Introduction
Mesenchymal stem cells (MSCs) are a type of
multipotent adult stem cells that have the potential to form
different cell types, including adipocytes, osteocytes,
chondrocytes, cardiomyocytes and neurons (1). Human umbilical cord MSCs (hUCMSCs), a
novel type of MSCs, have various phenotypes and characteristics in
common with MSCs (2). The
umbilical cords, particularly the Wharton's jelly tissues are rich
in hUCMSCs (1). hUCMSCs have been
broadly studied in the field of transplant therapy (2). Compared with other types of MSCs,
hUCMSCs are easily obtained and the method used to isolate them is
not traumatic to the donors (3)
and the umbilical cord is a rich and easily available source of
hUCMSCs (3). The benefits of
hUCMSCs has led to their wide use in transplantation medicine.
Previous studies have revealed that hUCMSCs have been used to treat
neurodegeneration, neuronal injury, cardiac infarction, diabetes
mellitus, kidney and lung injury (4–9).
The most important factor determining the
therapeutic efficiency of the transplanted hUCMSCs is their
proliferative ability in vitro or in vivo in the
recipient bodies (10). High
proliferative ability ensures a higher hUCMSCs survival rate in the
host organs or tissues following the transplantation. Previous
studies indicated that additional supplements of the growth
factors, such as insulin-like growth factor 1 (IGF-1), fibroblast
growth factor, epidermal growth factor, basic fibroblast growth
factor (bFGF) and platelet-derived growth factor may promote the
proliferation of MSCs (11–13).
IGF-1 is a 70-amino acid protein in humans and has
multiple biological functions in cell physiology. IGF-1, binding to
its receptor (IGF-1R), mediates the phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt)/mechanistic target of rapamycin
(mTOR) and mitogen-activated protein kinase
(MAPK)/extracellular-signal regulated kinase (ERK) signaling
pathways that contribute to regulation of cell proliferation,
differentiation and apoptosis (14–17).
Previous studies indicated that MSCs are able to secrete growth
factors, including IGF-1, bFGF, hepatocyte growth factor and
vascular endothelial growth factor, or increase the expression
levels of these growth factors in the host cells, tissues or organs
following transplantation (18–20).
The growth factors secreted by the transplanted
hUCMSCs have been demonstrated to stimulate growth of the host
cells in the recipients. Imberti et al (21) reported that IGF-1 secreted by bone
marrow MSCs promoted proliferation of proximal tubular epithelial
cells (PTEC) and inhibited cisplatin-induced PTEC apoptosis in the
in vitro co-culture state (21). Blocking IGF-1R with a specific
antibody attenuated PTEC proliferation and increased their
apoptotic rate (21). These
findings were confirmed by Morigi et al (22) in an in vivo study, which
revealed that the release of IGF-1 from the transplanted MSCs may
stimulate tubular cell proliferation, limit renal cell apoptosis
and accelerate mice recovery from acute renal injury (22). A previous study indicated that the
transplanted MSCs stimulated the osteoblast proliferation and the
formation of new bone through paracrine IGF-1 and promoted their
differentiation into osteoblasts through the autocrine IGF-1 in the
recipients (23). These previous
findings suggest that MSCs may affect their own physiological
functions via autocrine IGF-1. Additionally, a previous in
vitro study also indicated that treatment with exogenous IGF-1
may increase MSC viability (13).
Therefore, the present study hypothesized that the autocrine IGF-1
may affect the viability of MSCs. In order to verify this
hypothesis, hUCMSCs were treated with αIR-3, a specific
IGF-1R-blocking antibody, to block the action of the autocrine
IGF-1. Subsequently cell viability, cell cycle and apoptosis of
hUCMSCs were quantified and the underlying molecular mechanisms
were investigated.
Materials and methods
hUCMSCs culture
A total of 12 umbilical cords from full-term
deliveries were obtained from the Affiliated Hospital of Guizhou
Medical University (Guiyang, China) from January-December 2012. The
gender ratio of the collected umbilical cords was 1:1. Written
informed consent was obtained from parents and the experiments were
performed and approved by the Ethical Committee of Guizhou Medical
University (Guiyang, China). The isolation and culture of hUCMSCs
was performed as previously described (24). The umbilical cords were washed
twice with pre-cooled PBS at 4°C, the umbilical vessels were
removed and the Wharton's jelly was minced into ~2.5 mm3
sections. The small sections were plated in 100-mm dishes
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) in 5-mm wide
gaps and cultured in Dulbecco's modified Eagle's medium/Nutrient
mixture F12 (DMEM/F12; GE Healthcare Life Sciences, Chalfont, UK)
supplemented with 20% fetal bovine serum (FBS; GE Healthcare Life
Sciences), 100 U/ml penicillin (Beyotime Institute of
Biotechnology, Beijing, China) and 100 mg/ml streptomycin (Beyotime
Institute of Biotechnology) at 37°C with 5% CO2. Cells
at 85% confluence were digested with 0.25% trypsin (GE Healthcare
Life Sciences) and transferred into 75 cm2 cell culture
flasks (Sigma-Aldrich; Merck Millipore) and cultured with DMEM/F12
medium with 10% FBS at 37°C with 5% CO2.
Treatment groups
The passage 4 of hUCMSCs was plated into 6-well
(5×104 cells/well) or 24-well (1×104
cells/well) plates and randomly divided into two groups: i) Control
group; and ii) experimental group. The cells were treated with 5
µg/ml αIR-3 (cat. no. MABS192; Merck Millipore) in the experimental
group and without αIR-3 in the control group for 24 h. Cell
viability, apoptosis, cell cycle and levels of Akt/glycogen
synthase kinase (GSK)-3β activation were subsequently
quantified.
Immunocytochemistry staining
hUCMSCs (1×104) cultured on coverslips
were fixed in 4% paraformaldehyde for 10 min and permeabilized with
0.1% Triton X-100 for 10 min at room temperature. Following washing
with PBS, the cells were incubated with 3% hydrogen peroxide for 10
min at room temperature to block the endogenous peroxidase
activity. Following washing twice with PBS, the cells were blocked
with 10% goat serum (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) for 20 min and then incubated with rabbit anti-human
polyclonal IGF-1 antibody (cat. no. ab9572; 1:200; Abcam,
Cambridge, MA, USA;) and rabbit anti-human polyclonal IGF-1
receptor β antibody (cat. no. 3027; 1:200; Cell Signaling
Technology, Inc., Danvers, MA, USA) overnight at 4°C. The cells
were washed with PBS three times and then incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibody (cat. no. A0208; 1:5,000; Beyotime Institute of
Biotechnology) for 30 min at room temperature. The cells were
washed four times with PBS and incubated with streptavidin biotin
complex (SABC) solution (Beyotime Institute of Biotechnology) for
20 min at room temperature and subsequently washed with PBS four
times. The cells were incubated with 100 µl 3,3′-diaminobenzidine
substrate solution (Beyotime Institute of Biotechnology) for 5 min
and observed under an inverted microscope (NIKON TS100; Nikon
Corporation, Tokyo, Japan), followed by dehydration with 95%
ethanol, clearing with xylene and sealing with neutral gum
(Beyotime Institute of Biotechnology).
Cell cycle analysis
Following treatment with αIR3, hUCMSCs were digested
with 0.25% trypsin, collected, washed with pre-cold PBS and fixed
with cold 70% ethanol. The cells were washed with pre-cold PBS
three times, resuspended in 500 µl PBS and treated with 50 µl of
RNase-A (100 µg/ml; Sigma-Aldrich; Meck Milipore) and 200 µl of
propidium iodide (50 µg/ml; Beyotime Institute of Biotechnology)
solution with 0.1% Triton-X 100 in the dark. Cell cycle was
analyzed using a flow cytometer at 488 nm (BD Biosciences, San
Jose, CA, USA).
Quantification of cell apoptosis
Apoptosis of hUCMSCs was quantified using a flow
cytometry assay using a Dead Cell Apoptosis kit with fluorescein
isothiocyanate-Annexin V/(Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) following the manufacturer's protocol.
Analysis of apoptosis (Annexin V positivity) was conducted using a
flow cytometer at 488 and 535 nm.
Western blotting
Proteins were extracted from the treated hUCMSCs
using RIPA lysis buffer (Sigma-Aldrich; Merck Millipore)
supplemented with protease inhibitor (Sigma-Aldrich; Merck
Millipore), phosphatase inhibitor (Sigma-Aldrich; Merck Millipore)
and phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich; Merck
Millipore). The protein concentration was quantified using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Proteins (30 µg/lane) were diluted in 2X SDS-PAGE
protein loading buffer (Beyotime Institute of Biotechnology),
heated at 95°C for 5 min, loaded into 10% SDS-PAGE gel (Beyotime
Biotechnology) and separated by electrophoresis. Following
electrophoresis, the proteins were transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 5% non-fat milk in Tris-buffered saline
with Tween-20 (TBST) for 4 h at room temperature and subsequently
incubated with rabbit anti-human phosphorylated (p)-Akt polyclonal
antibody (cat. no. ab18206; 1:1,000; Abcam), rabbit anti-human
p-glycogen synthase kinase 3β (GSK-3β) polyclonal antibody (cat.
no. 9336; 1:1,000; Cell Signaling Technology, Inc.), rabbit
anti-human p21 polyclonal antibody (cat. no. ab7960; 1:1,000;
Abcam), rabbit anti-human cyclin D1 polyclonal antibody (cat. no.
2922; 1:1,000; Cell Signaling Technology, Inc.), rabbit anti-human
p-p70 S6 kinase (P70S6K) polyclonal antibody (cat. no. 9025;
1:1,000; Cell Signaling Technology, Inc.), rabbit anti-human
p-ERK1/2 monoclonal antibody (cat. no. 4370; 1:1,000; Cell
Signaling Technology, Inc.) in blocking solution at 4°C overnight.
The blots were washed 3 times with TBST and incubated with
HRP-conjugated goat anti-rabbit secondary antibody (cat. no.
BA1054; 1:5,000, Wuhan Boster Biological Technology) in a blocking
solution for 1 h at room temperature. The immunoreactive bands were
washed with TBST, visualized with western blotting enhanced
chemiluminescence reagent (EMD Millipore) and subsequently exposed
to X-ray film (Thermo Fisher Scientific, Inc.). The blots were
normalized to the expression of β-actin, which was detected by the
same blots through washing with a stripping buffer (Beyotime
Institute of Biotechnology) and subsequent incubation with rabbit
anti-mouse β-actin polyclonal antibody (cat. no. ab8227; 1:2,000;
Abcam). All protein bands were quantified by densitometry using
ImageJ software (version 1.48h3; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 15.0 software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation from 4–6 independent
experiments. Univariate comparisons of means were evaluated using
the Student t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphology of hUCMSCs and expression
levels of IGF-1 and IGF-1R
The morphology of primary and passaged hUCMSCs was
determined at 2 and 7 days after the Wharton's jelly sections were
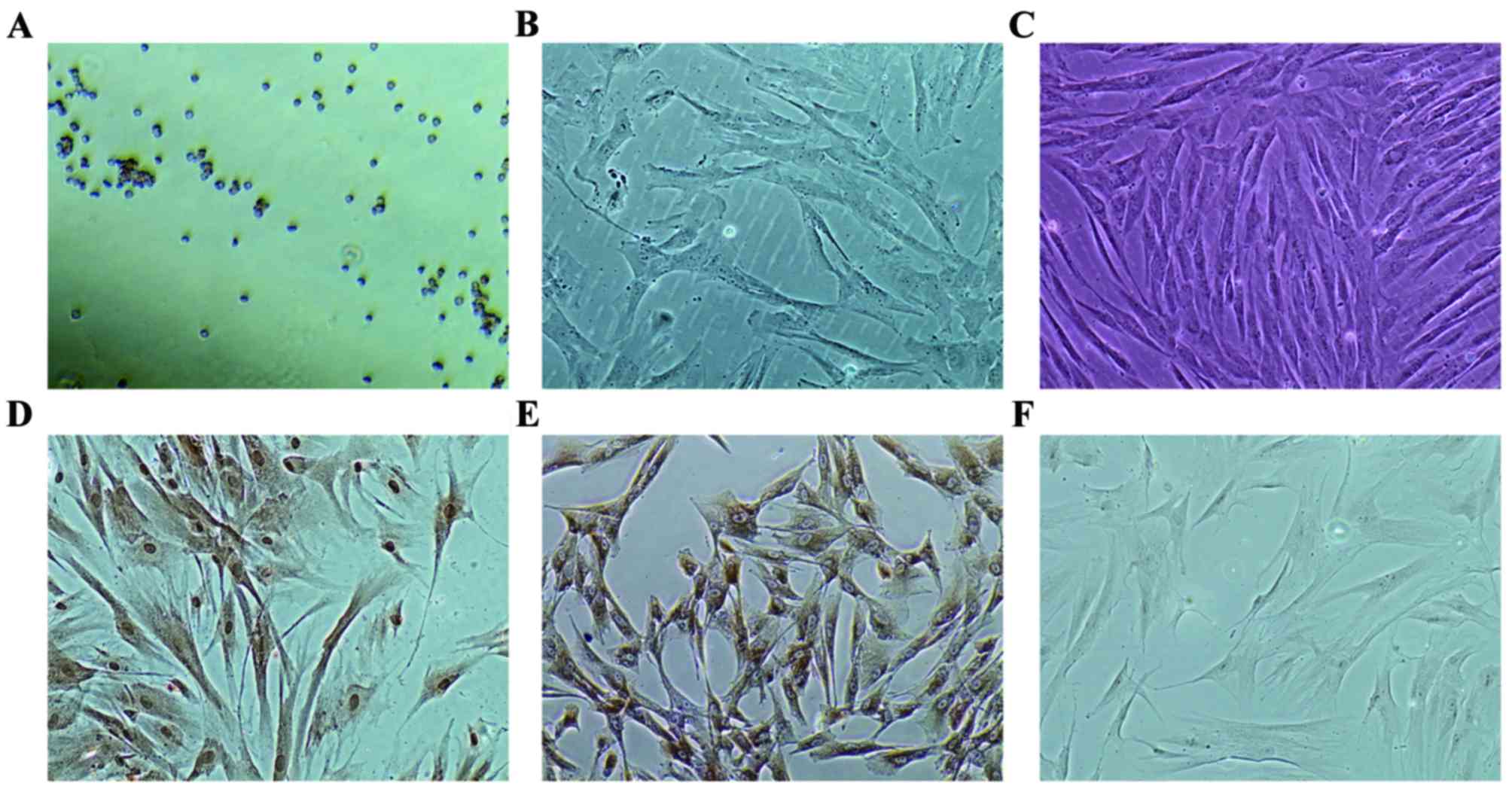
plated. As presented in Fig. 1A,
round cells dissociated from the Wharton's jelly sections and
attached to the bottom of the culture dishes 2 days after the small
sections were plated. By day 7, cells assumed triangular and
spindle shape and a number of cells had long dendrites (Fig. 1B). Cells at passage 4 exhibited
long spindle shape and upon reaching confluence, formed a pattern
similar to a whirlpool (Fig. 1C).
Immunocytochemistry staining revealed that IGF-1 was expressed in
hUCMSCs, particularly in the nuclei (Fig. 1D). IGF-1R was also positively
expressed in hUCMSCs, primarily located on the membrane and absent
in the nuclei (Fig. 1E).
Blockade of autocrine IGF-1 reduces
hUCMSCs cell viability
The present study quantified cell viability using an
MTT assay. As presented in Table
I, treatment with the IGF-1R-specific blocker αIR-3 for 24 h
significantly reduced the hUCMSCs cell viability when compared with
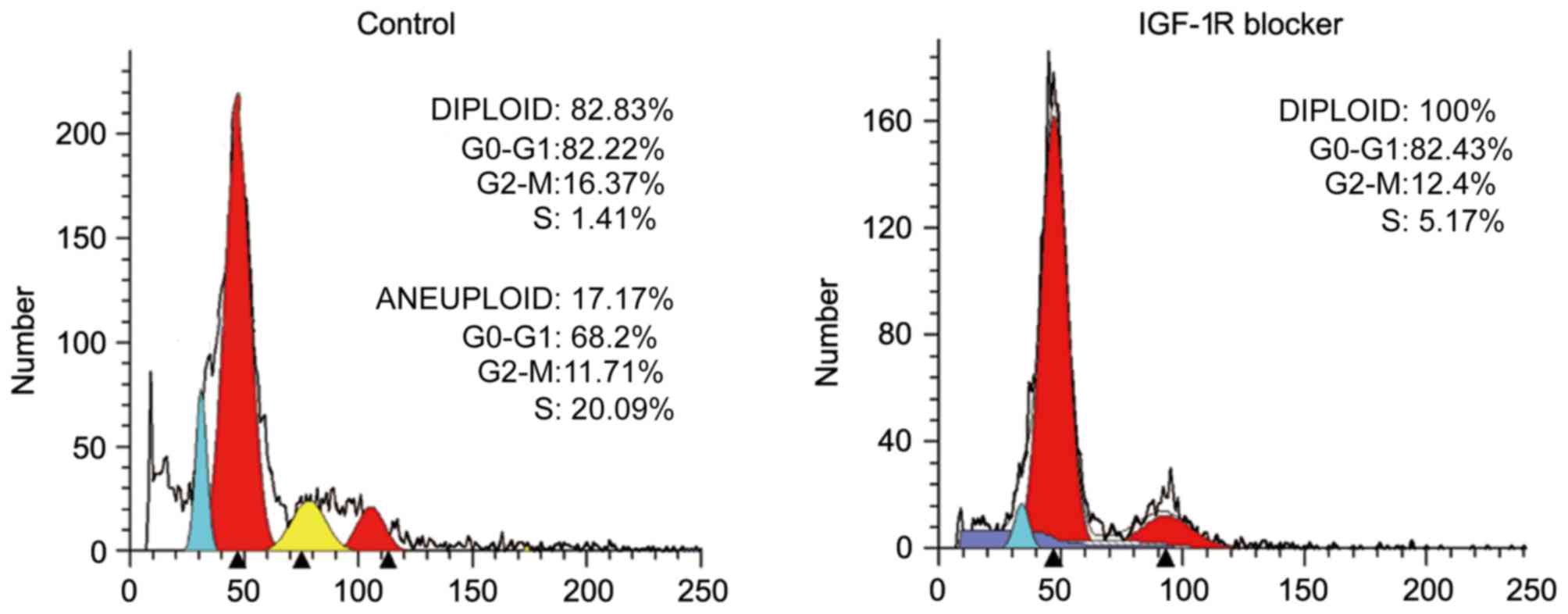
the control group (P<0.05). Additionally, cell cycle analysis
revealed that the number of cells in the G2/M phase was reduced in
the experimental group compared with the control group, which also
indicated a reduced proliferation of hUCMSCs following treatment
with αIR-3 (Fig. 2).
 | Table I.Cell viability of human umbilical cord
mesenchymal stem cells following treatment with 5 µg/ml αIR-3 for
24 h. |
Table I.
Cell viability of human umbilical cord
mesenchymal stem cells following treatment with 5 µg/ml αIR-3 for
24 h.
| Group | Cell viability |
|---|
| Control | 0.591±0.111 |
| αIR-3 (5 µg/ml) |
0.431±0.104a |
Blockade of autocrine IGF-1 induces
hUCMSCs apoptosis
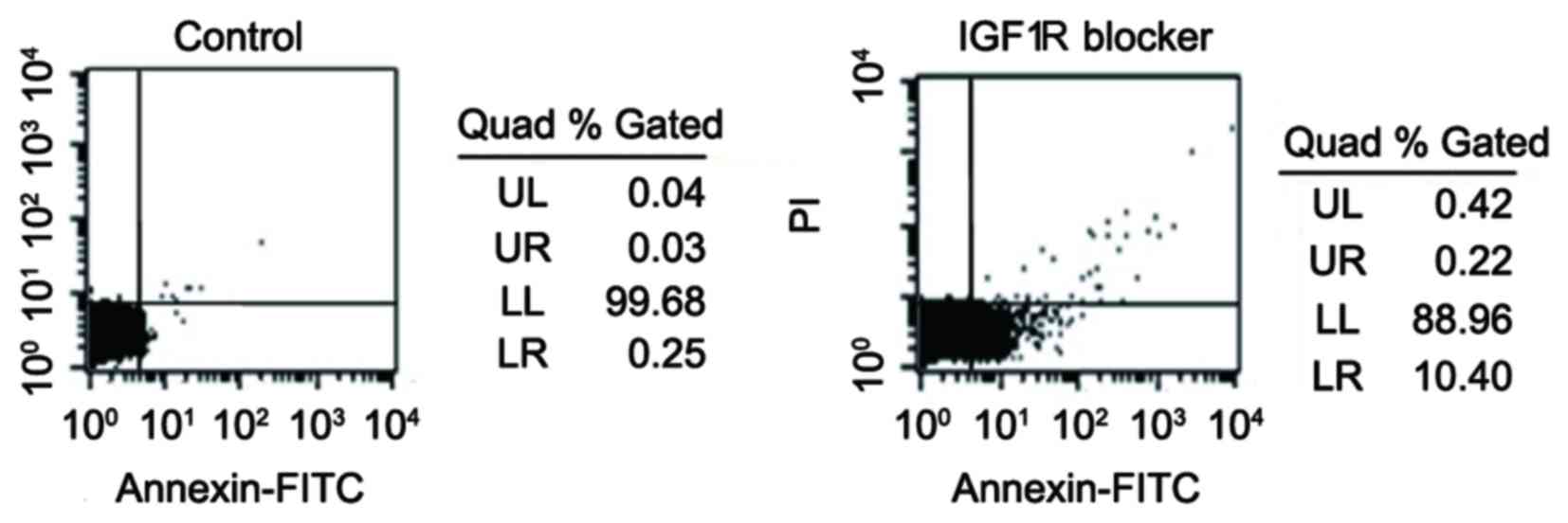
Cell apoptosis was quantified by analysis of Annexin
V activity using flow cytometry. The increase of Annexin V activity
indicated an early stage of apoptosis (25). As presented in Fig. 3, treatment with the IGF-1R-specific
blocker, αIR-3, markedly increased Annexin V positivity (from 0.25%
in the control group to 10.40% in the experimental group).
Effect of blockade of autocrine IGF-1
on the expression levels of p-Akt, p-ERK1/2, p-Gsk-3b, p-P70S6K,
cyclin D1 and p21 in hUCMSCs
Previous studies have reported that the binding of
IGF-1 to its receptor may mediate the activation of the PI3K/Akt
and MAPK/ERK signaling pathways (15,16).
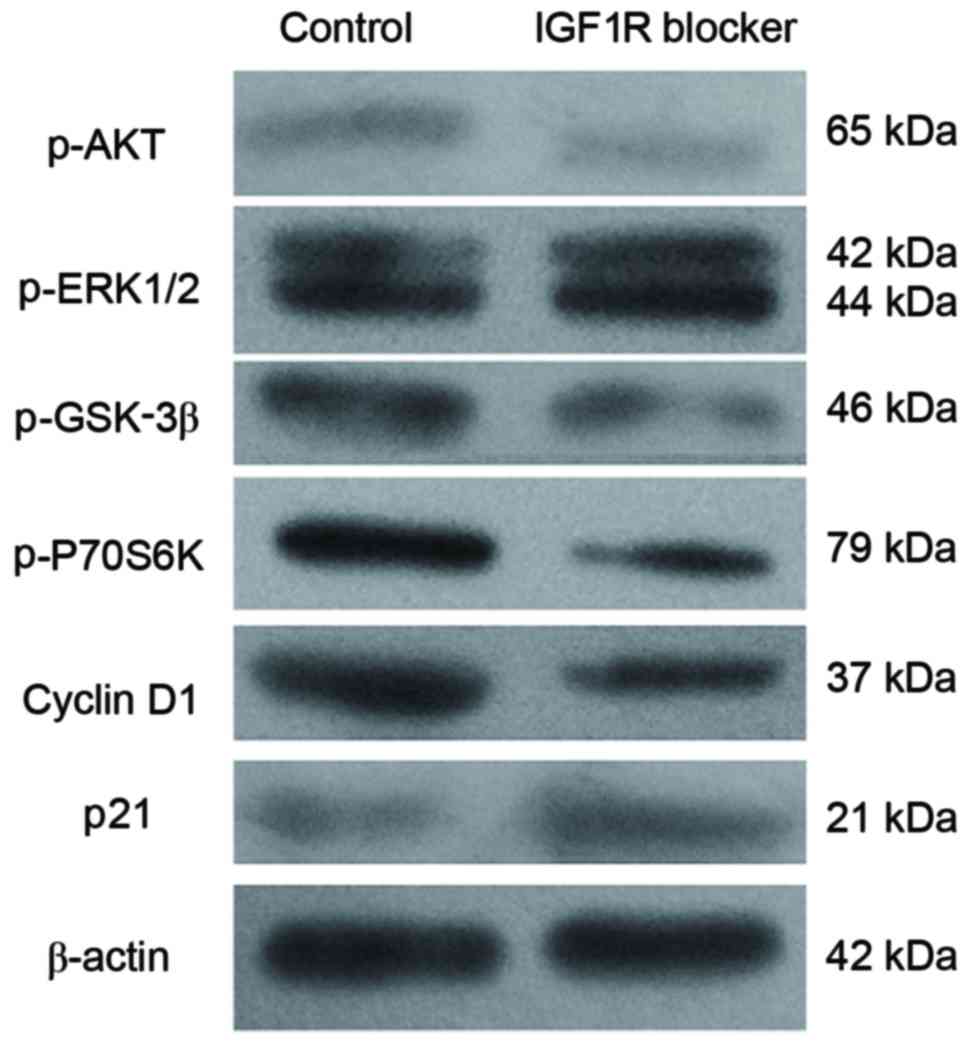
The findings of the present study revealed that treatment with the
IGF-1R-specific blocker αIR-3 significantly reduced the expression
levels of p-Akt, p-Gsk-3β and p-P70S6K (P<0.05; Fig. 4; Table II), However, no significant
difference was identified in p-ERK1/2 expression when the
experimental group was compared with the control group (P>0.05;
Fig. 4; Table II). These findings indicated that
the effect of autocrine IGF-1 on hUCMSCs cell viability may be due
to the activation of the Akt/Gsk-3β/P70S6K signaling pathway;
however, not the activation of MAPK/ERK signaling pathway. In
addition, treatment with αIR-3 also significantly reduced cyclin D1
expression levels and increased p21 expression levels (P<0.05;
Fig. 4; Table II). These two proteins are
important factors determining cell viability and apoptosis
(26).
 | Table II.Protein expression values of p-Akt,
p-ERK1/2, p-GSK-3β, p-P70S6K, cyclin D1 and p21, relative to
β-actin in human umbilical cord mesenchymal stem cells following
treatment with 5 µg/ml αIR-3 for 24 h. |
Table II.
Protein expression values of p-Akt,
p-ERK1/2, p-GSK-3β, p-P70S6K, cyclin D1 and p21, relative to
β-actin in human umbilical cord mesenchymal stem cells following
treatment with 5 µg/ml αIR-3 for 24 h.
| Target proteins | Control | αIR-3 (5 µg/ml) |
|---|
| p-Akt | 0.3017±0.0198 |
0.1121±0.0078a |
| p-ERK1 | 0.3321±0.0338 | 0.3977±0.0206 |
| p-ERK2 | 0.5605±0.0303 | 0.6012±0.0382 |
| p-GSK-3β | 0.5589±0.0417 |
0.2769±0.0154a |
| p-P70S6K | 0.8187±0.05074 |
0.4139±0.0215a |
| Cyclin D1 | 0.8302±0.0412 |
0.3241±0.0146a |
| p21 | 0.1384± 0.0079 |
0.2983±0.0131a |
Discussion
A previous report has suggested that MSCs are able
to secrete IGF-1 (18). To the
best of our knowledge, this is the first study to investigate
whether MSCs may be able to alter their own proliferation through
autocrine IGF-1. Therefore, the present study examined the effect
of autocrine IGF-1 on cell viability and apoptosis of hUCMSCs. The
expression levels of IGF-1 and IGF-1R in hUCMSCs were quantified
and it was determined that both were expressed in hUCMSCs. The
hUCMSCs were treated with 5 µg/ml αIR-3 (an IGF-1R-specific
blocker) for 24 h in order to block the autocrine IGF-1. It was
determined that treatment with αIR-3 significantly reduced cell
viability and increased apoptosis of hUCMSCs. Additionally, cell
cycle analysis revealed that the number of cells in the G2/M phase
was reduced in the experimental group compared with the control
group, which also indicated a low viability of hUCMSCs following
treatment with αIR-3. Treatment with αIR-3 significantly reduced
p-Akt, p-Gsk-3β, p-P70S6K and cyclin D1 expression levels, whereas
the expression of p21 was significantly increased in the
experimental group compared with the control. However, αIR-3
treatment did not significantly affect p-ERK1/2 expression levels.
These findings indicated that the mechanism by which autocrine
IGF-1 altered hUCMSCs cell viability and apoptosis of may be via
the activation of the Akt/Gsk-3β/P70S6K signaling pathway.
The human umbilical cord is a promising source of
MSCs and the transplantation of hUCMSCs has revealed a novel area
for stem cell therapy (27).
hUCMSCs may be readily isolated and the collection procedure is
painless to the donors, unlike bone marrow-derived MSCs.
Additionally, hUCMSCs have the increased self-renewal properties
and potentials to differentiate into multiple cell lineages
(27). These unique
characteristics allow for hUCMSCs to be widely applied in
regenerative medicine. Their proliferative ability is an important
factor affecting the efficiency of MSCs-based cell transplantation
therapy.
IGF-1 is a peptide hormone that has been
demonstrated to stimulate the growth of several of cell lineages
in vivo and in vitro. IGF-1 exerts the majority of
its effect via binding to its receptor IGF-1R (28). Previous studies suggested that MSCs
may be able to secrete a series of growth factors, including IGF-1
(18–20). The current study determined that
IGF-1 and IGF-1R are expressed in hUCMSCs. This suggested that the
auto-secreted IGF-1 by hUCMSCs has the potential to affect cell
viability of hUCMSCs by binding to the membrane-bound IGF-1R and
subsequently initiating the downstream intracellular signals. The
present study used αIR-3, an IGF-1R-specific antibody, to block the
autocrine IGF-1 binding to IGF-1R. As expected, the blockade of the
autocrine IGF-1 with αIR-3 significantly reduced cell viability of
hUCMSCs, and reduced the number of G2/M phase cells. Additionally,
blocking the autocrine IGF-1 also markedly increased the hUCMSCs
apoptotic rate, which indirectly indicated a low cell viability
following treatment with αIR-3. IGF-1 is a critical stimulator of
cell proliferation and a potent inhibitor of programmed cell death
(28,29). Blocking autocrine IGF-1 inhibited
cell growth and initiated apoptosis of hUCMSCs.
The binding of IGF-1 to IGF-1R may lead to the
activation of critical downstream targets, through the Akt/mTOR and
MAPK/ERK signaling pathways, which mediate cell cycle progression
and prevent cell apoptosis (30).
The present study determined that blockade of the autocrine IGF-1
markedly reduced the expression of p-Akt; however, the expression
of p-ERK1/2 was not affected. Previous studies have determined that
IGF-1 is a potent natural activator of the Akt signaling pathway
(28,30). GSK-3β is one of the downstream
signals of Akt. The findings of the present study revealed that the
blockade of the autocrine IGF-1 significantly inhibited the
expression of p-GSK-3β. These findings indicated that the
inhibitory effect of blocking autocrine IGF-1 on cell viability of
hUCMSCs may depend on inactivation of the Akt/GSK-3β signaling
pathway, not the ERK1/2 signaling pathway. A recent study also
reported that inhibition of Akt/GSK-3β signaling reduced cell
viability and induced apoptosis in colorectal cancer cell lines
(31). In addition, the present
study also determined that blockade of the autocrine IGF-1 reduced
the expression of p-P70S6K. P70S6K is a serine/threonine kinase
that contributes to the downstream signaling in the
PI3K/Akt//GSK-3β pathway (32).
P70S6K may promote cell cycle progression and cell growth through
regulating the organization of cytoskeleton (33).
Cell cycle proteins, such as cell division cycle 42
and cyclins are important regulators for the activation of P70S6K
(34). The current study
determined that blockade of the autocrine IGF-1 reduced cyclin D1
expression and increased p21 expression. These findings confirmed
the low viability of hUCMSCs following treatment with the IGF-1R
blocker, αIR-3. Cyclin D1 is important for cell cycle progression,
as it controls the progression from G1 to S phase (35). P21 is a potent cyclin-dependent
kinase inhibitor, which binds of cyclin-cyclin-dependent kinase
complexes, suppresses their activity and therefore inhibits cell
cycle progression at the G1 and S phase (36).
In conclusion, the present study demonstrated that
hUCMSCs may affect their own viability through autocrine IGF-1.
Blocking the autocrine IGF-1 using a IGF-1R-specific blocker
markedly reduced hUCMSCs viability and induced apoptosis. The
information presented in the current study provides evidence that
the self-characteristics of MSCs may be used to regulate their
physiological functions.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81160099
and 31401246; Beijing, China).
References
|
1
|
Watson N, Divers R, Kedar R, Mehindru A,
Mehindru A, Borlongan MC and Borlongan CV: Discarded Wharton jelly
of the human umbilical cord: A viable source for mesenchymal
stromal cells. Cytotherapy. 17:18–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li DR and Cai JH: Methods of isolation,
expansion, differentiating induction and preservation of human
umbilical cord mesenchymal stem cells. Chin Med J (Engl).
125:4504–4510. 2012.PubMed/NCBI
|
|
3
|
Li T, Xia M, Gao Y, Chen Y and Xu Y: Human
umbilical cord mesenchymal stem cells: An overview of their
potential in cell-based therapy. Expert Opin Biol Ther.
15:1293–1306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang H, Xie Z, Wei Z, Yang H, Yang S, Zhu
Z, Wang P, Zhao C and Bi J: Human umbilical cord mesenchymal stem
cell-derived neuron-like cells rescue memory deficits and reduce
amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem
Cell Res Ther. 4:762013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui B, Li E, Yang B and Wang B: Human
umbilical cord blood-derived mesenchymal stem cell transplantation
for the treatment of spinal cord injury. Exp Ther Med. 7:1233–1236.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santos Nascimento D, Mosqueira D, Sousa
LM, Teixeira M, Filipe M, Resende TP, Araújo AF, Valente M, Almeida
J, Martins JP, et al: Human umbilical cord tissue-derived
mesenchymal stromal cells attenuate remodeling after myocardial
infarction by proangiogenic, antiapoptotic, and endogenous
cell-activation mechanisms. Stem Cell Res Ther. 5:52014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang G, Li Y, Wang Y, Dong Y, Wang FS,
Ding Y, Kang Y and Xu X: Roles of the co-culture of human umbilical
cord Wharton's jelly-derived mesenchymal stem cells with rat
pancreatic cells in the treatment of rats with diabetes mellitus.
Exp Ther Med. 8:1389–1396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye
S, Peng X, Li W and Xu W: Hepatocyte growth factor modification
promotes the amelioration effects of human umbilical cord
mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev.
20:103–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Min F, Gao F, Li Q and Liu Z: Therapeutic
effect of human umbilical cord mesenchymal stem cells modified by
angiotensin-converting enzyme 2 gene on bleomycin-induced lung
fibrosis injury. Mol Med Rep. 11:2387–2396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee S, Choi E, Cha MJ and Hwang KC: Cell
adhesion and long-term survival of transplanted mesenchymal stem
cells: A prerequisite for cell therapy. Oid Med Cell Longev.
2015:6329022015.
|
|
11
|
Tsutsumi S, Shimazu A, Miyazaki K, Pan H,
Koike C, Yoshida E, Takagishi K and Kato Y: Retention of
multilineage differentiation potential of mesenchymal cells during
proliferation in response to FGF. Biochem Biophys Res Commun.
288:413–419. 2011. View Article : Google Scholar
|
|
12
|
Chieregato K, Castegnaro S, Madeo D,
Astori G, Pegoraro M and Rodeghiero F: Epidermal growth factor,
basic fibroblast growth factor and platelet-derived growth
factor-bb can substitute for fetal bovine serum and compete with
human platelet-rich plasma in the ex vivo expansion of mesenchymal
stromal cells derived from adipose tissue. Cytotherapy. 13:933–943.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huat TJ, Khan AA, Pati S, Mustafa Z,
Abdullah JM and Jaafar H: IGF-1 enhances cell proliferation and
survival during early differentiation of mesenchymal stem cells to
neural progenitor-like cells. BMC Neurosi. 15:912014. View Article : Google Scholar
|
|
14
|
Bertrand FE, Steelman LS, Chappell WH,
Abrams SL, Shelton JG, White ER, Ludwig DL and McCubrey JA: Synergy
between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR
pathway inhibitors in suppressing IGF-1R-mediated growth in
hematopoietic cells. Leukemia. 20:1254–1260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lau MT and Leung PC: The PI3K/Akt/mTOR
signaling pathway mediates insulin-like growth factor 1-induced
E-cadherin down-regulation and cell proliferation in ovarian cancer
cells. Cancer Lett. 326:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao Y, Zhou X, Liang C, Li H, Han B, Li F
and Chen Q: TGF-β3 and IGF-1 synergy ameliorates nucleus pulposus
mesenchymal stem cell differentiation towards the nucleus pulposus
cell type through MAPK/ERK signaling. Growth Factors. 33:326–336.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Wei A, Liu Y, He G, Zhou Z and Yu
Z: IGF-1 protects retinal ganglion cells from hypoxia-induced
apoptosis by activating the Erk-1/2 and Akt pathways. Mol Vis.
19:1901–1912. 2013.PubMed/NCBI
|
|
18
|
Zhu SF, He YL and Fu XF: Biological
features and ultrastructure of human umbilical cord mesenchymal
stem cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 33:382–386.
2011.(In Chinese). PubMed/NCBI
|
|
19
|
Yamahara K, Harada K, Ohshima M, Ishikane
S, Ohnishi S, Tsuda H, Otani K, Taguchi A, Soma T, Ogawa H, et al:
Comparison of angiogenic, cytoprotective, and immunosuppressive
properties of human amnion- and chorion-derived mesenchymal stem
cells. PLoS One. 9:e883192014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shalaby RH, Rashed LA, Ismaail AE, Madkour
NK and Elwakeel SH: Hematopoietic stem cells derived from human
umbilical cord ameliorate cisplatin-induced acute renal failure in
rats. Am J Stem Cells. 3:83–96. 2014.PubMed/NCBI
|
|
21
|
Imberti B, Morigi M, Tomasoni S, Rota C,
Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J,
et al: Insulin-like growth factor-1 sustains stem cell mediated
renal repair. J Am Soc Nephrol. 18:2921–2928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morigi M, Introna M, Imberti B, Corna D,
Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, et al:
Human bone marrow mesenchymal stem cells accelerate recovery of
acute renal injury and prolong survival in mice. Stem Cells.
26:2075–2082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Granero-Moltó F, Myers TJ, Weis JA,
Longobardi L, Li T, Yan Y, Case N, Rubin J and Spagnoli A:
Mesenchymal stem cells expressing insulin-like growth factor-I
(MSCIGF) promote fracture healing and restore new bone formation in
Irs1 knockout mice: Analyses of MSCIGF autocrine and paracrine
regenerative effects. Stem Cells. 29:1537–1548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang F, Hong Y, Liang W, Ren T, Jing S
and Lin J: Co-culture with Sertoli cells promotes proliferation and
migration of umbilical cord mesenchymal stem cells. Biochem Biophys
Res Commun. 427:86–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang G, Gurtu V, Kain SR and Yan G: Early
detection of apoptosis using a fluorescent conjugate of annexin V.
Biotechniques. 23:525–531. 1997.PubMed/NCBI
|
|
26
|
Wang J, Zheng T, Chen X, Song X, Meng X,
Bhatta N, Pan S, Jiang H and Liu L: MDM2 antagonist can inhibit
tumor growth in hepatocellular carcinoma with different types of
p53 in vitro. J Gastroenterol Hepatol. 26:371–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding DC, Chang YH, Shyu WC and Lin SZ:
Human umbilical cord mesenchymal stem cells: A new era for stem
cell therapy. Cell Transplant. 24:339–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ashare A, Nymon AB, Doerschug KC, Morrison
JM, Monick MM and Hunnighake GW: Insulin-like growth factor-1
improves survival in sepsis via enhanced hepatic bacterial
clearance. Am J Respir Crit Care Med. 178:149–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galvan V, Logvinova A, Sperandio S, Ichijo
H and Bredesen DE: Type 1 insulin-like growth factor receptor
(IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1
(ASK1). J Biol Chem. 278:13325–13332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shelton JG, Steelman LS, White ER and
McCubrey JA: Synergy between PI3K/Akt and Raf/MEK/ERK pathways in
IGF-1R mediated cell cycle progression and prevention of apoptosis
in hematopoietic cells. Cell Cycle. 3:372–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang G, Feng CC, Chu SJ, Zhang R, Lu YM,
Zhu JS and Zhang J: Toosendanin inhibits growth and induces
apoptosis in colorectal cancer cells through suppression of
AKT/GSK-3β/β-catenin pathway. Int J Oncol. 47:1767–1774. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park ES, Kang DH, Yang MK, Kang JC, Jang
YC, Park JS, Kim SK and Shin HS: Cordycepin, 3′-deoxyadenosine,
prevents rat hearts from ischemia/reperfusion injury via activation
of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression.
Cardiovasc Toxicol. 14:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Khaidakov M, Ding Z, Dai Y,
Mercanti F and Mehta JL: LOX-1 in the maintenance of cytoskeleton
and proliferation in senescent cardiac fibroblasts. J Mol Cell
Cardiol. 60:184–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chou MM, Masuda-Robens JM and Gupta ML:
Cdc42 promotes G1 progression through p70 S6 kinase-mediated
induction of cyclin E expression. J Biol Chem. 278:35241–35247.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Resnitzky D, Gossen M, Bujard H and Reed
SI: Acceleration of the G1/S phase transition by expression of
cyclins D1 and E with an inducible system. Mol Cell Biol.
14:1669–1679. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gartel AL and Radhakrishnan SK: Lost in
transcription: p21 repression, mechanisms, and consequences. Cancer
Res. 65:3980–3985. 2005. View Article : Google Scholar : PubMed/NCBI
|