Introduction
Inflammatory bowel disease (IBD) is an idiopathic
inflammatory disorder involving the mucosa and submucosa of the
colon. The main clinical manifestations of IBD include abdominal
pain, diarrhea and purulent stools. IBD provokes an abnormal,
exacerbated immune response in the intestine (1), the initiation and progression of
which is likely to be associated with certain internal and external
environmental factors (1). As a
result, the synthesis and release of various proinflammatory
mediators, including reactive oxygen and nitrogen metabolites,
eicosanoids, cytokines and chemokines are upregulated in IBD. There
is currently no cure for this disorder, and clinical management
focuses on downregulation of the exacerbated immune response, using
drugs such as aminosalicylates, glucocorticoids and
immunosuppressants, such as sulfasalazine; however, long-term use
of these drugs often results in serious side effects. Biological
drugs, such as infliximab and adalimumab, have shown great
potential as therapeutics for IBD; however, their exorbitant costs
and potential for serious adverse effects limit their long-term
use. Recently, much attention has been focused on the inhibitory
effects of some active ingredients from natural herbs on
inflammatory responses in human IBD or experimental colitis
(2).
Cortex Magnoliae officinalis is an herbal
supplement in traditional Chinese medicine, which is commonly used
to ameliorate microbial infection, inflammation and
gastrointestinal disorders (3).
Phytochemical studies have demonstrated that this herb is rich in a
large number of bioactive substances, including magnolol and
honokiol. Magnolol possesses various biological activities,
including antioxidative (4,5),
antibacterial (6),
anti-inflammatory (7), and
antiulcer activities (8). An
increasing amount of evidence has suggested that oxidative damage
serves a key role in the development of tissue destruction in
patients with IBD, and modulating free radical production may
represent a novel direction for IBD therapy (9,10).
Besides the antioxidative activity, previous studies have also
shown an interest in using magnolol for the treatment of acute
inflammatory conditions (11,12).
The present study aimed to investigate whether magnolol exhibits
protective effects in TNBS-induced colitis through its
anti-inflammatory activity.
Materials and methods
Chemicals
Sulfasalazine was purchased from Shanghai Xinyi
Jiahua Pharmaceutical Co., Ltd. (Shanghai, China); magnolol
(purity, 98%) was purchased from Phytomarker, Ltd. (Tianjin,
China); and 2,4,6-trinitrobenzenesulfonic acid (TNBS) was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). All other
chemicals used were of analytical grade.
Animals and colitis modeling
A total of 48 male Wistar rats (age, 6–8 weeks;
weight, 200±20 g), of specific-pathogen-free grade, were obtained
from the Military Medical Science Academy (Tianjin, China). All
rats were provided with tap water and food ad libitum, and
maintained at a controlled temperature (22±2°C) and humidity
(50–70%). The animals were maintained in a 12 h light/dark cycle
throughout the experiment in the Animal Center of the Radiation
Medicine Institute, Chinese Academy of Medical Sciences (Tianjin,
China). This study was conducted in compliance with the National
Institutes of Health Guidelines for the Care and Use of Laboratory
Animals (8th edition, China) and received approval from the Animal
Care and Use Committee of Tianjin Medical University (Tianjin,
China).
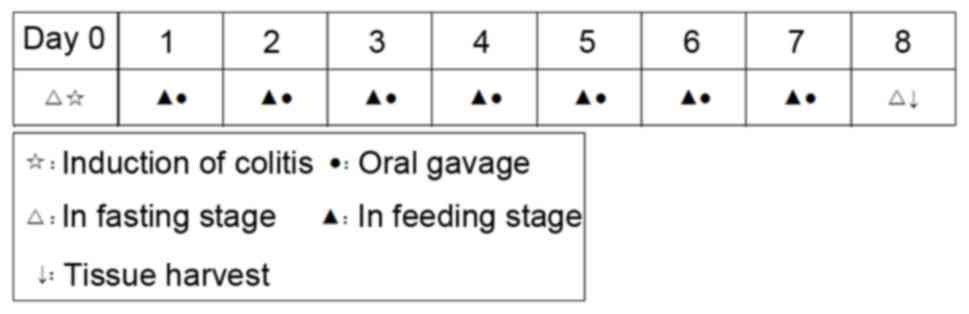
A schematic representation of the experimental
process is presented in Fig. 1.
Colitis was induced on day 0 according to the method described by
Morris et al (13).
Briefly, all animals were fasted, with ad libitum access to
tap water, for 24 h prior to the induction of colitis. Rats were
kept in light narcosis by the administration of 10% chloral hydrate
[300 mg/kg, intraperitoneal (i.p.)]. A modified urinary catheter
with an external diameter of 2 mm was inserted through the rectum
and into the colon ~8 cm proximal to the anus. TNBS dissolved in
50% ethanol was introduced into the colon through the catheter at a
dose of 100 mg/kg in the colitis groups. Saline with vehicle
(ethanol) was given to normal control rats. Animal behavior, body
weight, stool consistency and fecal occult blood tests were
monitored daily throughout the experiment.
Animals were assigned to six groups using a random
number table method (n=8/group). A total of 24 h after the
induction of colitis, doses equivalent to 15, 30 and 60 mg/kg body
weight (bw) magnolol were suspended in 5% sodium carboxymethyl
cellulose (CMC-Na) solution. Low-, medium- and high-doses magnolol
groups received doses of 15, 30 and 60 mg/kg bw magnolol,
respectively. Rats in the normal control and TNBS groups received a
comparable volume of the 5% CMC-Na vehicle solution. Positive
control rats received sulfasalazine (500 mg/kg bw). Drugs were
administered once daily by oral gavage (10 µl/g bw). All
intervention regimens lasted for 7 days. On the 8th day, after a 24
h fast, the abdominal cavity was opened via a middle incision under
chloral hydrate (300 mg/kg bw) anesthesia, and blood was collected
using the abdominal aortic method, as previously described
(13), from which the serum was
separated after centrifugation at 1,500 × g at 4°C and stored at
−80°C until further analysis. All animals were anaesthetized by 10%
chloral hydrate and were sacrificed by cervical dislocation, and
the intestine between the ileocecal junction and anus was excised.
The weight/length (mg/cm) ratio was calculated and the colon was
immediately cut into sections. One segment (1 cm) was fixed in 4%
buffered formaldehyde for histopathological examination; an
additional 1 cm longitudinal piece was immediately snap-frozen in
liquid nitrogen and stored at −80°C for further biochemical
studies. The spleen and thymus were also excised and weighed.
Evaluation of disease activity index
(DAI)
The DAI score was determined by combining scores of:
i) Body weight loss, ii) stool consistency, and iii) fecal occult
blood as previously described (13). The average of the three values was
defined as the DAI. The body weight recorded on day 0 was
considered as the baseline, and body weight loss was calculated as
the percent difference between the initial body weight at baseline
and the body weight on the measurement day. Fecal occult blood
testing of stool samples was performed to detect occult bleeding
using a commercial kit (BA-2020B; Baso Diagnostics, Inc., Zhuhai,
China) following the manufacturer's instructions.
Evaluation of visceral indexes
The visceral index (spleen index/thymus index) was
defined as the ratio of the spleen/thymus weight (mg) to body
weight (g), whereas the colon weight/length ratio was calculated as
the ratio of colonic sample weight (mg) to length (cm).
Macroscopic scoring and
histopathological study
The colon mucosal damage index (CMDI) was scored on
a 0–8 scale by two observers blinded to treatments, according to
the criteria previously described (14), to evaluate any macroscopic colonic
damage caused by inflammation. Cross-sections were selected and
embedded in paraffin, and 5 µm serial sections were obtained and
stained with hematoxylin and eosin. The tissue damage index (TDI)
was assessed in accordance with previously described criteria
(14). Five different regions were
selected at random and examined under a Leica DM3000 light
microscope (Leica Microsystems GmbH, Wetzlar, Germany) with ×100
magnification, and images were captured using a Leica DFC-420C
Digital Camera system (Leica Microsystems GmbH).
Measurement of myeloperoxidase (MPO)
activity
The protein was extracted by RIPA cell lysate
(containing PMSF; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Protein concentration was detected using a modified
bicinchoninic acid method with a commercially available protein
assay kit (Sangon Biotech Co., Ltd., Shanghai, China), according to
the manufacturer's instructions. An MPO detection kit (A044;
Nanjing Jiancheng Biochemical Engineering Research Institute,
Nanjing, China) was used to determine MPO activity following the
instruction of the kit, using an Evolution 201 UV-Visible
spectrophotometer (Thermo Fisher Scientific, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
Serum levels of interleukin (IL)-6 and IL-17 were
quantified using ELISA (IL-16, D1600; IL-17, DY177; R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 50–100 mg colonic
tissue using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The concentrations and purity of samples were determined
using a NanoDrop™ 2000 spectrophotometer (NanoDrop;
Thermo Fisher Scientific, Inc., Wilmington, DE, USA), and 2 µg RNA
was reverse transcribed using a HiFi-MMLV cDNA kit (CW0744; Beijing
ComWin Biotech Co., Ltd., Beijing, China) following the
manufacturer's instructions. qPCR amplification and detection was
performed using a 7500 fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The SYBR®
Premix Ex Taq™ II (Takara Bio, Inc., Otsu, Japan)
and specific primers were annealed at 60°C. A protocol of 94°C
denaturation 45 sec, 59°C annealing 45 sec, 72°C extension 60 sec
for 32 cycles followed. The specific primers used for inducible
nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), Toll-like
receptor-4 (TLR-4), nuclear factor-κB p65 (NF-κB p65) and the
β-actin endogenous control are presented in Table I. Melting curve analysis was
performed on each sample to ensure single amplification, and
quantification cycle (Cq) values were measured. The ΔCq value was
calculated for each sample by subtracting the Cq value of the
endogenous control from the Cq value of the targeted gene (iNOS,
COX-2, TLR-4 and NF-κB p65). The relative expression levels of
targeted genes were calculated using the 2−ΔΔCq method
(15).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| iNOS | F:
ACATCAGGTCGGCCATCACT | 87 |
|
| R:
CGTACCGGATGAGCTGTGAATT |
|
| COX-2 | F:
TGTATGCTACCATCTGGCTTCGG | 94 |
|
| R:
GTTTGGAACAGTCGCTCGTCATC |
|
| TLR-4 | F:
AATCCCTGCATAGAGGTACTTCCTAAT | 107 |
|
| R:
CTCAGATCTAGGTTCTTGGTTGAATAAG |
|
| NF-κB p65 | F:
ACCTGGAGCAAGCCATTAGC | 100 |
|
| R:
CGGACCGCATTCAAGTCATA |
|
| β-actin | F:
GTCAGGTCATCACTATCGGCAAT | 147 |
|
| R:
AGAGGTCTTTACGGATGTCAACGT |
|
Immunohistochemistry
Immunohistochemical analysis was performed using the
streptavidin-biotin-peroxidase method as previously described
(16). Briefly, colonic segments
were fixed in 4% buffered formaldehyde, dehydrated in graded
ethanol, embedded in paraffin, and cut into 5 µm sections. All
sections were incubated with a buffered blocking solution
containing normal goat serum (Beyotime Institute of Biotechnology,
Haimen, China) for 15 min, which was followed by co-incubation with
the primary antibody (TLR-4, cat. no. BA1717, rabbit polyclonal;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) diluted
1:100, in a humidity chamber at 37°C for 3 h. After washing the
sections with phosphate-buffered saline, the sections were
incubated with secondary antibody (cat. no. BA1003, biotinylated
goat anti-rabbit; Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China) at 37°C for 15 min. Subsequently,
sections were rinsed with PBS and co-incubated with horseradish
peroxidase-conjugated streptavidin (cat. no. BA1088; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) at 37°C for 15 min.
Sections were counterstained with Harris hematoxylin for 2 min as
previously described (13) and
then images were captured under a Leica DM3000 microscope (Leica
Microsystems GmbH). Five random microscopic fields per section were
captured at ×400 magnification, and a threshold optical density was
obtained using the Image-Pro Plus 5.1 software (Media Cybernetics,
Inc., Rockville, MD, USA). For all analyses, total pixel intensity
was determined, and data were presented as optical densities.
Statistical analysis
The results were expressed as the mean ± standard
error. Statistical analysis was performed using SPSS 13.0 (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance and least
significant difference multiple comparison tests were used for data
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Magnolol protects against TNBS-induced
colitis
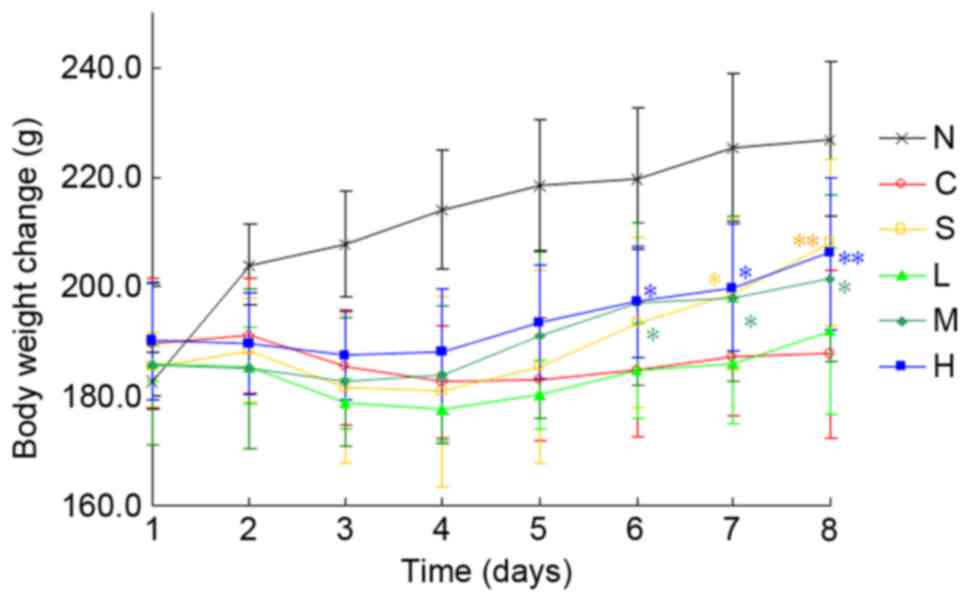
A total of 24 h after intracolonic instillation of
TNBS/ethanol, rats exhibited piloerection, hypomotility and
debilitation. Furthermore, TNBS/ethanol-treated rats suffered from
severe anorexia, which was followed by a sharp decline in food
intake and a gradual loss of body weight from day 1 to 3 (Fig. 2). These rats developed severe
diarrhea on day 2 after TNBS challenge, and rectal bleeding was
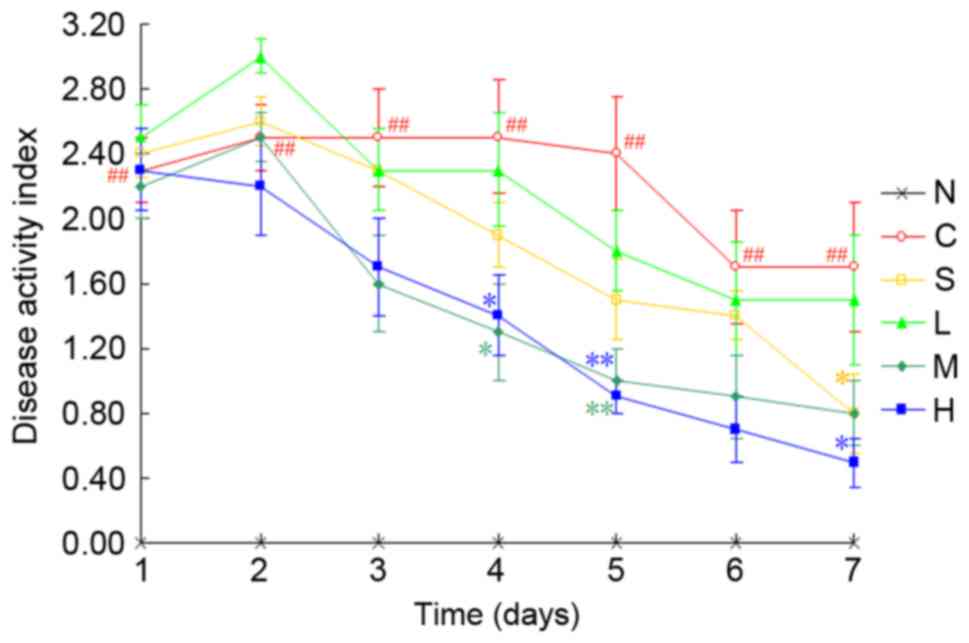
occasionally observed. A marked increase in DAI score from day 1
was observed in TNBS/ethanol-treated rats compared with the normal
control group (P<0.01; Fig. 3).
When compared with the TNBS control group, administration of
medium- or high-dose magnolol, or 500 mg/kg sulfasalazine, resulted
in a significant reduction in DAI scores (P<0.01 or
P<0.05).
Intracolonic administration of TNBS/ethanol markedly
increased the colonic weight/length ratio (P<0.01; Table II). All drug treatments exhibited
inhibitory effects on the TNBS-induced increase in colonic
weight/length ratio, however only high-dose magnolol significantly
reduced the elevated colonic weight/length ratio compared with the
TNBS control group (P<0.05). None of the drugs counteracted the
increase in spleen index (P>0.05), whereas the thymus index was
reduced by ~50% in the TNBS control rats compared with normal
control rats. Notably, all interventions, with the exception of
low-dose magnolol, resulted in a marked amelioration of the thymus
index (P<0.01).
 | Table II.Effects of magnolol on visceral
indexes and colonic weight/length ratios in rats, 7 days after the
induction of colitis with TNBS. |
Table II.
Effects of magnolol on visceral
indexes and colonic weight/length ratios in rats, 7 days after the
induction of colitis with TNBS.
| Treatment
group | Spleen index | Thymus index | Colonic
weight/length ratio (mg/cm) |
|---|
| N | 0.231±0.015 | 0.126±0.016 |
8.03±0.57 |
| C | 0.234±0.051 |
0.063±0.021a |
15.43±2.54a |
| S | 0.254±0.027 |
0.103±0.027b |
13.14±2.89a |
| L | 0.228±0.034 |
0.083±0.025a |
14.10±1.36a |
| M | 0.249±0.033 |
0.102±0.020b |
13.09±2.19a |
| H | 0.251±0.036 |
0.117±0.037b |
11.19±1.30a,c |
Macroscopic observation demonstrated colonic mucosal
damage with edema, hyperemia, deep ulcerations and focal adhesions
to adjacent organs in the TNBS control group (Fig. 4). Conversely, no macroscopic damage
was detected in the normal control group. Intracolonic instillation
of TNBS/ethanol markedly enhanced CMDI scores (P<0.01 vs. normal
control group) (Fig. 4G). CMDI was
improved substantially by magnolol when administered at doses of 30
and 60 mg/kg (P<0.05). However, 15 mg/kg magnolol and 500 mg/kg
sulfasalazine failed to mitigate macroscopic damage compared with
the TNBS control group (P>0.05).
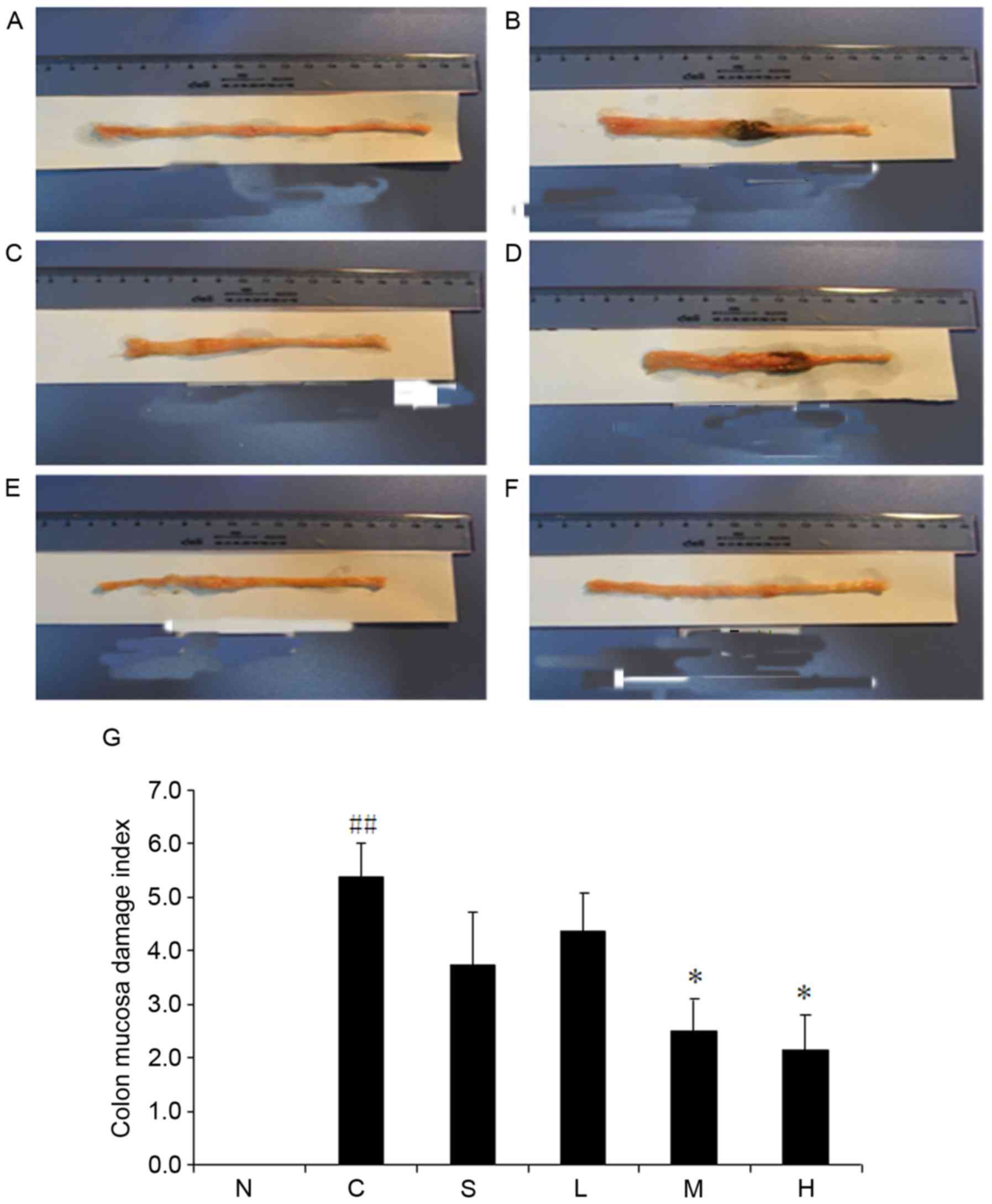 | Figure 4.Magnolol ameliorates
2,4,6-trinitrobenzenesulfonic acid/ethanol-induced upregulation of
colonic weight/length ratio. (A) Normal control, (B) model control
and (C) sulfasalazine groups, and (D) low dose (15 mg/kg), (E)
medium dose (30 mg/kg) and (F) high dose (60 mg/kg)
magnolol-treated groups. (G) Pooled data from each treatment group.
*P<0.05 vs. model control group; ##P<0.01 vs.
normal control group. N, normal control; C, model control; S,
sulfasalazine group; L, low (15 mg/kg) magnolol dose; M, medium (30
mg/kg) magnolol dose; H, high (60 mg/kg) magnolol dose. |
Compared with the normal group (Fig. 5A), the colonic samples from the
TNBS control group (Fig. 5B)
revealed extensive ulceration and inflammation involving all of the
intestinal layers. This inflammatory process was also associated
with severe goblet cell depletion. Different degrees of remission
were observed in all intervention groups with respect to the TNBS
control group. The 60 mg/kg magnolol group demonstrated the lowest
TDI compared with the TNBS control group (Fig. 5G); however, no difference was
observed in TDI between the 15 mg/kg magnolol group and the TNBS
control group (P>0.05).
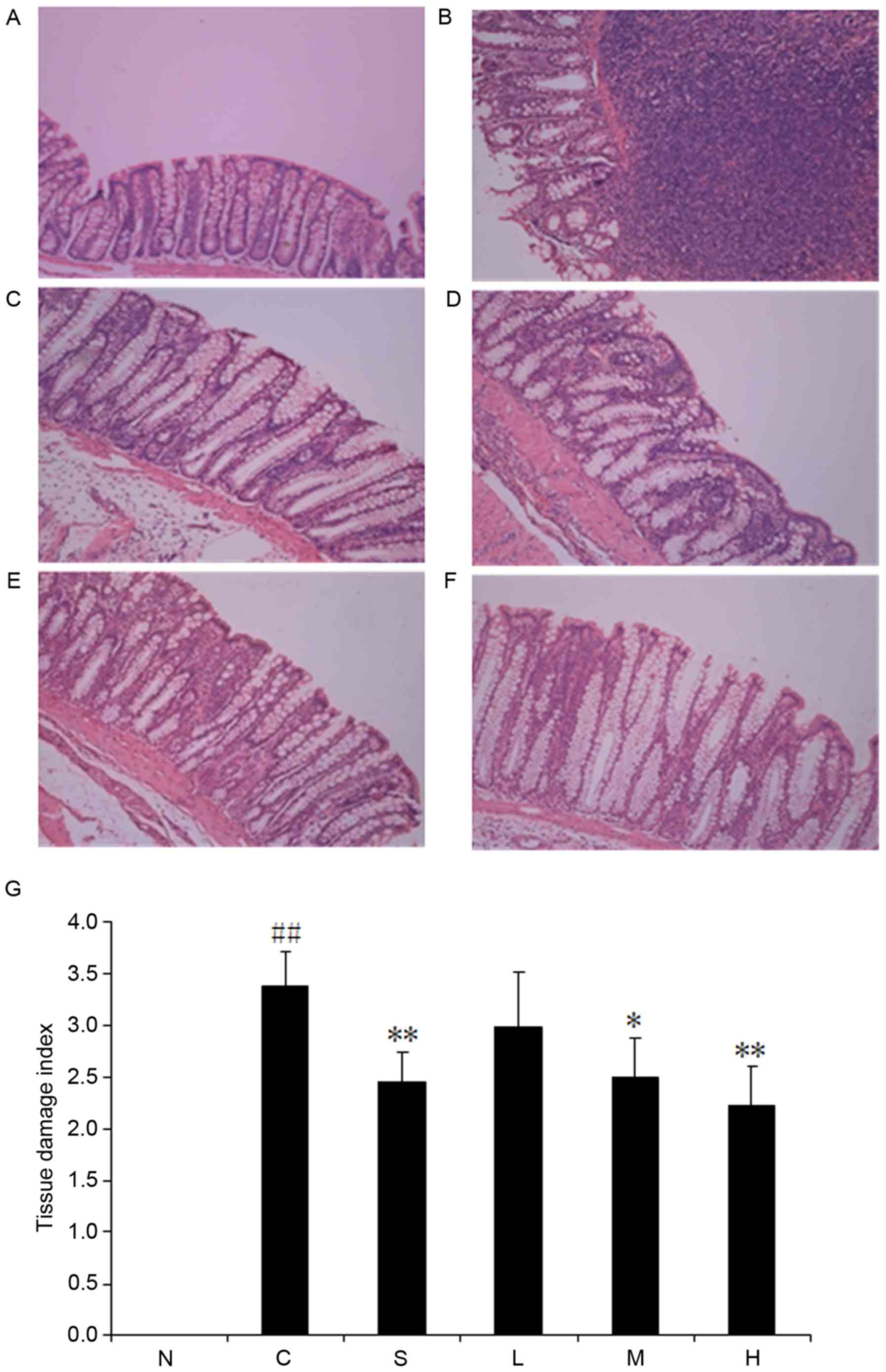 | Figure 5.Magnolol ameliorates
2,4,6-trinitrobenzenesulfonic acid/ethanol-induced ulceration and
inflammation. (A) Normal control, (B) model control and (C)
sulfasalazine groups, and (D) low dose (15 mg/kg), (E) medium dose
(30 mg/kg) and (F) high dose (60 mg/kg) magnolol-treated groups.
(G) Pooled data from each treatment group. *P<0.05, **P<0.01
vs. model control group; ##P<0.01 vs. normal control
group. N, normal control; C, model control; S, sulfasalazine group;
L, low (15 mg/kg) magnolol dose; M, medium (30 mg/kg) magnolol
dose; H, high (60 mg/kg) magnolol dose. |
Effects of magnolol on colonic MPO
activity in TNBS-induced colitis
MPO activity was significantly elevated upon
stimulation with TNBS/ethanol in colonic tissues (P<0.05;
Table III). Treatment with 15,
30 or 60 mg/kg magnolol, or 500 mg/kg sulfasalazine, resulted in
decreased MPO activity (P<0.05 vs. TNBS control group).
 | Table III.Effects of magnolol on colonic MPO
and serum cytokine activity in rats with
2,4,6-trinitrobenzenesulfonic acid-induced colitis. |
Table III.
Effects of magnolol on colonic MPO
and serum cytokine activity in rats with
2,4,6-trinitrobenzenesulfonic acid-induced colitis.
| Treatment
group | MPO activity (U/g
protein) | IL-6 (ng/l) | IL-17 (pg/l) |
|---|
| N | 14.99±1.39 | 41.44±6.06 | 26.05±1.62 |
| C |
21.07±3.74a |
64.37±4.21a |
33.07±1.29a |
| S |
15.95±2.41a |
48.82±10.95b |
26.78±2.62b |
| L |
18.43±2.55b |
49.52±9.73b |
28.37±3.22b |
| M |
15.38±1.76b |
46.71±10.07b |
27.28±2.30c |
| H |
14.74±2.15b |
45.41±3.61c |
27.07±2.06c |
Effects of magnolol on serum IL-6 and
IL-17 levels in rats with TNBS-induced colitis
Colonic injury by TNBS administration was
characterized by an increase in the production of the
proinflammatory cytokines IL-6 and IL-17. The levels of these
cytokines were significantly reduced in rats treated with all doses
of magnolol, or 500 mg/kg sulfasalazine (Table III).
Effects of magnolol on iNOS, COX-2,
TLR-4 and NF-κB p65 mRNA expression levels
The expression of inflammation-related genes was
detected following magnolol treatment. iNOS, COX-2, TLR-4 and NF-κB
p65 were all detected at the mRNA level (Fig. 6). TLR-4 gene expression in the TNBS
control group was considerably upregulated compared with the normal
control group (Fig. 6C), and all
tested drugs significantly decreased the expression of TLR-4 after
TNBS instillation (P<0.05). Similarly, intracolonic instillation
of TNBS enhanced the gene expression of iNOS and COX-2 compared
with the normal control group (Fig. 6A
and B). All tested drugs statistically inhibited the expression
of these two genes (P<0.05), with the exception of 15 mg/kg
magnolol (P>0.05). TNBS stimulation also elevated NF-κB p65 mRNA
expression, however only 30 or 60 mg/kg magnolol, or 500 mg/kg
sulfasalazine, downregulated the increase in NF-κB p65 gene
expression (P<0.05).
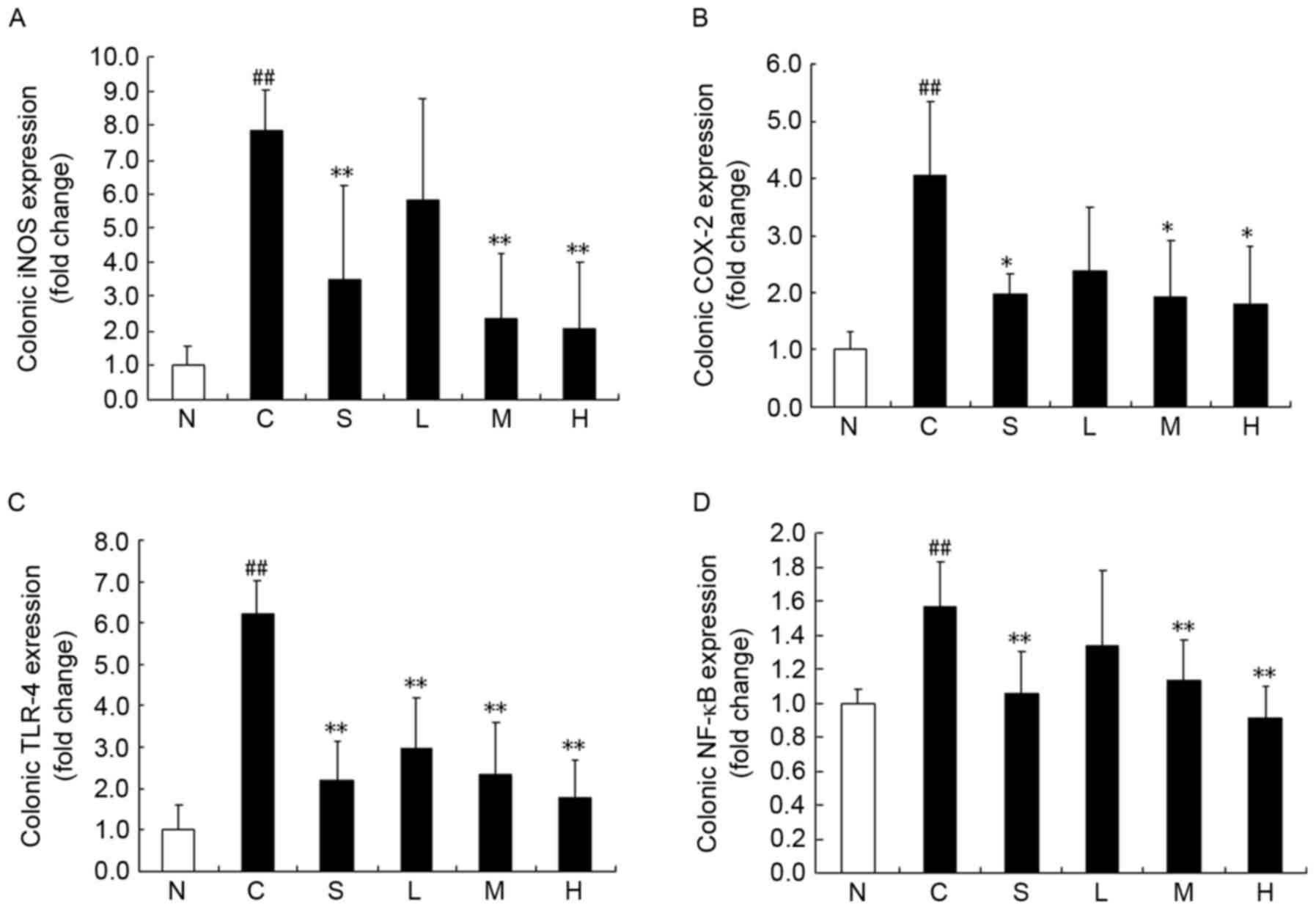 | Figure 6.Magnolol ameliorates
2,4,6-trinitrobenzenesulfonic acid/ethanol-induced upregulation of
inflammation-related genes. (A) iNOS, (B) COX-2, (C) TLR-4, and (D)
NF-κB p65 mRNA levels. *P<0.05 and **P<0.01 vs. model control
group; ##P<0.01 vs. normal control group. N, normal
control; C, model control; S, sulfasalazine group; L, low (15
mg/kg) magnolol dose; M, medium (30 mg/kg) magnolol dose; H, high
(60 mg/kg) magnolol dose. iNOS, inducible nitric oxide synthase;
COX-2, cyclooxygenase-2; TLR-4, Toll-like receptor-4; NF-κB p65,
nuclear factor-κB p65. |
Effects of magnolol on TLR-4 protein
expression in colonic tissue
TLR-4 expression was further investigated using
immunohistochemistry. Protein expression of TLR-4 was predominantly
localized within the cell membrane of the colonic mucosal
epithelial cells (Fig. 7). In
parallel with its gene expression profile, expression of the TLR-4
protein in the TNBS control group was significantly upregulated
compared with the normal control group (P<0.01). However, 30 or
60 mg/kg magnolol, or 500 mg/kg sulfasalazine, reduced the protein
expression of TLR-4 (P<0.05).
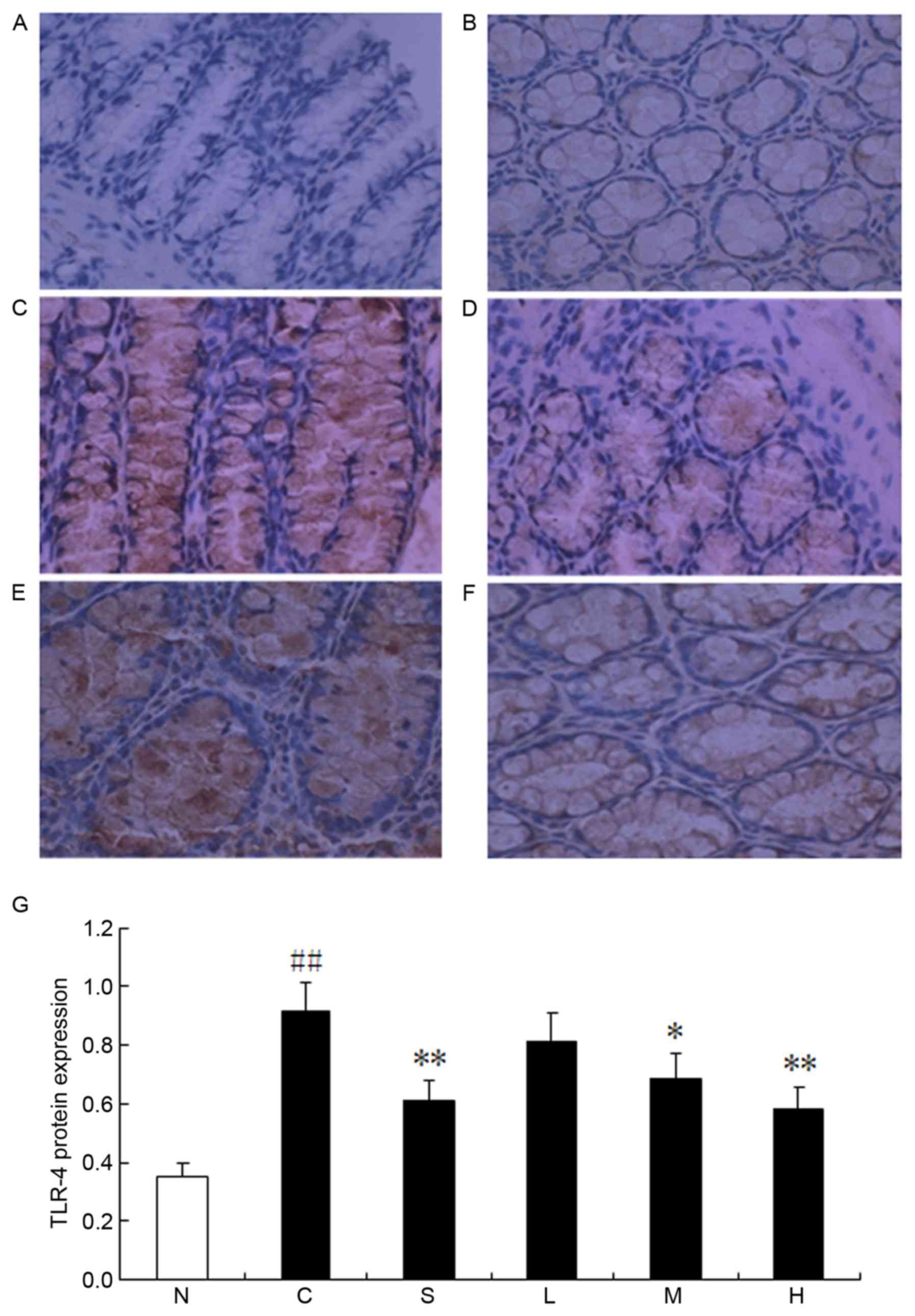 | Figure 7.Magnolol ameliorates
2,4,6-trinitrobenzenesulfonic acid/ethanol-induced upregulation of
TLR-4 protein expression. (A) Normal control, (B) model control and
(C) sulfasalazine groups, and (D) low dose (15 mg/kg), (E) medium
dose (30 mg/kg) and (F) high dose (60 mg/kg) magnolol-treated
groups. (G) Pooled data from each treatment group. *P<0.05,
**P<0.01 vs. model control; ##P<0.01 vs. normal
control group. N, normal control; C, model control; S,
sulfasalazine group; L, low (15 mg/kg) magnolol dose; M, medium (30
mg/kg) magnolol dose; H, high (60 mg/kg) magnolol dose; TLR-4,
Toll-like receptor-4. |
Discussion
The present study demonstrated that magnolol exerts
beneficial effects in a dose-dependent manner on TNBS-induced
colitis. Anti-inflammatory effects were exerted by magnolol at 30
and 60 mg/kg doses, and these effects were comparable to those
obtained with 500 mg/kg sulfasalazine, one of the drugs commonly
employed in the treatment of human IBD.
Prior to the measurement of biochemical parameters,
the clinical effects of magnolol were evaluated by assessing DAI,
viscera indexes, macroscopic colonic scores (CMDI) and colonic
histopathological scores (TDI). Treating the rats with magnolol for
7 consecutive days resulted in significant amelioration, to varying
degrees, in DAI, thymus index, colonic weight/length ratio, CMDI
and TDI values. TNBS-induced colonic inflammation is frequently
associated with a significant increase in the colonic weight/length
ratio, which serves as a reliable indicator of tissue edema
(17). In the present study, an
inhibitory effect on colonic weight/length ratio was only obtained
in the 60 mg/kg magnolol group; no significant reduction in this
ratio was observed in the 500 mg/kg sulfasalazine group. Therefore,
it may be hypothesized that a high dose of magnolol may exert a
prominent inhibitory effect on edema and/or fibrosis in the colonic
submucosa, which would limit the increase in the colonic
weight/length ratio.
The spleen and the thymus, two main immune organs,
serve vital roles in inflammatory and immune reactions. Spleen and
thymus indexes were therefore calculated to evaluate the
immunomodulatory effect of magnolol on TNBS-stimulated rats.
Previous research reported that the acute inflammatory process
induced a reduction in thymus weight and an increase in spleen
weight in TNBS-induced experimental colitis (18). Furthermore, in severe cases, the
thymus almost disappeared in terms of size and cell numbers, which
could be associated with an immunosuppressive state in TNBS-induced
colitis (19), whereas spleen
enlargement may represent a systemic inflammatory reaction that is
correlated with the severity of colitis (18). In this present study, there were no
significant changes regarding spleen index. However, it was
demonstrated that medium and high doses of magnolol effectively
improved immune function by alleviating the reduction in thymus
weight, in rats with TNBS-induced colitis.
MPO is an enzyme specific to granulocyte lysosomes,
which is directly correlated with the number of neutrophils
(20), and is therefore used as a
biochemical marker of neutrophil infiltration. Therefore,
determination of MPO activity has been widely employed to detect
intestinal inflammatory processes. In the present study, colonic
MPO activity was significantly increased following TNBS
instillation. As expected, colonic biopsies from animals that were
treated with any dose of magnolol or 500 mg/kg sulfasalazine
exhibited lower MPO activity than that in the TNBS control group,
which indicated that magnolol treatment ameliorated neutrophil
infiltration.
IBD is associated with an enhanced production of the
reactive metabolites of oxygen and nitrogen (21). Previous research has suggested that
in human IBD, the overproduction of NO via the upregulation of iNOS
may be responsible for intestinal hypomotility and subsequent
bacterial overgrowth (22).
Likewise, upregulation of iNOS and COX-2 expression has been
demonstrated in experimental colitis (23), and was further supported in the
present study by the amplification of iNOS and COX-2 mRNA
expression, in colonic tissue from rats with TNBS-induced colitis.
Magnolol has previously been reported to inhibit
lipopolysaccharide-induced iNOS and COX-2 expression in murine
macrophages, by blocking intracellular signaling pathways (24). The present study indicated that 30
and 60 mg/kg doses of magnolol significantly attenuated the
elevated levels of iNOS and COX-2 gene expression, which was
observed after TNBS induction.
As one of the classical indicators of the T-helper
(Th)1 response, IL-6 has a fundamental role in IBD pathogenesis
(25). In addition, a previous
study confirmed an important role for IL-6 in the suppression of
regulatory T-cell function, and the development of pathogenic Th17
cells (26). Notably, a previous
study using IL-17 receptor-A knockout mice demonstrated that IL-17
is necessary for the development of acute gut inflammation, induced
by the intrarectal administration of TNBS (27). In the present study, treatment with
all doses of magnolol and sulfasalazine resulted in a marked
decline in serum levels of IL-6 and IL-17, demonstrating the
ability of magnolol to downregulate proinflammatory cytokines.
It has previously been reported that IBD is not
induced to develop and does not progress in TLR-4 knockout animals
(28). Furthermore, TLR-4 is
upregulated in the intestinal mucosa of patients suffering from
active IBD (29). In the present
study, TLR-4 mRNA and protein levels were markedly increased upon
TNBS stimulation, and were significantly reduced following
treatment with the 30 and 60 mg/kg doses of magnolol.
NF-κB activation is increased in mucosal macrophages
and epithelial cells in the inflamed colon of patients with IBD and
animal models of IBD (30).
Inhibition of NF-κB activation has therefore been proposed as a
promising strategy for treating IBD. In the present study, TNBS
provoked a marked elevation in NF-κB p65 gene expression, and this
alteration was significantly suppressed by medium and high doses of
magnolol, suggesting that magnolol may effectively inhibit
TLR-4-associated NF-κB activation in IBD.
Finally, no drug-induced changes in food intake or
in clinical, macroscopic, microscopic or biochemical parameters
(data not shown) were observed during the 1 week course of
treatment, suggesting that magnolol may be a safe product for use
in rats. The strong anti-inflammatory activity of magnolol and the
absence of apparent toxicity, combined with its anticancer activity
(31), have made it a promising
therapeutic candidate for the treatment of IBD.
In conclusion, magnolol appears to ameliorate
TNBS-induced colitis in a dose-dependent manner. The beneficial
effects of this compound likely involve a decrease in
proinflammatory mediators (including iNOS and COX-2) and cytokines
(such as IL-6 and IL-17), by inhibiting TLR-4-associated NF-κB
activation. These findings suggest that magnolol may be a promising
therapeutic agent in the clinical management of IBD.
Acknowledgements
The authors would like to gratefully acknowledge
Professor Hai-Dong Li in the Department of Biochemistry and
Molecular Biology at Tianjin Medical University, for providing the
facilities to perform this study.
References
|
1
|
Algieri F, Zorrilla P, Rodriguez-Nogales
A, Garrido-Mesa N, Bañuelos O, González-Tejero MR, Casares-Porcel
M, Molero-Mesa J, Zarzuelo A, Utrilla MP, et al: Intestinal
anti-inflammatory activity of hydroalcoholic extracts of Phlomis
purpurea L. and Phlomis lychnitis L. in the
trinitrobenzenesulphonic acid model of rat colitis. J
Ethnopharmacol. 146:750–759. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo BJ, Bian ZX, Qiu HC, Wang YT and Wang
Y: Biological and clinical implications of herbal medicine and
natural products for the treatment of inflammatory bowel disease.
Ann N Y Acad Sci. 1401:37–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernaskova M, Kretschmer N, Schuehly W,
Huefner A, Weis R and Bauer R: Synthesis of tetrahydrohonokiol
derivates and their evaluation for cytotoxic activity against
CCRF-CEM leukemia, U251 glioblastoma and HCT-116 colon cancer
cells. Molecules. 19:1223–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai YC, Cheng PY, Kung CW, Peng YJ, Ke
TH, Wang JJ and Yen MH: Beneficial effects of magnolol in a rodent
model of endotoxin shock. Eur J Pharmacol. 641:67–73. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chuang DY, Chan MH, Zong Y, Sheng W, He Y,
Jiang JH, Simonyi A, Gu Z, Fritsche KL, Cui J, et al: Magnolia
polyphenols attenuate oxidative and inflammatory responses in
neurons and microglial cells. J Neuroinflammation. 10:152013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park J, Lee J, Jung E, Park Y, Kim K, Park
B, Jung K, Park E, Kim J and Park D: In vitro antibacterial and
anti-inflammatory effects of honokiol and magnolol against
Propionibacterium sp. Eur J Pharmacol. 496:189–195. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M, Long Y, Sun Y, Wang Y, Li Q, Wu
H, Guo Z, Li Y, Niu Y, Li C, et al: Evidence for the complementary
and synergistic effects of the three-alkaloid combination regimen
containing berberine, hypaconitine and skimmianine on the
ulcerative colitis rats induced by trinitrobenzene-sulfonic acid.
Eur J Pharmacol. 651:187–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang YJ, Park HJ, Chung HJ, Min HY, Park
EJ, Lee MA, Shin Y and Lee SK: Wnt/β-catenin signaling mediates the
antitumor activity of magnolol in colorectal cancer cells. Mol
Pharmacol. 82:168–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witaicenis A, Luchini AC, Hiruma-Lima CA,
Felisbino SL, Garrido-Mesa N, Utrilla P, Gálvez J and Di Stasi LC:
Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a
natural coumarin: Comparison with prednisolone and sulphasalazine.
Chem Biol Interact. 195:76–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonçalves CC, Hernandes L, Bersani-Amado
CA, Franco SL, Silva JF and Natali MR: Use of propolis
hydroalcoholic extract to treat colitis experimentally induced in
rats by 2,4,6-trinitrobenzenesulfonic acid. Evid Based Complement
Alternat Med. 2013:8539762013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YH, Lin FY, Liu PL, Huang YT, Chiu
JH, Chang YC, Man KM, Hong CY, Ho YY and Lai MT: Antioxidative and
hepatoprotective effects of magnolol on acetaminophen-induced liver
damage in rats. Arch Pharm Res. 32:221–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang TC, Zhang SW, Sun LN, Wang H and Ren
AM: Magnolol attenuates sepsis-induced gastrointestinal dysmotility
in rats by modulating inflammatory mediators. World J
Gastroenterol. 14:7353–7360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morris GP, Beck PL, Herridge MS, Depew WT,
Szewczuk MR and Wallace JL: Hapten-induced model of chronic
inflammation and ulceration in the rat colon. Gastroenterology.
96:795–803. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsune I, Ikejima K, Hirose M, Yoshikawa M,
Enomoto N, Takei Y and Sato N: Dietary glycine prevents
chemical-induced experimental colitis in the rat. Gastroenterology.
125:775–785. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X and Wang J: Anti-inflammatory
effects of iridoid glycosides fraction of Folium syringae leaves on
TNBS-induced colitis in rats. J Ethnopharmacol. 133:780–787. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peran L, Camuesco D, Comalada M, Bailon E,
Henriksson A, Xaus J, Zarzuelo A and Galvez J: A comparative study
of the preventative effects exerted by three probiotics,
Bifidobacterium lactis, Lactobacillus casei and Lactobacillus
acidophilus, in the TNBS model of rat colitis. J Appl Microbiol.
103:836–844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oz HS, Zhong J and de Villiers WJ:
Osteopontin ablation attenuates progression of colitis in TNBS
model. Dig Dis Sci. 57:1554–1561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fritsch Fredin M, Elgbratt K, Svensson D,
Jansson L, Melgar S and Hultgren Hörnquist E: Dextran sulfate
sodium-induced colitis generates a transient thymic
involution-impact on thymocyte subsets. Scand J Immunol.
65:421–429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xing JF, Sun JN, Sun JY, You CY, Dong K,
Lv J and Dong YL: Protective effects of
3,4-oxo-isopropylidene-shikimic acid on experimental colitis
induced by trinitrobenzenesulfonic acid in rats. Dig Dis Sci.
57:2045–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Conner EM, Brand SJ, Davis JM, Kang DY and
Grisham MB: Role of reactive metabolites of oxygen and nitrogen in
inflammatory bowel disease: Toxins, mediators, and modulators of
gene expression. Inflamm Bowel Dis. 2:133–147. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szalai Z, Szász A, Nagy I, Puskás LG,
Kupai K, Király A, Berkó AM, Pósa A, Strifler G, Baráth Z, et al:
Anti-inflammatory effect of recreational exercise in TNBS-induced
colitis in rats: Role of NOS/HO/MPO system. Oxid Med Cell Longev.
2014:9259812014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun P, Zhou K, Wang S, Li P, Chen S, Lin
G, Zhao Y and Wang T: Involvement of MAPK/NF-κB signaling in the
activation of the cholinergic anti-inflammatory pathway in
experimental colitis by chronic vagus nerve stimulation. PLoS One.
8:e694242013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai CS, Lee JH, Ho CT, Liu CB, Wang JM,
Wang YJ and Pan MH: Rosmanol potently inhibits
lipopolysaccharide-induced iNOS and COX-2 expression through
downregulating MAPK, NF-kappaB, STAT3 and C/EBP signaling pathways.
J Agric Food Chem. 57:10990–10998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du L, Tang H, Ma Z, Xu J, Gao W, Chen J,
Gan W, Zhang Z, Yu X, Zhou X and Hu X: The protective effect of the
recombinant 53-kDa protein of Trichinella spiralis on experimental
colitis in mice. Dig Dis Sci. 56:2810–2817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiong J, Lin YH, Bi LH, Wang JD, Bai Y and
Liu SD: Effects of interleukin-4 or interleukin-10 gene therapy on
trinitrobenzenesulfonic acid-induced murine colitis. BMC
gastroenterol. 13:1652013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caprioli F, Pallone F and Monteleone G:
Th17 immune response in IBD: A new pathogenic mechanism. J Crohns
Colitis. 2:291–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joh EH, Lee IA, Han SJ, Chae S and Kim DH:
Lancemaside A ameliorates colitis by inhibiting NF-kappaB
activation in TNBS-induced colitis mice. Int J Colorectal Dis.
25:545–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Himmel ME, Hardenberg G, Piccirillo CA,
Steiner TS and Levings MK: The role of T-regulatory cells and
Toll-like receptors in the pathogenesis of human inflammatory bowel
disease. Immunology. 125:145–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pasparakis M: IKK/NF-kappaB signaling in
intestinal epithelial cells controls immune homeostasis in the gut.
Mucosal Immunol. 1 Suppl 1:S54–S57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seo JU, Kim MH, Kim HM and Jeong HJ:
Anticancer potential of magnolol for lung cancer treatment. Arch
Pharm Res. 34:625–633. 2011. View Article : Google Scholar : PubMed/NCBI
|