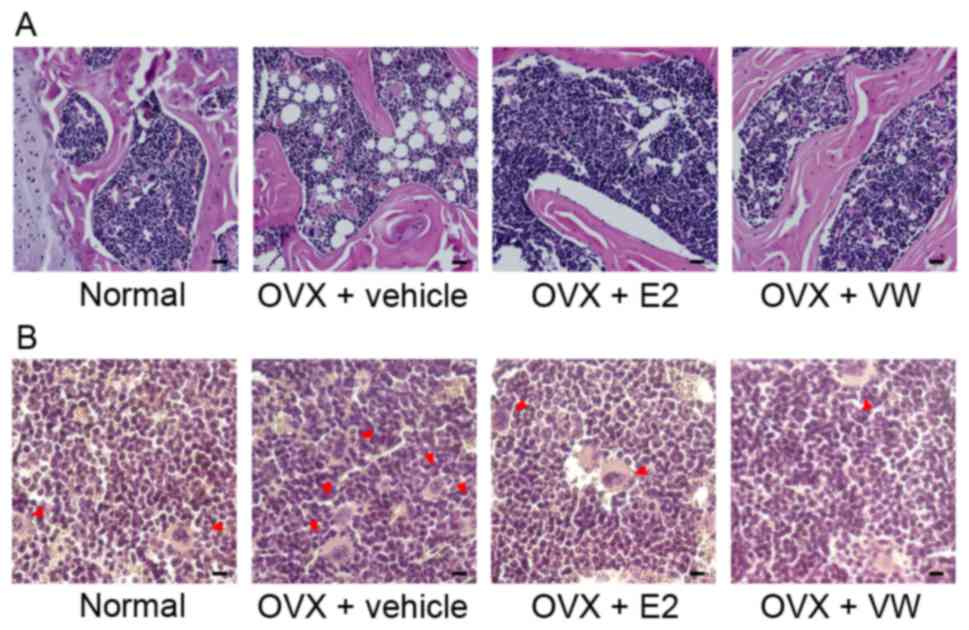
Effect of CW on histological changes
of epicondyles
In the OVX + vehicle group, the pores within
interstitial cells filling the lateral and medial epicondyles were
markedly increased in comparison with the normal group. E2
injection as a positive control drug ameliorated histopathological
changes of epicondyles. As recoveries in the E2 injected group,
CW-treated mice demonstrated dense and well-formed bone marrow
cells. Additionally, CW treatment reduced the bone marrow pores in
the lateral and medial epicondyles (Fig. 3A).
Effect of CW on TRAP-positive
osteoclasts in epicondyles
The OVX + vehicle group demonstrated substantial
increases of TRAP-stained multinucleated osteoclasts compared with
normal mice. In lateral and medial epicondyles of the
CW-administrated group, stained TRAP-positive cells were fewer
compared with the OVX + E2 group (Fig.
3B).
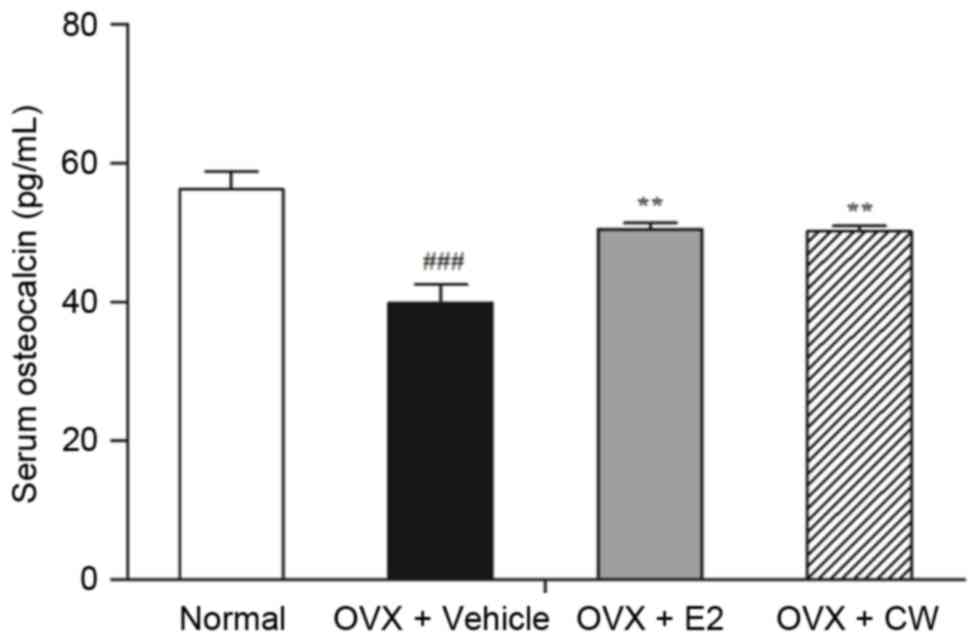
Effect of CW on serum osteocalcin
concentration
The value of serum osteocalcin concentration was
significantly lower in the OVX + vehicle group compared with the
normal group. While the serum concentration of osteocalcin in
normal group was 56.26±2.6 pg/ml, that in the OVX + vehicle group
was 39.91±2.69 pg/ml; a significant ~29.06% decrease. Following
treatment by CW, serum osteocalcin level demonstrated a 25.93%
recovery (50.26±0.7 pg/ml; Fig.
4).
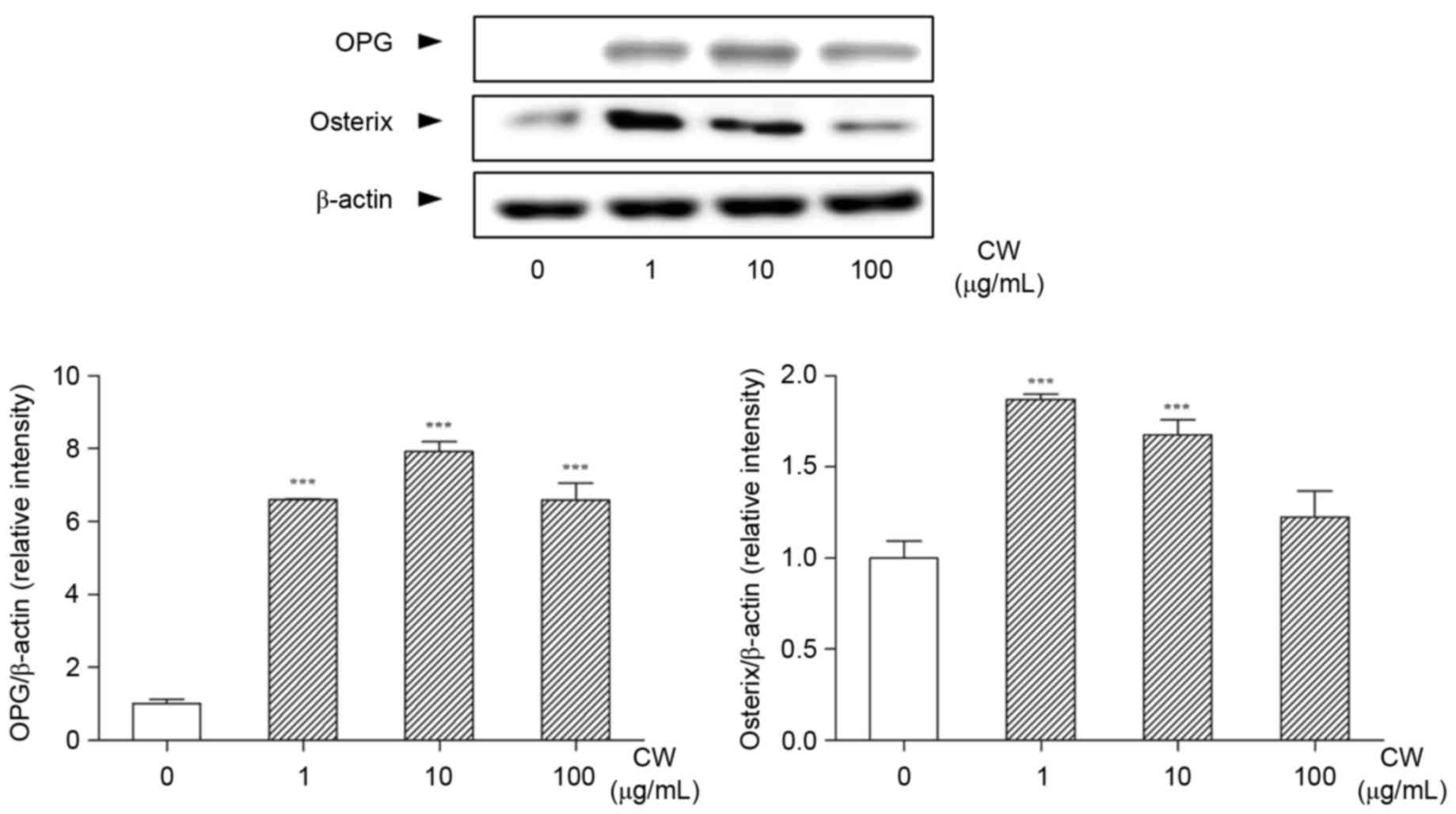
Effect of CW on bone
differentiation-related markers in Saos-2 osteoblast cells
When the cells were treated with various
concentrations of CW, the expression of OPG was significantly
increased (Fig. 5). In addition,
the expression of osterix in Saos-2 cells was increased by CW
treatment compared with non-treated cells (Fig. 5).
Discussion
Osteoporosis is a skeletal disorder characterized by
low BMD level and microarchitectural deterioration of bone tissue
(19). Several prospective studies
have demonstrated that decline in BMC or BMD is strongly associated
with an increased risk of fragility fractures (20–22).
The present study demonstrated that treatment with CW significantly
decreased the loss of BMC and BMD in the femur. In addition, the
improvement in bone mass was accompanied by complete normalization
of bone structure in CW-treated mice. Histological analysis
demonstrated that the bone marrow cells were restored to dense and
well-formed tissue with reduced bone marrow pores by CW treatment,
indicating that CW is efficacious in the maintenance of bone
integrity in osteoporosis and with the enhancement of BMD.
Osteoporotic bone results from a homeostatic
imbalance between bone resorption and bone formation (23). Continuous and well-balanced bone
remodeling by bone-resorbing osteoclasts and bone-forming
osteoblasts is essential to retain bone homeostasis, which is
fundamentally controlled by proliferation and maturation of
precursors of these two cells (24,25).
Accordingly, bone metabolism and activities of osteoclast and
osteoblast were examined to determine the improvement of
osteoporosis by CW treatment in vivo and in
vitro.
Osteoclasts secrete TRAP, a biomarker of osteoclast
differentiation, during bone resorption and its secretion is
identified to correlate positively with resorptive behavior
(26,27). Histopathological examination
demonstrated that OVX increased the number of TRAP-positive
multinucleated osteoclasts, as expected. There was a significant
decrease in the number of mature osteoclasts following CW
administration in OVX-induced osteoporotic mice. Osteoblastic
lineage cells are responsible for production of OPG, a secreted
member of the tumor-necrosis factor receptor family, which is a
crucial inhibitor of osteoclastogenesis (28). To elucidate the further mechanism
of CW on osteoclasts formation, the possible effects of CW on OPG
production in Saos-2 osteoblast cells was evaluated. In the present
study, the expression of OPG was notably increased by CW treatment
in vitro, which meant that the development, function and
survival rate of osteoclasts can be inhibited by CW. Together,
these results appear to suggest that CW treatment can inhibit bone
resorption by exerting an anti-osteoclastic effect due to the
increase of OPG.
Osteocalcin, also known as bone
gamma-carboxyglutamic acid-containing protein, is the most abundant
non-collagenous extracellular matrix protein (29). Serum osteocalcin concentration is
regarded as a bone-turnover marker closely associated with bone
formation (30). In particular,
osteocalcin is expressed in high amounts by osteoblasts during bone
matrix mineralization (31). In
the present study, serum osteocalcin was decreased by OVX and
partially recovered by CW treatment in vivo. To confirm the
effect of CW on osteoblast differentiation, the expression of
osterix was evaluated in Saos-2 cells. Osterix, an indispensable
transcription factor that regulates osteoblastogenesis and
osteogenesis, serves a critical role in the commitment and
differentiation of osteoblast precursor cells to osteoblasts
(32). The expression of osterix
in Saos-2 cells demonstrated a noticeable increase following CW
treatment, which provides evidence to support that CW promoted bone
formation by upregulating the osteoblast differentiation. These
results clearly indicate that CW treatment can expedite recovery
from bone loss by inducing the development of mature osteoblasts
and the formation of bone tissue.
Taken together, treatment of CW maintained the bone
integrity, inhibited the osteoclast formation and induced the
osteoblast differentiation. These results suggest that CW has
ameliorative effects on osteoporosis and could be used as a
treatment for osteoporosis. Further studies are required to clarify
the molecular mechanisms through which CW ameliorates
osteoporosis.
Acknowledgements
This work was supported by Samik Dairy & Food
Co., Ltd. (Seoul, Korea).
References
|
1
|
Cooper C, Campion G and Melton LJ III: Hip
fractures in the elderly: A world-wide projection. Osteoporos Int.
2:285–289. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melton LJ III, Chrischilles EA, Cooper C,
Lane AW and Riggs BL: Perspective. How many women have
osteoporosis? J Bone Miner Res. 7:1005–1010. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holroyd C, Cooper C and Dennison E:
Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab.
22:671–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zallone A: Direct and indirect estrogen
actions on osteoblasts and osteoclasts. Ann N Y Acad Sci.
1068:173–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nelson HD, Humphrey LL, Nygren P, Teutsch
SM and Allan JD: Postmenopausal hormone replacement therapy:
Scientific review. JAMA. 288:872–881. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishida Y and Kawai S: Comparative efficacy
of hormone replacement therapy, etidronate, calcitonin,
alfacalcidol, and vitamin K in postmenopausal women with
osteoporosis: The Yamaguchi osteoporosis prevention study. Am J
Med. 117:549–555. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross RK, Paganini-Hill A, Wan PC and Pike
MC: Effect of hormone replacement therapy on breast cancer risk:
Estrogen versus estrogen plus progestin. J Natl Cancer Inst.
92:328–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grady D, Gebretsadik T, Kerlikowske K,
Ernster V and Petitti D: Hormone replacement therapy and
endometrial cancer risk: A meta-analysis. Obstet Gynecol.
85:304–313. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McClung M, Harris ST, Miller PD, Bauer DC,
Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK and Lewiecki EM:
Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug
holiday. Am J Med. 126:13–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Russell RG and Rogers MJ: Bisphosphonates:
From the laboratory to the clinic and back again. Bone. 25:97–106.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Udell JA, Fischer MA, Brookhart MA,
Solomon DH and Choudhry NK: Effect of the women's health initiative
on osteoporosis therapy and expenditure in Medicaid. J Bone Miner
Res. 21:765–771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banu J, Varela E and Fernandes G:
Alternative therapies for the prevention and treatment of
osteoporosis. Nutr Rev. 70:22–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee BJ and Lee K: Discrimination and
proper use of polygoni multiflori radix, cynanchi wilfordii radix,
and cynanchi auriculati radix in Korea: A descriptive review. Evid
Based Complement Alternat Med. 2015:8273802015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi DH, Lee YJ, Kim JS, Kang DG and Lee
HS: Cynanchum wilfordii ameliorates hypertension and endothelial
dysfunction in rats fed with high fat/cholesterol diets.
Immunopharmacol Immunotoxicol. 34:4–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HS, Choi JH, Kim YE, Kim IH, Kim BM
and Lee CH: Effects of the cynanchum wilfordii ethanol extract on
the serum lipid profile in hypercholesterolemic rats. Prev Nutr
Food Sci. 18:157–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shan L, Liu RH, Shen YH, Zhang WD, Zhang
C, Wu DZ, Min L, Su J and Xu XK: Gastroprotective effect of a
traditional Chinese herbal drug ‘Baishouwu’ on experimental gastric
lesions in rats. J Ethnopharmacol. 107:389–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MS, Baek JH, Park JA, Hwang BY, Kim
SE, Lee JJ and Kim KW: Wilfoside K1N isolated from Cynanchum
wilfordii inhibits angiogenesis and tumor cell invasion. Int J
Oncol. 26:1533–1539. 2005.PubMed/NCBI
|
|
18
|
Chang A, Kwak BY, Yi K and Kim JS: The
effect of herbal extract (EstroG-100) on pre-, peri-and
post-menopausal women: A randomized double-blind,
placebo-controlled study. Phytother Res. 26:510–516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Compston J: Clinical and therapeutic
aspects of osteoporosis. Eur J Radiol. 71:388–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hui SL, Slemenda CW and Johnston CC Jr:
Age and bone mass as predictors of fracture in a prospective study.
J Clin Invest. 81:1804–1809. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cummings SR, Black DM, Nevitt MC, Browner
W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J and Vogt TM:
Bone density at various sites for prediction of hip fractures. The
Study of Osteoporotic Fractures Research Group. Lancet. 341:72–75.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melton LJ III, Atkinson EJ, O'Fallon WM,
Wahner HW and Riggs BL: Long-term fracture prediction by bone
mineral assessed at different skeletal sites. J Bone Miner Res.
8:1227–1233. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suda T, Nakamura I, Jimi E and Takahashi
N: Regulation of osteoclast function. J Bone Miner Res. 12:869–879.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kirstein B, Chambers TJ and Fuller K:
Secretion of tartrate-resistant acid phosphatase by osteoclasts
correlates with resorptive behavior. J Cell Biochem. 98:1085–1094.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Minkin C: Bone acid phosphatase:
Tartrate-resistant acid phosphatase as a marker of osteoclast
function. Calcif Tissue Int. 34:285–290. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hauschka PV, Lian JB, Cole DE and Gundberg
CM: Osteocalcin and matrix Gla protein: Vitamin K-dependent
proteins in bone. Physiol Rev. 69:990–1047. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brown JP, Delmas PD, Malaval L, Edouard C,
Chapuy MC and Meunier PJ: Serum bone Gla-protein: A specific marker
for bone formation in postmenopausal osteoporosis. Lancet.
1:1091–1093. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stein GS, Lian JB, Van Wijnen AJ, Stein
JL, Montecino M, Javed A, Zaidi SK, Young DW, Choi JY and Pockwinse
SM: Runx2 control of organization, assembly and activity of the
regulatory machinery for skeletal gene expression. Oncogene.
23:4315–4329. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|